Abstract
Over the last several decades, the growth of lung transplantation has been hindered by a much higher demand for donor lungs than can be supplied, leading to considerable waiting time and mortality among patients waiting for transplant. This has led to the search for an alternative bridging strategy in patients with end-stage lung disease. The use of extracorporeal membrane oxygenation (ECMO) as a bridge to lung transplantation as well as a rescue strategy post-transplant for primary graft dysfunction (PGD) has been studied previously, however due to initially poor outcomes, its use was not heavily instituted. In recent years, with significant improvement in technologies, several single and multi-center studies have shown promising outcomes related to the use of ECMO as a bridging strategy as well as a therapy for patients suffering from PGD post-transplant. These results have challenged our current notion on ECMO use and hence forced us to reexamine the utility, efficacy and safety of ECMO in conjunction with lung transplantation. Through this review, we will address the various aspects related to ECMO use as a bridge to lung transplantation as well as a rescue post-transplant in the treatment of PGD. We will emphasize newer technologies related to ECMO use, examine recent observational studies and randomized trials of ECMO use before and after lung transplantation, and reflect upon our own institutional experience with the use of ECMO in these difficult clinical situations.
Keywords: Lung transplantation, extracorporeal membrane oxygenation (ECMO), primary graft dysfunction (PGD)
Introduction
The prevalence of lung transplantation has increased significantly over the last few decades, especially in the treatment of end stage lung diseases such as chronic obstructive pulmonary disease (COPD), interstitial lung disease (ILD), and cystic fibrosis (CF) (1). In 2012, over 3,640 lung transplants were recorded in the registry of the International Society for Heart and Lung Transplantation, up from 3,395 the year before (1). Early survival following lung transplantation has improved over the years with 1-year survival approaching 79% (1). Unfortunately, the long-term success of lung transplantation has only seen a modicum of improvement, with median survival for the most recent era averaging 6.1 years (1).
Due to its modest successes and changing demographics, waiting time for lung transplantation continues to be an issue as the need for donor organs far exceeds their availability (2). While the implementation in the United States of the lung allocation score (LAS) in 2005 has helped to prioritize patients in the most urgent need for transplantation, roughly 500 patients continue to die while awaiting a lung transplant every year (3-5). The resulting estimates of mortality for patients on the waitlist is concerning, and has raised considerable interest in looking for alternative bridging strategies for patients with end-stage lung disease awaiting transplantation (2).
Utility of ECMO
Extracorporeal membrane oxygenation (ECMO) is a complex technique that allows for respiratory and/or cardiac support in critically ill patients (6). There are many indications for the implementation of ECMO, including adult respiratory distress syndrome (ARDS), inability to wean from cardiopulmonary bypass, and cardiogenic shock, among others (7). It can be used both in a veno-venous (VV) circuit for pure pulmonary support as well as a veno-arterial (VA) circuit for concomitant cardiac support (8-10). Cannulation strategies and implantation techniques vary tremendously based upon the local environment, resources and patient needs (6-8). Because of the technical expertise required and considerable financial costs, its use has been limited to patients with a high risk of mortality and whose underlying disease process is either reversible or as a short-term “bridge” to more definitive therapy (11).
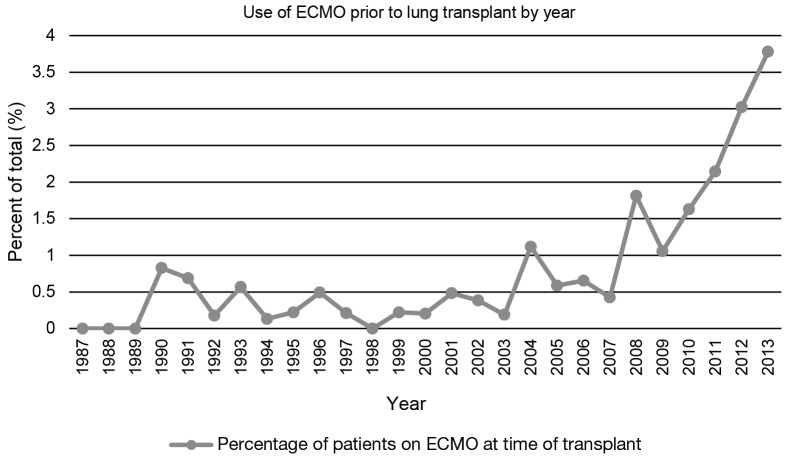
Over the last several years, the use of ECMO as a bridge to lung transplantation has gained significant attention in the management of patients with severe end-stage lung disease (9,12). Historically, ECMO use in this setting has been associated with poor outcomes which led many to condemn the practice (13,14). However, in recent years, technical advances have resulted in the extended use of various extracorporeal life support (ECLS) devices, such as ECMO, in the management of patients presenting with acute respiratory failure with significant improvement in outcomes (15). Furthermore, the implementation of the LAS has led to decreasing waiting times for lung transplantation (16). Combined, this has also led to a reinvigoration in the use of ECMO as a bridge to lung transplantation. In a study of more than 9,000 patients from the UNOS database from 2005 to 2011, roughly 1% of pulmonary patients were bridged to transplant with ECMO support (5). These numbers have continued to grow since then as an increasing number of single-center studies have demonstrated the utility and successful outcomes associated with ECMO as a bridging strategy to lung transplantation (Figure 1) (17-23).
Figure 1.
Percentage of patients on ECMO at time of transplant by year. Data obtained from the United Network for Organ Sharing (UNOS) database 1987-2013. Only patients with no previous transplant were included. ECMO, extracorporeal membrane oxygenation.
Historical challenges
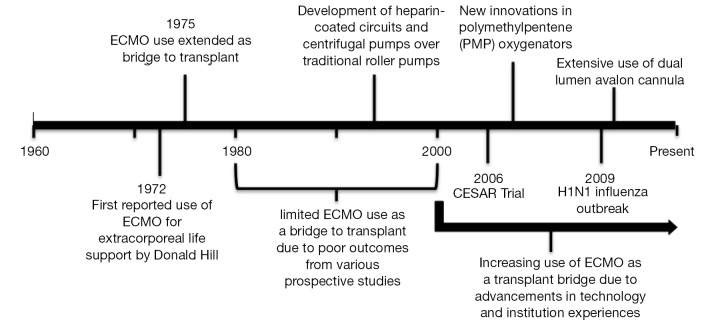
There has been significant variability in the use of ECMO as a means of bridging patients to lung transplantation over its short history (Figure 2). Hill et al. first reported the use of ECMO as a treatment modality for the management of cardiopulmonary failure in 1972 (24). Shortly thereafter in 1975, ECMO was described as a means of bridging a patient to lung transplantation, however further use was impeded by poor initial outcomes (13,14,25). Unacceptable post-transplant survival following pre-operative ECMO was likely related to both the severity of the patient’s illness and the technological inadequacies of early ECMO systems (17). Furthermore, it was traditionally regarded that ECMO use pre-transplant was associated with impaired bronchial anastomotic healing that contributed to the morbidity and mortality in lung transplant recipients (25). In addition, the results of a randomized, prospective study in 1979 demonstrating no survival benefit from ECMO in a non-lung transplant cohort of patients with acute respiratory failure further contributed to this declining use (18).
Figure 2.
Historical points of interest in the use of ECMO as a bridge to lung transplantation. ECMO, extracorporeal membrane oxygenation.
For the next two decades, the use of ECMO as a bridge to lung transplant was only sporadically used and limited to a few centers with mixed outcomes. However, significant improvements in ECMO-related technologies were made during this time period and data accrued slowly that challenged earlier preconceptions about the utility of ECMO (19,20). For example, during the 2009 H1N1 influenza outbreak, ECMO gained special attention by successfully managing a significant proportion of patients with severe acute respiratory failure (19). Furthermore, the Conventional Ventilation or ECMO for Severe Adult Respiratory Failure (CESAR) trial was conducted in the United Kingdom, and demonstrated a significant survival benefit of ECMO compared to conventional management for patients with severe ARDS (20).
ECMO as a rescue strategy post-transplant
Background
In lung transplantation a renewal of interest in ECMO was first seen for severe primary graft dysfunction (PGD) following lung transplantation and this remains the most common indication for its use after transplant (21,22). PGD is a syndrome consisting of lung injury during the first 72 hours following lung transplant defined as a decreased PaO2/FiO2 ratio and the presence of diffuse infiltrates on chest X-ray (23,26). As institutional experience with ECMO accrued, several isolated studies and case reports explored the use of ECMO as a rescue strategy in the treatment of PGD post lung transplantation, and as a bridge to redo lung transplant in select patients with intermittent successes (27,28). About 5% of lung transplant procedures require ECMO support for PGD or early complications (21). Many interventions have been studied to try and ameliorate the effects of PGD after transplant, including experimentation with inhaled nitric oxide and prostaglandins (29,30). However, none of these have been successful in significantly altering the rates of clinically important Grade 3 PGD, which hovers at about 17% according to results of the Lung Transplant Outcomes Group (26). According to this multi-institutional study, grade 3 PGD was associated with a 23% absolute increase in the risk of death within one year of transplant, indicating it’s continued overall impact on transplant survival (26).
Indications
An important question remains regarding when to employ ECMO after transplantation. Enhanced safety combined with increased experience has led to earlier deployment of ECMO circuits to support patients after lung transplantation (31). The goal should be to avoid or minimize the detrimental effects of ventilator support for PGD secondary to elevated airway pressures or high inspired oxygen concentrations. Firm guidelines vary from center to center, but we recommend initiating ECMO support when ventilatory requirements reach a peak inspiratory pressure of 35 cm H2O or FiO2 surpasses 60% in order to minimize lung injury from aggressive mechanical ventilation and oxidative stress. When necessary, the delayed initiation of ECMO after transplantation greater than 48 hours has been associated with worse outcomes, and this is consistent with our own experience that favors prompt initiation of ECMO (32).
Outcomes
Our group and others have reported on utilizing ECMO to support the recipients who suffer from severe PGD. Survival in this group of patients was surprisingly good when supported with VV ECMO, especially considering the lethality of severe PGD (22). The mean reported ECMO duration post-transplant is varied, but most studies have reported between 2 to 8 days (21,28,33). One study demonstrated successful use of ECMO for 3 weeks prior to a redo lung transplantation, however others have demonstrated that prolonged ECMO duration post-transplant is associated with high mortality (28,34). Nonetheless, it provides a means of treatment in patients who suffer from PGD post-operatively who would otherwise succumb quickly. We have reported a 96% success rate in weaning recipients from VV ECMO following transplant, with a 30-day survival of 82% and 1-year survival of 64% (22). Some centers report success with both VA and VV ECMO for these patients. However, our experience has been that VV ECMO should be preferred due to a decrease in complications and greater survival when compared to patients supported with VA ECMO (21).
ECMO as a bridge to transplant
Background
In the last several years, there has also been a continued push at individual centers to reexamine lung transplantation in patients on ECMO. Numerous reasons are cited for this including a benefit in weaning patients off of mechanical ventilation (which is also associated with increased post-operative mortality) as well as allowing patients with acute respiratory failure to be transported to centers with transplant services from those without (35-37). Others use recent advancements in technology as an argument for reexamining this issue. For instance Jackson et al. list three major recent advancements in ECMO: the development of the polymethylpentene (PMP) oxygenator, the use of heparin coated circuits, and the use of centrifugal pumps over traditional roller pumps (25). We would add portability and the dual-lumen cannula to this list and emphasize that together these advances have led to the ability to minimize anticoagulation needs and likely result in much less hemolysis and activation of blood components traveling through the circuit.
Indications
Although multiple centers have published with regards to their successes transplanting patients following the use of VV ECMO, there are no universally accepted indications for this practice (10,27,38). Careful patient selection for lung transplantation after ECMO is imperative to maximize outcomes and ensure appropriate resource allocation of scarce donor lungs. Current recommendations are based on institutional experience. Much of the earliest use of ECMO as a bridge to lung transplantation was for patients with PGD requiring retransplantation, and therefore this was seen as an early indication (39). Since that time however, improving outcomes have led to the use of ECMO bridging in patients without prior transplantation (13,39). Most studies recommend the use of this practice primarily in younger patients who suffer an acute decompensation in a chronic pulmonary process, not for acute respiratory distress syndrome (9,39). Furthermore, these patients should have had reasonable functional status prior to their acute episode (35,39). However, there has been anecdotal success in bridging previously healthy young patients to transplant when they suffer irreversible lung injury acutely.
Contraindications
Current contraindications for lung transplantation following ECMO are also based on institutional experience (Table 1). For instance, Lafarge et al. recommended that renal failure be considered a contraindication for transplantation following ECMO due to the intraoperative death of a patient who had pre-transplant anuric renal failure (10). Toyoda et al. recommended this be expanded to any organ failure including liver failure (9). Other studies including those by Bermudez et al. and Mason et al. discuss how pre-transplant ECMO populations tend to be younger, likely demonstrating inherent selection biases (9,37,39). Further research is necessary to determine if increased age is an absolute or relative contraindication. Multiple studies have also documented that their institutional outcomes have improved over time, likely secondary to a mixture of newer technology/protocols as well as extensive experience (39). As lower-volume centers are often limited in experience, ECMO use as a bridge to transplant should likely be limited at these centers until standardized best-practice protocols have been developed to optimize outcomes (9). Lastly, traditional contraindications to lung transplantation including uncontrolled or untreated infection, recent malignancy, significant coronary artery disease, and active substance abuse among others continue to be contraindications to the use of ECMO-bridged transplantation (40).
Table 1. Contraindications (both absolute and relative) to bridging to lung transplant with ECMO (9,10,37,39,40).
| Absolute contraindication |
| Untreated infection |
| Organ failure (other than pulmonary) |
| Recent malignancy |
| Active substance abuse |
| Poor social support system |
| History of nonadherence |
| Relative contraindication |
| Advancing age |
| Small institutional experience |
| Poor pre-ECMO functional status |
| Severe obesity (BMI >30) |
ECMO, extracorporeal membrane oxygenation.
Outcomes
Although several trials have evaluated the outcomes of ECMO in severe respiratory failure, very few have examined in isolation, the utility and role of ECMO as a bridge to lung transplantation. Current literature is limited to several single center retrospective studies advocating for the use of ECMO as an alternative “salvage” therapy in patients with end-stage lung disease (9,10,12,13,27,35,36,38,41-44). Most of these analyses were composed of a mixture of ambulatory/extubated patients and sedated/intubated patients. A summary of these studies can be found in Table 2. One year survivals ranged from 33-93%, many of which are better than that reported previously (9,10,12,13,17,27,37,38,43,44). Moreover, diagnoses in these groups varied, but overall CF and idiopathic pulmonary fibrosis (IPF) had a higher prevalence while COPD had a lower prevalence than that of the general lung transplant population (1,9,10,12,35,36,38,41,42). As an indication of the changing times, there has been over a 200% increase in lung transplantation in patients on ECMO between 2009-2013 (Figure 1).
Table 2. Overview of recent single and multi-institution studies reviewing outcomes following lung transplantation after ECMO.
| Study | Number of patients | 1-year survival (%) |
|---|---|---|
| Toyoda et al. [2013] (9) | 24 | 74 |
| Hoopes et al. [2013] (12) | 31 | 93 |
| Anile et al. [2013] (44) | 7 | 85.7 |
| Nosotti et al. [2013] (43) | 11 | 85.7 |
| Lafarge et al. [2013] (10) | 30 | 66.5 |
| Bittner et al. [2012] (27) | 27 | 33 |
| Gottlieb et al. [2012] (42) | 60 | 57 |
| Lang et al. [2012] (38) | 34 | 60 |
| Hämmäinen et al. [2011] (13) | 13 | 92 |
ECMO, extracorporeal membrane oxygenation.
The discrepancy noted in survival outcomes among the above referenced studies is unclear; however, we speculate that this may be attributable to the nature of ECMO used, institutional differences in cannulation strategies, disparate wait list times among centers, and the extent and severity of post-transplant complications such as PDG. Furthermore, several studies have also shown that although high acuity lung transplant patients who are bridged with ECMO have increased risk for short-term mortality compared to the average lung transplant recipient, these high-risk recipients have better overall outcomes when performed at high volume centers (5). It is likely that this success is secondary to the extensive experience and technological capabilities in managing the complexities associated with ECMO at high volume centers. It may also be secondary to shorter waiting times at these high volume centers subsequently leading to a shorter pre-transplant ECMO duration and improved survival.
Newer treatment modalities
With the advancement of technology and increase in institutional experience in the past few years, newer and more promising strategies of incorporating the use of ECMO as a bridge to transplant have been developed. For instance, Fuehner et al. examined outcomes using ECMO as a bridge to transplantation in patients who were awake and spontaneously breathing. Compared to the conventional mechanical ventilation strategy, patients who received “awake” ECMO as a bridge to transplant and made it to transplantation had significantly better survival at 6 months (80% versus 50%), and had shorter postoperative hospital stays (although not to statistical significance) (41). The authors hypothesize that the main benefit of this “awake” ECMO is the avoidance of prolonged sedation and intubation and its associated complications (41). The authors further postulate that future successes in this arena could lead to a “destination therapy” much like that seen with left ventricular assist devices (41).
Other recent advances including low-resistance gas exchange membranes, high-durability centrifugal blood pumps, heparin-coated tubing, and improved cannulation strategies have resulted in a much safer medical device compared to those in use a few years ago (45,46). Newer devices are also increasingly smaller and lightweight. The new “Cardiohelp” by Maquet Cardiopulmonary is light enough to be carried by the patient, and also can simultaneously measure patient vitals, venous oxygen concentration, and hemoglobin (47,48). Haneya et al. reported on its use in 22 patients with a survival rate of 68.2% (47). Further advantages of these smaller systems include easier inter-facility transport of patients, which once again can allow transport of a patient to a transplant center when indicated (48).
Our institutional experience
Taking the concept of awake ECMO one step further we recently published on our institutional experience with pre-operative ECMO in bridged patients able to perform active rehabilitation. This experience included nine patients, all of whom survived through 1-year post-transplant (17). The patients who were able to undergo active rehabilitation while awaiting lung transplantation on ECMO demonstrated shorter post-transplant ventilator duration and hospital lengths of stay. This is due to the absence of post-transplant myopathy secondary to participation in active rehab. Our rehab protocol begins with the weaning of sedation and ventilator settings. Most of the patients will require tracheostomy, which is performed early in the process or at the time of ECMO cannulation. A few patients may be extubated while on ECMO. Resistance and stretching exercises follow once awake. The patients then progress through sitting, standing, and eventually ambulation. At least two formal rehab sessions are performed each day with staffing consisting of a physical therapist, ECMO specialist, respiratory therapist and 1-2 bedside nurses. Although resource intensive, patients have demonstrated the ability to walk up to 400 meters during one session and outcomes appear to be considerably improved.
Technical aspects of ECMO
Historically, extracorporeal support required dual cannulation, such as the femoral and internal jugular veins for VV or femoral vein and artery for VA ECMO. Femoral cannulation sites may increase the risk of infection and impede patient mobility. Therefore, whether it is for bridging to transplant or support after transplant, our most commonly employed ECMO strategy now involves a VV technique utilizing a dual-lumen cannula (Avalon Maquet) in the right internal jugular vein (Figure 3) (49). However, many other cannulation strategies are possible for both VV as well as VA ECMO and are oftentimes dictated by patient anatomic limitations or other factors (6,9,35). For active rehabilitation on VA ECMO, our most common approach is to sew a 6 to 8 mm vascular graft to the right axillary artery with a 21 to 23 mm venous cannula in the right internal jugular vein for drainage. Based on our experience, if at all possible we recommend a cannulation and ICU management strategy that will allow for active rehabilitation while awaiting lung transplantation on ECMO support.
Figure 3.

Demonstration of a patient ambulating on VV ECMO with a dual lumen cannula in the right internal jugular vein. VV, veno-venous; ECMO, extracorporeal membrane oxygenation.
Complications related to ECMO
Complications resulting from ECMO use are common, and depend on the type of ECMO technique (VA or VV) as well as the cannulation strategy used (7,9). Usual complications include bleeding, infection, and renal failure as well as less common complications including gas embolism, stroke, and limb ischemia (8,11,50,51). Bleeding is perhaps the most commonly reported complication ranging from 5-79% in the literature (11,52). Its cause is multifactorial, both secondary to iatrogenic anticoagulation necessary for ECMO as well as thrombocytopenia and fibrinolysis occurring because of contact with the ECMO circuit (11,53). Treatment is best performed through prevention, and modern circuits as described above allow users to reduce the requirement for systemic anticoagulation (11,53).
Although difficult to predict, it is pertinent to quickly identify and treat these complications to reduce associated mortality. Limb ischemia is a specific complication for which prompt diagnosis and action can improve outcomes. Occurring in 13-25% of VA ECMO patients cannulated through the femoral artery, its incidence can be reduced through use of a secondary distal catheter to increase distal limb perfusion, or through reliance on VV ECMO whenever possible to avoid arterial cannulation (54,55). Proper anticoagulation can also prevent emboli formation in the ECMO circuit. When limb ischemia is diagnosed early, prompt treatment can avoid permanent limb injury and reduce the amputation rate (54,56).
Conclusions
Lung transplantation is now considered an appropriate therapeutic option for the treatment of patients with end-stage lung disease (5). However, given the paucity of available donors, there is still significant mortality for patients on the waiting list (2). Historically, the use of extracorporeal circulatory support such as ECMO was considered to be a contraindication to lung transplantation due to poor outcomes (14). However, in recent years, this trend is evolving as more institutions look to optimize the safety and efficacy of their ECMO strategies as a means of bridging high-risk and high-acuity patients for lung transplant (44).
As larger institutional studies are performed, a clearer picture as to the outcomes of ECMO use is emerging. Some are already calling for a randomized multicenter controlled trial to help give an answer to this question (57). However, there are still many unanswered questions remaining and a randomized trial of adequate size is unlikely to ever be successfully performed. Therefore, it will be up to the lung transplant community to determine issues such as how the need for pre-transplant ECMO should weigh in to organ allocation, or what the appropriate indications and patient populations to bridge to lung transplantation should be. No doubt that as technologies continue to improve we will be obliged to revisit these questions, as well as many others periodically.
Modern experience with ECMO and reported institutional experiences on survival challenge historical assumptions about the treatment of end-stage lung disease and suggest that “bridging” to transplant with ECMO is both technically feasible and logistically viable. What is clear at this point in time is that continued advances in the technologies and further research will help determine how best to include ECMO as a bridging strategy for lung transplantation.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Yusen RD, Christie JD, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Adult Lung and Heart-Lung Transplant Report---2013; focus theme: age. J Heart Lung Transplant 2013;32:965-78 [DOI] [PubMed] [Google Scholar]
- 2.Gottlieb J.Update on lung transplantation. Ther Adv Respir Dis 2008;2:237-47 [DOI] [PubMed] [Google Scholar]
- 3.Yusen RD, Shearon TH, Qian Y, et al. Lung transplantation in the United States, 1999-2008. Am J Transplant 2010;10:1047-68 [DOI] [PubMed] [Google Scholar]
- 4.Egan TM, Murray S, Bustami RT, et al. Development of the new lung allocation system in the United States. Am J Transplant 2006;6:1212-27 [DOI] [PubMed] [Google Scholar]
- 5.George TJ, Beaty CA, Kilic A, et al. Outcomes and temporal trends among high-risk patients after lung transplantation in the United States. J Heart Lung Transplant 2012;31:1182-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sidebotham D, Allen SJ, McGeorge A, et al. Venovenous extracorporeal membrane oxygenation in adults: practical aspects of circuits, cannulae, and procedures. J Cardiothorac Vasc Anesth 2012;26:893-909 [DOI] [PubMed] [Google Scholar]
- 7.Allen S, Holena D, McCunn M, et al. A review of the fundamental principles and evidence base in the use of extracorporeal membrane oxygenation (ECMO) in critically ill adult patients. J Intensive Care Med 2011;26:13-26 [DOI] [PubMed] [Google Scholar]
- 8.Sidebotham D, McGeorge A, McGuinness S, et al. Extracorporeal membrane oxygenation for treating severe cardiac and respiratory failure in adults: part 2-technical considerations. J Cardiothorac Vasc Anesth 2010;24:164-72 [DOI] [PubMed] [Google Scholar]
- 9.Toyoda Y, Bhama JK, Shigemura N, et al. Efficacy of extracorporeal membrane oxygenation as a bridge to lung transplantation. J Thorac Cardiovasc Surg 2013;145:1065-70; discussion 1070-1 [DOI] [PubMed] [Google Scholar]
- 10.Lafarge M, Mordant P, Thabut G, et al. Experience of extracorporeal membrane oxygenation as a bridge to lung transplantation in France. J Heart Lung Transplant 2013;32:905-13 [DOI] [PubMed] [Google Scholar]
- 11.Lafç G, Budak AB, Yener AÜ, et al. Use of extracorporeal membrane oxygenation in adults. Heart Lung Circ 2014;23:10-23 [DOI] [PubMed] [Google Scholar]
- 12.Hoopes CW, Kukreja J, Golden J, et al. Extracorporeal membrane oxygenation as a bridge to pulmonary transplantation. J Thorac Cardiovasc Surg 2013;145:862-7; discussion 67-8 [DOI] [PubMed] [Google Scholar]
- 13.Hämmäinen P, Schersten H, Lemström K, et al. Usefulness of extracorporeal membrane oxygenation as a bridge to lung transplantation: a descriptive study. J Heart Lung Transplant 2011;30:103-7 [DOI] [PubMed] [Google Scholar]
- 14.Veith FJ. Lung Transplantation. Transplant Proc 1977;9:203-8 [PubMed] [Google Scholar]
- 15.Müller T, Philipp A, Luchner A, et al. A new miniaturized system for extracorporeal membrane oxygenation in adult respiratory failure. Crit Care 2009;13:R205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi SM, Garrity ER. The impact of the lung allocation score. Semin Respir Crit Care Med 2010;31:108-14 [DOI] [PubMed] [Google Scholar]
- 17.Rehder KJ, Turner DA, Hartwig MG, et al. Active rehabilitation during extracorporeal membrane oxygenation as a bridge to lung transplantation. Respir Care 2013;58:1291-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA 1979;242:2193-6 [DOI] [PubMed] [Google Scholar]
- 19.Noah MA, Peek GJ, Finney SJ, et al. Referral to an Extracorporeal Membrane Oxygenation Center and Mortality among Patients with Severe 2009 Influenza a(H1n1). JAMA 2011;306:1659-68 [DOI] [PubMed] [Google Scholar]
- 20.Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009;374:1351-63 [DOI] [PubMed] [Google Scholar]
- 21.Hartwig MG, Appel JZ 3rd, Cantu E 3rd, et al. Improved results treating lung allograft failure with venovenous extracorporeal membrane oxygenation. Ann Thorac Surg 2005;80:1872-9; discussion 1879-80. [DOI] [PubMed]
- 22.Hartwig MG, Walczak R, Lin SS, et al. Improved survival but marginal allograft function in patients treated with extracorporeal membrane oxygenation after lung transplantation. Ann Thorac Surg 2012;93:366-71 [DOI] [PubMed] [Google Scholar]
- 23.Suzuki Y, Cantu E, Christie JD. Primary graft dysfunction. Semin Respir Crit Care Med 2013;34:305-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill JD, O’Brien TG, Murray JJ, et al. prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). Use of the bramson membrane lung. N Engl J Med 1972;286:629-34 [DOI] [PubMed] [Google Scholar]
- 25.Jackson A, Cropper J, Pye R, et al. Use of Extracorporeal membrane oxygenation as a bridge to primary lung transplant: 3 consecutive, successful cases and a review of the literature. J Heart Lung Transplant 2008;27:348-52 [DOI] [PubMed] [Google Scholar]
- 26.Diamond JM, Lee JC, Kawut SM, et al. Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med 2013;187: 527-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bittner HB, Lehmann S, Rastan A, et al. Outcome of extracorporeal membrane oxygenation as a bridge to lung transplantation and graft recovery. Ann Thorac Surg 2012;94:942-9; author reply 949-50 [DOI] [PubMed] [Google Scholar]
- 28.Meyers BF, Sundt TM, 3rd, Henry S, et al. Selective use of extracorporeal membrane oxygenation is warranted after lung transplantation. J Thorac Cardiovasc Surg 2000;120:20-6 [DOI] [PubMed] [Google Scholar]
- 29.Moreno I, Vicente R, Mir A, et al. Effects of inhaled nitric oxide on primary graft dysfunction in lung transplantation. Transplant Proc 2009;41:2210-2 [DOI] [PubMed] [Google Scholar]
- 30.Ruberto F, Bergantino B, Testa MC, et al. Low-flow venovenous CO2 removal in association with lung protective ventilation strategy in patients who develop severe progressive respiratory acidosis after lung transplantation. Transplant Proc 2013;45:2741-5 [DOI] [PubMed] [Google Scholar]
- 31.Diaz-Guzman E, Davenport DL, Zwischenberger JB, et al. Lung function and ECMO after lung transplantation. Ann Thorac Surg 2012;94:686-7; author reply 687 [DOI] [PubMed] [Google Scholar]
- 32.Marasco SF, Vale M, Preovolos A, et al. Institution of extracorporeal membrane oxygenation late after lung transplantation - a futile exercise? Clin Transplant 2012;26:E71-7 [DOI] [PubMed] [Google Scholar]
- 33.Aigner C, Jaksch P, Taghavi S, et al. Pulmonary retransplantation: is it worth the effort? A long-term analysis of 46 cases. J Heart Lung Transplant 2008;27:60-5 [DOI] [PubMed] [Google Scholar]
- 34.Shigemura N, Bermudez C, Bhama J, et al. Successful lung retransplantation after extended use of extracorporeal membrane oxygenation as a bridge. Transplant Proc 2011; 43:2063-5 [DOI] [PubMed] [Google Scholar]
- 35.Javidfar J, Brodie D, Iribarne A, et al. Extracorporeal membrane oxygenation as a bridge to lung transplantation and recovery. J Thorac Cardiovasc Surg 2012;144:716-21 [DOI] [PubMed] [Google Scholar]
- 36.Haneya A, Philipp A, Mueller T, et al. Extracorporeal circulatory systems as a bridge to lung transplantation at remote transplant centers. Ann Thorac Surg 2011;91:250-5 [DOI] [PubMed] [Google Scholar]
- 37.Mason DP, Thuita L, Nowicki ER, et al. Should lung transplantation be performed for patients on mechanical respiratory support? The US experience. J Thorac Cardiovasc Surg 2010;139:765-773.e1. [DOI] [PubMed]
- 38.Lang G, Taghavi S, Aigner C, et al. Primary lung transplantation after bridge with extracorporeal membrane oxygenation: a plea for a shift in our paradigms for indications. Transplantation 2012;93:729-36 [DOI] [PubMed] [Google Scholar]
- 39.Bermudez CA, Rocha RV, Zaldonis D, et al. Extracorporeal membrane oxygenation as a bridge to lung transplant: midterm outcomes. Ann Thorac Surg 2011;92:1226-31; discussion 31-2 [DOI] [PubMed] [Google Scholar]
- 40.Orens JB, Estenne M, Arcasoy S, et al. International guidelines for the selection of lung transplant candidates: 2006 update--a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2006;25:745-55 [DOI] [PubMed] [Google Scholar]
- 41.Fuehner T, Kuehn C, Hadem J, et al. Extracorporeal membrane oxygenation in awake patients as bridge to lung transplantation. Am J Respir Crit Care Med 2012;185:763-8 [DOI] [PubMed] [Google Scholar]
- 42.Gottlieb J, Warnecke G, Hadem J, et al. Outcome of critically ill lung transplant candidates on invasive respiratory support. Intensive Care Med 2012;38:968-75 [DOI] [PubMed] [Google Scholar]
- 43.Nosotti M, Rosso L, Tosi D, et al. Extracorporeal membrane oxygenation with spontaneous breathing as a bridge to lung transplantation. Interact Cardiovasc Thorac Surg 2013;16:55-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anile M, Diso D, Russo E, et al. Extracorporeal membrane oxygenation as bridge to lung transplantation. Transplant Proc 2013;45:2621-3 [DOI] [PubMed] [Google Scholar]
- 45.Diaz-Guzman E, Hoopes CW, Zwischenberger JB. The evolution of extracorporeal life support as a bridge to lung transplantation. ASAIO J 2013;59:3-10 [DOI] [PubMed] [Google Scholar]
- 46.Riley JB, Scott PD, Schears GJ. Update on safety equipment for extracorporeal life support (ECLS) circuits. Semin Cardiothorac Vasc Anesth 2009;13:138-45 [DOI] [PubMed] [Google Scholar]
- 47.Haneya A, Philipp A, Foltan M, et al. First experience with the new portable extracorporeal membrane oxygenation system Cardiohelp for severe respiratory failure in adults. Perfusion 2012;27:150-5 [DOI] [PubMed] [Google Scholar]
- 48.Philipp A, Arlt M, Amann M, et al. First experience with the ultra compact mobile extracorporeal membrane oxygenation system Cardiohelp in interhospital transport. Interact Cardiovasc Thorac Surg 2011;12:978-81 [DOI] [PubMed] [Google Scholar]
- 49.Reeb J, Falcoz PE, Santelmo N, et al. Double lumen bi-cava cannula for veno-venous extracorporeal membrane oxygenation as bridge to lung transplantation in non-intubated patient. Interact Cardiovasc Thorac Surg 2012;14:125-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smedira NG, Moazami N, Golding CM, et al. Clinical experience with 202 adults receiving extracorporeal membrane oxygenation for cardiac failure: survival at five years. J Thorac Cardiovasc Surg 2001;122:92-102 [DOI] [PubMed] [Google Scholar]
- 51.Mols G, Loop T, Geiger K, et al. Extracorporeal membrane oxygenation: a ten-year experience. Am J Surg 2000;180:144-54 [DOI] [PubMed] [Google Scholar]
- 52.Brodie D, Bacchetta M.Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med 2011;365:1905-14 [DOI] [PubMed] [Google Scholar]
- 53.Mair P, Hoermann C, Moertl M, et al. Percutaneous venoarterial extracorporeal membrane oxygenation for emergency mechanical circulatory support. Resuscitation 1996;33:29-34 [DOI] [PubMed] [Google Scholar]
- 54.Bisdas T, Beutel G, Warnecke G, et al. Vascular complications in patients undergoing femoral cannulation for extracorporeal membrane oxygenation support. Ann Thorac Surg 2011;92:626-31 [DOI] [PubMed] [Google Scholar]
- 55.Foley PJ, Morris RJ, Woo EY, et al. Limb ischemia during femoral cannulation for cardiopulmonary support. J Vasc Surg 2010;52:850-3 [DOI] [PubMed] [Google Scholar]
- 56.Cheng R, Hachamovitch R, Kittleson M, et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1,866 adult patients. Ann Thorac Surg 2014;97:610-6 [DOI] [PubMed] [Google Scholar]
- 57.Del Sorbo L, Ranieri VM, Keshavjee S. Extracorporeal membrane oxygenation as “bridge” to lung transplantation: what remains in order to make it standard of care? Am J Respir Crit Care Med 2012;185:699-701 [DOI] [PubMed] [Google Scholar]