Abstract
Since the first successful lung transplant performed three decades ago, the technique of lung transplantation has evolved with acceptable short- and long-term outcomes such that it has become the standard for those with end stage pulmonary disease. Herein, we describe our current favored approach and discuss some of the current areas in need of further investigation as they relate to the technical aspects of the operation.
Keywords: Lung transplantation, surgical technique, end-stage lung disease, bilateral sequential lung transplantation, single lung transplantation
Introduction
Lung transplantation is the most effective treatment modality for end-stage pulmonary disease (1-4). The number of procedures performed continues to increase every year with an estimated 3,000 transplants being performed annually (1). The majority of transplants performed today are bilateral sequential procedures (3). This is supported by evidence within the literature that has identified a long-term survival benefit from bilateral as opposed to single lung transplantation (5,6). Operative techniques and critical care, however, continue to evolve and 1- and 5-year outcomes continue to improve (5,7).
Operative approach
The decision as to whether to use extracorporeal support during bilateral lung transplantation varies with institutional experience and with patient selection. The bulk of the decision-making should be made preoperatively and can be modified based on intraoperative hemodynamic stability. Recipients are prepared for the operating room (OR) well in advance by completing their routine studies. I personally ensure that all recipient studies are confirmed using a standard checklist for our program that includes the detailed review of all pre-operative studies. We also have a pre-operative safety checklist in addition to our institutional OR standards that ensures blood type and serology acknowledgement prior to entering the OR. This is specific to our organ transplant program and is a “hard stop” in the OR flow if the documentation is not completed correctly.
Following appropriate donor selection and communication with the procurement team at the donor site, it is paramount to engage in constructive dialogue with the anesthesiology, perfusion, and OR teams so that intraoperative needs are anticipated ahead of time. This involves a discussion regarding selection of antimicrobial prophylaxis, preoperative inhaled pulmonary vasodilators (such as nitric oxide), the likelihood of requiring cardiopulmonary support, immunosuppression induction, intravascular access, and the availability of blood products. Additionally, any patient or donor-specific nuances are reviewed.
Prior to intubation, two intravenous lines and a radial arterial line are placed. The patient is intubated with a double lumen endotracheal tube that is positioned using fiberoptic bronchoscopy. The time of induction can be very destabilizing and I make it a point to be in the room ready to intervene in case of cardiopulmonary instability. A left femoral arterial line is placed. Venous access is established in the right neck and left groin. If the patient is high risk or the donor lungs of marginal quality, it is prudent for the team to place the right venous neck line in the left neck in the event that post-operative extracorporeal membrane oxygenation (ECMO) may be required (the right neck would be used for a cannula during veno-venous ECMO). Placement of a pulmonary artery (PA) catheter is performed. A transesophageal echo (TEE) probe is placed in the esophagus and routine evaluation performed.
The patient is positioned supine with arms abducted, supported and padded above the head to expose both the chest and the axillary regions (Figure 1). The entire neck, chest, abdomen, and bilateral groins are prepped in the sterile field to allow for access to the femoral vessels in the event of the need for rapid extracorporeal support. The traditional incision used for bilateral lung transplantation is the clamshell incision, but the procedure may also be performed using separate bilateral sternal sparing anterior thoracotomies. I prefer the bilateral thoracosternotomy because of the ability to intervene with central cannulation rapidly if there is any hemodynamic compromise during the operation (Figure 2). This sternal-sparing anterior thoracotomy incision is a nice approach for single lung transplantation as you can easily place the patient on ECMO/CPB via the groin. Early in my practice I performed single lung transplants through posterolateral thoracotomies, but subsequently have switched to the anterior approach because of the ease of cannulation access when the patient is positioned supine.
Figure 1.
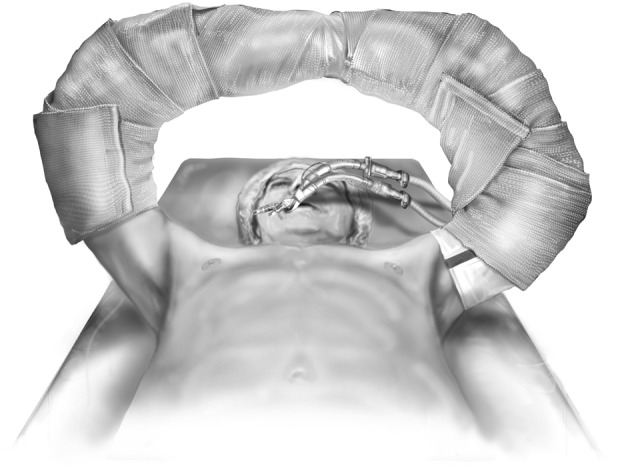
Patient positioning for bilateral sequential lung transplantation.
Figure 2.
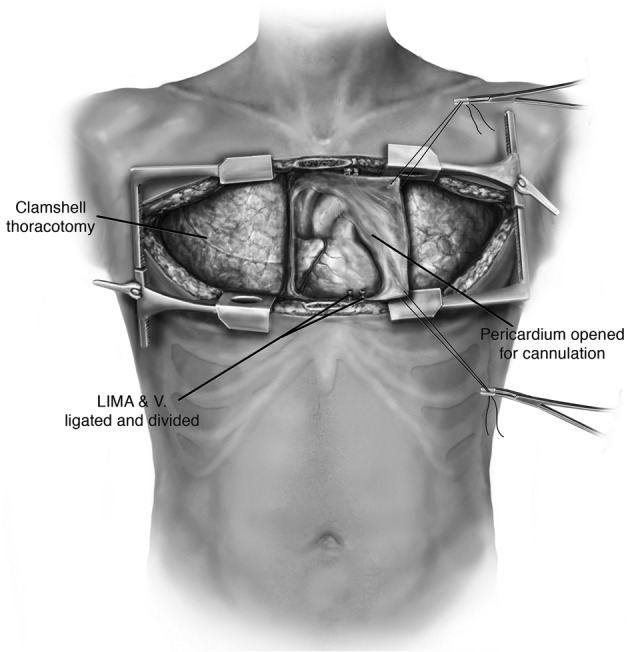
The approach for bilateral thoracosternotomy.
Each of the commonly used incisions is performed by convention in the fourth (idiopathic pulmonary fibrosis) or fifth (emphysema, cystic fibrosis) intercostal space. When a clamshell incision or bilateral thoracosternotomy is performed, special care must be taken to ligate the internal mammary arteries as they can be an inconvenient source of bleeding postoperatively. Once the chest is entered, the internal thoracotomy is completed posteriorly sparing the latissimus dorsi and serratus anterior muscles. Chest retractors are placed. The mediastinal pleura is divided superiorly to the level of the mammary vein and inferiorly to the level of the pericardium.
The choice of which side should be transplanted first may be determined preoperatively by split function testing in which the worse side is transplanted first. There may, however, be other donor and recipient characteristics that dictate this decision. The lungs and chest cavity are inspected for pathologic findings. A figure-of-eight traction suture (0-silk) is placed on the dome of the diaphragm and brought out infero-medially on the external to the body. This is secured with a small clamp. The pericardium may be opened at this point or later in the case in preparation for central cannulation, to aid with hilar dissection, or to allow for intentional cardiac shifting for optimizing hemodynamics (especially for left sided anastomoses). Adhesions encountered within the chest are liberated with electrocautery. The inferior pulmonary ligament is released. The hilar dissection is then carried out and the phrenic nerve is left uninjured. Pneumonectomy is performed in a standard fashion beginning with the division of the inferior pulmonary ligament, the sequential encircling of the PA and pulmonary veins (PV) followed by multiple firings of an endo GIA stapler staying as peripheral as possible. Before stapling the PA, it is snared down using a tourniquet for 5 to 10 minutes to assess hemodynamic stability. In the event of escalating PA pressure, the decision to use cardiopulmonary support should be made. Regardless of the circumstance, I give a small dose of Heparin (100 U/kg) systemically and keep the activated clotting times (ACTs) 160-200 once the PA is clamped. If ECMO is used then I run the ACTs 180-250. If CPB is used, the ACTs are that for standard CPB. Generally, for CPB, my preference is for central cannulation that includes an aortic cannula and a two-stage venous cannula. Of course, cannulae size and other variables are adjusted to the patient characteristics and potential need for additional cardiothoracic procedures (patent foramen ovale closure, coronary artery bypass, etc.).
For the pneumonectomy, the pulmonary vessels are divided first followed by the bronchus. On the right side the bronchus is divided immediately proximal to the takeoff of the right upper lobe. On the left side, I divide the bronchus immediately proximal to the secondary carina. During the division of the bronchus, the fraction of inspired oxygen (FiO2) should be decreased to less than 30% and suction applied to the ipsilateral side through the double lumen ET tube so as to minimize the entrainment of high flow oxygen that could result in sparking a fire due to the simultaneous use of electrocautery. We also flood the field with CO2. Once the pneumonectomy has been performed, the recipient lung is cultured and then sent for permanent fixation, sectioning, and pathological examination.
The hilum is then prepared by circumferentially opening the pericardium (Figure 3). This affords mobilization of the PVs and PA to admit clamps. The bronchus is prepared centrally and cut with an angled scalpel at the desired length. On the right side I prefer to cut at 2 rings from the carina. During this preparation the mediastinal lymph nodes are liberated such that a safe anastomosis may be performed. Bronchial arteries are ligated with cautery and clips to prevent significant bleeding. Denudation of the recipient bronchus should be avoided to prevent ischemic complications (8-10). Any secretions within the bronchus are suctioned liberally and the double lumen endotracheal tube is adjusted appropriately. The pleural space and bronchus are irrigated liberally with antibiotic-containing solution. The amount and content of irrigation is typically recipient and center-dependent.
Figure 3.
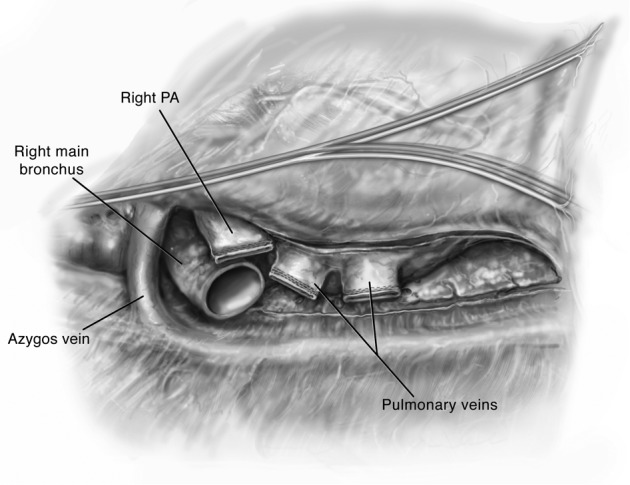
The view of the right hilum following recipient pneumonectomy (right side is shown).
Back table preparation is performed to ready the donor lungs for implantation. With the graft on ice, the bronchus, PVs, and PA are prepared. The donor bronchus is cultured. Extra tissue from the procurement is removed sharply or with electrocautery. The donor bronchus is trimmed to within approximately 1-2 rings from the lobar takeoffs. We use crushed ice to keep the recipient thoracic cavity cool during the implantation with a “phrenic pad” placed in situ to protect graft from warming and from direct contact with the body wall. The implantation is then conducted sequentially beginning with the most posterior anatomical structure, the bronchial anastomosis (Figure 4). The bronchial anastomosis is completed using a running 3-0 polypropylene suture which begins with the membranous portion of the airway and ends anteriorly on the cartilaginous portion. The anastomosis is performed in an end-to-end fashion taking great care to achieve membranous-to-membranous and cartilaginous-to-cartilaginous apposition. My preference is to reinforce the suture line at 10 and 2 o’clock with two additional 3-0 polypropylene stitches thereby locking the continuous suture line in place. The anastomosis is immediately inspected using bronchoscopy. In our experience, we routinely tack an edge of intervening donor pericardium to separate the bronchus from the PA.
Figure 4.
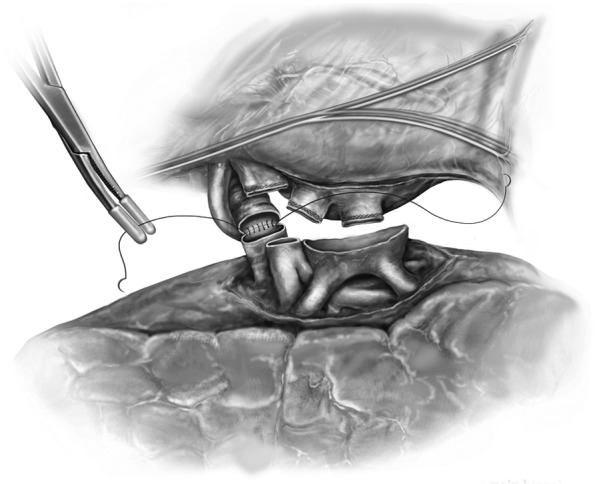
The bronchial anastomosis.
The PA anastomosis is fashioned next following the infusion of 500-700 mL of pulmoplegia into the PA using a handheld antegrade cannula. This flows from retrograde exiting through the PV and is recirculated using “cell saver”. A Satinsky clamp is placed proximally on the PA and the staple line is removed. The donor PA is trimmed to an appropriate length. Care must be taken to not leave the donor PA too long or too short such that problems with kinking or tearing are avoided respectively. The PAs are aligned and anastomosed using a continuous 5-0 polypropylene suture (Figure 5). At the completion of the suture line, they are clamped and not secured until later.
Figure 5.
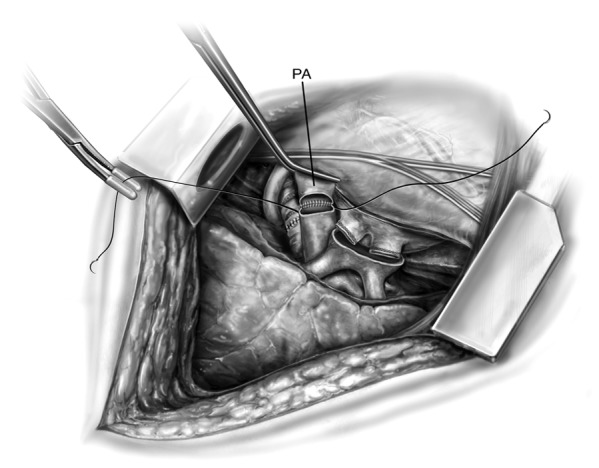
The pulmonary artery (PA) anastomosis.
The left atrial anastomosis is next and this is aided by circumferential mobilization of the left atrium within the pericardium. A large Satinsky clamp is placed on the body of the left atrium. The staple lines of the superior PV and the inferior PV are excised and connected creating a recipient cuff for anastomosis. An endothelial to endothelial, end-to-end anastomosis is then performed using a running 4-0 polypropylene suture (Figure 6). Attention is taken to include the intima and exclude the muscle from the suture line. As the anastomosis nears completion, the anesthesiologist should administer 250-500 mg IV methylprednisolone.
Figure 6.

The left atrial anastomosis.
We do not immediately knot down the anastomoses and instead allow for flushing and de-airing using 500-700 mL of “hotshot pulmoplegia” administered using a handheld cardioplegia cannula in antegrade fashion, thereby reperfusing the allograft. The Satinsky clamp on the PV is then partially opened to deair and the PV knot is tied. The PA is then unclamped over the course of 5-15 minutes and the suture line is secured. This affords controlled low pressure perfusion of the lung. Ventilation with minimal FiO2 (preferably less than 30%) is initiated by hand and then by mechanical ventilation. A gentle Valsalva may be performed to overcome atelectatic de-recruitment and allow for efficient expansion of the lung. At this point the chest is irrigated and the bronchus tested for leak under saline immersion to a pressure of 25-35 cm H2O. Once satisfied with this, positive end-expiratory pressure (PEEP) is set at 8-10 cm H2O and the patient is ventilated under pressure control or with tidal volumes approximately 5-7 mL/kg donor weight. Intraoperative TEE is utilized to evaluate for de-airing and gradient measurement across the PVs and PA. The suture lines are inspected for hemostasis and once satisfied with this, the patient is allowed to recover during this time for 10-15 minutes before the opposite side is addressed in an exact analogous fashion.
By convention, we place three chest tubes in each pleural cavity. A large bore chest tube is positioned anteriorly in the chest. A 24F Blake drain is placed along the diaphragm and posteriorly towards the apex in the chest. A third large bore right angled chest tube is placed posterolaterally. This is the same for each chest. If the pericardium was opened as I do in the vast majority of transplants, a 24F Blake drain is placed in the pericardium. The bilateral thoracotomy incision is closed using interrupted #5 Poly (ethylene, terephthalate) suture in a figure-of-eight fashion. The sternum approximated using three number 6 sternal wires. The pectoral fascial layer, the subcutaneous layer, the subdermal layer, and the skin are reapproximated with absorbable suture. Recently, we have been much more liberal with staples for skin closure. If the lungs are oversized, there is significant PGD, or hemodynamic instability, we leave the chest open according to that method previously described (11).
The double lumen endotracheal tube is exchanged for a single lumen endotracheal tube and bronchoscopy is performed for pulmonary toilet immediately post procedure. During this time a nasoenteric feeding tube is also placed with the added benefit of performing this under endoscopic control of the airway to avoid the inadvertent placement of the feeding tube within the airway. We typically use conservative FiO2 concentration of 40% in the immediate postoperative phase to avoid theoretical risk of free radical-induced oxygen toxicity and PEEP of 10. Adjuncts such as Nitric oxide and epoprostenol should be weaned off expeditiously in the first 12-24 hours postoperatively to allow for prompt extubation.
Areas of debate related to technique
There have been a number of unsuccessful efforts in the past at reaching a consensus regarding various technical aspects of lung transplantation. Attempts, for example, made to reduce the incidence of the risk of airway complications have resulted in the varying popularity of a number of techniques (12-14). This has included telescoping of the bronchial anastomosis, the use of vascularized pedicle flaps, and even bronchial artery revascularization (15-21). We believe that the increased technical detail and variation in experience with these various steps has not allowed for any consensus beyond what we have described in this report. We also recognize that the intraoperative use of pulmoplegia before and after the fashioning of the pulmonary anastomoses is not a universally accepted practice. I have performed transplants both ways and observed no distinct differences. Thus, the use of pulmoplegia is an area deserving of further investigation.
The debate between the use of interrupted versus continuous suture techniques for the bronchial anastomosis continues to garner supporters on either side of the argument. FitzSullivan and colleagues described the use of continuous suture on the membranous bronchus and interrupted figure-of-eight suturing of the cartilaginous bronchus (14). Weder and colleagues on the other hand, prescribe the use of interrupted suture circumferentially around the entire anastomoses (22). Both groups, as is typically the case, reported satisfactory results and a reduction in airway complications. I perform a modified version of the continuous anastomosis placing two additional interrupted sutures for two reasons. One, it allows me to rest more easily knowing that there are additional sutures and the continuous suture line does not depend on one single running polypropylene suture. Two, if the anastomosis falls apart, I can blame myself such that the trainee that typically sews the continuous suture line is alleviated of the responsibility for this complication.
There has also been a growing trend in the use of lobar lung transplantation which has been fueled by the paucity of donors and the increasing need to match larger donors with smaller recipients. This has resulted in an increased consideration for lobar lung transplantation and outcomes have been acceptable where the simpler procedure of graft reduction was not considered a durable option (23-25).
Conclusions
The technique of bilateral sequential lung transplantation has evolved over the years to make it relatively safe operation when combined with careful pre-operative candidate selection, careful donor selection, and advances in critical care. The improvement in early patient survival has been achieved by a reduction in the overall rate of PGD to 5-15%. Post-operatively, severe PGD as marked by hypoxia, pulmonary edema, elevated PA pressures, and poor compliance needs to be recognized early and intervened on. We advocate for early institution of veno-venous (V-V) ECMO when recipients are deteriorating and require FiO2 >70% to better manage the patient and avoid further injury from barotrauma.
The hallmark of post-operative care is a team approach which should mirror that of the team approach to patient selection. This includes involving anesthesia with relationship to pain control as the placement of paravertebral catheters may be of substantial benefit in recovery. There is no doubt that a dedicated intensivist with experience in cardiothoracic surgery is critical to managing fluids, hemodynamics, and optimizing the outcome of end organ perfusion. We have also instituted a clinical pathway to aim towards early extubation and recovery that involves multidisciplinary input from pulmonary medicine, pharmacists, transplant infectious disease, cardiopulmonary rehab, etc. This has led to tremendous progress in lung transplantation over the past several years with 1-year and 5-year survival rates comparable to those of other solid organs. Although organ supply is remains limited, the current era of ex vivo lung perfusion (EVLP) holds great promise for increasing the number of organs available, (re)assessment of graft performance, and potentially repair/reconditioning of donor organs (26,27). This is an exciting time for lung transplantation and investigations into some of the newer areas of lung transplantation, such as EVLP, should afford improved understanding of the nuances of the surgical technique and ultimately translate into improved early and late outcomes for patients with end-stage lung disease.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Christie JD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult lung and heart-lung transplant report--2010. J Heart Lung Transplant 2010;29:1104-18 [DOI] [PubMed] [Google Scholar]
- 2.DeMeo DL, Ginns LC. Lung transplantation at the turn of the century. Annu Rev Med 2001;52:185-201 [DOI] [PubMed] [Google Scholar]
- 3.Cai J, Mao Q, Ozawa M, et al. Lung transplantation in the United States: 1990-2005. Clin Transpl 2005:29-35 [PubMed] [Google Scholar]
- 4.Arcasoy SM, Kotloff RM. Lung transplantation. N Engl J Med 1999;340:1081-91 [DOI] [PubMed] [Google Scholar]
- 5.Yusen RD. Survival and quality of life of patients undergoing lung transplant. Clin Chest Med 2011;32:253-64 [DOI] [PubMed] [Google Scholar]
- 6.Weiss ES, Allen JG, Merlo CA, et al. Survival after single versus bilateral lung transplantation for high-risk patients with pulmonary fibrosis. Ann Thorac Surg 2009;88:1616-25; discussion 1625-6 [DOI] [PubMed] [Google Scholar]
- 7.Yusen RD, Shearon TH, Qian Y, et al. .Lung transplantation in the United States, 1999-2008. Am J Transplant 2010;10:1047-68 [DOI] [PubMed] [Google Scholar]
- 8.Puri V, Patterson GA. Adult lung transplantation: technical considerations. Semin Thorac Cardiovasc Surg 2008;20:152-64 [DOI] [PubMed] [Google Scholar]
- 9.Boasquevisque CH, Yildirim E, Waddel TK, et al. Surgical techniques: lung transplant and lung volume reduction. Proc Am Thorac Soc 2009;6:66-78 [DOI] [PubMed] [Google Scholar]
- 10.Aigner C, Klepetko W.Bilateral lung transplantation. Operative Tech Thorac Cardiovasc Surg 2012;17:181-93 [Google Scholar]
- 11.D’Cunha J, Rueth NM, Belew B, et al. The effectiveness of the “open chest” for the unstable patient after bilateral sequential lung transplantation. J Heart Lung Transplant 2010;29:894-7 [DOI] [PubMed] [Google Scholar]
- 12.Kshettry VR, Kroshus TJ, Hertz MI, et al. Early and late airway complications after lung transplantation: incidence and management. Ann Thorac Surg 1997;63:1576-83 [DOI] [PubMed] [Google Scholar]
- 13.Santacruz JF, Mehta AC. Airway complications and management after lung transplantation: ischemia, dehiscence, and stenosis. Proc Am Thorac Soc 2009;6:79-93 [DOI] [PubMed] [Google Scholar]
- 14.FitzSullivan E, Gries CJ, Phelan P, et al. Reduction in airway complications after lung transplantation with novel anastomotic technique. Ann Thorac Surg 2011;92:309-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fell SC, Mollenkopf FP, Montefusco CM, et al. Revascularization of ischemic bronchial anastomoses by an intercostal pedicle flap. J Thorac Cardiovasc Surg 1985;90:172-8 [PubMed] [Google Scholar]
- 16.Chhajed PN, Malouf MA, Tamm M, et al. Interventional bronchoscopy for the management of airway complications following lung transplantation. Chest 2001;120:1894-9 [DOI] [PubMed] [Google Scholar]
- 17.Higgins R, McNeil K, Dennis C, et al. Airway stenoses after lung transplantation: management with expanding metal stents. J Heart Lung Transplant 1994;13:774-8 [PubMed] [Google Scholar]
- 18.Mizobuchi T, Sekine Y, Yasufuku K, et al. Comparison of surgical procedures for vascular and airway anastomoses that utilize a modified non-suture external cuff technique for experimental lung transplantation in rats. J Heart Lung Transplant 2004;23:889-93 [DOI] [PubMed] [Google Scholar]
- 19.Nicolls MR, Zamora MR. Bronchial blood supply after lung transplantation without bronchial artery revascularization. Curr Opin Organ Transplant 2010;15:563-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siegelman SS, Hagstrom JW, Koerner SK, et al. Restoration of bronchial artery circulation after canine lung allotransplantation. J Thorac Cardiovasc Surg 1977;73:792-5 [PubMed] [Google Scholar]
- 21.Veith FJ, Richards K. Improved technic for canine lung transplantation. Ann Surg 1970;171:553-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weder W, Inci I, Korom S, et al. Airway complications after lung transplantation: risk factors, prevention and outcome. Eur J Cardiothorac Surg 2009;35:293-8; discussion 298 [DOI] [PubMed] [Google Scholar]
- 23.Shigemura N, Bhama J, Bermudez C, et al. Lobar lung transplantation: emerging evidence for a viable option. Semin Thorac Cardiovasc Surg 2013;25:95-6 [DOI] [PubMed] [Google Scholar]
- 24.Inci I, Schuurmans MM, Kestenholz P, et al. Long-term outcomes of bilateral lobar lung transplantation. Eur J Cardiothorac Surg 2013;43:1220-5 [DOI] [PubMed] [Google Scholar]
- 25.Shigemura N, D’Cunha J, Bhama JK, et al. Lobar lung transplantation: a relevant surgical option in the current era of lung allocation score. Ann Thorac Surg 2013;96:451-6 [DOI] [PubMed] [Google Scholar]
- 26.Munshi L, Keshavjee S, Cypel M.Donor management and lung preservation for lung transplantation. Lancet Respir Med 2013;1:318-28 [DOI] [PubMed] [Google Scholar]
- 27.Whitson BA, D’Cunha J. Ex vivo lung perfusion and extracorporeal life support in lung transplantation. Semin Thorac Cardiovasc Surg 2011;23:176-7 [DOI] [PubMed] [Google Scholar]


