Abstract
High-density lipoproteins (HDL) are a target for drug development because of their proposed anti-atherogenic properties. In this review, we will briefly discuss the currently established drugs for increasing HDL-C, namely niacin and fibrates, and some of their limitations. Next, we will focus on novel alternative therapies that are currently being developed for raising HDL-C, such as CETP inhibitors. Finally, we will conclude with a review of novel drugs that are being developed for modulating the function of HDL based on HDL mimetics. Gaps in our knowledge and the challenges that will have to be overcome for these new HDL based therapies will also be discussed.
Keywords: HDL, Cholesterol, Atherosclerosis, Cardiovascular disease, Drugs
1. HDL biology and rationale for HDL raising drugs
High-density lipoproteins (HDL) are one of the four major classes of lipoproteins and based on abundant epidemiological studies,1 as well as animal studies,2,3 they are believed to be atheroprotective unlike low-density lipoproteins (LDL).4 Similar to LDL, we routinely quantify the amount of HDL by determining its cholesterol content (HDL-C).5 The main protein content of HDL is ApoA-I, and it is ∼50% by weight lipid, which mostly consist of phospholipids, free cholesterol, and cholesteryl esters.5
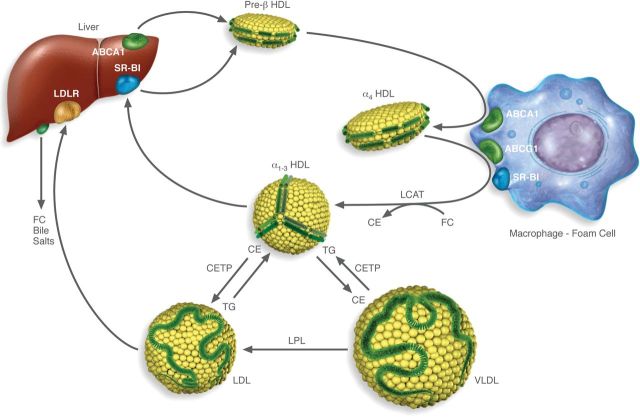
The biology of HDL is complex and a number of putative beneficial anti-atherogenic properties of HDL have been proposed.4 The best understood mechanism for the atheroprotective property of HDL is the Reverse Cholesterol Transport pathway.4 As will be discussed in more detail in the following sections, HDL mediates the removal of excess cellular cholesterol from peripheral cells in concert with ABC transporters and other cell membrane proteins by this pathway and delivers excess cholesterol to the liver and possibly the intestine for excretion (Figure 1). Although the pathophysiological relevance of this pathway in the pathogenesis of atherosclerosis has not been definitively established,4 it still currently serves as the framework for the development and understanding of most HDL drugs.
Figure 1.
Diagram of the Reverse Cholesterol Transport (RCT) pathway. The first step beings with the formation of nascent Preβ-HDL, which largely occurs in the liver and to a lesser degree in the intestine, when apoA-I acquires phospholipid and a small amount of cholesterol by the ABCA1 transporter. Preβ-HDL is then transformed into a larger discoidal species of HDL called α4 HDL when it acquires additional lipid by ABCA1 transporters in the periphery. HDL is then transformed into spherical α1-3 forms of HDL after acquiring additional lipid by other transporters and proteins on cell membranes, such as ABCG1 or SR-BI, or by a passive diffusion process. LCAT is involved in this process by converting cholesterol to cholesteryl esters, which migrate into the core of HDL. Cholesterol on HDL can be delivered directly to the liver after uptake by SR-BI, which then regenerates Preβ-HDL. Alternatively, cholesteryl ester is transferred in exchange for triglycerides to VLDL and LDL by CETP and LDL is eventually delivered to the liver by the LDL-receptor. Cholesterol is then excreted by the liver either as free cholesterol or is converted to a bile salt.
In this review, we will first discuss the current approved drugs for increasing HDL, as well as those being developed, which were primarily designed for raising the cholesterol content of HDL (HDL-C). Because HDL-C may be an imperfect measure of HDL function,2,4 we will also discuss a new class of drugs that were designed based on strategies for modulating the function of HDL.
2. Established drugs for increasing HDL-C levels
The two main classes of drugs in current use that raise HDL-C are nicotinc acid and fibrates. Nicotinic acid is more effective in raising HDL-C, whereas fibrates are more effective in decreasing elevated triglyceride (TG) levels, which are often inversely associated to HDL levels.6,7 Both drugs present some weaknesses, uricosuria, increased glucose tolerance, flushing for nicotinc acid, and problematic pharmacokinetic interactions for fibrates, which limits their use.
Nicotinic acid increases HDL-C plasma levels by inducing hepatic production of apoA-I8 and by inhibiting HDL particle uptake and catabolism in the liver.9 Nicotinic acid also has broad lipid-modulating actions and has been for many years the principal available therapy besides fibrates for raising HDL-C. Following nicotinic acid therapy, HDL-C increases in a dose-dependent manner up to ∼25%, and typically reduces both LDL-C by 15–18% and TG by 20–40%. Nicotinic acid is also the only currently available drug that decreases Lp(a) levels by as much as 30%.10 Several formulations of nicotinic acid have been developed for the purpose of reducing the side effects related to flushing. Recent large clinical trials, however, have failed to show an incremental benefit for these agents in patients with established cardiovascular disease that were also treated with statins.11 The combination drug containing extended-release niacin along with laropiprant also did not significantly reduce the risk of the combination of coronary deaths, non-fatal MI, strokes, or coronary revascularizations compared with statin therapy.12 At the same time, it appeared to significantly increase the risk of non-fatal but serious side effect, which prompted the European Medicines Agency (EMA) to withdraw the authorization for niacin/laropiprant.
The effect of fibrates on plasma lipoproteins and, more specifically on HDL, is mostly attributed to its PPARα-mediated induction of the transcription of apoA-I, as well as other key players in the HDL system, such as ABCA1.13 The induction of also apoA-I and apoA-II synthesis by fibrates leads to a modest increase of HDL-C levels, up to 10–15% in short-term studies and <5% in the long-term intervention trials.14 Fibrates also promote a shift in the LDL-C particle size distribution towards larger, more buoyant particles, which are less susceptible to oxidation and bind with high affinity the LDL receptor.15,16 Due to these effects, fibrates are commonly used in subjects with significant hypertriglyceridaemia, but their long-term effect in reducing cardiovascular events is uncertain.
3. Novel drugs for increasing HDL-C levels
An extensive analysis of HDLomics, as well as human genetic and animal studies, have led to the identification of specific targets for raising HDL-C levels by either modulating CETP activity, increasing ApoA-I expression, and/or interfering with HDL catabolism, which will be discussed in the following section.
3.1. Cholesteryl ester transfer protein inhibitors
As shown in Figure 1, the transfer of cholesteryl esters from HDL to LDL and VLDL is mediated by the cholesteryl ester transfer protein (CETP); as a consequence of this activity, VLDL-C and LDL-C levels increase and HDL-C decrease. CETP also causes the HDL particles to undergo a progressive remodelling as they become enriched in TGs, which are mainly transferred from apo B containing lipoproteins. These TG-rich HDL are a better substrate for hepatic lipase and are then rapidly cleared from the circulation.17
CETP deficiency is associated with increased HDL-C levels, while pathological conditions, such as atherosclerosis were associated with increased CETP activity.18 These findings supported the hypothesis of developing CETP inhibitors to increase HDL-C levels, reduce VLDL-C and LDL-C levels. This strategy, however, is controversial because as shown in Figure 1, much of the cholesterol that returns to the liver in the last part of the RCT pathway is through the uptake of cholesterol from LDL and is dependent upon CETP. The first two developed compounds, torcetrapib and dalcetrapib, were discontinued19,20 either for off-target effects, such as the stimulation of aldosterone synthesis by torcetrapib, thus leading to increased systolic blood pressure,21 or for lack of cardiovascular benefit in the case of dalcetrapib.22,23 Other CETP inhibitors, such as anacetrapib and evacetrapib with even higher CETP inhibitory activity are still under development. Large phase III trials are on-going for these other compounds and the results are expected by 2017.
New CETP-inhibitors are still being developed and investigated. Recently, data from phase 1 study of a newly developed CETP inhibitor called DEZ001/TA-8895 were published.24 DEZ-001/TA-8995 showed nearly complete inhibition of CETP activity (92–99%), increased HDL-C by 96–140%, and decreased LDL-C by 40–53%. The doses used were well tolerated and a larger trial phase2b trial (TULIP) aiming at testing the compound in combination with statins is on-going. Another novel class of potent CETP inhibitors called BAY-38-1315 is based on chromanol derivatives,25 but no clinical studies have been reported.
It is important to note that the current CETP inhibitors in clinical development not only increase HDL-C levels but also reduce LDL-C levels,10,24 an effect which is additive to statin treatment. This raises the possibility that extensive CETP inhibition could be effective on CVD as a consequence of additional LDL-C and VLDL-C reduction rather than due to their ability to increase in HDL-C.
3.2. ApoA-I inducers
Apo-AI is the main constituent of HDL and nascent HDL, which are relatively lipid-poor, and is called preβ-HDL. This HDL subfraction promotes cholesterol efflux from the cell membrane via ABCA-1 and then progressively mature to large discoidal HDL (termed α-HDL) (Figure 1). The latter process is further potentiated by the activity of the Lecithin:Cholesterol Acyltransferase (LCAT) enzyme, which esterifies cholesterol and converts the discoidal shaped, preβ-HDL into larger spherical shaped alpha-HDL. This transformation occurs when cholesteryl esters formed by LCAT partitions into the core of HDL, because of its increased hydrophobicity. The larger spherical forms of HDL use different transporters, such as ATP-binding cassette subfamily G member 1 protein (ABCG-1) and scavenger receptor class B member 1 (SR-BI) to promote additional cholesterol efflux (Figure 1).
Based on our current understanding, the induction of Apo-AI, the main protein component of HDL, should lead to increase HDL-C, which is already discussed is thought to be at least, in part, the mechanism of action for fibrates and nicotinic acid.8 Recently, a novel compound called RVX-208 has been identified,26 which also induces apoA-I. This molecule has a unique mechanism of action related to influencing epigenetically the accessibility of ApoA-I gene by transcription machinery. This effect is mediated by the interaction of RVX208 with the BET proteins, which in turn affect the interaction with acetylated lysine residues on histones.26 While pre-clinical studies in non-human primates showed a strong effect of this compound on HDL-C levels, data in humans were less dramatic.27 More recently, the drug failed to meet its primary outcome on atheroma volume in statin-treated patients (http://www.escardio.org/about/press/press-releases/esc13-amsterdam/Pages/hotline-three-assure.aspx). Data from the SUSTAIN trial, which was aimed at addressing the percentage change in HDL-C levels following 24 weeks RVX-208 administration, were not published yet but will help to understand whether RVX-208 is still a viable pharmacological option for raising HDL-C.
3.3. SR-BI inhibitors
Based on the role of SR-BI in the hepatic uptake of cholesterol from HDL (Figure 1), another potential approach to increase HDL-C would be to inhibit SR-BI. SR-BI is a scavenger receptor that binds a wide variety of lipoproteins and mediates the selective uptake of HDL cholesterol ester (CE), as well as the bidirectional free cholesterol transport at cell membranes. Loss-of-function mutations of SR-BI in humans are associated with increased plasma HDL-C levels, but despite an associated reduction in serum cholesterol efflux potential, no impact of atherosclerosis was reported.28,29 Results from both over expression and knockout studies in mice have questioned, however, the value of the SR-BI inhibition strategy for the prevention of atherosclerosis.30
More recently, SR-BI has also emerged as a critical receptor affecting hepatitis C virus (HCV) entry,31–33 further linking HDL to the immune system.34,35 A human anti–SR-BI mAb has been reported to inhibit HDL binding, to interfere with cholesterol efflux and to decrease HCV entry during attachment steps without having a relevant impact on SR-BI–mediated post-binding steps.36,37 ITX5061, is a SR-BI inhibitor, which is in clinical development as an HCV entry inhibitor (phase I, http://clinicaltrials.gov/ct2/show/NCT01560468?term=ITX+5061&rank=3). This molecule was also tested in patients with low HDL-C and high triglycerides levels and resulted in a 20% increase of HDL-C without influencing other plasma lipids.38 No further development of this drug for cardiovascular applications has been described at this time.
4. Emerging drugs for improving HDL function
Although most of the HDL-related drugs that have been investigated to date have been based on strategies for raising HDL-C, there are several emerging drugs that are being developed for modulating or improving HDL function. This has occurred for several reasons. First as already described, none of the drugs that have been shown to raise HDL-C have been demonstrated to lower cardiovascular events in large clinical trials when used on top of statins. Secondly, although HDL-C levels are inversely related to cardiovascular risk, recent Mendelian randomization studies have questioned whether low HDL-C is causally linked to cardiovascular events.2,4 Other measures of HDL function, in fact, may be superior to HDL-C as cardiovascular risk markers, which has raised the possibility that alternative measures of HDL may be more closely related to its function and therefore be a better target for drug development.2,4
4.1. Increased cholesterol efflux to HDL
The ability of HDL to promote reverse cholesterol transport from the periphery to the liver is generally believed to be one of the main atheroprotective functions of HDL (Figure 1). This process relies on the interaction between HDL and a series of cellular membrane cholesterol transporters, including the ATP-binding cassette transporters A1 and G1 (ABCA1 and ABCG1). Factors that can induce the expression of genes in this pathway are currently being tested, with the rationale that this would lead to an overall increase in the flux of cholesterol through the RCT pathway.39 One of the first class of drugs tested for this are agonists of LXR, a potent transcription factor that regulates many of the genes in the RCT pathway.40 Several LXR agonists have been reported to be atheroprotective in animal models, however, LXR also promotes de novo lipidogenesis in the liver, leading to a fatty liver.40 This finding together with the result of a phase I trial in which central nervous system related adverse events were reported41 have so far limited the clinical development of these compounds.
As an alternative to LXR agonism, gene silencing approaches involving short non-coding RNAs, such as miRNAs, are being explored to control gene expression at the post-transcriptional level.42 MiRNAs are implicated in the pathogenesis of various cardiovascular diseases and hence are potential targets for therapeutic intervention.43 Among several miRNAs that have been shown to influence HDL metabolism, the possibility of interfering with miR-33a and miR-33b is under the most intense development. Pre-clinical studies clearly showed that miR33 inhibition improve hepatic ABCA1 expression and plasma HDL levels44–47 and favour atherosclerosis regression in LDL-R knockout mice.48 Clinical studies are expected to start soon.
4.2. Promote HDL maturation
As already mentioned, a key enzyme in the RCT pathway is LCAT (Figure 1). Genetic deficiency of LCAT is associated with low HDL levels but does not appear to be associated with a marked increase in atherosclerosis.49 LCAT deficiency, however, is associated with the development of chronic kidney disease (CKD).49 Whether impaired LCAT activity explains the prognostic value of reduced plasma HDL-C levels in predicting CKD progression50 is unknown but is under intensive investigation. The use of recombinant human LCAT normalizes plasma lipoprotein profile in LCAT deficiency in mice and in human plasma.51–53 Furthermore, the intravenous infusion in humans of ACP-501 (a form of recombinant LCAT) was shown to increase HDL-C in CAD patients (http://clinicaltrials.gov/ct2/show/NCT01554800?term=ACP-501&rank=1) and improves markers of renal function in an LCAT deficient patient.54 Translating LCAT replacement into larger trials may be challenging given its relatively short half-life of only a few days, but future clinical trials of recombinant LCAT are planned in patients with Familial LCAT Deficiency, as well as for patients with Acute Coronary Syndrome (ACS).
4.3. HDL mimetics
In addition to chronically raising HDL levels with the use of small molecule drugs, novel strategies have been developed for acutely raising HDL and likely its function with mimetics and this treatment is often referred to as HDL Replacement of HDL Infusion Therapy.55 The basis for such a therapy is that it has been shown in a wide variety of animal models that a relatively small number of HDL infusions, and in some cases a single infusion of HDL, can markedly reduce markers of inflammation and the lipid content in atherosclerotic plaque.55,56 Much of this benefit has been attributed to the ability of HDL to promote the efflux of cholesterol from plaque, but it may also relate to the other possible beneficial properties of HDL, such as its anti-inflammatory and anti-oxidant properties.4 Part of the rapid lipid mobilizing effect of HDL infusion can also possibly be related to the egress of macrophage foam cells from plaque, because HDL has been shown to stimulate the expression of chemokine receptors on macrophages.57
The potential patient population for HDL Infusion Therapy is ACS patients, who after first presenting with a myocardial infarction or unstable angina have a high likelihood of developing another event in the subsequent months. Besides the culprit lesion that causes the symptoms in ACS patients, they typically have atherosclerosis throughout their coronary vasculature, which puts them at a high risk of developing another event.58 The expectation is that acute HDL infusion therapy would reduce clinical events in ACS patients by systemically treating the whole coronary vasculature in a more rapid time frame than can be achieved with statins. Although the clinical benefit of HDL infusion therapy has yet been demonstrated, several clinical trials have shown by intravascular ultrasound that HDL infusion therapy can significantly reduce atherosclerotic plaque volume after 5 weeks in patients receiving weekly intravenous infusion therapy with reconstituted HDL.59–61 In one study in patients undergoing femoral atherectomy for peripheral vascular disease, a single infusion of reconstituted HDL markedly reduced histological markers of inflammation and lipid accumulation.62
The HDL mimetics used for this therapy are produced by taking recombinant apoA-I or apoA-I purified from plasma and reconstituting it with phosphatidylcholine and in one case also sphingomyelin.56 One therapeutic product in clinical development called MDCO-216 is produced with ApoA-I Milano instead of wild-type ApoA-I, because the Arg to Cys substitution at position 173 has been proposed to endow this particular form of apoA-I with superior anti-oxidant and cholesterol efflux properties.63 The other two clinical products being tested in early stage clinical trials, CSL-112 and CER-001, are made with wild-type ApoA-I either purified from plasma or produced recombinantly in CHO cells, respectively.56 These forms of reconstituted HDL have been shown to be particularly efficient in promoting cholesterol efflux from cells,64 because they are rich in phospholipid and deplete in cholesterol, but they have also been shown to have some of the other beneficial properties of HDL, such as anti-inflammation.64 Although this appears to be a promising new treatment approach for ACS, only early stage clinical trials have been completed, and there is still much unknown about the optimum formulation or delivery of HDL mimetics.
4.4. ApoA-I mimetic peptides
ApoA-I mimetics, which are short synthetic peptides, were first developed as structural probes of apolipoproteins.65 Later it was shown that these peptides can have biological properties like full-length apoA-I, and in particular can also promote the cholesterol efflux from cells.66 It is somewhat a misnomer, however, to call them apoA-I mimetic peptides, because some of these peptides are based on other apolipoproteins or do not have any primary sequence homology to any known apolipoprotein.67 The key structural motif that they all share and appears to be necessary for these peptides to efflux cholesterol from cells is the presence of an amphipathic helix, which enables these peptides to bind to lipids.66,68 In fact, reconstituting these peptides with phospholipids results in the formation of small discoidal HDL very similar in structure to preβ-HDL.67 Infusion of apoA-I mimetic peptides in animal models as either as the free peptide or as a complex with phospholipids has been shown to mobilize cholesterol from peripheral tissues and reduce atherosclerosis much like full-length ApoA-I.69
The rationale for the development of ApoA-I mimetic peptides is that they may be less expensive to produce and potentially safer than full-length apoA-I purified from plasma or produced recombinantly for HDL infusion therapy.67 In addition, unlike ApoA-I some of these peptides made with D-amino acids are potentially orally available and hence could possibly also be used chronically to raise and modulate HDL function.67 The first ApoA-I mimetic peptide tested in a clinical trial was the D4F peptide, which is synthesized with D-amino acids and contains 4 phenylalanines and hence its name. In a Phase I clinical trial, treatment with oral D4F had no significant effect on HDL-C levels, although it seemed to modestly improve the anti-inflammatory properties of HDL.70 Because only a relatively low plasma level of this peptide was achieved in this trial, another clinical trial involving the intravenous infusion of the same peptide made with L-amino acids called L4F was done, but surprisingly it showed no significant effect on HDL quantity and or function. Recently, it has been proposed that many of the potential beneficial effects of the D4F and L4F peptides on not only atherosclerosis but also on many other animal disease models related to inflammation71 may be due to their ability to alter the absorption and or formation of oxidized lipids from the intestine.72,73 Other ApoA-I mimetic peptides that are currently in pre-clinical stage development67 are either peptides based on ApoE74 or are variants of the L4F peptide, such as the 5A peptide,69 which were specifically designed to promote cholesterol efflux by the ABCA1 transporter.74,75
5. Conclusions
Based on epidemiological studies and animal studies, HDL is an attractive target for drug development for the treatment of cardiovascular disease. The two currently available drugs, niacin and fibrates, have limitations and recently clinical trials have questioned their utility and even the concept of raising HDL for cardiovascular prevention. It is becoming increasingly clear that raising the cholesterol content of HDL (HDL-C) may not be always the best metric for assessing the effect of these drugs and alternative biomarkers based on the other components of HDL and or its function may be needed. Finally, recent advances in our understanding of HDL biology have led to the development of several new approaches for improving or modulating HDL function, but much work remains to fully understand the beneficial properties of HDL and for assessing the clinical utility of these new drugs in large clinical trials based on clinical endpoints.
Conflict of interest: none declared.
Funding
A.T.R. research is supported by intramural research funds from the National Heart, Lung and Blood Institute, NIH. G.D.N. is supported by UNIMI Piano Sviluppo B 2014.
References
- 1.Barter P. HDL-C: role as a risk modifier. Atheroscler Suppl. 2011;12:267–270. doi: 10.1016/S1567-5688(11)70885-6. [DOI] [PubMed] [Google Scholar]
- 2.Rader DJ. Mechanisms of disease: HDL metabolism as a target for novel therapies. Nat Clin Pract Cardiovasc Med. 2007;4:102–109. doi: 10.1038/ncpcardio0768. [DOI] [PubMed] [Google Scholar]
- 3.Escola-Gil JC, Calpe-Berdiel L, Palomer X, Ribas V, Ordonez-Llanos J, Blanco-Vaca F. Antiatherogenic role of high-density lipoproteins: insights from genetically engineered-mice. Front Biosci. 2006;11:1328–1348. doi: 10.2741/1887. [DOI] [PubMed] [Google Scholar]
- 4.Rosenson RS, Brewer HB, Jr, Davidson WS, Fayad ZA, Fuster V, Goldstein J, Hellerstein M, Jiang XC, Phillips MC, Rader DJ, Remaley AT, Rothblat GH, Tall AR, Yvan-Charvet L. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation. 2012;125:1905–1919. doi: 10.1161/CIRCULATIONAHA.111.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenson RS, Brewer HB, Chapman MJ, Fazio S, Hussain MM, Kontush A, Krauss RM, Otvos JD, Remaley AT, Schaefer EJ. HDL measures, particle heterogeneity, proposed nomenclature, and relation to atherosclerotic cardiovascular events. Clin Chem. 2011;57:392–410. doi: 10.1373/clinchem.2010.155333. [DOI] [PubMed] [Google Scholar]
- 6.Pirillo A, Norata GD, Catapano AL. Treating high density lipoprotein cholesterol (HDL-C): quantity versus quality. Curr Pharma Design. 2013;19:3841–3857. doi: 10.2174/13816128113199990298. [DOI] [PubMed] [Google Scholar]
- 7.Pirillo A, Norata GD, Catapano AL. High-density lipoprotein subfractions—what the clinicians need to know. Cardiology. 2013;124:116–125. doi: 10.1159/000346463. [DOI] [PubMed] [Google Scholar]
- 8.Lamon-Fava S, Diffenderfer MR, Barrett PH, Buchsbaum A, Nyaku M, Horvath KV, Asztalos BF, Otokozawa S, Ai M, Matthan NR, Lichtenstein AH, Dolnikowski GG, Schaefer EJ. Extended-release niacin alters the metabolism of plasma apolipoprotein (Apo) A-I and ApoB-containing lipoproteins. Arterioscler Thromb Vasc Biol. 2008;28:1672–1678. doi: 10.1161/ATVBAHA.108.164541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamanna VS, Kashyap ML. Nicotinic acid (niacin) receptor agonists: will they be useful therapeutic agents? Am J Cardiol. 2007;100:S53–S61. doi: 10.1016/j.amjcard.2007.09.080. [DOI] [PubMed] [Google Scholar]
- 10.Norata GD, Ballantyne CM, Catapano AL. New therapeutic principles in dyslipidaemia: focus on LDL and Lp(a) lowering drugs. Eur Heart J. 2013;34:1783–1789. doi: 10.1093/eurheartj/eht088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 12.HPS2-THRIVE Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J. 2013;34:1279–1291. doi: 10.1093/eurheartj/eht055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chinetti G, Lestavel S, Bocher V, Remaley AT, Neve B, Torra IP, Teissier E, Minnich A, Jaye M, Duverger N, Brewer HB, Fruchart JC, Clavey V, Staels B. PPAR-alpha and PPAR-gamma activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat Med. 2001;7:53–58. doi: 10.1038/83348. [DOI] [PubMed] [Google Scholar]
- 14.Franceschini G, Calabresi L, Colombo C, Favari E, Bernini F, Sirtori CR. Effects of fenofibrate and simvastatin on HDL-related biomarkers in low-HDL patients. Atherosclerosis. 2007;195:385–391. doi: 10.1016/j.atherosclerosis.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 15.Fruchart JC, Duriez P. Mode of action of fibrates in the regulation of triglyceride and HDL-cholesterol metabolism. Drugs Today (Barc) 2006;42:39–64. doi: 10.1358/dot.2006.42.1.963528. [DOI] [PubMed] [Google Scholar]
- 16.Shah A, Rader DJ, Millar JS. The effect of PPAR-alpha agonism on apolipoprotein metabolism in humans. Atherosclerosis. 2010;210:35–40. doi: 10.1016/j.atherosclerosis.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Lewis GF, Rader DJ. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ Res. 2005;96:1221–1232. doi: 10.1161/01.RES.0000170946.56981.5c. [DOI] [PubMed] [Google Scholar]
- 18.Parini P, Rudel LL. Is there a need for cholesteryl ester transfer protein inhibition? Arterioscler Thromb Vasc Biol. 2003;23:374–375. doi: 10.1161/01.ATV.0000060447.25136.1C. [DOI] [PubMed] [Google Scholar]
- 19.Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 20.Forrest MJ, Bloomfield D, Briscoe RJ, Brown PN, Cumiskey AM, Ehrhart J, Hershey JC, Keller WJ, Ma X, McPherson HE, Messina E, Peterson LB, Sharif-Rodriguez W, Siegl PK, Sinclair PJ, Sparrow CP, Stevenson AS, Sun SY, Tsai C, Vargas H, Walker M, 3rd, West SH, White V, Woltmann RF. Torcetrapib-induced blood pressure elevation is independent of CETP inhibition and is accompanied by increased circulating levels of aldosterone. Br J Pharmacol. 2008;154:1465–1473. doi: 10.1038/bjp.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rader DJ. Illuminating HDL--is it still a viable therapeutic target? N Engl J Med. 2007;357:2180–2183. doi: 10.1056/NEJMe0707210. [DOI] [PubMed] [Google Scholar]
- 22.Luscher TF, Taddei S, Kaski JC, Jukema JW, Kallend D, Munzel T, Kastelein JJ, Deanfield JE. Vascular effects and safety of dalcetrapib in patients with or at risk of coronary heart disease: the dal-VESSEL randomized clinical trial. Eur Heart J. 2012;33:857–865. doi: 10.1093/eurheartj/ehs019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fayad ZA, Mani V, Woodward M, Kallend D, Abt M, Burgess T, Fuster V, Ballantyne CM, Stein EA, Tardif JC, Rudd JH, Farkouh ME, Tawakol A. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. Lancet. 2011;378:1547–1559. doi: 10.1016/S0140-6736(11)61383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ford J, Lawson M, Fowler D, Maruyama N, Mito S, Tomiyasu K, Kinoshita S, Suzuki C, Kawaguchi A, Round P, Boyce M, Warrington S, Weber W, van Deventer S, Kastelein JJ. Tolerability, pharmacokinetics and pharmacodynamics of TA-8995, a selective cholesteryl ester transfer protein (CETP) inhibitor, in healthy subjects. Br J Clin Pharmacol. 2014 doi: 10.1111/bcp.12380. doi:10.1111/bcp.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vakalopoulos A, Schmeck C, Thutewohl M, Li V, Bischoff H, Lustig K, Weber O, Paulsen H, Elias H. Chromanol derivatives--a novel class of CETP inhibitors. Bioorganic Med Chem Lett. 2011;21:488–491. doi: 10.1016/j.bmcl.2010.10.110. [DOI] [PubMed] [Google Scholar]
- 26.Picaud S, Wells C, Felletar I, Brotherton D, Martin S, Savitsky P, Diez-Dacal B, Philpott M, Bountra C, Lingard H, Fedorov O, Muller S, Brennan PE, Knapp S, Filippakopoulos P. RVX-208, an inhibitor of BET transcriptional regulators with selectivity for the second bromodomain. Proc Natl Acad Sci USA. 2013;110:19754–19759. doi: 10.1073/pnas.1310658110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicholls SJ, Gordon A, Johansson J, Wolski K, Ballantyne CM, Kastelein JJ, Taylor A, Borgman M, Nissen SE. Efficacy and safety of a novel oral inducer of apolipoprotein a-I synthesis in statin-treated patients with stable coronary artery disease a randomized controlled trial. J Am Coll Cardiol. 2011;57:1111–1119. doi: 10.1016/j.jacc.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 28.Vergeer M, Korporaal SJ, Franssen R, Meurs I, Out R, Hovingh GK, Hoekstra M, Sierts JA, Dallinga-Thie GM, Motazacker MM, Holleboom AG, Van Berkel TJ, Kastelein JJ, Van Eck M, Kuivenhoven JA. Genetic variant of the scavenger receptor BI in humans. N Engl J Med. 2011;364:136–145. doi: 10.1056/NEJMoa0907687. [DOI] [PubMed] [Google Scholar]
- 29.Brunham LR, Tietjen I, Bochem AE, Singaraja RR, Franchini PL, Radomski C, Mattice M, Legendre A, Hovingh GK, Kastelein JJ, Hayden MR. Novel mutations in scavenger receptor BI associated with high HDL cholesterol in humans. Clin Genet. 2011;79:575–581. doi: 10.1111/j.1399-0004.2011.01682.x. [DOI] [PubMed] [Google Scholar]
- 30.Mineo C, Shaul PW. Functions of scavenger receptor class B, type I in atherosclerosis. Curr Opin Lipidol. 2012;23:487–493. doi: 10.1097/MOL.0b013e328357ba61. [DOI] [PubMed] [Google Scholar]
- 31.Bartosch B, Verney G, Dreux M, Donot P, Morice Y, Penin F, Pawlotsky JM, Lavillette D, Cosset FL. An interplay between hypervariable region 1 of the hepatitis C virus E2 glycoprotein, the scavenger receptor BI, and high-density lipoprotein promotes both enhancement of infection and protection against neutralizing antibodies. J Virol. 2005;79:8217–8229. doi: 10.1128/JVI.79.13.8217-8229.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voisset C, Callens N, Blanchard E, Op De Beeck A, Dubuisson J, Vu-Dac N. High density lipoproteins facilitate hepatitis C virus entry through the scavenger receptor class B type I. J Biol Chem. 2005;280:7793–7799. doi: 10.1074/jbc.M411600200. [DOI] [PubMed] [Google Scholar]
- 33.von Hahn T, Lindenbach BD, Boullier A, Quehenberger O, Paulson M, Rice CM, McKeating JA. Oxidized low-density lipoprotein inhibits hepatitis C virus cell entry in human hepatoma cells. Hepatology. 2006;43:932–942. doi: 10.1002/hep.21139. [DOI] [PubMed] [Google Scholar]
- 34.Norata GD, Pirillo A, Ammirati E, Catapano AL. Emerging role of high density lipoproteins as a player in the immune system. Atherosclerosis. 2012;220:11–21. doi: 10.1016/j.atherosclerosis.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 35.Norata GD, Pirillo A, Catapano AL. HDLs, immunity, and atherosclerosis. Curr Opin Lipidol. 2011;22:410–416. doi: 10.1097/MOL.0b013e32834adac3. [DOI] [PubMed] [Google Scholar]
- 36.Catanese MT, Ansuini H, Graziani R, Huby T, Moreau M, Ball JK, Paonessa G, Rice CM, Cortese R, Vitelli A, Nicosia A. Role of scavenger receptor class B type I in hepatitis C virus entry: kinetics and molecular determinants. J Virol. 2010;84:34–43. doi: 10.1128/JVI.02199-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Catanese MT, Graziani R, von Hahn T, Moreau M, Huby T, Paonessa G, Santini C, Luzzago A, Rice CM, Cortese R, Vitelli A, Nicosia A. High-avidity monoclonal antibodies against the human scavenger class B type I receptor efficiently block hepatitis C virus infection in the presence of high-density lipoprotein. J Virol. 2007;81:8063–8071. doi: 10.1128/JVI.00193-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masson D, Koseki M, Ishibashi M, Larson CJ, Miller SG, King BD, Tall AR. Increased HDL cholesterol and apoA-I in humans and mice treated with a novel SR-BI inhibitor. Arterioscler Thromb Vasc Biol. 2009;29:2054–2060. doi: 10.1161/ATVBAHA.109.191320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Norata GD, Sala F, Catapano AL, Fernandez-Hernando C. MicroRNAs and lipoproteins: a connection beyond atherosclerosis? Atherosclerosis. 2013;227:209–215. doi: 10.1016/j.atherosclerosis.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calkin AC, Tontonoz P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat Rev Mol Cell Biol. 2012;13:213–224. doi: 10.1038/nrm3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katz A, Udata C, Ott E, Hickey L, Burczynski ME, Burghart P, Vesterqvist O, Meng X. Safety, pharmacokinetics, and pharmacodynamics of single doses of LXR-623, a novel liver X-receptor agonist, in healthy participants. J Clin Pharmacol. 2009;49:643–649. doi: 10.1177/0091270009335768. [DOI] [PubMed] [Google Scholar]
- 42.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 43.Condorelli G, Latronico MV, Dorn GW., II microRNAs in heart disease: putative novel therapeutic targets? Eur Heart J. 2010;31:649–658. doi: 10.1093/eurheartj/ehp573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gerin I, Clerbaux LA, Haumont O, Lanthier N, Das AK, Burant CF, Leclercq IA, Macdougald OA, Bommer GT. Expression of miR-33 from an SREBP2 intron inhibits cholesterol export and fatty acid oxidation. J Biol Chem. 2011;121:2921–2931. doi: 10.1074/jbc.M110.152090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marquart TJ, Allen RM, Ory DS, Baldan A. miR-33 links SREBP-2 induction to repression of sterol transporters. Proc Natl Acad Sci USA. 2010;107:12228–12232. doi: 10.1073/pnas.1005191107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Najafi-Shoushtari SH, Kristo F, Li Y, Shioda T, Cohen DE, Gerszten RE, Naar AM. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science. 2010;328:1566–1569. doi: 10.1126/science.1189123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rayner KJ, Suarez Y, Davalos A, Parathath S, Fitzgerald ML, Tamehiro N, Fisher EA, Moore KJ, Fernandez-Hernando C. MiR-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;328:1570–1573. doi: 10.1126/science.1189862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rayner KJ, Sheedy FJ, Esau CC, Hussain FN, Temel RE, Parathath S, van Gils JM, Rayner AJ, Chang AN, Suarez Y, Fernandez-Hernando C, Fisher EA, Moore KJ. Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J Clin Invest. doi: 10.1172/JCI57275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Calabresi L, Simonelli S, Gomaraschi M, Franceschini G. Genetic lecithin:cholesterol acyltransferase deficiency and cardiovascular disease. Atherosclerosis. 2012;222:299–306. doi: 10.1016/j.atherosclerosis.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 50.Baragetti A, Norata GD, Sarcina C, Rastelli F, Grigore L, Garlaschelli K, Uboldi P, Baragetti I, Pozzi C, Catapano AL. High density lipoprotein cholesterol levels are an independent predictor of the progression of chronic kidney disease. J Int Med. 2013;274:252–262. doi: 10.1111/joim.12081. [DOI] [PubMed] [Google Scholar]
- 51.Simonelli S, Tinti C, Salvini L, Tinti L, Ossoli A, Vitali C, Sousa V, Orsini G, Nolli ML, Franceschini G, Calabresi L. Recombinant human LCAT normalizes plasma lipoprotein profile in LCAT deficiency. Biologicals: J Int Assoc Biol Standard. 2013;41:446–449. doi: 10.1016/j.biologicals.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 52.Rousset X, Vaisman B, Stonik J, Duarte C, Remaley A. Effect of rapid infusion of LCAT on HDL metabolism and its possible utility for enzyme replacement therapy. Atherosclerosis. 2009 abstract e445. [Google Scholar]
- 53.Rousset X, Vaisman B, Auerbach B, Krause BR, Homan R, Stonik J, Csako G, Shamburek R, Remaley AT. Effect of recombinant human lecithin cholesterol acyltransferase infusion on lipoprotein metabolism in mice. J Pharmacol Exp Therap. 2010;335:140–148. doi: 10.1124/jpet.110.169540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shamburek R, Freeman L, Sampson M, Bakker-Arkema R, Krause BR, Auerbach B, Homan R, Shamburek A, Schwartz C, Amar M, Remaley AT. Human enzyme replacement therapy in a patient with familial lecithin cholesterol acyltransferase deficiency: rapid appearance of normal appearing HDL. Circulation. 2013;128:A18673. [Google Scholar]
- 55.Remaley AT, Amar M, Sviridov D. HDL-replacement therapy: mechanism of action, types of agents and potential clinical indications. Expert Rev Cardiovasc Ther. 2008;6:1203–1215. doi: 10.1586/14779072.6.9.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krause BR, Remaley AT. Reconstituted HDL for the acute treatment of acute coronary syndrome. Curr Opin Lipidol. 2013;24:480–486. doi: 10.1097/MOL.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 57.Trogan E, Feig JE, Dogan S, Rothblat GH, Angeli V, Tacke F, Randolph GJ, Fisher EA. Gene expression changes in foam cells and the role of chemokine receptor CCR7 during atherosclerosis regression in ApoE-deficient mice. Proc Natl Acad Sci USA. 2006;103:3781–3786. doi: 10.1073/pnas.0511043103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dobesh PP, Beavers CJ, Herring HR, Spinler SA, Stacy ZA, Trujillo TC. Key articles and guidelines in the management of acute coronary syndrome and in percutaneous coronary intervention: 2012 update. Pharmacotherapy. 2012;32:e348–e386. doi: 10.1002/phar.1225. [DOI] [PubMed] [Google Scholar]
- 59.Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, Eaton GM, Lauer MA, Sheldon WS, Grines CL, Halpern S, Crowe T, Blankenship JC, Kerensky R. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003;290:2292–2300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 60.Nicholls SJ, Uno K, Tuzcu EM, Nissen SE. Lessons from coronary intravascular ultrasound on the importance of raising high-density lipoprotein cholesterol. Curr Atherosclerosis Rep. 2010;12:301–307. doi: 10.1007/s11883-010-0125-4. [DOI] [PubMed] [Google Scholar]
- 61.Tardif JC GJ, L'Allier PL, Ibrahim R, Lesperance J, Heinonen TM, Kouz S, Berry C, Basser R, Lavoie MA GM, Rodes-Cabau J. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. JAMA. 2007;297:1675–1682. doi: 10.1001/jama.297.15.jpc70004. [DOI] [PubMed] [Google Scholar]
- 62.Shaw JA, Bobik A, Murphy A, Kanellakis P, Blombery P, Mukhamedova N, Woollard K, Lyon S, Sviridov D, Dart AM. Infusion of reconstituted high-density lipoprotein leads to acute changes in human atherosclerotic plaque. Circ Res. 2008;103:1084–1091. doi: 10.1161/CIRCRESAHA.108.182063. [DOI] [PubMed] [Google Scholar]
- 63.Calabresi L, Canavesi M, Bernini F, Franceschini G. Cell cholesterol efflux to reconstituted high-density lipoproteins containing the apolipoprotein A-IMilano dimer. Biochemistry. 1999;38:16307–16314. doi: 10.1021/bi991246n. [DOI] [PubMed] [Google Scholar]
- 64.Diditchenko S, Gille A, Pragst I, Stadler D, Waelchli M, Hamilton R, Leis A, Wright SD. Novel formulation of a reconstituted high-density lipoprotein (CSL112) dramatically enhances ABCA1-dependent cholesterol efflux. Arterioscler Thromb Vasc Biol. 2013;33:2202–2211. doi: 10.1161/ATVBAHA.113.301981. [DOI] [PubMed] [Google Scholar]
- 65.Segrest JP, De Loof H, Dohlman JG, Brouillette CG, Anantharamaiah GM. Amphipathic helix motif: classes and properties. Proteins. 1990;8:103–117. doi: 10.1002/prot.340080202. [DOI] [PubMed] [Google Scholar]
- 66.Remaley AT, Thomas F, Stonik JA, Demosky SJ, Bark SE, Neufeld EB, Bocharov AV, Vishnyakova TG, Patterson AP, Eggerman TL, Santamarina-Fojo S, Brewer HB. Synthetic amphipathic helical peptides promote lipid efflux from cells by an ABCA1-dependent and an ABCA1-independent pathway. J Lipid Res. 2003;44:828–836. doi: 10.1194/jlr.M200475-JLR200. [DOI] [PubMed] [Google Scholar]
- 67.Osei-Hwedieh DO, Amar M, Sviridov D, Remaley AT. Apolipoprotein mimetic peptides: mechanisms of action as anti-atherogenic agents. Pharmacol Therap. 2011;130:83–91. doi: 10.1016/j.pharmthera.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Remaley AT, Stonik JA, Demosky SJ, Neufeld EB, Bocharov AV, Vishnyakova TG, Eggerman TL, Patterson AP, Duverger NJ, Santamarina-Fojo S, Brewer HB., Jr Apolipoprotein specificity for lipid efflux by the human ABCAI transporter. Biochem Biophys Res Commun. 2001;280:818–823. doi: 10.1006/bbrc.2000.4219. [DOI] [PubMed] [Google Scholar]
- 69.Amar MJA, D'Souza W, Turner S, Demosky S, Sviridov D, Stonik J, Luchoomun J, Voogt J, Hellerstein M, Sviridov D, Remaley AT. 5A apolipoprotein mimetic peptide promotes cholesterol efflux and reduces atherosclerosis in mice. J Pharmacol Exp Therap. 2010;334:634–641. doi: 10.1124/jpet.110.167890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bloedon LT, Dunbar R, Duffy D, Pinell-Salles P, Norris R, DeGroot BJ, Movva R, Navab M, Fogelman AM, Rader DJ. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J Lipid Res. 2008;49:1344–1352. doi: 10.1194/jlr.P800003-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Van Lenten BJ, Navab M, Anantharamaiah GM, Buga GM, Reddy ST, Fogelman AM. Multiple indications for anti-inflammatory apolipoprotein mimetic peptides. Curr Opin Investig Drugs. 2008;9:1157–1162. [PMC free article] [PubMed] [Google Scholar]
- 72.Navab M, Hough G, Buga GM, Su F, Wagner AC, Meriwether D, Chattopadhyay A, Gao F, Grijalva V, Danciger JS, Van Lenten BJ, Org E, Lusis AJ, Pan C, Anantharamaiah GM, Farias-Eisner R, Smyth SS, Reddy ST, Fogelman AM. Transgenic 6F tomatoes act on the small intestine to prevent systemic inflammation and dyslipidemia caused by Western diet and intestinally derived lysophosphatidic acid. J Lipid Res. 2013;54:3403–3418. doi: 10.1194/jlr.M042051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Remaley AT. Tomatoes, lysophosphatidic acid, and the small intestine: new pieces in the puzzle of apolipoprotein mimetic peptides? J Lipid Res. 2013;54:3223–3226. doi: 10.1194/jlr.E045054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bielicki JK, Zhang H, Cortez Y, Zheng Y, Narayanaswami V, Patel A, Johansson J, Azhar S. A new HDL mimetic peptide that stimulates cellular cholesterol efflux with high efficiency greatly reduces atherosclerosis in mice. J Lipid Res. 2010;51:1496–1503. doi: 10.1194/jlr.M003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sethi AA, Stonik JA, Thomas F, Demosky SJ, Amar M, Neufeld E, Brewer HB, Davidson WS, D'Souza W, Sviridov D, Remaley AT. Asymmetry in the lipid affinity of bihelical amphipathic peptides. A structural determinant for the specificity of ABCA1-dependent cholesterol efflux by peptides. J Biol Chem. 2008;283:32273–32282. doi: 10.1074/jbc.M804461200. [DOI] [PMC free article] [PubMed] [Google Scholar]