Abstract
AIM: To demonstrated the combined effects of aging and carcinogen treatment on cancer stem/stem-like cells (CSCs) of gastric mucosa in an animal model.
METHODS: In this study we investigated the effects of aging and Helicobacter pylori (H. pylori) inflammation as a model for inflammation induced carcinogenesis in human and rat gastric mucosa samples. In aging studies, we compared 4-mo old (young) with 22 mo (aged) old Fischer-344 rats. For human studies, gastric biopsies and resection specimens representing normal mucosa or different stages of H. pylori gastritis and gastric adenocarcinomas were used for determining the expression of stem cell markers CD166, ALDH1 and LGR5. In addition we performed immunofluorescent double labeling for B-catenin and Lgr5 in both rat and human gastric tissues to examine the status of Wnt signaling in these cells.
RESULTS: CSC markers ALDH1, LGR5, and CD166 were expressed in very low levels in normal human gastric mucosa or young rat gastric mucosa. In contrast, level of expression for all three markers significantly increased in H. pylori gastritis and gastric adenocarcinomas as well as in normal gastric mucosa in aged rats. We also observed cytoplasmic B-catenin staining in both aged rat and human H. pylori inflamed gastric mucosa, which were found to be colocalized with Lgr5 immunoreactive cells. The increased number of ALDH1, CD166 and LGR5 positive cells in H. pylori gastritis indicates that increased number of stem-like cells in gastric mucosa is an early event, and may constitute an important step in the progression to neoplasia.
CONCLUSION: Our observation of the age-related increase in cancer stem/stem-like cells in the gastric mucosa may explain the increased incidence of gastric cancer during aging. Combination of aging and H. pylori infection may have additive effects in progression to neoplasia.
Keywords: Cancer stem cells, Aging, CD166, ALDH1, LGR5, Gastric cancer, Helicobacter pylori
Core tip: In this study we demonstrated an age-related increase in cancer stem/stem-like cells (CSCs) in normal appearing gastric mucosa with activated Wnt signaling. In addition, we have shown that gastric infection by Helicobacter pylori (H. pylori) induces an increase in CSC population in the gastric mucosa. Based on our observations we believe that aging and chronic inflammation with H. pylori are two significant factors that overlap and presumably exacerbate each other in gastric carcinogenesis.
INTRODUCTION
It has been well established that the incidences of cancer rise sharply with age and the majority of cancer cases are detected in patients over the age of 65 years[1]. Such a direct correlation between cancer incidence and advanced age in most cancers clearly suggests that the phenomenon of aging and cancer are intricately connected. Accumulating evidence also suggests that the increase in tumor incidence with advancing age is preceded in part by chronic disorders including inflammation[1,2]. The etiological causes of inflammation are many folds and include viruses, bacteria, environmental pollutants, and stress as well as food factors. Chronic inflammation as risk factor for most cancers is well recognized[2].
Aging and chronic inflammation are two factors associated with an increased risk for gastric cancer[1,2]. Within the gastrointestinal tract, inflammatory conditions such as gastroesophageal reflux disease, Helicobacter pylori infection (H. pylori), inflammatory bowel disease, and viral hepatitis are well known to be associated with cancers[1,2]. The possible explanations for the link between cancer and inflammation are accumulating gene mutations, inhibition of apoptosis, increased cell proliferation and pro-inflammatory cytokine release which creates a pro-carcinogenic microenvironment[1,2].
A growing body of evidence supports the contention that cancers, including the gastric cancer are diseases driven by a small set of self renewing cells, termed cancer stem cells (CSC) or cancer-initiating cells, that are distinct from the bulk of the cells in the tumor[3-9]. CSCs are widely believed to arise from the normal stem cells or progenitor cells upon mutations[7].
The putative progenitor/stem cell in the stomach is thought to reside in the isthmic region of the fundic epithelium[6,7,10]. In mice, granule free cells in the isthmus have been shown to act as stem cells[11].
The gastric progenitor/stem cells accomodate to acute and chronic injury to the gastric epithelium and replenish the destroyed epithelium during the lifetime of the organism of interest, which creates the risk of accumulating mutations and giving rise to gastric cancers[7,8,12]. H. pylori gastritis which is a known preneoplastic condition, is a good model to study the response of stem cells to chronic injury and mutagenesis[13-15]. A recent study has shown a direct interaction between H. pylori organisms and gastric stem cells[15].
We have recently demonstrated the combined effects of aging and carcinogen treatment on the colon CSCs in a rat model[16,17]. In this model, carcinogen treated rats had more dramatic increase in CSCs if they were also aged. Based on these and other relevant observations[1,9,17-21], we hypothesize that, aging and chronic inflammation are two parallel events leading to an increased incidence of cancers in the gastrointestinal tract, including colon and gastric cancers. We further hypothesize that the initiating factor in this scenario is the alteration of the CSC population in the normal appearing mucosa.
To test our hypothesis that combination of the effects of aging and inflammation on CSCs exacerbates cancer development, we made an attempt to identify gastric CSCs by using immunohistochemical (IHC) markers in young and old rat gastric mucosa samples. We then expanded our studies to human gastric mucosa with various degrees of H. pylori induced inflammation in order to show the alterations in CSC compartment during the course of H. pylori- induced disease.
MATERIALS AND METHODS
Animals
Male Fischer-344 rats, aged 4-6 (young) or 22-24 mo (old) were purchased from the National Institute on Aging (Bethesda, MD). All procedures were performed according to the standards for use of laboratory animals established by the Institute of Laboratory Animal Resources, National academy of Sciences, and were approved by the Animal Investigation Committee at Wayne State University School of Medicine. The details of animal handling have been previously published[16,20].
Human gastric tissues
Formalin fixed-paraffin embedded gastric tissue samples representing normal/uninfected mucosa (n = 10), helicobacter pylori gastritis (n = 12), helicobacter pylori gastritis with intestinal metaplasia (n = 10), dysplasia (n = 6) and gastric cancer (n = 12) were retrieved from the Pathology archives of John D. Dingell VA Medical Center, Detroit MI. The diagnoses were confirmed by three pathologists who are co-authors of this study. The study was approved by the IRB committee of Wayne State University, and the R&D committee of John D. Dingell VA Medical Center.
The mean age of the patients was 46 ± 6 (SD). They were all male, reflecting the population profile of the hospital. The difference of age between the control and the inflamed mucosa samples was not statistically significant (not shown).
Immunohistochemistry
The antibodies utilized for immunohistochemical stains were LGR5 (dilution at 1:200, ABGENT, San Diego CA), CD166 (dilution at 1:200 RD systems, Minneapolis MN), ALDH1 (dilution at 1:100 BD Biosciences, San Jose CA) and B-catenin (SCBT, Dallas TX at 1:100 dilution).
Immunohistochemistry was performed according to our standard protocol[8,13,19]. Briefly, the paraffin blocks of the fixed colon tissues were cut into 5 μm sections. The slides were deparaffinized. For antigen retrieval, tissues were microwaved for 15 min in Citrate pH = 6.0 buffer, then allowed to cool to room temperature. Endogenous peroxide was quenched by incubation of the sections with 3% hydrogen peroxide. Non specific binding was blocked application of 5% horse serum. Primary antibodies were applied overnight at 4 °C and antibody detection was completed utilizing the Vecstatin Elite ABC system detection kit from Vector (Burlingame CA). AEC was used as chromogen.
We defined positivity in normal and H. pylori cases as membranous and cytoplasmic staining in number of cells per gland (CPG). For cancer cases we used percentage of tumor cells to define positivity.
Immunofluorescence double labeling of B-catenin and LGR5
We have performed double labeling for B-catenin and Lgr5 on sections from rat gastric mucosa and human gastric epithelium by using immunofluorescent secondary antibodies to demonstrate the co-expression of these markers. For B-catenin primary antibody (Santa Cruz BT) anti-mouse IgG TRITC (Sigma, St Louis MO) secondary antibody was used. For LGR5 (ABGENT) antibody, anti-rabbit IgG FITC (Sigma) antibody was used. The slides were evaluated by a fluorescent microscope with green and red filters. Gastric cancer specimens were used as positive controls. For negative controls, we omitted the primary antibody, and applied only secondary antibody.
Statistical analysis
For statistical analysis we assessed the CSC expression as low vs high expression, with 0 and 1-2 CSC considered as low expression and ≥ 3-4 CSC as high expression. This cut off value was based on the observation that in normal mucosa we rarely encountered more than 1-2 CSC per gland counted. Statistical significance was assessed by χ2 test.
RESULTS
Rat gastric mucosa
In the 4 mo old (young) rats ALDH1, CD166 and LGR5 staining were rare events. Very few cells in the isthmic region of the fundic mucosa demonstrated cytoplasmic staining for the three markers (Figure 1). The results are summarized in Table 1.
Figure 1.
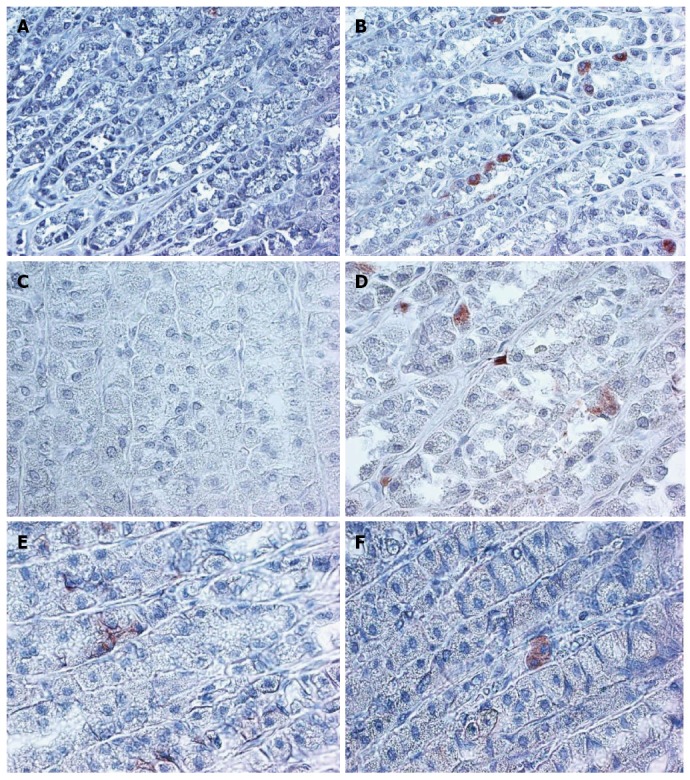
Higher expression of B-catenin, CD166, and LGR5 in normal aged rat gastric mucosa, compared to young rat normal gastric mucosa is demonstrated. × 200 magnification. A: 4 m CD166; B: 24 m CD166; C: 4 m LGR5; D: 24 m LGR5; E: 4 m B-catenin; F: 24 m B-catenin.
Table 1.
Staining scores for stem cell markers in human gastric mucosa and rat gastric mucosa
| n | ALDH | CD166 | LGR5 | |
| Normal | 10 | 1-2 | 0 | 1-2 |
| H. pylori without IM | 12 | 5-6a | 3-4a | 3-4a |
| H. pylori with IM | 10 | 7-8a | 5-6a | 5-6a |
| Gastric adenocarcinoma | 12 | 85% | 75% | 70% |
| 4-mo-old rat | 6 | 1-2 | 1-2 | 0 |
| 24-mo-old rat | 6 | 3-4 | 5-6a | 3-4a |
The numbers are cells expressing the marker per a crypt counted. We grouped the cell counts as 0; 1-2; 3-4; 5-6; 7-8; etc. For evaluation of gastric adenocarcinoma, we expressed the percentage of cells staining with the related marker.
P < 0.05 vs normal human gastric mucosa or 4-mo-old rat gastric mucosa. Gastric adenocarcinoma was not included in statistical analysis.
The expression of LGR5 and CD166 was significantly increased in the 24 mo old rats (Figure 1 and Table 1). The staining location was still in the isthmus region. The B-catenin staining was limited to few stem cells and was membranous in the young rat mucosa and cytoplasmic in the aged mucosa (Figure 1).
B-catenin LGR5 double labeling
We also investigated the expression of B-catenin and LGR5 in a double immunofluorescent labeling study. B-catenin is normally expressed in the cell membrane in the inactive state. Activated B-catenin pathway can be detected by nuclear or cytoplasmic staining. We therefore investigated the status of B-catenin signaling in the LGR5 expressing putative stem cells. As shown in Figure 2A, a rare cell with LGR5 expression (green signal) also revealed cytoplasmic B-catenin immunoreactivity (red signal).
Figure 2.
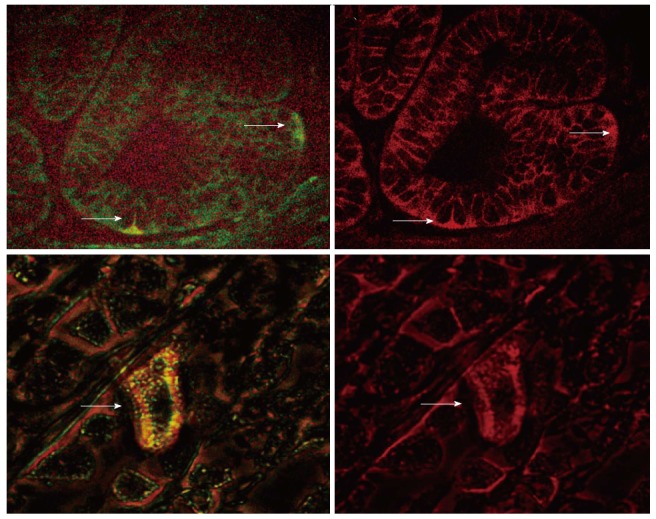
Double labeling for LGR5 (green, left panel) and B-catenin (red, right panel) in aged rat gastric mucosa (Lower Panel, × 600 magnification), H. pylori infected human gastric mucosa (Upper Panel, × 400 magnification) shows cytoplasmic localization of B-catenin in an LGR5 expressing cell.
Human gastric mucosa
We first investigated the expression of CSC markers in normal mucosa which included both antral and fundic mucosa. The staining of the cells was localized to the isthmic region of the fundic mucosa (Figure 3). In antrum, the staining was present in the base of the glands.
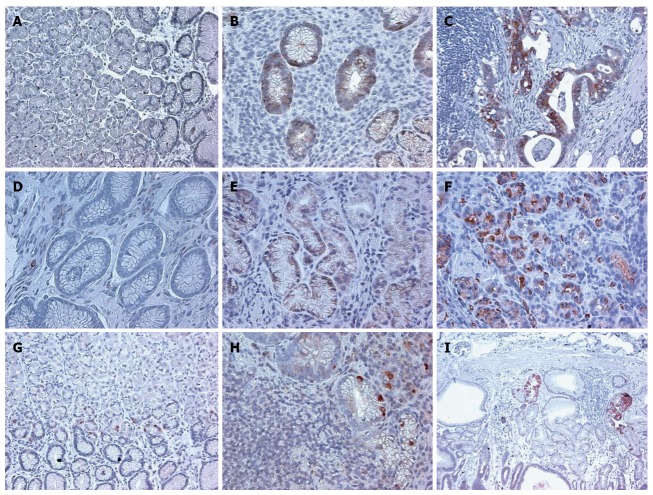
Figure 3.
Immunohistochemical staining of stem cell markers ALDH1, CD166, LGR5 in human normal gastric mucosa, gastric mucosa with H. pylori gastritis, and gastric adenocarcinoma, demonstrates increased expression of each of the markers over the normal controls. × 200 magnification. A: ALDH1 Normal; B: ALDH1 HP; C: ALDH1 CA; D: CD166 Normal; E: CD166 HP; F: CD166 CA; G: LGR5 Normal; H: LGR5 HP; I: LGR5 CA.
In H. pylori infected gastric mucosa, the expression of CSC markers was significantly increased for all three markers examined (Table 1 and Figure 3).
In the H. pylori infected gastric mucosa with intestinal metaplasia and also in gastric cancer, the level of staining was also significantly increased (Table 1).
In addition, we performed a double labeling immunofluorescent staining for LGR5 and B-catenin in H. pylori infected gastric mucosa. We observed that in the LGR5 expressing cells in the gastric mucosa; the expression of B-catenin is cytoplasmic, implying an activation of B-catenin signaling (Figure 2B).
DISCUSSION
In this study we demonstrated an age-related in CSCs in normal appearing gastric mucosa with activated WNT signaling. In addition, we have shown that, gastric infection by H. pylori induces an increase in CSC population in the gastric mucosa. Based on our observations we believe that aging and chronic inflammation with H. pylori are two significant factors that overlap and presumably exacerbate each other in gastric carcinogenesis.
Studies of the murine gastrointestinal tract have shown that cells from old mice at or near the position of the stem cells within the crypts of Lieberkuhn are more susceptible to apoptosis under stress[18] and exhibit reduced regenerative potential despite an age dependent increase in the number of crypt cells. Similar age related decline in functional properties of stem cells have been shown in other tissues particularly in hematopoietic stem cells[9]. It is very likely that the increase in the number of stem cells is a compensatory event to replenish the destroyed cells in the target tissue and is a reflection of the decreased functional capacity of these cells. This situation is analogous to myelodysplastic syndrome, which is commonly present in the elderly and is characterized by a hypercellular bone marrow despite a peripheral cytopenia.
Stem cells are subject to the similar array of insults as somatic cells and are therefore susceptible to genetic damage. The accumulating damage, unlike that of somatic cells is propagated to the daughter cells and to downstream lineages through the process of self renewal and differentiation. The nature of accumulating mutations and genetic damage determines the fate of the CSCs. The outcome could be senescence, apoptosis, or transformation[9].
In this context, the survival pathways utilized by the stem cells are very critical in their maintenance and possibly transformation. The important signaling pathways involved in gastric CSCs survival include the Wnt/B-catenin, sonic hedgehog (shh), Notch, and fibroblastic growth factor/bone morphogenic protein (FGF/BMP) pathways[8,9,12,22].
Wnt signaling pathway releases B-catenin from the AXIN/GSK3b degradation complex. Activation of the Wnt signaling results in the translocation of B-catenin from the cell membrane to the cytoplasm and subsequent translocation to the nucleus. Accumulation of B-catenin in the nucleus results in the transcriptional activation of target genes that play critical roles in regulating cell proliferation[22].
H. pylori infection results in the activation of the stem cell signaling networks such as Wnt, Notch, FGF/BMP, and Hh/SHH oncogenic signaling pathways[2,8,22]. In a previous study, H. pylori infection has been shown to be associated with an increased expression of CD44 in gastric mucosa[10]. We also investigated CD44 in the gastric mucosa and found that CD44 expression is markedly increased in H. pylori infected mucosa (data not shown).
LGR5 is an orphan G-protein coupled receptor and Wnt target gene, and is a putative marker for gastrointestinal stem cells. Barker et al[3] first identified a subpopulation of Lgr5+ stem cells at the base of the crypts in mouse small intestine and colon. Since then, several studies have confirmed the utility of Lgr5 as a putative stem cell/progenitor marker[3-7]. Our studies highlight an Lgr5 positive population in normal human fundic epithelium, localized to the isthmic region, in concordance with the anatomic localization of the progenitor cells. In addition, we demonstrate that Lgr5 expressing cells are increased during aging and in response to H. pylori infection.
Our double labeling studies demonstrate that B-catenin is relocated to cytoplasm in the stem cells in aging and H. pylori infection. Wnt signaling may preferentially influence the expansion of progenitor cells in the gastrointestinal system and may be the driving force behind the early increase in CSCs in the colon and stomach. However, we do not know whether the activation of B-Catenin is entirely normal or an early phase of neoplastic transformation. Since WNT signaling can support both the normal and CSCs renewal and maintenance, it is a potential target for therapeutic interventions or preventative measures.
Our current findings are very similar to our previous data from colonic mucosa of aged and carcinogen exposed rats[14,16]. We propose that in gastrointestinal cancers, aging and chronic inflammation leading to cytokine activation is a critical factor. The increase in CSCs is probably one of the early events in this process. Further studies are needed to directly observe the link between increase in CSCs and acquisition of cancer phenotype.
The finding of increase in CSCs in otherwise normal appearing mucosa has ramifications for diagnostic, prognostic, preventive, and therapeutic approaches to the gastrointestinal cancers. CSC markers can be used in gastric biopsies from patients with atrophic gastritis and intestinal metaplasia to see the status of CSC population. This can be used as a surrogate for increased risk for cancer. In addition, targeted therapies can be designed to specifically attack the stem cell population in cancers, and response to treatment can be monitored by observing the changes in the stem cell population.
COMMENTS
Background
Aging and chronic inflammation are two factors associated with gastric cancer. There is evidence suggesting a link between stem cells in gastric mucosa and increased risk for cancer. Aging and chronic inflammation may cause alterations in stem cells thus causing cancer.
Research frontiers
Cancer stem cells can be detected by using specific markers and demonstrated by immunohistochemistry. This approach allows the authors to demonstrate changes in cancer stem cells associated with aging and inflammation.
Innovations and breakthroughs
In this study the authors demonstrated that aging and chronic inflammation are associated with an increased stem cell population in gastric mucosa.
Applications
Gastric cancer stem cell markers can be utilized as prognostic markers or can be used to monitor response to treatment. They can also help the authors understand tumor pathobiology.
Terminology
Cancer stem cells are thought to be normal resident stem cells or specialized cells which acquire cancer initiating properties. Cancer stem cell hypothesis assumes that cancers arise by alterations in cancer initiating subpopulations of cells in a given tissue.
Peer review
The reviewers think that the study provides data identifying and quantifying stem cells in gastric mucosa of rats and humans. According to their data the number of stem cells is increased by chronic inflammation and aging.
Footnotes
Supported by Grants to Dr. Majumdar from NIH/NIA, No. AG014343; and the Department of Veterans Affairs (VA Merit Review)
P- Reviewer: Langner C S- Editor: Qi Y L- Editor: A E- Editor: Lu YJ
References
- 1.Majumdar APN, Basson MD. Effect of Aging on the Gastrointestinal Tract. In: Physiology of the Gastrointestinal tract., editor. Johnson LR, Barrett K, Ghishan F, Merchant JI, Said HM, Wood JD, editors. New York: Academic Press; 2006. pp. 405–433. [Google Scholar]
- 2.Quante M, Wang TC. Inflammation and stem cells in gastrointestinal carcinogenesis. Physiology (Bethesda) 2008;23:350–359. doi: 10.1152/physiol.00031.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 4.Boman BM, Huang E. Human colon cancer stem cells: a new paradigm in gastrointestinal oncology. J Clin Oncol. 2008;26:2828–2838. doi: 10.1200/JCO.2008.17.6941. [DOI] [PubMed] [Google Scholar]
- 5.Boman BM, Fields JZ, Cavanaugh KL, Guetter A, Runquist OA. How dysregulated colonic crypt dynamics cause stem cell overpopulation and initiate colon cancer. Cancer Res. 2008;68:3304–3313. doi: 10.1158/0008-5472.CAN-07-2061. [DOI] [PubMed] [Google Scholar]
- 6.Fukuda K, Saikawa Y, Ohashi M, Kumagai K, Kitajima M, Okano H, Matsuzaki Y, Kitagawa Y. Tumor initiating potential of side population cells in human gastric cancer. Int J Oncol. 2009;34:1201–1207. [PubMed] [Google Scholar]
- 7.Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355:1253–1261. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 8.Pilpilidis I, Kountouras J, Zavos C, Katsinelos P. Upper gastrointestinal carcinogenesis: H. pylori and stem cell cross-talk. J Surg Res. 2011;166:255–264. doi: 10.1016/j.jss.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell. 2008;132:681–696. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 10.Takaishi S, Okumura T, Tu S, Wang SS, Shibata W, Vigneshwaran R, Gordon SA, Shimada Y, Wang TC. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells. 2009;27:1006–1020. doi: 10.1002/stem.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. Anat Rec. 1993;236:259–279. doi: 10.1002/ar.1092360202. [DOI] [PubMed] [Google Scholar]
- 12.Saikawa Y, Fukuda K, Takahashi T, Nakamura R, Takeuchi H, Kitagawa Y. Gastric carcinogenesis and the cancer stem cell hypothesis. Gastric Cancer. 2010;13:11–24. doi: 10.1007/s10120-009-0537-4. [DOI] [PubMed] [Google Scholar]
- 13.Kato K, Hasui K, Wang J, Kawano Y, Aikou T, Murata F. Homeostatic mass control in gastric non-neoplastic epithelia under infection of Helicobacter pylori: an immunohistochemical analysis of cell growth, stem cells and programmed cell death. Acta Histochem Cytochem. 2008;41:23–38. doi: 10.1267/ahc.07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDonald SA, Greaves LC, Gutierrez-Gonzalez L, Rodriguez-Justo M, Deheragoda M, Leedham SJ, Taylor RW, Lee CY, Preston SL, Lovell M, et al. Mechanisms of field cancerization in the human stomach: the expansion and spread of mutated gastric stem cells. Gastroenterology. 2008;134:500–510. doi: 10.1053/j.gastro.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 15.Oh JD, Kling-Bäckhed H, Giannakis M, Engstrand LG, Gordon JI. Interactions between gastric epithelial stem cells and Helicobacter pylori in the setting of chronic atrophic gastritis. Curr Opin Microbiol. 2006;9:21–27. doi: 10.1016/j.mib.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 16.Levi E, Misra S, Du J, Patel BB, Majumdar AP. Combination of aging and dimethylhydrazine treatment causes an increase in cancer-stem cell population of rat colonic crypts. Biochem Biophys Res Commun. 2009;385:430–433. doi: 10.1016/j.bbrc.2009.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nautiyal J, Du J, Yu Y, Kanwar SS, Levi E, Majumdar APN: EGFR regulation of colon cancer stem-like cells during aging and in response to the colonic carcinogen dimethylhydrazine. Am J Physiol Gastrointest Liver Physiol. 2012;302:G655–63. doi: 10.1152/ajpgi.00323.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin K, Potten CS, Roberts SA, Kirkwood TB. Altered stem cell regeneration in irradiated intestinal crypts of senescent mice. J Cell Sci. 1998;111(Pt 16):2297–2303. doi: 10.1242/jcs.111.16.2297. [DOI] [PubMed] [Google Scholar]
- 19.Patel BB, Yu Y, Du J, Levi E, Philip PA, Majumdar AP. Age related increase in colorectal cancer stem cells in macroscopically normal mucosa of patients with adenomas: A risk factor for colon cancer. Biochem Biophys Res Commun. 2009;378:344–347. doi: 10.1016/j.bbrc.2008.10.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmelz EM, Levi E, Du J, Xu H, Majumdar AP. Age-related loss of EGF-receptor related protein (ERRP) in the aging colon is a potential risk factor for colon cancer. Mech Ageing Dev. 2004;125:917–922. doi: 10.1016/j.mad.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Xiao ZQ, Moragoda L, Jaszewski R, Hatfield JA, Fligiel SE, Majumdar AP. Aging is associated with increased proliferation and decreased apoptosis in the colonic mucosa. Mech Ageing Dev. 2001;122:1849–1864. doi: 10.1016/s0047-6374(01)00323-2. [DOI] [PubMed] [Google Scholar]
- 22.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]