Abstract
Helicobacter pylori (H. pylori), a Gram-negative bacterium, is one of the most frequent causes of gastrointestinal infections worldwide. It has been associated as a pathogen for the human body with many systemic diseases, including different eye diseases. We will focus on a specific eye disease called idiopathic central serous chorioretinopathy (ICSCR). This disease is characterized by a serous detachment of the neurosensory retina in the macular region, which affects the vision to different degrees. Currently, the pathophysiology of ICSCR is not clear and there is no effective treatment. However, several potential risk factors have been elucidated. One of the factors that has more frequently been associated with ICSCR is stress. As H. pylori was identified as a possible etiological factor for occlusive arterial diseases in young people who were particularly stressed, it was thought that H. pylori might also be present in ICSCR. Therefore, some physicians started to test its presence in patents with ICSCR. If H. pylori happened to be associated with ICSCR, the treatment of gastrointestinal infection could also improve visual symptoms and help to remediate this eye disease. Although H. pylori is highly prevalent in the general population, a true correlation seems to exist. We present a review on the relationship between ICSCR and H. pylori.
Keywords: Helicobacter pylori, Idiopathic central serous chorioretinopathy, Retina, Eye disease, Occlusive arterial disease
Core tip: Helicobacter pylori (H. pylori) has been associated with many systemic diseases. We focus on a specific eye disease called idiopathic central serous chorioretinopathy (ICSCR), which is characterized by a serous detachment of the neurosensory retina in the macular region and affects vision to different degrees. One factor frequently associated with ICSCR is stress. As H. pylori was identified as a possible etiological factor for occlusive arterial diseases in young people who were particularly stressed, it was thought that H. pylori might also be present in ICSCR. We present a review on the relationship between ICSCR and H. pylori.
INTRODUCTION
Helicobacter pylori (H. pylori), a Gram-negative bacterium, is one of the most frequent causes of gastrointestinal infections worldwide. It has been associated as a pathogen for the human body with many systemic diseases, including vascular (atherosclerosis and cardiovascular diseases, Raynaud’s syndrome, primary headache), autoimmune (Sjögren syndrome, autoimmune thyroiditis, idiopathic arrythmias, Parkinson’s disease, nonarterial anterior optic ischemic neuropathy) and skin diseases (urticaria, rosacea), iron deficiency anemia, growth retardation, late menarche, extra-gastric MALT lymphoma, duodenal ulcer, gastric cancer, gastro-oesophageal reflux disease, diabetes mellitus, hepatic encephalopathy, sudden infant death syndrome, and anorexia of aging[1-4].
H. pylori has also been associated with eye diseases such as Sjögren syndrome, blepharitis, glaucoma, uveitis and idiopathic central serous chorioretinopathy (ICSCR)[5-9].
Our review focuses on the relation between ICSCR and H. pylori. ICSCR was first described by von Graefe in 1886[10]. ICSCR affects middle-aged adults (between 25-45 years old), predominantly men, and is characterized by a serous detachment of the neurosensory retina in the macular region. It is usually unilateral (90% of the patients). Patients may develop metamorphopsia, central positive scotoma, micropsia, and impaired color vision. Additional retinal findings include retinal pigment epithelium (RPE) detachment, RPE atrophic tracks, capillary telangiectasia, retinal or choroidal neovascularisation, and intraretinal deposits[11-13], which may be visualized with fluorescein and indocyanine green angiography, and optical coherence tomography (OCT). (Figures 1 and 2).
Figure 1.
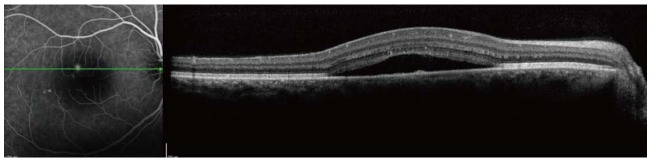
Optical coherence tomography image showing separation of the sensory retina from the retinal pigment epithelium.
Figure 2.
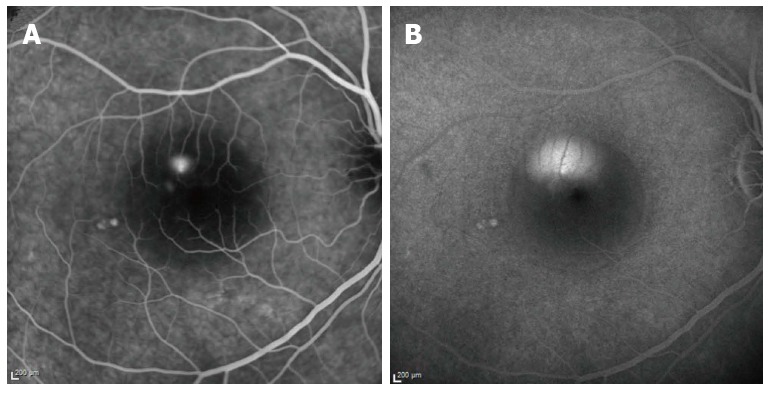
Fluorescein angiography at 2 (A) and 20 (B) min. A: The early phase shows a hyperfluorescent spot due to leakage of dye through the RPE; B: During the late venous phase, fluorescein passes into the subretinal space and spreads until the entire area is filled with dye.
Most of the cases spontaneously resolve with recovery of good visual function. However, recurrences have been observed in 50% or more of the cases[14]. A small percentage of subjects experience chronic decompensation of the RPE and develop severe vision loss.
The pathophysiology of ICSCR is poorly understood. It is thought that damage to the RPE active fluid transport mechanisms that usually dehydrate the subretinal space may play a role[14]. Cigarette smoking, systemic hypertension, pregnancy, allergic respiratory disease, antibiotic or alcohol ingestion[15], sildenafil citrate[16] or systemic corticosteroids[17], sympathomimetic agents[18], antiphospholipid antibodies[19], retinitis pigmentosa[20], psoriasis[21], and endogenous mineralcorticoid dysfunction[17] have been cited as potential risk factors for this disease. ICSCR has also been reported in patients with a benign tumor of the adrenal gland[22], cryoglobulinemia[23], systemic lupus erythematosus[24], or after bone marrow transplantation[25]; and has been strongly associated with individuals with type A personality[26].
Currently, there is no effective treatment for ICSCR. Photodynamic therapy with verteporfin has been used in the last few years. Although it decreases serous detachment and improves visual acuity, it results in scotomas in some patients. A new treatment has recently been proposed based on oral eplerenone. Experimental data has shown that central chorioretinopathy could result from an overactivity of the mineralcorticoid receptor pathway in choroid vessels. Eplerenone is a mineralcorticoid receptor antagonist and has therefore been considered as a potential treatment for ICSCR. Randomized controlled trials are needed to confirm if this therapy could help in the treatment of ICSCR[17].
DISCUSSION
HP was first associated with ICSCR in 2000. A French team (Mauget-Faÿse et al[7]) presented their first results on a poster at the Association for Research in Vision and Ophthalmology (ARVO) congress. Knowing that H. pylori was identified as a possible etiological factor for occlusive arterial diseases in young people who were particularly stressed[27], the authors of this study decided to test the presence of HP infection in ICSCR patients. As occlusive arterial disease shared some characteristics with ICSCR (i.e., associated with type A personality and ischemia), it was believed that HP infection might be a common factor.
This prospective pilot study of 16 patients affected by active ICSCR or by its variant, diffuse retinal epitheliopathy, found that the prevalence of H. pylori infection; determined by one of more of the following methods: histology of gastric biopsy specimens, C-urea breath test, or serology test (Boehringer Mannhein test); was significantly higher in subjects with ICSCR[7]. A complementary study including more patients and confirming the results was published in 2004[28]. A few months earlier, a case report of a 43-year-old man suggested that ICSCR recurrences were associated with the presence of H. pylori. Resolution of ICSCR was correlated with the eradication of the bacterium using the conventional triple-therapy regimen (amoxicillin, clarithromycin, omeprazole)[29].
Some further studies confirmed this relationship. A Spanish team observed that 68.75% of ICSCR patients were infected with H. pylori, compared with 30% of the control population[30]. Recently, Casella et al[18] suggested that chronic ICSCR patients could be infected with H. pylori and that the treatment of the infection could have a positive impact on the outcome of chronic ICSCR regarding the improvement of final best-corrected visual acuity and resolution of the serous detachment. Lastly, Dang et al[17] reported that, although H. pylori eradication does not increase visual acuity and does not diminish subretinal fluid, it could benefit central retinal sensitivity in ICSCR patients. A statistical difference was observed in central retinal sensitivity at 3 mo after HP eradication therapy. Macular sensitivity was measured using microperimeter-1 (Nidek, Vigonza, Italy) after pupil dilatation and not with contrast sensitivity charts. Thirty-three stimulus points located in the area of the central 15° diameter around the macula were examined. The average sensitivity of the 33 points was defined as the central retinal sensitivity[17].
It is difficult to determine the potential role of H. pylori in the pathogenesis of ICSCR. Giusti elucidated several hypotheses regarding pathogenesis[31]. A possible explanation might be the link between H. pylori infection and atherosclerosis. A cross reactivity of anti-Cag A antibodies, whose presence is more frequently associated in atherosclerosis, and the presence of immunoglobulin-G (Ig-G) antibody have been considered as risk factors for endothelial dysfunction[32]. Another mechanism is the role of heat shock proteins expressed by several pathogens, e.g., H. pylori. It has been hypothesized that an immune response against antigens located on pathogenic organisms would cross-react with homologous host proteins, e.g., with the endothelial vascular wall[33].
Further implications of H. pylori infections have lately been proposed: increase of lipids and fibrinogen levels[32], upregulation of endothelial adhesion molecules and increase of polymorphonuclear leucocyte adhesion[34], and increase of platelet activation and aggregation[35].
CONCLUSION
Several studies indicate that many ICSCR patients could be infected with H. pylori and that the treatment of the infection could have a positive impact on the outcome of the disease. Due to the high prevalence of H. pylori infection in the general population, it is difficult to establish a true correlation. Prospective and masked clinical trials are necessary to confirm the relationship between ICSCR and H. pylori, as well as the benefits to ICSCR patients from receiving H. pylori treatment.
Footnotes
P- Reviewer: Velez-Montoya R S- Editor: Wen LL L- Editor: A E- Editor: Lu YJ
References
- 1.De Koster E, De Bruyne I, Langlet P, Deltenre M. Evidence based medicine and extradigestive manifestations of Helicobacter pylori. Acta Gastroenterol Belg. 2000;63:388–392. [PubMed] [Google Scholar]
- 2.Carloni E, Cremonini F, Di Caro S, Padalino C, Gerardino L, Santoliquido A, Colasanti S, Pola P, Gasbarrini A. Helicobacter pylori-related extradigestive diseases and effects of eradication therapy. Dig Liver Dis. 2000;32 Suppl 3:S214–S216. doi: 10.1016/s1590-8658(00)80282-0. [DOI] [PubMed] [Google Scholar]
- 3.Zbinden R. Expanding the spectrum of Helicobacter pylori-associated diseases. Infection. 2005;33:49. doi: 10.1007/s15010-005-7205-3. [DOI] [PubMed] [Google Scholar]
- 4.Roussos A, Philippou N, Gourgoulianis KI. Helicobacter pylori infection and respiratory diseases: a review. World J Gastroenterol. 2003;9:5–8. doi: 10.3748/wjg.v9.i1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Miedany YM, Baddour M, Ahmed I, Fahmy H. Sjogren’s syndrome: concomitant H. pylori infection and possible correlation with clinical parameters. Joint Bone Spine. 2005;72:135–141. doi: 10.1016/j.jbspin.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Saccà SC, Pascotto A, Venturino GM, Prigione G, Mastromarino A, Baldi F, Bilardi C, Savarino V, Brusati C, Rebora A. Prevalence and treatment of Helicobacter pylori in patients with blepharitis. Invest Ophthalmol Vis Sci. 2006;47:501–508. doi: 10.1167/iovs.05-0323. [DOI] [PubMed] [Google Scholar]
- 7.Mauget-Faÿsse M, Kodjikian L, Quaranta M, Ben Ezra D, Trepsat C, Mion F, Mégraud F. [Helicobacter pylori in central serous chorioretinopathy and diffuse retinal epitheliopathy. Results of the first prospective pilot study] J Fr Ophtalmol. 2002;25:1021–1025. [PubMed] [Google Scholar]
- 8.Otasevic L, Zlatanovic G, Stanojevic-Paovic A, Miljkovic-Selimovic B, Dinic M, Djordjevic-Jocic J, Stankovic A. Helicobacter pylori: an underestimated factor in acute anterior uveitis and spondyloarthropathies? Ophthalmologica. 2007;221:6–13. doi: 10.1159/000096515. [DOI] [PubMed] [Google Scholar]
- 9.Izzotti A, Saccà SC, Bagnis A, Recupero SM. Glaucoma and Helicobacter pylori infection: correlations and controversies. Br J Ophthalmol. 2009;93:1420–1427. doi: 10.1136/bjo.2008.150409. [DOI] [PubMed] [Google Scholar]
- 10.Von Graefe A. Ueber centrale rezidivierende keratitis. Albrecht von Graefes Arch Klin Ophthalmol. 1886;12:211. [Google Scholar]
- 11.Iida T, Yannuzzi LA, Spaide RF, Borodoker N, Carvalho CA, Negrao S. Cystoid macular degeneration in chronic central serous chorioretinopathy. Retina. 2003;23:1–7; quiz 137-138. doi: 10.1097/00006982-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Teschner S, Noack J, Birngruber R, Schmidt-Erfurth U. Characterization of leakage activity in exudative chorioretinal disease with three-dimensional confocal angiography. Ophthalmology. 2003;110:687–697. doi: 10.1016/S0161-6420(02)01972-3. [DOI] [PubMed] [Google Scholar]
- 13.Spaide RF. Deposition of yellow submacular material in central serous chorioretinopathy resembling adult-onset foveomacular vitelliform dystrophy. Retina. 2004;24:301–304. doi: 10.1097/00006982-200404000-00019. [DOI] [PubMed] [Google Scholar]
- 14.Albert DM, Jakobiec FA. Principles and practice of ophthalmology. Philadelpia: Saunders; 1994. [Google Scholar]
- 15.Haimovici R, Koh S, Gagnon DR, Lehrfeld T, Wellik S. Risk factors for central serous chorioretinopathy: a case-control study. Ophthalmology. 2004;111:244–249. doi: 10.1016/j.ophtha.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 16.Allibhai ZA, Gale JS, Sheidow TS. Central serous chorioretinopathy in a patient taking sildenafil citrate. Ophthalmic Surg Lasers Imaging. 2004;35:165–167. [PubMed] [Google Scholar]
- 17.Dang Y, Mu Y, Zhao M, Li L, Guo Y, Zhu Y. The effect of eradicating Helicobacter pylori on idiopathic central serous chorioretinopathy patients. Ther Clin Risk Manag. 2013;9:355–360. doi: 10.2147/TCRM.S50407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casella AM, Berbel RF, Bressanim GL, Malaguido MR, Cardillo JA. Helicobacter pylori as a potential target for the treatment of central serous chorioretinopathy. Clinics (Sao Paulo) 2012;67:1047–1052. doi: 10.6061/clinics/2012(09)11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Costen MT, Parkin BT, Davison CR, Crick MP. Central serous chorioretinopathy and antiphospholipid antibodies--results of a pilot study. Eye (Lond) 2004;18:938. doi: 10.1038/sj.eye.6701348. [DOI] [PubMed] [Google Scholar]
- 20.Dorenboim Y, Rehany U, Rumelt S. Central serous chorioretinopathy associated with retinitis pigmentosa. Graefes Arch Clin Exp Ophthalmol. 2004;242:346–349. doi: 10.1007/s00417-003-0819-1. [DOI] [PubMed] [Google Scholar]
- 21.Nucci C, Corsi A, Mancino R, Macri G. Central serous chorioretinopathy in patients with psoriasis. Acta Ophthalmol Scand. 2004;82:105–107. doi: 10.1111/j.1600-0420.2004.00189c.x. [DOI] [PubMed] [Google Scholar]
- 22.Katsimpris JM, Vandoros M, Petropoulos IK, Andrikopoulos P. Central serous chorioretinopathy associated with adrenal myelolipoma. Klin Monbl Augenheilkd. 2003;220:199–203. doi: 10.1055/s-2003-38187. [DOI] [PubMed] [Google Scholar]
- 23.Zamir E, Chowers I. Central serous chorioretinopathy in a patient with cryoglobulinaemia. Eye (Lond) 1999;13(Pt 2):265–266. doi: 10.1038/eye.1999.67. [DOI] [PubMed] [Google Scholar]
- 24.Cunningham ET, Alfred PR, Irvine AR. Central serous chorioretinopathy in patients with systemic lupus erythematosus. Ophthalmology. 1996;103:2081–2090. doi: 10.1016/s0161-6420(96)30385-0. [DOI] [PubMed] [Google Scholar]
- 25.Karashima K, Fujioka S, Harino S. Two cases of central serous chorioretinopathy treated with photocoagulation after bone marrow transplantation. Retina. 2002;22:651–653. doi: 10.1097/00006982-200210000-00022. [DOI] [PubMed] [Google Scholar]
- 26.Spahn C, Wiek J, Burger T, Hansen L. Psychosomatic aspects in patients with central serous chorioretinopathy. Br J Ophthalmol. 2003;87:704–708. doi: 10.1136/bjo.87.6.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Libby P, Egan D, Skarlatos S. Roles of infectious agents in atherosclerosis and restenosis: an assessment of the evidence and need for future research. Circulation. 1997;96:4095–4103. doi: 10.1161/01.cir.96.11.4095. [DOI] [PubMed] [Google Scholar]
- 28.Ahnoux-Zabsonre A, Quaranta M, Mauget-Faÿsse M. [Prevalence of Helicobacter pylori in central serous chorioretinopathy and diffuse retinal epitheliopathy: a complementary study] J Fr Ophtalmol. 2004;27:1129–1133. doi: 10.1016/s0181-5512(04)96281-x. [DOI] [PubMed] [Google Scholar]
- 29.Giusti C. [Central serous chorioretinopathy: a new extragastric manifestation of Helicobacter pylori?: Analysis of a clinical case] Clin Ter. 2001;152:393–397. [PubMed] [Google Scholar]
- 30.Asensio-Sánchez VM, Rodríguez-Delgado B, García-Herrero E, Cabo-Vaquera V, García-Loygorri C. [Central serous chorioretinopathy as an extradigestive manifestation of Helicobacter pylori gastric infection] Arch Soc Esp Oftalmol. 2008;83:177–182. [PubMed] [Google Scholar]
- 31.Giusti C. Association of Helicobacter pylori with central serous chorioretinopathy: hypotheses regarding pathogenesis. Med Hypotheses. 2004;63:524–527. doi: 10.1016/j.mehy.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 32.Franceschi F, Sepulveda AR, Gasbarrini A, Pola P, Silveri NG, Gasbarrini G, Graham DY, Genta RM. Cross-reactivity of anti-CagA antibodies with vascular wall antigens: possible pathogenic link between Helicobacter pylori infection and atherosclerosis. Circulation. 2002;106:430–434. doi: 10.1161/01.cir.0000024100.90140.19. [DOI] [PubMed] [Google Scholar]
- 33.Lamb DJ, El-Sankary W, Ferns GA. Molecular mimicry in atherosclerosis: a role for heat shock proteins in immunisation. Atherosclerosis. 2003;167:177–185. doi: 10.1016/s0021-9150(02)00301-5. [DOI] [PubMed] [Google Scholar]
- 34.Byrne MF, Corcoran PA, Atherton JC, Sheehan KM, Murray FE, Fitzgerald DJ, Murphy JF. Stimulation of adhesion molecule expression by Helicobacter pylori and increased neutrophil adhesion to human umbilical vein endothelial cells. FEBS Lett. 2002;532:411–414. doi: 10.1016/s0014-5793(02)03728-6. [DOI] [PubMed] [Google Scholar]
- 35.Byrne MF, Kerrigan SW, Corcoran PA, Atherton JC, Murray FE, Fitzgerald DJ, Cox DM. Helicobacter pylori binds von Willebrand factor and interacts with GPIb to induce platelet aggregation. Gastroenterology. 2003;124:1846–1854. doi: 10.1016/s0016-5085(03)00397-4. [DOI] [PubMed] [Google Scholar]


