Abstract
Lung transplantation (LTx) is a treatment option for end-stage lung disease that would be otherwise fatal for specific patient populations. The most common indications for LTx in adults remain to be chronic obstructive pulmonary disease, idiopathic pulmonary fibrosis, cystic fibrosis, alpha-1 antitrypsin deficiency, and idiopathic pulmonary arterial hypertension. Recent trends include performing re-transplantation while more patients over the age of 65 years are undergoing LTx. Even with these tendencies, slight improvements in survival have occurred. This article briefly reviews recent developments in adults undergoing LTx.
Keywords: Adults, indications, outcomes, lung transplantation (LTx)
Introduction
Lung transplantation (LTx) is the only therapeutic option for end-stage parenchymal lung diseases or pulmonary vascular disorders. In 1963, Hardy et al. (1) performed the first lung transplant in a 58-year-old male patient who died of nephrotoxicity. Since then, significant advancements have occurred regarding organ preservation, extracorporeal support of both donor organs and recipients, surgical techniques, immunosuppressive therapeutic agents, and allograft surveillance, along with the advent of multidisciplinary, collaborative medical and surgical teams to provide care to patients after LTx. The purpose of this brief review is to review indications for LTx in adult patients and to present clinical outcomes.
Recent trends in lung transplant numbers
The International Society for Heart and Lung Transplantation (ISHLT) Registry provides detailed annual information on patients who have undergone LTx. The most recent report in 2013 summarized data from 43,428 adult lung and 3,703 adult heart-lung transplant recipients and their donors through June 30, 2012 (2). The number of lung transplants has continued to rise, especially over the last 5 years (Figure 1); however, this increase in demand for organs has coincided with a reduction in number of available donor lungs (2,3). Coinciding with the increase in total lung transplants, patients who are older than 65 years undergoing LTx are on the rise (Figure 1) (2,3). Similarly, the age of donor lung allografts is on the rise (4).
Figure 1.

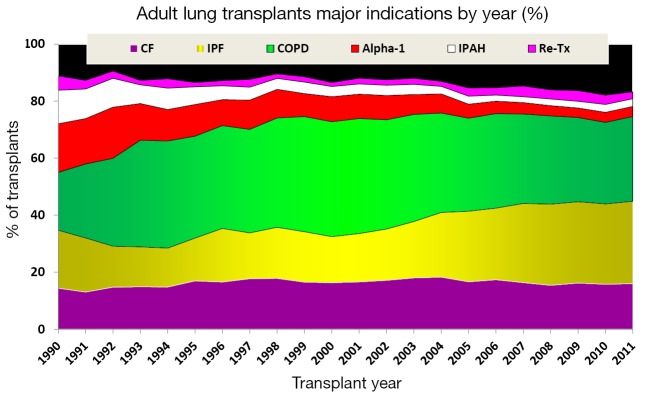
Major indications for lung transplants by year (%) from 1990 to 2011, modified with permission (2). The age distribution of lung transplant recipients was compared between eras using a chi-square test. A significant P value means that at least one of the groups is different than the others but it doesn’t identify which group it is.
Indications for lung transplantation (LTx) in adults
The decision to perform LTx is a complex treatment that carries considerable surgical risks. Table 1 shows the indications for lung transplants in adults performed between January 1995 and June 2012, while Figure 2 provides the major indications by year from 1990 to 2011 (2). Revision of international guidelines for lung transplant candidates was last published in 2006 by Orens et al. (5) with a revised update being published soon, which will include pediatric recommendations for the first time.
Table 1. Indications for adult lung transplants between January 1995 to June 2012, modified with permission (2).
| Diagnosis | Single lung (N=14,197) No. (%) | Bilateral lung (N=23,384) No. (%) | Total (N=37,581) No. (%) |
|---|---|---|---|
| COPD* (without alpha-1 antitrypsin deficiency) | 6,312 (44.5) | 6,290 (26.9) | 12,602 (33.5) |
| COPD* (with alpha-1 antitrypsin deficiency) | 753 (5.3) | 1,429 (6.1) | 2,182 (5.8) |
| Interstitial lung disease (with idiopathic pulmonary fibrosis) | 4,872 (34.3) | 4,032 (17.2) | 8,904 (23.7) |
| Bronchiectasis associated with cystic fibrosis | 229 (1.6) | 6,002 (25.7) | 6,231 (16.6) |
| Idiopathic pulmonary arterial hypertension | 87 (0.6) | 1,073 (4.6) | 1,160 (3.1) |
| Pulmonary fibrosis, other | 563 (4.0) | 820 (3.5) | 1,383 (3.7) |
| Bronchiectasis | 59 (0.4) | 956 (4.1) | 1,015 (2.7) |
| Retransplant (obliterative bronchiolitis) | 276 (1.9) | 292 (1.2) | 568 (1.5) |
| Retransplant (not obliterative bronchiolitis) | 182 (1.3) | 220 (0.9) | 402 (1.1) |
| Sarcoidosis | 265 (1.9) | 689 (2.9) | 954 (2.5) |
| Connective tissue disease | 156 (1.1) | 332 (1.4) | 488 (1.3) |
| Obliterative bronchiolitis (not retransplant) | 98 (0.7) | 298 (1.3) | 396 (1.1) |
| Lymphangioleiomyomatosis | 136 (1.0) | 255 (1.1) | 391 (1.0) |
| Congenital heart disease | 56 (0.4) | 269 (1.2) | 325 (0.9) |
| Cancer | 7 (0.0) | 29 (0.1) | 36 (0.1) |
| Other | 146 (1.0) | 398 (1.7) | 544 (1.4) |
*, COPD, chronic obstructive pulmonary disease.
Figure 2.
Adult lung transplants recipient age distribution by era from 1985 to 2012, modified with permission (2).
Table 2 lists the major disease categories that should be considered for LTx. Patients with these pulmonary disorders should be referred for consideration for LTx at any point if these characteristics exist or if the patient or primary healthcare provider has further questions regarding the potential benefit of LTx. Tables 3,4 outlines both absolute and relative contraindications for LTx as recently recommended. In short, LTx should not be considered in a patient with a florid infection, recent malignant tumor, continued addictive behavior, or lacks reliable social support. Infectious issues are different in cystic fibrosis with controversy continuing with most centers generally not offering transplant in patients colonized with Burkholderia cenocepacia and extreme caution used in offering transplant in the presence of Mycobacterium abscessus. Relative contraindications are determined by the individual centers with updated recommendations under development to be soon available.
Table 2. Indications for lung transplantation according to underlying major diseases.
| Chronic obstructive pulmonary disease (with or without alpha-1 antitrypsin deficiency) |
| BODE (body-mass index, airflow obstruction, dyspnea, and exercise) index >5 |
| FEV1 <20% of predicted |
| Diffusion capacity <20% of predicted |
| Pulmonary hypertension or cor pulmonale despite oxygen therapy |
| Hypercapnia, PaCO2 >50 mmHg |
| Fibrotic lung disease |
| Histologic or radiographic evidence suggestive of usual interstitial pneumonia (UIP) or nonspecific interstitial pneumonia (NSIP) |
| FVC <60% of predicted |
| Diffusion capacity <39% of predicted (UIP) or <35% of predicted (NSIP) |
| Drop in FVC by ≥10% or diffusion capacity by ≥15% over a 6-month period |
| Drop in SaO2 on pulse oximetry by <88% on 6-minute walk test |
| High-resolution CT imaging with honeycombing (fibrosis score >2) |
| Pulmonary hypertension |
| Cystic fibrosis |
| FEV1 <30% of predicted |
| PaO2 <55 mmHg |
| PaCO2 >50 mmHg |
| Exacerbations requiring intensive care unit stay |
| Increasing frequent of pulmonary exacerbations requiring antibiotic therapy |
| Recurrent and/or refractory pneumothorax |
| Recurrent hemoptysis not controlled by bronchial artery embolization |
| Pulmonary hypertension |
| Progressive weight loss, body mass index <18 kg/m2 |
| Idiopathic pulmonary arterial hypertension |
| Low or declining 6-minute walk test at <380 |
| Maximum oxygen intake <10.4 mL/min/kg |
| World Health Organization functional stage III or IV on maximal medical therapy |
| Cardiac index <2 L/min/m2 |
| Right atrial pressure >15 mmHg |
| Failure of intravenous epoprostenol therapy or equivalent |
BODE, body-mass index, airflow obstruction, dyspnea, and exercise; FEV1, forced expiratory volume in one second; FVC, forced vital capacity.
Table 3. Absolute contraindications for lung transplantation.
| Malignancy in the last 2 years except for cutaneous squamous and basal cell tumors, 5-year disease-free interval is prudent |
| Dysfunction of another major organ system (heart, liver, or kidney) that is not amenable to treatment |
| Noncurable xtrapulmonary infection (active viral hepatitis B, hepatitis C, human immunodeficiency virus) |
| Significant chest wall/spinal deformity |
| Nonadherence and/or inability to follow through with medical therapy or office follow-up |
| Untreatable psychiatric or psychologic condition(s) associated with the inability to cooperate or comply with medical therapy |
| Lack of dependable social support system |
| Substance addiction (alcohol, tobacco, or narcotics) within the last 6 months |
Table 4. Relative contraindications for lung transplantation.
| Age older than 65 years |
| Critical or unstable clinical condition |
| Severely limited functional status with poor rehabilitation potential |
| Colonization with highly resistant or highly virulent bacteria, fungi, or mycobacteria |
| Severe obesity defined as a body mass index (BMI) exceeding 30 kg/m2 |
| Severe or symptomatic osteoporosis |
| Mechanical ventilation |
| Other medical conditions that have not resulted in end-stage organ damage, such as diabetes mellitus, systemic hypertension, peptic ulcer disease, or gastroesophageal reflux should be optimally treated before transplantation |
Clinical outcomes
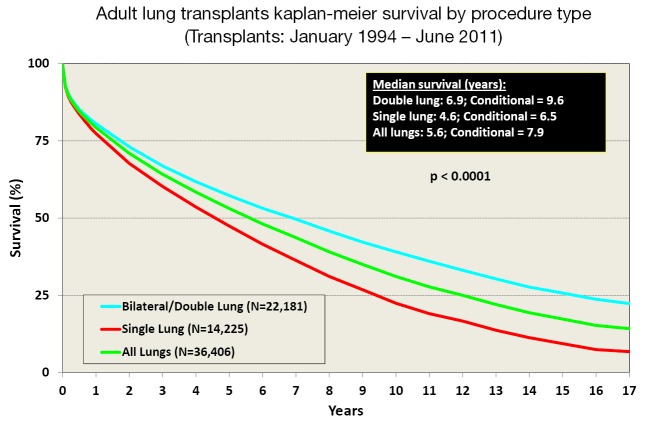
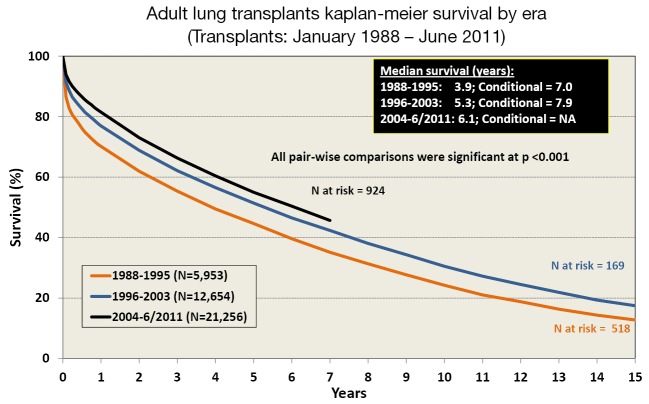
Survival after LTx in adult patients has slowly improved over the last 30 years (2). One contributing factor is the increasing number of bilateral lung transplants being performed, especially in the younger patient population (Figure 3). The improvement in survival has improved in a stepwise fashion as outlined in Figure 4.
Figure 3.
Adult lung transplants Kaplan-Meier survival by procedure type (single or bilateral) from January 1994 to June 2011, modified with permission (2). Survival was calculated using the Kaplan-Meier method, which incorporates information from all transplants for whom any follow-up has been provided. Since many patients are still alive and some patients have been lost to follow-up, the survival rates are estimates rather than exact rates because the time of death is not known for all patients. The median survival is the estimated time point at which 50% of all of the recipients have died. The conditional median survival is the estimated time point at which 50% of the recipients who survive to at least 1 year have died. Because the decline in survival is greatest during the first year following transplantation, the conditional survival provides a more realistic expectation of survival time for recipients who survive the early post-transplant period. Survival rates were compared using the log-rank test statistic.
Figure 4.
Adult lung transplants Kaplan-Meier survival by era from January 1988 to June 2011, modified with permission (2). Survival was calculated using the Kaplan-Meier method, which incorporates information from all transplants for whom any follow-up has been provided. Since many patients are still alive and some patients have been lost to follow-up, the survival rates are estimates rather than exact rates because the time of death is not known for all patients. The median survival is the estimated time point at which 50% of all of the recipients have died. The conditional median survival is the estimated time point at which 50% of the recipients who survive to at least 1 year have died. Because the decline in survival is greatest during the first year following transplantation, the conditional survival provides a more realistic expectation of survival time for recipients who survive the early post-transplant period. Survival rates were compared using the log-rank test statistic. Adjustments for multiple comparisons were done using Scheffe’s method.
Innovations
Hardy et al. were clearly innovative in 1963 when they performed the first lung transplant. Novel discoveries continue to influence the outcomes of patients with advanced lung disease regarding LTx. The use of extracorporeal support has made an immediate impact as it is commonplace for patients to be bridged to LTx with extracorporeal membrane oxygenation (ECMO) (6-16), but ECMO remains to be a relative contraindication in the current published guidelines, thus the need for an update. The use of ECMO as a means to bridge was recently reported with similar outcomes as lung retransplantation (6). A major innovation with the advent of normothermic ex vivo lung perfusion by the group at the University of Toronto has resulted in the successful transplantation of donor lungs that would have been previously discarded (17,18). This technology uses extracorporeal means to support donor organs. More recently, induction immunosuppression was shown to have a significantly positive effect on survival (19). Discoveries continue to include modifications of currently available treatments as best practice still continues to evolve in LTx.
Conclusions
Based on the recent advancements, the future is very bright in the care of patients with advanced lung disease who require LTx. Despite recent novel discoveries and innovations, further work is needed to improve and enhance not only the current technologies and treatments, but how we use them and in what clinical situation. Multi-center studies are badly needed in order to even further improve outcomes in LTx.
Acknowledgements
No funding was required to complete this work which was completed at The Ohio State University and Nationwide Children’s Hospital.
Disclosure: The authors declare no conflict of interest.
References
- 1.Hardy JD, Webb WR, Dalton ML, Jr, et al. Lung homotransplantation in man. JAMA 1963;186:1065-74 [DOI] [PubMed] [Google Scholar]
- 2.Yusen RD, Christie JD, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Adult Lung and Heart-Lung Transplant Report--2013; focus theme: age. J Heart Lung Transplant 2013;32:965-78 [DOI] [PubMed] [Google Scholar]
- 3.Organ Procurement and Transplantation Network and Scientific Registry of Transplant Recipients. OPTN/SRTR 2010 Annual Data Report, 2011 [May 17, 2014]. Available online: http://srtr.transplant.hrsa.gov/annual_reports/2011/default.aspx
- 4.Abecassis M, Bridges ND, Clancy CJ, et al. Solid-organ transplantation in older adults: current status and future research. Am J Transplant 2012;12:2608-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orens JB, Estenne M, Arcasoy S, et al. International guidelines for the selection of lung transplant candidates: 2006 update--a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2006;25:745-55 [DOI] [PubMed] [Google Scholar]
- 6.Hayes D, Jr, Higgins RS, Kilic A, et al. Extracorporeal Membrane Oxygenation and Retransplantation in Lung Transplantation: An Analysis of the UNOS Registry. Lung 2014;192:571-6 [DOI] [PubMed] [Google Scholar]
- 7.Fischer S, Simon AR, Welte T, et al. Bridge to lung transplantation with the novel pumpless interventional lung assist device NovaLung. J Thorac Cardiovasc Surg 2006;131:719-23 [DOI] [PubMed] [Google Scholar]
- 8.Ricci D, Boffini M, Del Sorbo L, et al. The use of CO2 removal devices in patients awaiting lung transplantation: an initial experience. Transplant Proc 2010;42:1255-8 [DOI] [PubMed] [Google Scholar]
- 9.Haneya A, Philipp A, Mueller T, et al. Extracorporeal circulatory systems as a bridge to lung transplantation at remote transplant centers. Ann Thorac Surg 2011;91:250-5 [DOI] [PubMed] [Google Scholar]
- 10.Hämmäinen P, Schersten H, Lemström K, et al. Usefulness of extracorporeal membrane oxygenation as a bridge to lung transplantation: a descriptive study. J Heart Lung Transplant 2011;30:103-7 [DOI] [PubMed] [Google Scholar]
- 11.Bermudez CA, Rocha RV, Zaldonis D, et al. Extracorporeal membrane oxygenation as a bridge to lung transplant: midterm outcomes. Ann Thorac Surg 2011;92:1226-31; discussion 1231-2 [DOI] [PubMed] [Google Scholar]
- 12.Hayes D, Jr, Kukreja J, Tobias JD, et al. Ambulatory venovenous extracorporeal respiratory support as a bridge for cystic fibrosis patients to emergent lung transplantation. J Cyst Fibros 2012;11:40-5 [DOI] [PubMed] [Google Scholar]
- 13.Bittner HB, Lehmann S, Rastan A, et al. Outcome of extracorporeal membrane oxygenation as a bridge to lung transplantation and graft recovery. Ann Thorac Surg 2012;94:942-9; author reply 949-50 [DOI] [PubMed] [Google Scholar]
- 14.Fuehner T, Kuehn C, Hadem J, et al. Extracorporeal membrane oxygenation in awake patients as bridge to lung transplantation. Am J Respir Crit Care Med 2012;185:763-8 [DOI] [PubMed] [Google Scholar]
- 15.Lang G, Taghavi S, Aigner C, et al. Primary lung transplantation after bridge with extracorporeal membrane oxygenation: a plea for a shift in our paradigms for indications. Transplantation 2012;93:729-36 [DOI] [PubMed] [Google Scholar]
- 16.Hoopes CW, Kukreja J, Golden J, et al. Extracorporeal membrane oxygenation as a bridge to pulmonary transplantation. J Thorac Cardiovasc Surg 2013;145:862-7; discussion 867-8 [DOI] [PubMed] [Google Scholar]
- 17.Cypel M, Yeung JC, Liu M, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med 2011;364:1431-40 [DOI] [PubMed] [Google Scholar]
- 18.Cypel M, Yeung JC, Machuca T, et al. Experience with the first 50 ex vivo lung perfusions in clinical transplantation. J Thorac Cardiovasc Surg 2012;144:1200-6 [DOI] [PubMed] [Google Scholar]
- 19.Whitson BA, Lehman A, Wehr A, et al. To induce or not to induce: a 21st century evaluation of lung transplant immunosuppression’s effect on survival. Clin Transplant 2014;28:450-61 [DOI] [PubMed] [Google Scholar]