Abstract
Heart failure remains a major global problem with approximately 6 million individuals suffering from heart failure in the United States alone. The surgical technique of heart transplantation, popularized by Dr. Norman Shumway, has led to its success and currently remains the best treatment options for patients with end-stage. However, with the continued limitation of donor organs and the rapid development of ventricular assist device technology, the number of patients bridged to transplant with mechanical circulatory support has increased significantly. This has created some new technical challenges for heart transplantation. Therefore, it is now important to be familiar with multiple new technical challenges associated with the surgical techniques of heart transplantation with an ultimate goal in reducing donor heart ischemic time, recipient cardiopulmonary bypass time and post-operative complications. In this review, we described our technique of heart transplantation including the timing of the operation, recipient cardiectomy and donor heart implantation.
Keywords: Heart transplantation
Introduction
Heart transplantation remains the gold standard therapy for end-stage congestive heart failure despite the continued development of the new treatments including biventricular pacing, stem cell therapies and mechanical circulatory support. Dr. Shumway paved the way for the widespread clinical acceptance of cardiac transplantation starting with his experimental work in the laboratory dating back to the 1950s. Beginning with his early work including topical hypothermia and bi-atrial anastomosis (1-3), the surgical technique and medical management of heart transplantation has continued to evolve and with the improved success has resulted in over 5,000 heart transplantations worldwide per year. At our center, we prefer the bicaval orthotopic heart transplant technique. The bicaval approach preserves normal atrial morphology, sinus node and valvular function. Previous studies have shown the bicaval technique was associated with reduced hospital stay, decreased incidence of atrial dysrhythmias and conduction disturbances, less mitral and tricuspid incompetence secondary to atrioventricular geometry distortion and right ventricular failure (4-6). With the increased and successful use of left ventricular assist devices (LVADs), as a bridge-to-transplant, it has dramatically influenced not only the number of patients receiving heart transplant with a LVAD but it has also increased the technical challenges in heart transplantation. This new challenge of LVAD removal/explant, prior to proceeding with the donor heart implantation, has added new challenges not only technically but frequently with the timing of the operation and the sequence of the anastomoses. The ultimate goal is to limit donor heart ischemic time, recipient cardiopulmonary bypass time, post-operative complications and maintain the overall success that heart transplantation has achieved over the years. In this chapter, we review our technique in heart transplantation including the timing of the operation, recipient cardiectomy and donor heart implantation.
Technique
Timing of the operation
The timing of donor and recipient cardiectomy is critical in minimizing the allograft ischemic time and recipient cardiopulmonary bypass time. The donor ischemic time should be less than 6 hours but more preferably and routinely less than 4 hours. Frequent communication between the procurement and implanting teams is necessary and will allow optimal coordination of the procedures. The recipient operation should be started sufficiently in advance of the arrival of the donor heart to minimize ischemic time. We usually allow at least 1 hour from skin incision to the arrival of the donor’s heart for recipients who have not undergone a previous sternotomy. In patient with prior sternotomy or LVAD placement, this period is extended to 2 hours to allow adequate time to complete the dissection of the recipient’s heart. Factors that have to be considered when coordinating timing of the operations include: time the abdominal organ procurement teams need to complete their dissection before cross-clamping; organ transportation time, time required for the anesthesia team for induction and monitoring lines placement in the recipient, and time required for recipient heart dissection and explantation, especially in patient with LVAD and prior sternotomies. The perfect coordination of the donor and recipient operations is one of the key components in the attempt to reduce donor heart ischemic time and recipient cardiopulmonary bypass time.
Recipient cardiectomy
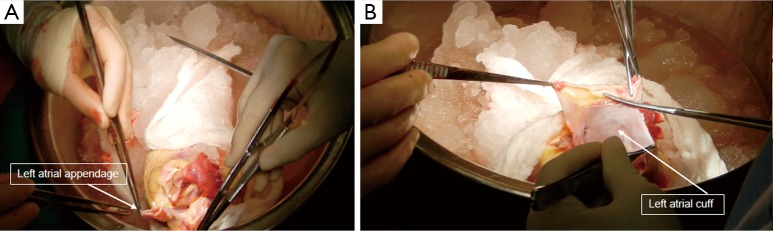
Median sternotomy is performed and pericardial cradle is created. The aorta, pulmonary artery, superior vena cava (SVC) and inferior vena caca (IVC) are dissected and isolated away from their adjacent structures. Umbilical tape snares are passed around the SVC and IVC (Figure 1). After heparinization, the distal ascending aorta (just proximal to the innominate artery), SVC and ICV are cannulated for cardiopulmonary bypass. In redo sternotomy, enough native heart is dissected so that at the minimal the SVC, IVC and the ascending aorta are accessible for cannulation and establishing cardiopulmonary bypass. If needed, the remainder of the dissection can then be completed once on cardiopulmonary bypass. In patients with a previous sternotomy, the femoral vein can be percutaneously cannulated for IVC drainage (Figure 2) which will also allow more room for the IVC anastomosis during heart implantation. After cardiopulmonary bypass is initiated, the recipient aorta is cross-clamped and the snares around the SVC and IVC are tightened. For patients with a LVAD, the LVAD outflow graft should be clamped and the device should be turned off before the initiation of cardiopulmonary bypass (Figure 3). The aorta and pulmonary artery are divided just above the semilunar valves. The right atrium is excised completely by transecting the SVC and then the IVC near its their junction with the main body of the right atrium. On opening the SVC, the swan ganz catheter should be removed and preserved. Any pacing leads are placed on tension and then divided. The heart is retracted inferiorly to expose the left atrial dome. The left atrial dome is opened and the incision is extended towards the mitral valve annulus in a circumferential fashion. At this point, the native heart can be removed. After removing the native heart, the left atrial appendage can be removed and the left atrial cuff trimmed. The aorta, pulmonary artery, IVC and SVC are usually individually trimmed to appropriate lengths after the donor heart has been inspected and the left atrial anastomosis completed cuff. For patients with a LVAD, we remove the LVAD with the native heart. The driveline is dissected, mobilized and divided from within the chest cavity (Figure 3). The driveline exit site is contaminated, and hence the remaining driveline should be removed at the end of the procedure after the recipient chest is closed.
Figure 1.
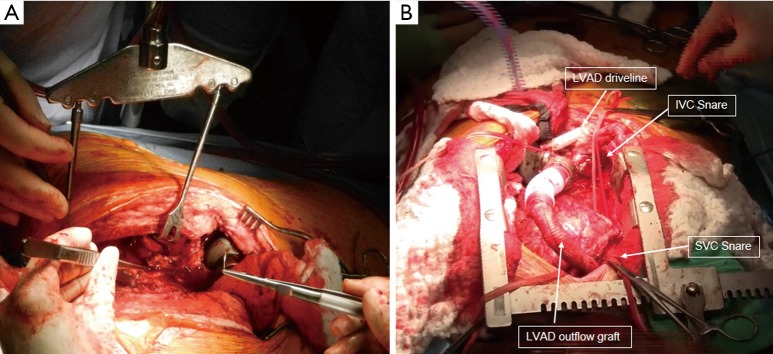
(A) A redo-sternotomy in a patient with previous LVAD placement. Rult retractor (Rultract, Cleveland, OH) was used to assist in exposure for the dissection of the left ventricular apex and LVAD. (B) Completion of the dissection with LVAD outflow graft and LVAD driveline exposed. Snares were placed around the SVC and IVC. LVAD, left ventricular assist device; SVC, superior vena cava; IVC, inferior vena caca.
Figure 2.
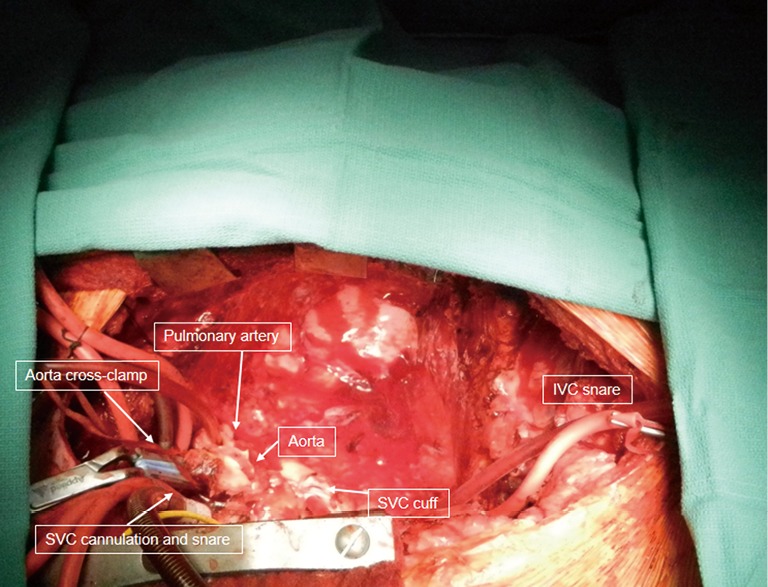
Completion of the recipient cardiectomy with aorta cross-clamped and vena cavae snared. IVC cannulation was done via the right common femoral vein. SVC, superior vena cava; IVC, inferior vena caca.
Figure 3.
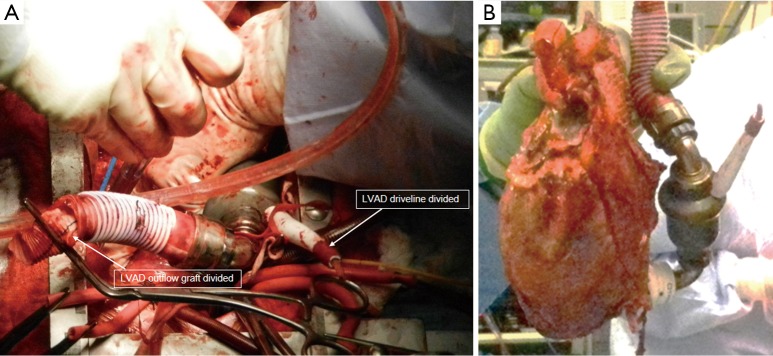
(A) LVAD driveline was divided and outflow graft was clamped and divided; (B) Recipient heart was resected along with the LVAD. LVAD, left ventricular assist device.
At this point, the donor heart is removed from the procurement container and is the start of the “warm ischemic” time. The donor heart is examined on the back table for a patent foreman ovale, valve defects or congenital anomaly. If the lungs were not procured, the left atrial cuff is then created by connecting the orifices of the pulmonary veins. The left atrial appendage incision should be closed if it was used for venting during the procurement (Figure 4).
Figure 4.
Back table donor heart preparation. The left atrial appendage incision was closed and the left atrial cuff was created. The donor heart was examined for patent ductus ovale, valvular abnormality and congenital anomaly.
Implantation of the donor heart
At our center, the bicaval technique is the preferred approach. We perform our anastomoses in the following order: left atrium, pulmonary artery, aorta, IVC and SVC. If necessary, the aortic cross-clamp can be removed immediately after the left atrial and aortic anastomosis in order to allow earlier allograft re-perfusion. However, more commonly, we remove the aortic cross-clamp after completing the left atrial, pulmonary artery, aortic and SVC anastomoses.
The anastomosis of the donor and recipient left atrium is performed first with a long double-armed running 3-0 polypropylene suture. The first stitch is placed across the recipient atrial cuff at the level of the left superior pulmonary vein and then to the donor atrial cuff at the base of the left atrial appendage (Figure 5). After 3-4 stitches sewing towards the left inferior pulmonary vein, the donor heart is then parachuted down into the recipients’ chest (Figure 6). The donor heart is wrapping in a sponge with slushed ice for cooling and to insulate it from direct warming from the adjacent thoracic structures. Stay stitches can be placed at the septum at the level of the right superior pulmonary vein and right inferior pulmonary vein for retraction and exposure. The suture line is continued around the superior and inferior borders of the left atrium and then tied. It is important to continually assess size discrepancy between donor and recipient atria so that appropriate plication of excess tissue may be performed. The surgeon should also be sensitive to the respective positions of the recipient and donor IVC and SVC while constructing the left atrial suture line. A left ventricular vent should be placed through the right superior pulmonary vein for de-airing and to avoid accumulation of venous return from the lungs, which can lead to warming of the donor heart during implantation. The pulmonary arteries are trimmed and tailored to the appropriate length and the anastomosis starts on the back wall of the artery with a double-armed 4-0 polypropylene suture. It is crucial that the pulmonary artery ends be trimmed to eliminate any redundancy in the vessel that might cause kinking. The suture line is continued on the front wall and the sutures are tied at the anteromedial aspect of the pulmonary artery. The anastomosis of the aorta is then performed in the same manner with a double-armed 4-0 polypropylene suture in a running fashion (Figures 5,7). It should be noted that, unlike the pulmonary artery, some redundancy in length is desired because it can allow better visualization of the posterior aortic suture line when necessary. With the patient in the trendelenburg position, a dose of steroids are administered and the aortic cross-clamp is removed. The SVC and IVC anastomoses are performed on a beating heart. Intravenous methylprednisolone (500 mg) is administered before the removal of the aortic cross-clamp. An aortic root vent can be placed into the ascending aorta for de-airing (Figure 8). The IVC anastomosis is performed with a double-armed 4-0 polypropylene suture in an end-to-end fashion starting with the posterior wall. The SVC anastomosis is performed in the same fashion. The length of the SVC should be tailored to avoid extra length and possible kinking (Figure 5). Before the weaning from cardiopulmonary bypass, sufficient time is needed to allow adequate reperfusion. Roughly, approximately 15 minutes reperfusion for each hour of ischemic time, the patient is gradually weaned from cardiopulmonary bypass. Preexisting pulmonary hypertension and the effects of cardiopulmonary bypass on pulmonary vascular resistance may give rise to perioperative right ventricular dysfunction following heart transplantation. We routinely use inhaled flolan or nitric oxide as an adjunct to lower pulmonary vascular resistance before coming off cardiopulmonary bypass and to help prevent right heart failure. Pulmonary artery venting can also be used to assist in the weaning of cardiopulmonary bypass and to avoid acute dilation of the right ventricle. It is also important to keep the pCO2 between 30-35 torr with minute ventilation adjustment to maximize pulmonary vasodilation. The heart rate of the newly implanted heart should be maintained between 100-120 beats per minutes with isoproterenol or pacing wires to allow adequate cardiac output from both the left and right ventricles. Intraoperative transesophageal echocardiogram is utilized to inspect all valves, anastomotic orientation and right and left ventricular function.
Figure 5.
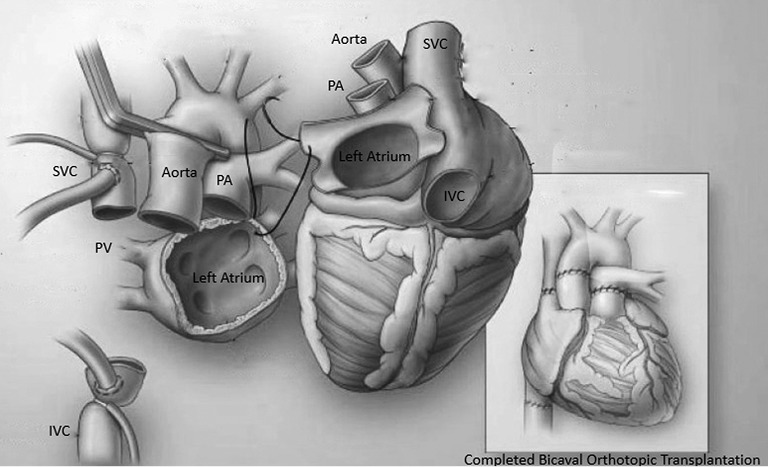
An illustration of the recipient mediastinum and the donor heart with the first stitch at the level of the donor left atrial appendage and recipient left superior pulmonary vein. The illustration on the right showed the completion of a bicaval orthotopic heart transplantation. PA, pulmonary artery; PV, pulmonary vein; SVC, superior vena cava; IVC, inferior vena caca.
Figure 6.
The donor heart was placed into the recipient left chest after 3-4 stiches was placed in the left atrial cuff at the level of the left superior pulmonary vein (recipient) and left atrial appendage (donor).
Figure 7.
The aorta anastomosis. Aortic cross-clamp was removed after the aorta anastomosis.
Figure 8.
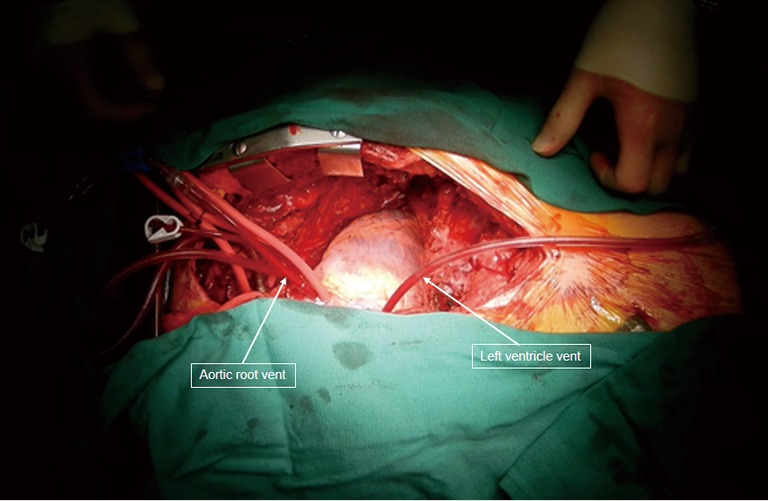
Completion of the allograft implantation. Aortic root and left ventricular vent were used for de-airing.
Conclusions
The advancement in the surgical technique of heart transplantation has contributed to its success and it remains the gold standard therapy for end-stage congestive heart failure with demonstrated improvement in patient survival and quality of life. Our technique of heart transplantation has evolved over time and specific modifications for including the explant of a LVAD now included with the primary goals of reduction in allograft ischemic time, recipient cardiopulmonary bypass time and post-operative complications. With the persistent limitation of suitable donor hearts, continued future developments will be needed in the areas of donor allocation, use of “marginal” donor hearts and the technological advancement in mechanical circulatory assist devices.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Lower RR, Stofer RC, Shumway NE. Homovital transplantation of the heart. J Thorac Cardiovasc Surg 1961;41:196-204 [PubMed] [Google Scholar]
- 2.Lower RR, Shumway NE. Studies on orthotopic homotransplantation of the canine heart. Surg Forum 1960;11:18-9 [PubMed] [Google Scholar]
- 3.Lower RR, Dong E, Jr, Shumway NE. Long-term survival of cardiac homografts. Surgery 1965;58:110-9 [PubMed] [Google Scholar]
- 4.Weiss ES, Nwakanma LU, Russell SB, et al. Outcomes in bicaval versus biatrial techniques in heart transplantation: an analysis of the UNOS database. J Heart Lung Transplant 2008;27:178-83 [DOI] [PubMed] [Google Scholar]
- 5.Milano CA, Shah AS, Van Trigt P, et al. Evaluation of early postoperative results after bicaval versus standard cardiac transplantation and review of the literature. Am Heart J 2000;140:717-21 [DOI] [PubMed] [Google Scholar]
- 6.Aziz T, Burgess M, Khafagy R, et al. Bicaval and standard techniques in orthotopic heart transplantation: medium-term experience in cardiac performance and survival. J Thorac Cardiovasc Surg 1999;118:115-22 [DOI] [PubMed] [Google Scholar]


