Abstract
The developing central nervous system may be particularly sensitive to bisphenol A (BPA)-induced alterations. Here, pregnant Sprague Dawley rats (n = 11–12/group) were gavaged daily with vehicle, 2.5 or 25.0 μg/kg BPA, or 5.0 or 10.0 μg/kg ethinyl estradiol (EE2) on gestational days 6–21. The BPA doses were selected to be below the no-observed-adverse-effect level (NOAEL) of 5 mg/kg/day. On postnatal days 1–21, all offspring/litter were orally treated with the same dose. A naïve control group was not gavaged. Body weight, pubertal age, estrous cyclicity, and adult serum hormone levels were measured. Adolescent play, running wheel activity, flavored solution intake, female sex behavior, and manually elicited lordosis were assessed. No significant differences existed between the vehicle and naïve control groups. Vehicle controls exhibited significant sexual dimorphism for most behaviors, indicating these evaluations were sensitive to sex differences. However, only EE2 treatment caused significant effects. Relative to female controls, EE2-treated females were heavier, exhibited delayed vaginal opening, aberrant estrous cyclicity, increased play behavior, decreased running wheel activity, and increased aggression toward the stimulus male during sexual behavior assessments. Relative to male controls, EE2-treated males were older at testes descent and preputial separation and had lower testosterone levels. These results suggest EE2-induced masculinization/defeminization of females and are consistent with increased volume of the sexually dimorphic nucleus of the preoptic area (SDN-POA) at weaning in female siblings of these subjects (He, Z., Paule, M. G. and Ferguson, S. A. (2012) Low oral doses of bisphenol A increase volume of the sexually dimorphic nucleus of the preoptic area in male, but not female, rats at postnatal day 21. Neurotoxicol. Teratol. 34, 331–337). Although EE2 treatment caused pubertal delays and decreased testosterone levels in males, their behaviors were within the range of control males. Conversely, BPA treatment did not alter any measured endpoint. Similar to our previous reports (Ferguson, S. A., Law, C. D. Jr and Abshire, J. S. (2011) Developmental treatment with bisphenol A or ethinyl estradiol causes few alterations on early preweaning measures. Toxicol. Sci. 124, 149–160; Ferguson, S. A., Law, C. D. and Abshire, J. S. (2012) Developmental treatment with bisphenol A causes few alterations on measures of postweaning activity and learning. Neurotoxicol. Teratol. 34, 598–606), the BPA doses and design used here produced few alterations.
Keywords: bisphenol A, ethinyl estradiol, developmental, behavior, estrous cycle, puberty
Bisphenol A (BPA) is a high volume industrial chemical. More than 90% of the U.S. and Canadian populations exhibit detectable urinary levels of BPA (Bushnik et al., 2010; Calafat et al., 2008). Although there may be other routes of exposure (e.g., dermal), the most common exposure route appears to be oral (Geens et al., 2012). Estimated daily BPA intake is higher for infants and children and highest for infants that are exclusively formula-fed. Formula-fed infants could receive 0.2–0.4 μg BPA/kg body weight/day (Food and Drug Administration, 2009).
Despite the many studies of BPA exposure in laboratory animals, there are widely differing views on the potential relevance of such studies to humans (Beronius et al., 2010). The general consensus is that there may be some risks of BPA exposure during development, particularly central nervous system exposure (National Toxicology Program, 2008). Further, the behaviors potentially altered by developmental BPA exposure may be those that are not required for assessment as detailed in typical test guidelines (e.g., OECD guidelines). For example, in a detailed review of 44 developmental neurotoxicity studies of BPA treatment, Beronius et al. (2013) reported that a higher number of significant BPA effects were detected in anxiety, social, and sexual endpoints than in conventional motor activity endpoints, suggesting the need to assess a wide range of endpoints in behavioral studies of BPA exposure.
Previously, we described few BPA effects on pre- and postweaning behaviors that are not generally thought to be sexually dimorphic (Ferguson et al., 2012, 2011). Our study design incorporated test methods and testing considerations that have been suggested for BPA research (recently reviewed in Shelnutt et al., 2013 and see also Hunt et al., 2009; Li et al., 2008; Richter et al., 2007). Specifically, BPA was orally administered, a reference estrogen-treated group was included, exogenous estrogens were carefully controlled, and potential litter effects were statistically controlled by assessing no more than 1 pup/sex/litter. Here, from that same study design, we describe the results of assessments of behaviors typically thought to be sexually dimorphic as well as pubertal landmark age, adult serum hormone levels, estrous cyclicity, and body weight. Because BPA is a weak estrogen and because androgens/estrogens are critical for sexual differentiation of the brain and behavior of rodents, we tested the hypothesis that BPA could influence sexually dimorphic behaviors when administered during the developmental period that is known to be affected by estrogens in the rat.
MATERIALS AND METHODS
Detailed descriptions of the animals, physical environment, diet, breeding, and treatment have been published (Ferguson et al., 2011, 2012) and are only briefly described below.
Animals
The National Center for Toxicological Research (NCTR) Breeding Colony supplied female (total n = 364) and male (total n = 180) postnatal day (PND) 21 Sprague Dawley rats. At breeding age (see below), those animals became the dams and sires (i.e., F0) of subsequent litters. Ad libitum food (see below for diet) and Millipore filtered water were provided throughout. Housing rooms were maintained on a 12/12-h light/dark cycle (on at 6 a.m.–off at 6 p.m.) at 22 ± 1°C (mean ± SE) and 45–55% humidity. All animal procedures followed the “Guide for the care and use of laboratory animals” (National Research Council, 1996) and were approved in advance by the NCTR Institutional Animal Care and Use Committee.
Housing environment
Rats were housed in polysulfone cages with polysulfone microfilter tops and glass water bottles. All chow was stored in metal containers. This environment began for the F0 generation at PND 21 and continued throughout.
Diet
Upon arrival from the NCTR Breeding Colony and throughout the study, all rats (i.e., F0 and F1) were maintained on a low phytoestrogen chow (TestDiet 5K96 (irradiated pellets), Verified Casein Diet 10 IF, TestDiet, Richmond, IN). Levels of daidzein, genistein, and BPA in six lots of this diet were reported in Delclos et al. (2014) and averaged 0.249 ± 0.064 ppm, 0.374 ± 0.118 ppm, and 2.6 ± 0.8 ppb (mean ± standard deviation), respectively.
Breeding and treatment assignment
Each of the nine breeding rounds was separated by 2 weeks and consisted of a 24 h pairing of an F0 PNDs 87–90 male and female. Following pairing, the female and the cage bottom were visually examined for a sperm plug. Breeding occurred prior to any treatment; thus, success or failure was not treatment-related. Females for which a sperm plug was detected were randomly assigned to one of the six treatment groups within their body weight stratum. This assignment yields approximately equal dam body weight distributions across treatments.
BPA and EE2 treatment
Bisphenol A (2,2-bis(4-hydroxyphenyl)propane, Product no. B0494, TCI America, Portland, OR) and EE2 (17α-ethinyl estradiol, Product no. E4876, Sigma, St Louis, MO) were each dissolved in a 0.3% (by weight) aqueous solution of carboxymethylcellulose sodium salt (CMC, high viscosity) (Product no. C5013, Sigma). CMC was selected as vehicle, rather than an oil, because certain oils may possess estrogenic activity (Ashby and Lefevre, 1997; Ryan, 2005).
Beginning on the morning of gestational day (GD) 6, females were gavaged with 5.0 ml 0.3% CMC/kg/day (vehicle control), 2.5 μg BPA/kg/day (2.5 BPA), 25.0 μg BPA/kg/day (25.0 BPA), 5.0 μg EE2/kg/day (5.0 EE2), or 10.0 μg EE2/kg/day (10.0 EE2) using a Hamilton Microlab 500 system (Hamilton Company, Reno, NV) interfaced with a weight scale and data collection software. An automated algorithm calculated the necessary volume based on each rat's daily body weight. Daily gavage continued through GD 21 (the day prior to parturition). Dams in the naïve control group were removed daily (GDs 6–21) from their home cage, weighed, restrained in the gavage position, but were not gavaged. Thus, there were six treatment groups: (1) naïve control, (2) vehicle control, (3) 2.5 BPA, (4) 25.0 BPA, (5) 5.0 EE2, and (6) 10.0 EE2.
On the day after parturition (PND 1), the eight offspring in each litter (after randomly culling to 4 pups/sex/litter) were orally treated with the same dose and volume (5 ml/kg) as their dams had received. Daily treatment of offspring continued through PND 21. Offspring in the naïve control group were removed daily from their home cage, weighed, and restrained in the gavage position, but were not gavaged.
The two doses of BPA (2.5 and 25.0 μg/kg/day) were specifically selected to be below the no-observed-adverse-effect level (NOAEL) of 5 mg/kg/day. Many of the developmental neurotoxicity effects of BPA have been reported at BPA doses less than the NOAEL; thus, it was essential to investigate doses within that range. The two doses of EE2 (5.0 and 10 μg/kg/day) were selected based on our previous study of dietary EE2 exposure (Ferguson et al., 2003) in which male and female offspring of dams consuming chow containing 200 ppb EE2 (≈10–13 and 18–38 μg/kg/day during pregnancy and lactation, respectively) exhibited reduced body weight but few behavioral alterations.
Postweaning environment
On PND 21, two offspring/sex/litter were weaned, tail tattooed for identification, and pair-housed in caging and with access to ad libitum water and food as described above. With the exception of residential running wheel assessments on PNDs 58–69 and a brief 24 h separation prior to play behavior assessments on PND 35, the rats remained pair-housed with their same-sex sibling throughout the study. Both siblings had been assessed for early preweaning behaviors (Ferguson et al., 2011) and certain postweaning behaviors (Ferguson et al., 2012). For each same-sex sibling pair, one rat was designated as the major behavioral assessment subject and is referred to here as male no. 1 or female no. 1. With the exception of flavored solution intake and female sex behavior, that subject underwent all behavioral assessments as well as measurement of serum hormone levels. The other subject underwent female sex behavior assessments (females only) and flavored solution intake tests (both sexes) and is referred to here as male no. 2 or female no. 2. For each endpoint, typical group subject numbers were 12 naïve controls (i.e., 12/sex), 12 vehicle controls, eleven 2.5 BPA, twelve 25.0 BPA, eleven 5.0 EE2, and twelve 10.0 EE2.
Body weight, food, and water intake
All rats were weighed once weekly beginning at PND 27, except during residential running wheel assessments (PNDs 58–69). Because rats were pair-housed with a same-sex sibling, both rats were weighed and the average of the two body weights was used for statistical analyses. If measured more than once weekly, food and water intakes were first averaged for each week and then divided by two in order to approximate the average intake/rat. Group sizes for these endpoints were: naïve control n = 12/sex; vehicle control n = 12/sex; 2.5 BPA n = 11/sex; 25.0 BPA n = 12/sex; 5.0 EE2 n = 11/sex; 10.0 EE2 n = 12/sex.
Pubertal landmarks
Beginning on PND 1, all offspring/litter were examined daily for developmental and pubertal landmarks. Preweaning landmarks (i.e., ear canal and eye opening) for the subjects of this study have been reported (Ferguson et al., 2011). Postweaning, daily examinations continued in the remaining 2 pups/sex/litter for preputial separation and testes descent (males) and vaginal opening (females) until the landmark was present or until the end of the study. Group sizes for these endpoints were: naïve control n = 12/sex; vehicle control n = 12/sex; 2.5 BPA n = 11/sex; 25.0 BPA n = 12/sex; 5.0 EE2 n = 11/sex; 10.0 EE2 n = 12/sex.
During euthanasia procedures, it was noted that some females appeared to exhibit abnormal genitalia. These were photographed and evaluated by a veterinary pathologist. Sample photos are presented here; however, not all females were examined and the number affected in each treatment group is not known.
Estrous cyclicity
Female no. 1 underwent daily vaginal lavage on PNDs 82–109 for vaginal cytology after manual lordosis assessments (described below). Vaginal cytology slides were air-dried, immersion-fixed in methanol, stained with toluidine blue, coverslipped, and evaluated by light microscopy for determination of estrous phase by experienced personnel. Group sizes for these endpoints were: naïve control n = 12; vehicle control n = 12; 2.5 BPA n = 11; 25.0 BPA n = 12; 5.0 EE2 n = 5; 10.0 EE2 n = 7. EE2 group sizes were smaller as some females in those two groups never experienced vaginal opening.
Serum hormone measures
Serum hormone levels were measured in male and female no. 1 (i.e., the subject that underwent the majority of behavioral assessments). Levels were measured in males of replicates 1–3 at PND 110 and the remaining replicates (i.e., 4–9) at PND 90. Levels were measured in all females at PND 110. Each rat was lightly anesthetized with CO2, after which blood was collected via cardiac puncture. All samples were allowed to clot and then centrifuged. Serum was removed and stored at −80°C until analysis. Most samples were collected between 8:15 a.m. and 12:00 p.m. Through an inadvertent error, samples from females in replicates 6 (n = 8) and 9 (n = 7) were collected between 12:45 and 2:30 p.m. Several hormones measured here exhibit circadian rhythms (e.g., Kalra and Kalra, 1977; Mock et al., 1978). Thus, hormone levels were statistically analyzed twice: once including all subjects and once without the 15 females for which samples were obtained in the afternoon. Further, several hormones measured here are associated with estrous phase. Although estrous phase was not measured on the day of euthanasia (i.e., PND 110), it was measured the day prior as part of the estrous cyclicity assessment. Group sizes for these endpoints were: naïve control n = 12/sex; vehicle control n = 12/sex; 2.5 BPA n = 11/sex; 25.0 BPA n = 12/sex; 5.0 EE2 n = 11 males, 10 females; 10.0 EE2 n = 12/sex.
Total thyroxine (T4), total triiodothyronine (T3), total estradiol (E2), total testosterone, corticosterone, and luteinizing hormone (LH) were analyzed in duplicate with Siemens radioimmunoassay reagents (Los Angeles, CA) and counted on a Perkin Elmer Cobra 5005 gamma counter (Shelton, CT). Leptin (Millipore rat leptin ELISA, Billerica, MA) and total ghrelin (Linco rat/mouse ghrelin ELISA, St Charles, MO) were analyzed via ELISA and read on a BioTek EL808 microplate reader (Winooski, VT). ELISA results were calculated with the instrument's data reduction software using a sigmoidal 4-parameter logistic equation. The radioimmunoassay results were calculated with the instrument's data reduction software from a logit-log representation of the linear calibration curve. Two levels of assayed controls were included in each assay as internal controls.
Whole and regional brain weights
Whole and regional brain weights were measured in those males that were assessed for play behavior and flavored solution intake (i.e., male no. 2). Their paired female sibling (i.e., female no. 2) was not measured as this subject had been assessed for sex behavior and may have been pregnant. Each male was euthanized with CO2 and the whole brain was removed and weighed. Frontal cortex and hippocampus were then dissected as previously described (Ferguson et al., 1993) and weighed. Absolute and body weight adjusted (ratio) whole brain, frontal cortex, and hippocampal weights were subjected to analysis. Group sizes for these endpoints were: naïve control n = 11; vehicle control n = 11; 2.5 BPA n = 10; 25.0 BPA n = 12; 5.0 EE2 n = 9; 10.0 EE2 n = 9.
Play behavior
On PND 34, each same-sex sibling pair was individually housed in clean cages. After 24 h and during the lighted portion of the light cycle, the pair was reunited in their original home cage and behavior was videorecorded for 7 min. A single tester blind to treatment status scored all sessions after training to reliability coefficients of 0.94 or better on the two behaviors. Frequency of pins and dorsal contacts were measured during the last 5 min of the session only as initially there is increased environmental exploration and little play behavior. Group sizes for these endpoints were: naïve control n = 12 pairs/sex; vehicle control n = 12 pairs/sex; 2.5 BPA n = 11 pairs/sex; 25.0 BPA n = 12 pairs/sex; 5.0 EE2 n = 10 male pairs, 11 female pairs; 10.0 EE2 n = 12 pairs/sex.
Residential running wheel activity
On PNDs 58–69, one offspring/sex/litter (male and female no. 1) was housed in a residential running wheel cage (34.3 cm diameter, Mini-Mitter, Sunriver, OR) interfaced with a computer. Number of wheel revolutions was recorded by 10 min intervals and the lighting cycle in the testing room was identical to that of the housing room (12/12-h; 6 a.m.–6 p.m.). On PND 63, each rat was removed briefly for normal husbandry routines. Group sizes for these endpoints were: naïve control n = 12/sex; vehicle control n = 12/sex; 2.5 BPA n = 11/sex; 25.0 BPA n = 12/sex; 5.0 EE2 n = 11/sex; 10.0 EE2 n = 12/sex.
Intake of flavored solutions
On PNDs 59–62, two bottles were placed on the home cage of the remaining offspring/sex/litter (i.e., male and female no. 2). One bottle contained regular water and the other contained a 0.05% (0.0027M) saccharin solution. On PNDs 63–66, a similar procedure was conducted with one bottle of regular water and the other containing a 0.3% (0.0513M) sodium chloride solution. Bottles were weighed daily to determine intake (ml consumed). Prior to statistical analyses, each daily intake (ml) was divided by PND 59 (for saccharin intake data) or PND 63 body weight (for sodium intake data). We have previously shown this endpoint (ml intake/g body weight) to be sexually dimorphic in adult Sprague Dawley rats (Ferguson and Boctor, 2010; Ferguson et al., 2007; Flynn et al., 2005). In addition, saccharin and sodium preference for each of the 3 days was calculated as 100 × (flavored solution intake/total intake) and subjected to analysis as well. Group sizes for these endpoints were: naïve control n = 12/sex for saccharin intake and n = 11/sex for sodium intake; vehicle control n = 12/sex for saccharin intake and n = 11/sex for sodium intake; 2.5 BPA n = 11/sex for saccharin intake and n = 9/sex for sodium intake; 25.0 BPA n = 12/sex for saccharin intake and n = 10/sex for sodium intake; 5.0 EE2 n = 11/sex for saccharin intake and n = 10/sex for sodium intake; 10.0 EE2 n = 12/sex for saccharin and sodium intake.
Manual lordosis measures
On PNDs 96–100, female no. 1 was assessed for manual elicitation of the lordosis response similar to that previously described (Gans and McClintock, 1993; Gans et al., 1995; Hoffman et al., 2002). This assessment was conducted in a test room under dim red light, although during the light period of the light cycle. The home cage was gently opened and an experienced tester attempted to elicit lordosis by using their dominant hand and gently gripping the female on the dorsal side near the middle of the back while allowing the rat's feet to remain firmly on the cage floor. The tester then gently stroked their hand toward the rat's tail using very light pressure on the flanks while at the same time, using one finger to lightly touch the perineal area under the tail. Each female was palpated three times in succession and all palpations were done by the same tester who was blind to treatment status. Each of those three trials was scored on a 0–3 scale: 0 = no reflex at all (back was hunched and head parallel with the bottom of the cage or lower), 1 = marginal reflex (slight flex of spine, slightly raised head and hips with tail base elevated from cage floor), 2 = normal reflex (spine was flexed, head at approximate angle of 30° with horizontal, front paws placed slightly forward and hind legs straightened up stiffly), 3 = intense/exaggerated reflex (pronounced spinal flex, head at an angle of 45° or more with horizontal). Some 5.0 and 10.0 EE2 females scheduled for manual lordosis assessments never experienced vaginal opening (see Results section). Manual lordosis assessments were conducted on those females and the dataset was analyzed twice: once including all females and once without those females that did not experience vaginal opening. Group sizes for these endpoints were: naïve control n = 12; vehicle control n = 12; 2.5 BPA n = 11; 25.0 BPA n = 12; 5.0 EE2 n = 11; 10.0 EE2 n = 12.
Female sex behavior
Female no. 2 was assessed for male-elicited sex behavior on PNDs 96–100. Fifteen experienced Sprague Dawley breeders obtained from the NCTR Breeding Colony were used as stimulus males. Seven were used with females of replicates 1–4 and the remaining eight were used with females of replicates 5–9. Each female subject was placed with a different stimulus male for each of the five test days. Sessions were conducted under dim red lighting. Between 12:30 and 1:00 p.m., the stimulus male was placed into one of four Plexiglas chambers (38.5 cm × 22.0 cm × 30.0 cm) which contained a small amount of bedding. After 10 min, the female was placed into the chamber with the stimulus male and behavior was videorecorded for 20 min. Videorecordings were later scored by a single experienced tester blind to treatment status of the female. For each session, frequency of lordosis postures and hopping or darting episodes exhibited by the female were recorded. In addition, each session was scored for the presence or absence of the following behaviors: female investigation of male anogenital area, male investigation of female anogenital area, female aggression directed at male, and male aggression directed at female. These aggressive behaviors were generally biting (by either sex), jumping on top of male by female (or vice versa), or the female being held down by the male. Some 5.0 and 10.0 EE2 females that were scheduled for female sex behavior assessments never experienced vaginal opening (see Results section). Those females were not tested; thus, group numbers are somewhat smaller for the EE2 groups. Group sizes for these endpoints were: naïve control n = 12; vehicle control n = 12; 2.5 BPA n = 11; 25.0 BPA n = 12; 5.0 EE2 n = 5; 10.0 EE2 n = 7.
Statistical analyses
All analyses were conducted using SAS (version 9.2, SAS Institute Inc., Cary, NC) or SigmaPlot (version 11.0, Systat Software, Inc., San Jose, CA). Significant effects (i.e., those at p < 0.050) were further detailed using appropriate comparisons to the vehicle control group. Litter was the experimental unit in all analyses. Data were analyzed separately by sex for those endpoints in which the variances for males and females were not similar as the analysis of variance (ANOVA) utilizes pooled estimates of variance. Thus, body weight, and food and water intake were analyzed separately by sex, each using a repeated measures (RM) ANOVA with factors of treatment, PND (or week), and the interaction.
Initially, the behavior of the vehicle control group was assessed for sexual dimorphism. For play behavior, t-tests determined the effect of sex in the vehicle control group for frequency of dorsal contacts and pins. For residential running wheel activity, a single datapoint was calculated for each vehicle control subject by averaging over days for each 12-h light period wheel activity and for each 12-h dark period wheel activity. Similarly, averages of the 3 days of saccharin-flavored solution intake/preference and sodium-flavored solution intake/preference were calculated. Each of those datapoints (i.e., light period wheel activity, dark period wheel activity, saccharin-flavored solution intake/preference, and sodium-flavored solution intake/preference) was subjected to a t-test. Where the data were not normally distributed, a Mann-Whitney/Wilcoxon test assessed sex differences.
Because 2/sex/litter were examined for pubertal landmarks (i.e., preputial separation, testes descent, and vaginal opening), litter correlation was accounted for by using a RM ANOVA with a compound symmetric variance structure. If one same-sex sibling did not achieve the landmark, the other sibling's datapoint was used. If both same-sex siblings did not achieve the landmark, that litter was not included in the analysis.
Proportion of days in each estrous phase (proestrus, estrus, and diestrus) for female no. 1 (measured daily on PNDs 82–109) was analyzed via a multivariate analysis of variance (MANOVA) on the arcsine-square root transformed estrous phase relative frequencies. Pairwise comparisons to the vehicle control group were adjusted for multiple comparisons using Dunnett's test. Analysis of aberrant estrous cycles was conducted using a method similar to that described by Girard and Sager (1987). This method uses a Markov chain to model transitions between estrous states. Here, aberrant states were defined as extended diestrus (4 or more consecutive days) or extended estrus (3 or more consecutive days). All other states were defined to be normal. A chi square analysis of transition matrices compared the relative frequencies of transitions involving aberrant states (any transition involving extended diestrus or estrus). Pairwise comparisons to the vehicle control group were adjusted using a Hochberg adjustment.
Serum hormone levels were analyzed via two-way ANOVA with factors of treatment, sex, and the interaction. For subjects in which a level was below the limit of detection, that value (i.e., the limit of detection) was used in the analysis.
Absolute and weight adjusted whole and regional brain weights of male no. 2 were analyzed via one-way ANOVAs with a factor of treatment. Play behaviors were analyzed via two-way ANOVAs with factors of treatment, sex, and the interaction.
Because the estrous cycle can affect residential running wheel activity (Dixon et al., 2003; Eckel et al., 2000; Steiner et al., 1982) and EE2 treatment had a substantial effect on estrous cyclicity (see Results section), running wheel revolutions were first summed for each 12-h light and dark period and then averaged for each subject, resulting in two datapoints for each subject: average light period revolutions and average dark period revolutions. Those data were analyzed separately, each using an ANOVA with treatment, sex, and the interaction as factors. In addition to those typical analyses, running wheel data were modeled using a sigmoidally transformed cosine curve described by Marler et al. (2006) in which it is assumed that activity is cyclical over a 24-h period. This running wheel model was previously shown to be sensitive to cerebellar development alterations (Kim et al., 2010). The parameters of interest were: (1) initial amplitude (on day 1), (2) daily increase in amplitude (the rate at which amplitude increased each day), (3) tmax (the time each day at which maximum activity occurred), (4) beta or activity slope (how quickly activity increased or decreased from the inactive or active portion of the cycle), (5) t1/2 up (the time each day at which increasing activity reached half the amplitude), (6) t1/2 down (the time each day at which decreasing activity reached half the amplitude), (7) duration of maximum activity (the number of hours each day during which the rat had higher activity levels; calculated as t1/2 down − t1/2 up), and (8) duration of minimum activity (the number of hours each day during which the rat had lower activity levels; calculated as t1/2 up on the next day − t1/2 down). The model was modified to account for the increase in activity over days. A “slope” term was included in the amplitude parameter to allow for the probability that amplitude increases over days. Thus, amplitude was split into an intercept and a slope: a0 (baseline amplitude at day 0) and a1 (rate of increase in amplitude/day). The modeled parameters were estimated for each subject and then subjected to exact Mann-Whitney tests. The “time” parameters of tmax, t1/2 up, and t1/2 down are actual clock times on a 24-h clock. Each day, the dark cycle began at approximately 18:30 (6:30 p.m.) and ended at 06:30 a.m. For many rats, the time that maximum activity was achieved, tmax, occurred near midnight. To avoid averaging premidnight values near 24 with postmidnight values near 0, 24 was added to the postmidnight values of tmax prior to averaging; thus, tmax values could range above 24:00.
Intake of regular water and flavored solutions were analyzed using four separate RM ANOVAs: (1) intake of regular water during the time saccharin-flavored solution was available, (2) intake of saccharin-flavored solution, (3) intake of regular water during the time sodium-flavored solution was available, and (4) intake of sodium-flavored solution. Preference was analyzed using two separate RM ANOVAs: (1) saccharin preference, and (2) sodium preference. Each RM ANOVA contained factors of treatment, sex, day, and all interactions as factors.
Manual lordosis of female no. 1 was assessed for five consecutive days. Thus, it was expected that on at least one of those days, the rat would be in estrus and expected to exhibit a lordosis response. Therefore, the number of females with a nonzero response on at least one of the three daily trials was subjected to a two-way RM logistic regression analysis with factors of treatment and days.
During sex behavior assessments of female no. 2, there was a relatively low occurrence of lordosis postures and hops/darts; thus, these endpoints were analyzed as binary variables. Any observation with a lordosis posture or a hop/dart was treated as an occurrence; those with no such behaviors were treated as a nonoccurrence. Data were analyzed using a categorical ANOVA-type model of these binary variables with factors of treatment, test day, and the interaction.
RESULTS
Differences between the Naïve and Vehicle Control Groups
The naïve control group was included as a potential control for stress induced by gavage, because prenatal stress can alter later behaviors (reviewed in Buynitsky and Mostofsky, 2009; Maccari and Morley-Fletcher, 2007; Weinstock, 2001). This became potentially relevant because an earlier study of siblings of the subjects of the current study described differences in the naïve and vehicle control groups (Cao et al., 2013). However, similar to the results of previous behavioral assessments in these subjects (Ferguson et al., 2011, 2012), there were no statistically significant comparisons between the naïve and vehicle control groups in any of the significant main effects of treatment or interactions with treatment described below.
Sexual Dimorphisms in the Vehicle Control Group
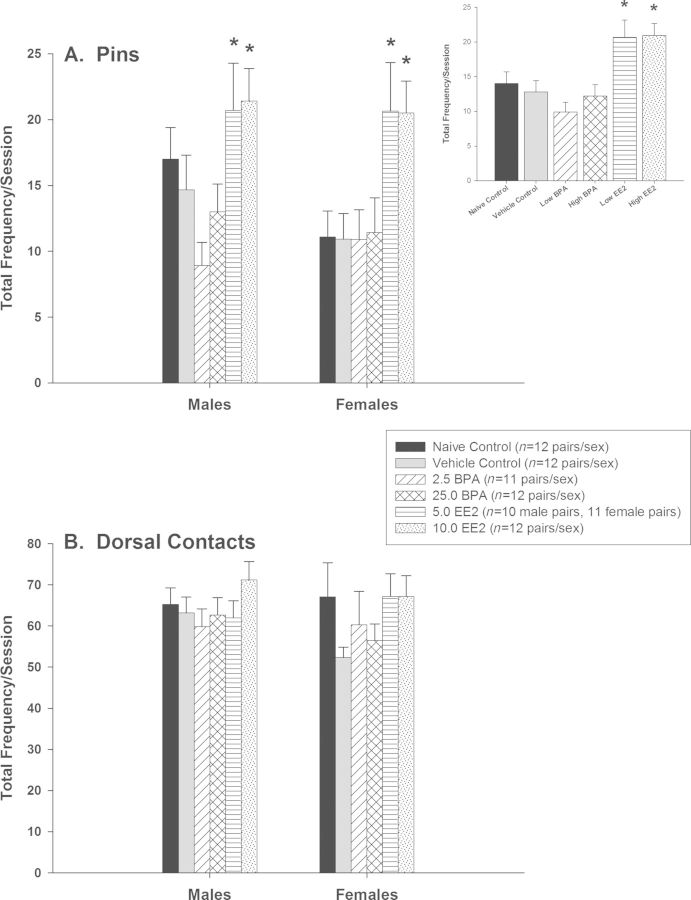
Table 1 shows mean ± SE for these endpoints for the vehicle control group. Vehicle control males performed significantly more dorsal contacts during play behavior assessments than females (df = 22, t = 2.36, p < 0.028). Although a statistically significant effect was not noted for number of pins (Mann-Whitney U = 44.5, p < 0.118), vehicle control males performed an average 34% more pins than vehicle control females. Average light period running wheel activity of vehicle control males was significantly less than that of vehicle control females (df = 22, t = 5.42, p < 0.001). Similarly, vehicle control males were significantly less active in running wheels than vehicle control females during the dark period (Mann-Whitney U = 3.0, p < 0.001). Relative to vehicle control females, vehicle control males consumed significantly less of the saccharin-flavored solution (df = 22, t = 4.12, p < 0.001) and the sodium-flavored solution (df = 20, t = 2.87, p < 0.010) on a ml/kg basis. However, saccharin preference averaged over the 3-day period did not differ between vehicle control males and females (df = 22, t = −0.81, p < 0.428), nor did sodium preference (df = 20, t = −1.35, p < 0.192).
TABLE 1. Sexual Dimorphisms in the Vehicle Control Group (Mean ± SE).
| Assessment | Endpoint | Males | Females |
|---|---|---|---|
| Play behavior | Dorsal contact frequency* | 63.2 ± 3.8 | 52.3 ± 2.5 |
| Pinning frequency | 14.7 ± 2.6 | 10.9 ± 2.0 | |
| Residential running wheel activity (revolutions/12 h) | Light period activity* | 216.7 ± 45.8 | 847.7 ± 107.2 |
| Dark period activity* | 1368.5 ± 246.7 | 5447.0 ± 620.4 | |
| Flavored solution intake (ml consumed/kg body weight) | Saccharin solution* | 0.1386 ± 0.0126 | 0.2426 ± 0.0219 |
| Sodium solution* | 0.0587 ± 0.0062 | 0.0950 ± 0.0110 | |
| Flavored solution preference | Saccharin preference | 88.01 ± 5.48 | 92.89 ± 2.55 |
| Sodium preference | 63.52 ± 6.10 | 73.82 ± 4.56 |
*Indicates a significant difference between vehicle control males and vehicle control females.
Body Weight, Food, and Water Intake
Analysis of male body weight indicated significant main effects of treatment (F(5, 64) = 5.35, p < 0.001) and PND (F(10, 538) = 3638.48, p < 0.001). However, pairwise comparisons did not indicate any significant differences between the vehicle control group and any other treatment group (see Fig. 1A). The main effect of PND indicated that all male groups gained weight with age. Analysis of female body weight indicated a significant interaction of treatment with PND (F(50, 640) = 4.65, p < 0.001). Pairwise comparisons indicated that, beginning at PND 41 and continuing through the end of the study, 5.0 EE2 females weighed more than vehicle control females (all comparisons p < 0.010) (see Fig. 1B). Beginning at PND 55 and continuing through the end of the study, 10.0 EE2 females weighed more than same-sex vehicle controls (all comparisons p < 0.011).
FIG. 1.
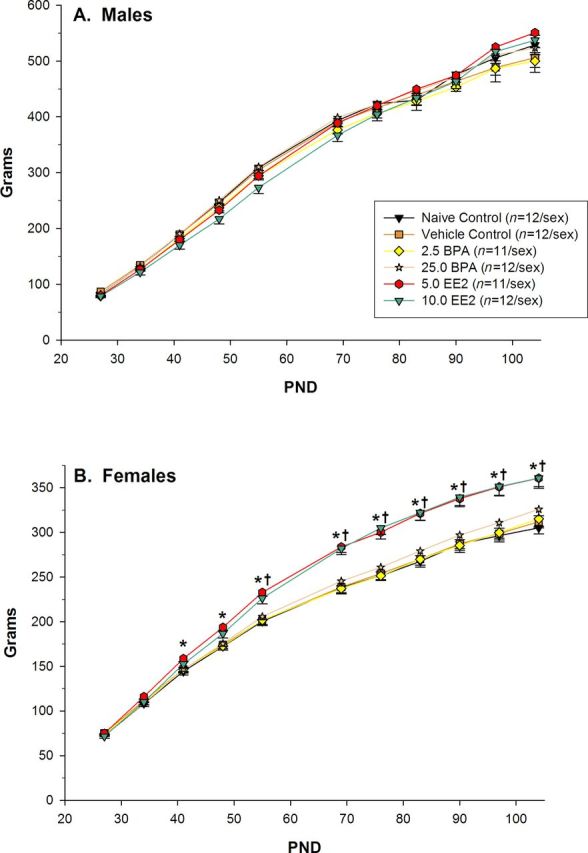
Offspring body weight (mean ± SE). (A) Males: Body weights of males in the BPA or EE2 groups did not differ significantly from same-sex vehicle controls at any age. (B) Females: (*) Body weights of 5.0 EE2 females were significantly more than vehicle control females beginning at PND 41 and continuing throughout the study. (†) Body weights of 10.0 EE2 females were significantly more than vehicle control females beginning at PND 55 and continuing throughout the study.
Analysis of food intake by females indicated a significant interaction of treatment with PND (F(50, 622) = 1.58, p < 0.008) as did the analysis of food intake by males (F(50, 489) = 1.44, p < 0.030) (data not shown). Pairwise comparisons indicated the 5.0 and 10.0 EE2 females consumed more than same-sex vehicle controls the week of PNDs 52–58 (p < 0.046) and the 10.0 EE2 males consumed less than same-sex vehicle controls the week of PNDs 45–51 (p < 0.009); however, there was nothing unusual about those 2 weeks. On any given week, females of the 5.0 EE2 group consumed 3–14% more than same-sex vehicle controls and females of the 10.0 EE2 group consumed −4 to 10% more than same-sex vehicle controls.
Analysis of water intake by females indicated a significant interaction of treatment with PND (F(55, 696) = 2.74, p < 0.001) as did the analysis of water intake by males (F(55, 563) = 1.50, p < 0.015) (data not shown). Pairwise comparisons indicated the 5.0 EE2 females consumed more water than same-sex vehicle controls the week of PNDs 52–58 (p < 0.009) and the 10.0 EE2 females consumed more water than same-sex vehicle controls the weeks of PNDs 66–72 and 73–79 (p < 0.031 for both). Pairwise comparisons of water intake by males indicated that the 5.0 EE2 males consumed more water than same-sex vehicle controls the weeks of PNDs 66–72 and 108–110 (the last week included only 2 days) (p < 0.046 for both).
Pubertal Landmarks
Table 2 shows mean ± SE by treatment group and sex. Testes descent occurred in all male subjects. However, there was a main effect of treatment on PND of testes descent (F(5, 64) = 41.16, p < 0.001). Pairwise comparisons indicated that the 5.0 and 10.0 EE2 groups experienced testes descent later than vehicle control males (p < 0.001 for both comparisons). There was a main effect of treatment on PND of preputial separation (F(5, 63) = 34.03, p < 0.001) (see Table 2). Pairwise comparisons indicated that the 5.0 and 10.0 EE2 groups experienced preputial separation later than vehicle control males (p < 0.020 for both comparisons). Further, three males from two different 10.0 EE2 litters never exhibited preputial separation. There was a main effect of treatment on PND of vaginal opening (F(5, 56) = 4.88, p < 0.001). Pairwise comparisons indicated that the 5.0 and 10.0 EE2 groups experienced vaginal opening later than vehicle control females (p < 0.023 for both comparisons). Further, several females of 5.0 and 10.0 EE2 litters never experienced vaginal opening (see Table 2 for details).
TABLE 2. Pubertal Landmark Age (PND) (Mean ± SE).
| Males | n | Testes descent | Preputial separation |
|---|---|---|---|
| Naïve control | 12 | 22.79 ± 0.19 | 44.88 ± 0.80 |
| Vehicle control | 12 | 23.13 ± 0.21 | 45.21 ± 0.75 |
| 2.5 BPA | 11 | 23.00 ± 0.32 | 44.45 ± 0.49 |
| 25.0 BPA | 12 | 22.88 ± 0.22 | 44.25 ± 0.52 |
| 5.0 EE2 | 11 | 27.27 ± 0.78* | 52.50 ± 1.35* |
| 10.0 EE2 | 11–12a | 32.88 ± 1.27* | 70.45 ± 4.24* |
| Females | n | Vaginal opening | |
| Naïve control | 12 | 35.96 ± 0.77 | |
| Vehicle control | 12 | 35.95 ± 0.88 | |
| 2.5 BPA | 11 | 34.18 ± 0.66 | |
| 25.0 BPA | 12 | 36.55 ± 0.69 | |
| 5.0 EE2 | 7b | 45.43 ± 5.98* | |
| 10.0 EE2 | 8c | 42.94 ± 3.39* |
aBoth male siblings from one 10.0 EE2 dam never experienced preputial separation. One male from a separate 10.0 EE2 dam also never experienced preputial separation, although its same-sex sibling did at PND 78. Thus, n = 11 for preputial separation.
bBoth female siblings from four 5.0 EE2 dams never experienced vaginal opening. One female each from three separate 5.0 EE2 dams also never experienced vaginal opening, although their same-sex sibling did. Thus, n = 7 for this treatment group.
cBoth female siblings from four 10.0 EE2 dams never experienced vaginal opening. One female from a separate 10.0 EE2 dam also never experienced vaginal opening, although its same-sex sibling did. Thus, n = 8 for this treatment group.
*Significantly later than same-sex vehicle controls.
Figure 2 shows examples of normal genitalia in a vehicle control female (Fig. 2A) and the abnormal genitalia noted in some EE2-treated females (Figs. 2B and 2C). Not all females were examined and the number affected in each treatment group is not known.
FIG. 2.
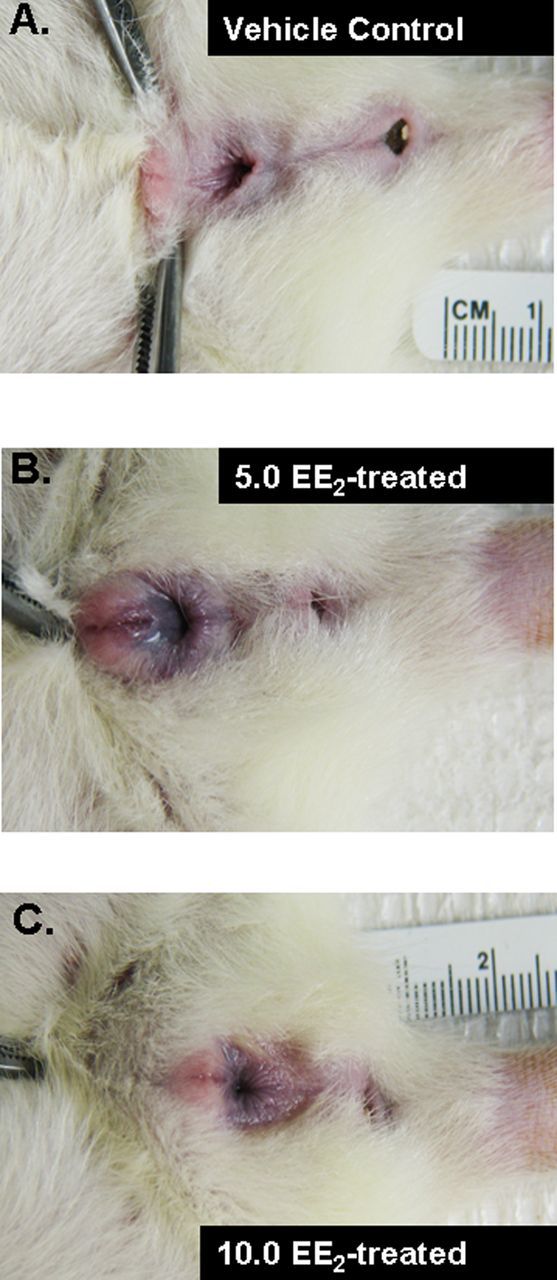
External genitalia of three adult female rats. (A) Vehicle control female. (B) 5.0 EE2-treated female. (C) 10.0 EE2-treated female. Genitalia of the EE2-treated females appear malformed and similar to what Sawaki et al. (see Figure 2 of Sawaki et al., 2003b) described as “excessive cleavage of urethral slit and insufficient raphe formation”.
Serum Hormone Levels
Table 3 shows mean ± SE by treatment group and sex. There were significant main effects of sex in analyses of corticosterone (F(1, 127) = 109.09, p < 0.001) and T4 (F(1, 127) = 45.68, p < 0.001) levels. Corticosterone levels of females were higher than males (350.6 ± 12.3 and 196.3 ± 8.5 ng/ml, respectively) and T4 levels of males were higher than females (4.3 ± 0.1 and 3.2 ± 0.1 μg/dl, respectively). Analysis of testosterone levels indicated a significant interaction of treatment with sex (F(5, 127) = 7.37, p < 0.001). Pairwise comparisons did not indicate any significant differences in female groups; however, testosterone levels of males of both EE2-treated groups were significantly lower than vehicle control males (p < 0.001 for both comparisons). There were no other significant main effects or interactions. Omitting data from the 15 females in which blood was obtained in the afternoon did not change the statistical results. Data from those subjects are included in Table 3.
TABLE 3. Serum Hormone Levels (Mean ± SE)*.
| Estradiol (pg/ml) | Testosterone (ng/dl) | T3 (ng/dl) | T4 (μg/dl)a | Corticosterone (ng/ml)a | Ghrelin (ng/ml) | Leptin (ng/ml) | |
|---|---|---|---|---|---|---|---|
| Males | |||||||
| Naïve control (n = 12) | 13.60 ± 2.67 | 359.020 ± 43.965 | 57.65 ± 3.72 | 4.198 ± 0.318 | 217.70 ± 17.00 | 1.01 ± 0.13 | 7.18 ± 1.50 |
| Vehicle control (n = 12) | 14.47 ± 3.15 | 339.730 ± 45.491 | 57.79 ± 4.22 | 4.180 ± 0.196 | 219.3 ± 19.0 | 0.99 ± 0.13 | 6.75 ± 1.32 |
| 2.5 BPA (n = 11) | 15.21 ± 2.05 | 339.313 ± 63.267 | 47.81 ± 4.92 | 3.724 ± 0.311 | 193.1 ± 23.6 | 0.90 ± 0.11 | 5.62 ± 0.79 |
| 25.0 BPA (n = 12) | 16.15 ± 2.50 | 442.343 ± 71.402 | 56.78 ± 4.43 | 4.578 ± 0.158 | 176.5 ± 22.4 | 1.13 ± 0.21 | 4.05 ± 0.57 |
| 5.0 EE2 (n = 11) | 15.23 ± 2.95 | 118.387 ± 26.074† | 46.61 ± 5.02 | 4.277 ± 0.362 | 160.5 ± 19.5 | 1.11 ± 0.21 | 5.61 ± 0.84 |
| 10.0 EE2 (n = 12) | 16.29 ± 3.20 | 108.779 ± 19.916† | 59.66 ± 4.67 | 4.764 ± 0.330 | 207.3 ± 18.5 | 0.99 ± 0.18 | 8.37 ± 1.75 |
| Females | |||||||
| Naïve control (n = 12) | 15.88 ± 3.17 | 4.346 ± 0.29 | 55.37 ± 3.44 | 2.914 ± 0.191 | 385.9 ± 20.5 | 1.13 ± 0.24 | 4.09 ± 0.94 |
| Vehicle control (n = 12) | 11.96 ± 1.65 | 4.00 ± 0.00 | 49.27 ± 4.84 | 2.914 ± 0.170 | 334.7 ± 35.7 | 1.28 ± 0.30 | 4.20 ± 1.40 |
| 2.5 BPA (n = 11) | 23.52 ± 4.57 | 4.97 ± 0.660 | 52.98 ± 5.45 | 3.029 ± 0.308 | 383.8 ± 19.7 | 1.22 ± 0.28 | 3.88 ± 0.83 |
| 25.0 BPA (n = 12) | 15.34 ± 2.45 | 4.41 ± 0.34 | 63.32 ± 5.46 | 3.473 ± 0.231 | 358.2 ± 34.2 | 0.86 ± 0.17 | 6.43 ± 1.62 |
| 5.0 EE2 (n = 10) | 16.39 ± 2.43 | 4.35 ± 0.35 | 64.48 ± 8.45 | 3.2707 ± 0.2933 | 339.7 ± 26.6 | 1.02 ± 0.20 | 7.59 ± 1.92 |
| 10.0 EE2 (n = 12) | 18.96 ± 3.24 | 4.00 ± 0.00 | 58.24 ± 4.78 | 3.7478 ± 0.2486 | 313.7 ± 29.6 | 1.08 ± 0.25 | 5.75 ± 1.60 |
aSignificant sex effects indicated higher T4 levels in males and higher corticosterone levels in females (neither interacted significantly with treatment).
*Data for LH are not shown as only one subject's level (a vehicle control female) was above the detection limit of 0.15 mIU/ml.
†Significantly lower than vehicle control males.
Estrous Cyclicity
Table 4 shows proportion of days in each phase and percentage of extended estrus transitions by treatment group. Analysis of those proportions indicated a significant effect of treatment (Wilks’ λ (15,141.2) = 2.533, p < 0.003), indicating that the distribution of phases differed between treatment groups. Contrasts indicated that the 10.0 EE2 group experienced a significantly lower proportion of days in diestrus than the vehicle control group (p < 0.002).
TABLE 4. Estrous Phase Proportions and Aberrant Transitions.
| Treatment | Proportion of days in each phase (mean ± SE) | % Extended estrus transitions | |||||
|---|---|---|---|---|---|---|---|
| Diestrus | Proestrus | Estrus | EX to EXb | EX to N | N to EX | Total | |
| Naïve control (n = 12) | 0.57 ± 0.03 | 0.22 ± 0.02 | 0.21 ± 0.03 | 0.72% | 1.44% | 1.44% | 3.60% |
| Vehicle control (n = 12) | 0.59 ± 0.04 | 0.18 ± 0.02 | 0.24 ± 0.04 | 3.54% | 0.39% | 0.79% | 4.72% |
| 2.5 BPA (n = 11) | 0.66 ± 0.04 | 0.16 ± 0.03 | 0.18 ± 0.03 | 0.00% | 0.42% | 0.42% | 0.84% |
| 25.0 BPA (n = 12) | 0.67 ± 0.03 | 0.17 ± 0.01 | 0.16 ± 0.02 | 0.00% | 0.00% | 0.00% | 0.00% |
| 5.0 EE2 (n = 5)a | 0.57 ± 0.07 | 0.18 ± 0.03 | 0.25 ± 0.07 | 0.93% | 3.74% | 4.67% | 9.34%† |
| 10.0 EE2 (n = 7)a | 0.34 ± 0.06* | 0.29 ± 0.10 | 0.37 ± 0.09 | 8.78% | 6.76% | 9.46% | 25.00%† |
aNot all females in the EE2 groups experienced vaginal opening, thus, the number of subjects in these groups is less.
bEX to EX indicates a transition from extended estrus to extended estrus; N to EX indicates a transition from normal to extended estrus.
*Significantly less than vehicle control group.
†Significantly more than vehicle control group.
Chi square analysis of all types of aberrant cycle transitions indicated significant differences between the vehicle control group and the two EE2 groups (5.0 EE2 group: χ2 = 18.64, df = 4, p < 0.004; 10.0 EE2 group: χ2 = 29.32, df = 4, p < 0.001). There were no significant differences between the vehicle control group and any treated group in the analysis of extended diestrus transitions; however, both EE2 groups had significantly more extended estrus transitions than the vehicle control group (p < 0.005 for both comparisons) (see Table 4).
Whole and Regional Brain Weights
Analyses of whole brain, frontal cortex, and hippocampal weights of males did not indicate any significant treatment effects, nor did analyses of ratios of those weights to body weight (data not shown).
Play Behavior
Analysis of frequency of pins/play behavior pair indicated a significant effect of treatment (F(5, 127) = 6.59, p < 0.001) (see Fig. 3A). Pairwise comparisons indicated that the 5.0 and 10.0 EE2 groups engaged in more frequent pins than vehicle controls (p < 0.012 for both comparisons). Analysis of frequency of dorsal contacts/play behavior pair did not indicate any significant effects (see Fig. 3B).
FIG. 3.
Play behavior of male and female offspring (mean ± SE). (A) Frequency of pins by treatment group and sex. A significant effect of treatment indicated that the 5.0 and 10.0 EE2 groups engaged in more pinning behavior than the vehicle control group. The treatment × sex interaction was not statistically significant (p = 0.691). Inset shows the effect by treatment group. (B) Frequency of dorsal contacts by treatment group and sex. There were no significant treatment group or sex differences on this play behavior endpoint.
Residential Running Wheel Activity
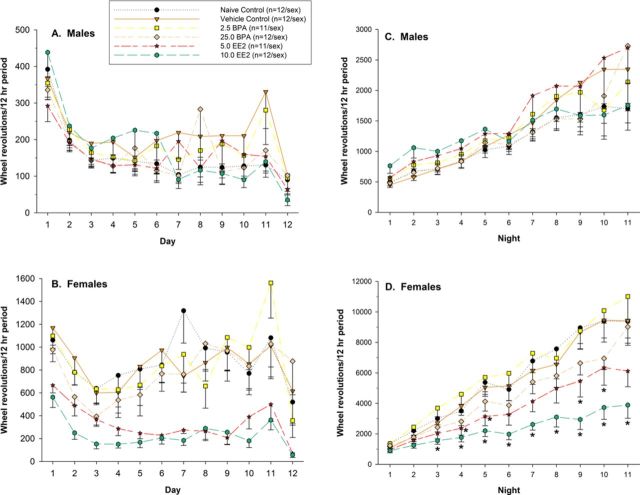
Although statistical analyses were conducted on averaged day or night activity, the online Supplementary data contains graphs of the activity by individual days and nights for each treatment group and sex to indicate the increasing activity with time. Analysis of average light period activity indicated a significant interaction of treatment with sex (F(5, 128) = 7.83, p < 0.001). There were no statistically significant comparisons between the vehicle control males and any same-sex BPA or EE2 group (see Fig. 4A). However, pairwise comparisons indicated that average light period activity of females of the 5.0 and 10.0 EE2 groups was less than that of vehicle control females (p < 0.001 for both comparisons) (see Fig. 4B).
FIG. 4.
Light and dark period residential running wheel activity (average number of wheel revolutions/12 h period) (mean ± SE). (A and B) Light period activity. *Pairwise comparisons of the significant interaction of treatment and sex indicated that females of the 5.0 and 10.0 EE2 groups were significantly less active than vehicle control females. (C and D) Dark period activity. (*) Pairwise comparisons of the significant interaction of treatment and sex indicated that females of the 5.0 and 10.0 EE2 groups were significantly less active than vehicle control females.
Analysis of average dark period activity indicated a significant interaction of treatment with sex (F(5, 128) = 6.75, p < 0.001). There were no significant comparisons among male treatment groups (see Fig. 4C). Pairwise comparisons indicated that females of the 5.0 and 10.0 EE2 groups were less active than vehicle control females (p < 0.012 for both comparisons) (see Fig. 4D).
Table 5 shows the mean ± SE for the parameter estimates from the sigmoidally transformed cosine model of running wheel activity. Analyses of these parameters indicated that the amplitude of activity generally increased across days in all groups. The daily increase in amplitude was significantly higher for females than males (F(1, 128) = 126.90, p < 0.001), regardless of treatment. Additionally, tmax (the time each day at which maximum activity occurred) was significantly earlier for males than females (F(1, 128) = 9.54, p < 0.002) and t1/2 down (the time at which activity began to slow down) was earlier for males than females (F(1, 128) = 10.47, p < 0.001). The daily increase in amplitude was significantly different among treatment groups (F(5, 128) = 5.30, p < 0.001), with a smaller increase in both EE2 groups, particularly at the 10.0 dose, compared with the vehicle control group (p < 0.050). This effect did not interact with sex.
TABLE 5. Parameters Modeled from Residential Running Wheel Activity Dataset (Mean ± SE).
| Naïve control | Vehicle control | 2.5 BPA | 25.0 BPA | 5.0 EE2 | 10.0 EE2 | |
|---|---|---|---|---|---|---|
| Males | ||||||
| Initial amplitude | 9.43 ± 2.57 | 2.57 ± 4.42 | 9.77 ± 4.49 | 4.21 ± 2.07 | 6.50 ± 4.10 | 18.87 ± 5.80 |
| Daily increase in amplitude* | 2.81 ± 0.46 | 4.99 ± 1.65 | 3.52 ± 1.18 | 4.31 ± 0.58 | 4.68 ± 1.79 | 1.96 ± 0.96 |
| tmax†a | 23.98 ± 0.32 | 23.33 ± 0.36 | 23.38 ± 0.49 | 24.22 ± 0.29 | 23.64 ± 0.27 | 24.52 ± 0.46 |
| Beta | 3.87 ± 1.98 | 4.87 ± 2.58 | 4.48 ± 1.36 | 2.09 ± 0.16 | 4.12 ± 1.64 | 3.35 ± 1.22 |
| t1/2 upb | 19.87 ± 0.30 | 19.24 ± 0.37 | 19.34 ± 0.46 | 20.11 ± 0.28 | 19.53 ± 0.27 | 20.44 ± 0.47 |
| t1/2 down#b | 4.10 ± 0.33 | 3.42 ± 0.35 | 3.43 ± 0.51 | 4.33 ± 0.29 | 3.75 ± 0.27 | 4.61 ± 0.46 |
| Maximum activity | 8.23 ± 0.04 | 8.18 ± 0.04 | 8.08 ± 0.10 | 8.22 ± 0.04 | 8.22 ± 0.06 | 8.17 ± 0.09 |
| Minimum activity | 15.77 ± 0.04 | 15.82 ± 0.04 | 15.92 ± 0.10 | 15.78 ± 0.04 | 15.78 ± 0.06 | 15.83 ± 0.09 |
| Females | ||||||
| Initial amplitude | 10.99 ± 9.30 | 7.39 ± 10.22 | 15.21 ± 11.25 | 5.90 ± 6.32 | 6.38 ± 6.76 | 13.22 ± 5.34 |
| Daily increase in amplitudea | 20.57 ± 2.66 | 20.54 ± 3.19 | 21.75 ± 2.69 | 17.13 ± 1.92 | 13.18 ± 2.64 | 6.63 ± 1.94 |
| tmax | 24.69 ± 0.46 | 24.97 ± 0.53 | 24.65 ± 0.53 | 24.95 ± 0.45 | 23.76 ± 0.34 | 24.58 ± 0.47 |
| Beta | 2.90 ± 0.83 | 1.97 ± 0.14 | 1.94 ± 0.14 | 6.52 ± 4.82 | 6.99 ± 4.05 | 7.55 ± 3.71 |
| t1/2 up | 20.55 ± 0.49 | 18.87 ± 1.62 | 20.53 ± 0.53 | 20.83 ± 0.45 | 19.63 ± 0.34 | 20.46 ± 0.48 |
| t1/2 down | 4.84 ± 0.44 | 5.07 ± 0.51 | 4.76 ± 0.53 | 5.06 ± 0.45 | 3.90 ± 0.34 | 4.71 ± 0.46 |
| Maximum activity | 8.29 ± 0.16 | 8.20 ± 0.05 | 8.23 ± 0.02 | 8.23 ± 0.04 | 8.27 ± 0.05 | 8.25 ± 0.08 |
| Minimum activity | 15.71 ± 0.16 | 15.80 ± 0.05 | 15.77 ± 0.02 | 15.77 ± 0.04 | 15.73 ± 0.05 | 15.75 ± 0.08 |
atmax was estimated as the time of day, on a 24-h clock, at which maximum activity occurred. The value, 24, was added to postmidnight tmax estimates to enable averaging with premidnight times. For example, a mean tmax = 24.5 is 30 min after midnight.
bt1/2 up and t1/2 down are estimated as the time of day, on a 24-h clock, at which half of the maximum activity was achieved, respectively, as activity increased or decreased.
*Significantly less for males relative to females (p < 0.001) and significantly less in the 5.0 and 10.0 EE2 groups (p < 0.050).
†Significantly earlier for males relative to females (p < 0.002).
#Significantly earlier for males relative to females (p < 0.001).
Intake of Flavored Solutions
Analyses were conducted on ml intake/g body weight and on preference endpoints. Table 6 shows saccharin and sodium preference by treatment group, sex and day. Analysis of regular water intake during the days that saccharin-flavored solution was available indicated a significant effect of day (F(2, 256) = 4.72, p < 0.010) (data not shown). Pairwise comparisons indicated that regular water intake on day 3 was higher than that on days 1 or 2 (both comparisons p < 0.046).
TABLE 6. Saccharin and Sodium Preference (Mean ± SE).
| Day 1 | Day 2 | Day 3 | Day 1 | Day 2 | Day 3 | |
|---|---|---|---|---|---|---|
| Males | Saccharin preference | Sodium preference | ||||
| Males | ||||||
| Naïve control | 91.04 ± 2.45 | 81.48 ± 7.91 | 71.12 ± 9.60 | 68.47 ± 3.99 | 80.07 ± 3.27 | 59.16 ± 6.89 |
| Vehicle control | 93.05 ± 1.62 | 86.29 ± 7.58 | 84.69 ± 7.82 | 63.10 ± 7.61 | 71.05 ± 7.61 | 54.64 ± 6.36 |
| 2.5 BPA | 87.92 ± 8.85 | 92.77 ± 3.86 | 82.37 ± 6.08 | 72.31 ± 8.60 | 73.81 ± 7.87 | 55.95 ± 11.63 |
| 25.0 BPA | 86.56 ± 8.29 | 85.46 ± 8.40 | 80.86 ± 9.65 | 68.77 ± 6.10 | 75.80 ± 3.98 | 59.39 ± 6.17 |
| 5.0 EE2 | 78.44 ± 10.91 | 68.69 ± 10.56 | 63.99 ± 11.06 | 72.10 ± 3.61 | 84.15 ± 1.85 | 67.95 ± 6.40 |
| 10.0 EE2 | 86.80 ± 7.45 | 83.57 ± 7.27 | 76.76 ± 7.78 | 66.24 ± 3.47 | 78.15 ± 3.79 | 64.92 ± 3.34 |
| Females | ||||||
| Naïve control | 91.79 ± 3.32 | 92.83 ± 2.40 | 83.17 ± 6.94 | 66.67 ± 7.05 | 80.73 ± 5.21 | 64.59 ± 5.96 |
| Vehicle control | 89.55 ± 7.12 | 96.39 ± 0.64 | 92.72 ± 1.31 | 76.74 ± 4.18 | 83.58 ± 3.89 | 61.15 ± 7.65 |
| 2.5 BPA | 88.51 ± 8.00 | 90.35 ± 6.12 | 86.84 ± 8.02 | 75.93 ± 5.37 | 86.64 ± 3.02 | 69.91 ± 6.88 |
| 25.0 BPA | 95.76 ± 0.89 | 95.27 ± 0.77 | 85.82 ± 6.82 | 84.20 ± 3.51 | 80.99 ± 3.99 | 77.69 ± 4.58 |
| 5.0 EE2 | 79.85 ± 10.90 | 80.21 ± 10.45 | 82.41 ± 8.42 | 79.13 ± 3.29 | 82.78 ± 2.50 | 68.41 ± 3.93 |
| 10.0 EE2 | 93.48 ± 3.02 | 92.52 ± 3.29 | 86.77 ± 6.49 | 62.71 ± 7.16 | 66.76 ± 6.85 | 53.26 ± 6.89 |
Analysis of saccharin-flavored intake indicated significant main effects of sex (F(1, 128) = 53.54, p < 0.001) and day (F(2, 256) = 69.46, p < 0.001) (see Fig. 5A). Females consumed significantly more than males on a ml/g basis. Intake on each of the 3 days differed from each other with the highest intake on the first day (all comparisons p < 0.001). Analysis of saccharin preference indicated a significant effect of day (F(2, 251) = 7.82, p < 0.001) and pairwise comparisons revealed that preference on days 1 and 2 was higher than on day 3 (p < 0.001).
FIG. 5.
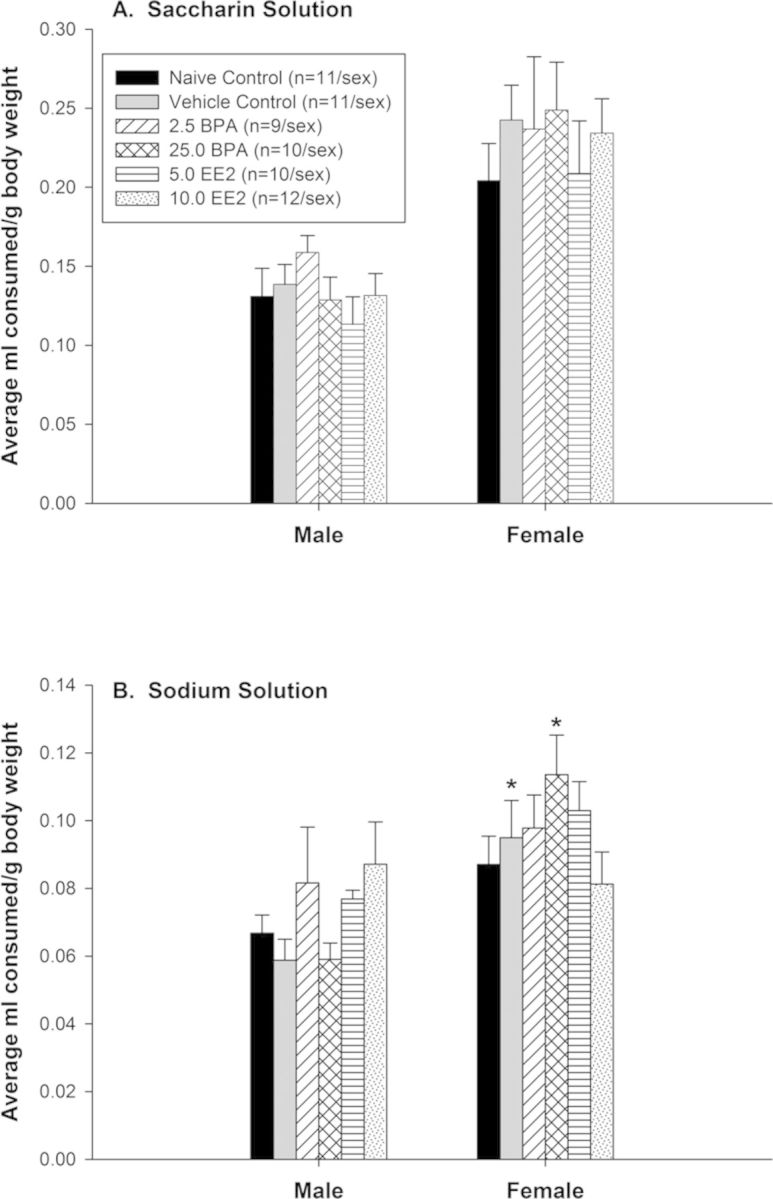
(A) Saccharin-flavored solution intake, (B) sodium-flavored solution intake (mean ± SE). Data were first averaged over the 3 days of intake for each subject. There were no significant treatment-related effects in saccharin-flavored solution intake; however, females consumed significantly more than males. (*) For sodium-flavored solution intake, vehicle control females and 25.0 BPA females consumed more than same-treatment males.
Due to an inadvertent error, the sodium solution concentration for animals in replicate 1 was 3.0% and not 0.3% as intended. Therefore, data for regular water and sodium-flavored solution intake for those 14 subjects were deleted prior to analyses. Analysis of regular water intake during the time that sodium-flavored solution was available indicated significant effects of treatment with sex F(5, 114) = 3.38, p < 0.008) and day (F(2, 226) = 16.73, p < 0.001) (data not shown). Pairwise comparisons indicated that the 10.0 EE2 females consumed more regular water than did vehicle control females (p < 0.001). Intake of regular water during each of the three test days was significantly different with the highest intake on day 3 (all comparisons p < 0.022).
Analysis of sodium-flavored intake indicated significant effects of treatment with sex (F(5, 114) = 2.96, p < 0.016) and day (F(2, 223) = 13.96, p < 0.001) (see Fig. 5B). Pairwise comparisons indicated that the vehicle control females consumed more of the sodium-flavored solution than did vehicle control males (p < 0.006). Similarly, 25.0 BPA females consumed more of the sodium-flavored solution than 25.0 BPA males (p < 0.001); no other comparisons of this effect were significant. Intake on each of the 3 days differed as well (for all comparisons p < 0.029). Analysis of sodium preference indicated a significant interaction of treatment with sex (F(5, 114) = 2.31, p < 0.049); however, there were no significant pairwise comparisons to either the male or the female vehicle control groups. In addition, analysis of sodium preference indicated a significant effect of day (F(2, 222) = 47.65, p < 0.001) and pairwise comparisons revealed that preference on day 1 was less than day 2 (p < 0.001) but higher than day 3 (p < 0.001). Preference on day 2 was higher than on day 3 (p < 0.001).
Female Sex Behavior
There were no significant effects of treatment, test day, or the interaction in the analyses of lordosis postures, hops/darks, male aggression directed at female, female investigation of male anogenital area, or male investigation of female anogenital area. There was, however, a significant effect of treatment on the behavior of female aggression directed at male (χ2 = 15.63, 5 df, p < 0.008). Contrasts of this effect indicated that 10.0 EE2 females exhibited more aggression directed at the male than did vehicle controls (p < 0.007). On average, 29% of the sessions with 10.0 EE2 females contained at least one event of female aggression directed at the male whereas only 7% of the vehicle control female sessions contained a similar event. Each of the seven EE2 females had at least one session in which there was female aggression directed at the male whereas only 4 of the 12 vehicle control female sessions exhibited a similar event.
Manual Lordosis
Analysis of lordosis responses did not indicate a significant treatment effect and excluding the EE2 females without vaginal opening did not change the results (data not shown). When all females were included, the number (and percentage) of females in each group with a nonzero response on at least one of the three trials on each of the 5 days was: 9/12 (75.0%) for the naïve control group, 7/12 (58.3%) for the vehicle control group, 7/11 (63.6%) for the 2.5 BPA group, 8/12 (66.7%) for the 25.0 BPA group, 8/11 (72.7%) for the 5.0 EE2 group, and 10/12 (83.3%) for the 10.0 EE2 group. When the 5.0 and 10.0 EE2 females that never experienced vaginal opening were excluded, the number (and percentage) of females in those two groups with a nonzero response on at least one of the three trials on each of the 5 days was: 3/5 (60.0%) for the 5.0 EE2 group and 5/7 (71.4%) for the 10.0 EE2 group.
DISCUSSION
Developmental BPA treatment at 2.5 or 25 μg/kg/day did not alter the physiological, pubertal, and sexually dimorphic behavioral endpoints measured here. However, those same endpoints were sensitive to developmental treatment with relatively high doses of the reference estrogen, ethinyl estradiol (EE2). Multiple endpoints in both sexes were significantly affected by EE2 treatment, although the number of affected endpoints and their severity were higher in females. Most of the alterations were consistent with previously reported effects of developmental estrogen treatment in laboratory rodents. Further, the EE2-induced behavioral effects described here concur with our previously reported neuroanatomical alterations (i.e., EE2-induced masculinization of females) collected from the same cohort as the current subjects (He et al., 2012). Additionally, the lack of significance for BPA treatment here is consistent with our previous descriptions from the same cohort (Ferguson et al., 2011, 2012).
The behavioral assessments in this study have been sensitive to sex differences in our laboratory (e.g., play behavior (Ferguson and Cada, 2004), light and dark period running wheel activity (Boctor and Ferguson, 2010; Ferguson et al., 2003; Flynn et al., 2000, 2001), saccharin solution intake (Ferguson and Boctor, 2010; Flynn et al., 2000), and sodium solution intake (Ferguson et al., 2003; Flynn et al., 2000, 2005)). Similar sex differences in the expected directions were apparent in the vehicle control group of the current study. The historical literature from our laboratory and the effects in the vehicle control group here provide support for the treatment-related effects described here.
Although the EE2 effects here replicate those previously described (discussed below), the lack of BPA effects at these doses is not entirely consistent with the literature. The methodology used here for assessing play behavior (i.e., a brief separation followed by reuniting a same-sex pair) is standard in the rodent literature, but others have used different methods for examining BPA-induced alterations. Porrini et al. (2005) examined social behavior of female Sprague Dawley rats at PNDs 35, 45, and 55 after orally treating their dams from mating until pup weaning with 40 μg BPA/kg. The subjects were housed with males and females postweaning and prior to assessment, there was no social deprivation. BPA treatment caused increased exploration at PNDs 35 and 45 and decreased play with males (but not females) and social grooming at PND 45. The same lab had earlier reported BPA-induced masculinization of females when behavior was analyzed from minutes 2 and 3 only of a 7-min test session (Dessi-Fulgheri et al., 2002). Dietary BPA exposure (≈5 μg BPA/day) during gestation and lactation appeared to increase the social behaviors of PND 21 female mice (Wolstenholme et al., 2011). However, in none of those three studies was the litter statistically controlled. The multiple reports describing the severity of ignoring litter effects (e.g., Haseman et al., 2001; Holson et al., 2008) clearly indicate that statistical significance may be falsely inflated if the litter is not the unit of analysis.
There appear to be no reports of BPA effects on long-term activity assessments, such as residential running wheel activity. Ryan et al. (2010) described no alterations in the 10 h figure 8maze activity levels of adult male and female rats developmentally treated with BPA; however, the females in that study were ovariectomized at the time of assessment. Thus, the lack of BPA-induced long-term activity alterations reported here awaits replication.
Intake of saccharin or sodium flavored solutions was not altered at the BPA doses administered here. Preference for highly concentrated 0.25 and 0.50% saccharin or 10–15% sucrose solutions has been reported to be altered by developmental BPA treatment at doses of 0.1 and 1.0 mg/l of drinking water (Xu et al., 2011) or subcutaneous injections of 40 μg BPA/kg during adolescence (Diaz Weinstein et al., 2013). However, oral BPA doses of 2, 20, or 200 μg/kg from GD 7 through lactational day 18 to rats did not alter the preference for a 0.25% saccharin solution in their adult male and female offspring (Ryan et al., 2010). There do not appear to be published descriptions of the effects of BPA treatment on intake or preference of sodium flavored solutions.
Developmental BPA treatment at these doses did not alter adult female sexual behavior. This is in contrast to the results of Naule et al. (2014) in which 0.050 (but not 5.0) mg/kg BPA administered orally to mice from GD 15 to lactational day 21 increased the lordosis quotient of their adult ovariectomized and hormonally primed female offspring on the first of two tests. However, similar to the results here, oral BPA doses of 20 or 200 μg/kg from GD 7 through lactational day 18 to rats did not alter the lordosis quotient of their adult ovariectomized and hormonally primed female offspring (Ryan et al., 2010).
Although BPA treatment did not produce substantial alterations, prenatal EE2 treatment followed by direct treatment from birth until weaning appeared to masculinize and/or defeminize females of the current study. These effects were expected given that estradiol, produced via aromatization of androgens, is essential for masculinization of the male rodent central nervous system (reviewed in Lenz and McCarthy, 2010). Here, the masculinization/defeminization of EE2-treated females was apparent in a heavier body weight, delayed or complete lack of vaginal opening, aberrant estrous cyclicity, increased play behavior, decreased running wheel activity, and increased male-directed aggression. Further, most of those effects occurred in both EE2 treatment groups (i.e., 5.0 and 10.0 μg/kg/day). With the possible exceptions of estrous cyclicity and dark period running wheel activity, there were no apparent indications of a dose-response as the effects seemed equally severe in both EE2-treated groups.
Previous studies have described abnormal estrous cyclicity after developmental treatment with estrogens or estrogen-like compounds (e.g., diethylstilbestrol (DES)) (Berretti et al., 2014; Delclos et al., 2009; Kwon et al., 2000; Levy et al., 1995; Willoughby et al., 2005). Here, EE2-treated rats exhibited a significantly lower proportion of days in diestrus and a higher percentage of extended estrus transitions, similar to that reported for dietary EE2 treatment in rats (Delclos et al., 2009) and PNDs 15–18 DES treatment in mice (Nikaido et al., 2005) as well as similar but chronic EE2 treatment (Delclos et al., 2014). Further, prenatal DES or neonatal estradiol benzoate treatment can produce increased body weight in female rats and mice (Donohoe and Stevens, 1983; Nikaido et al., 2004).
The delayed vaginal opening described for the EE2-treated females here was not unexpected as similar EE2 (e.g., 0.5 or 5.0 μg/kg/day) or estradiol benzoate treatment also resulted in vaginal opening delays (Berretti et al., 2014; Delclos et al., 2012, 2014; Sawaki et al., 2003a). Here, EE2 treatment significantly delayed vaginal opening such that EE2-treated rats experienced this landmark almost 7 days later than vehicle control females. Further, 4 of the 12 litters treated with 10.0 μg/kg/day EE2 never experienced vaginal opening. Still, many studies report accelerated vaginal opening as a result of developmental estrogen or estrogen-like compound treatment (Ashby et al., 1997; Nikaido et al., 2004, 2005; Odum et al., 2002; Rothschild et al., 1988; Ryan et al., 2010; Willoughby et al., 2005). However, the compounds, doses, treatment ages/durations, and administration routes of those studies overlap with similar studies in which treatment did not alter age at vaginal opening (e.g., Kwon et al., 2000; Shiorta et al., 2012; Yamamoto et al., 2003). As reviewed by Gorski (1986), even slight differences in the timing of exogenous estrogen treatment (e.g., PNDs 26–30 vs. PNDs 33–37) can affect vaginal opening.
The genital malformations in EE2-treated females of the current study were comparable to those previously reported to be caused by similar EE2 treatment in Long-Evans rats (see Figure 9 of Ryan et al., 2010) and Sprague Dawley rats (see Figure 2 of Sawaki et al., 2003b). Those abnormalities were described as cleft phallus with “excessive cleavage of urethral slit and insufficient raphe formation” (Sawaki et al., 2003b). Such malformations in females can also result from a single injection of estradiol or DES on GD 19 (Henry et al., 1984). Because not all females in the current study were carefully examined, it is not certain that similar abnormalities did not occur in BPA-treated females.
The behavioral masculinization of females by developmental EE2 treatment was reflected in all behaviors assessed here, except flavored solution intake and manual elicitation of lordosis. For example, EE2 treatment resulted in increased play behavior pinning frequencies by female rats similar to that caused by developmental androgen treatment (Meaney, 1989; Meaney and McEwen, 1986; Tonjes et al., 1987). Here, the pinning frequency of EE2-treated females was almost twice that of vehicle control females and nearly 40% more than that of vehicle control males, indicating pronounced masculinization. Masculinization of rodent play behavior had been thought to be due to direct effects on androgen receptors, rather than aromatization effects, as treatment of males with aromatase inhibitors or females with estradiol resulted in few play behavior alterations (Meaney and Stewart, 1981; Tonjes et al., 1987). A more recent study, however, has demonstrated that neonatal estradiol treatment of females at doses which approximate male-typical levels can cause increased play behavior, although that increase was more related to wrestling/boxing and pouncing behaviors than to pinning behavior (Olesen et al., 2005). A previous study of dietary EE2 in our laboratory found no treatment effects on play behavior, despite the use of higher external doses than in the current study (Ferguson et al., 2003). However, in that previous study, postnatal exposure to EE2 occurred via lactation and it is likely that the offspring were exposed to a lower dose postnatally relative to the direct treatment in the current study. Given that the play behavior of rodents is organized during the early postnatal period (Beatty et al., 1981), the direct EE2 treatment used here is more likely to have had an effect than lactational transfer of EE2.
Few studies have examined long-term activity levels, such as residential running wheel activity, after developmental estrogen treatment. In our previous study of dietary EE2 treatment, dark period running wheel activity of female offspring was only mildly decreased in a dose-response manner by 1–200 ppb EE2 in the diet (Ferguson et al., 2003). Again, however, the postnatal EE2 treatment occurred via lactational transfer in that study. Nevertheless, it is clear that loss of circulating estrogens (e.g., postovariectomy) drastically reduces running wheel activity in rats and mice, which can be restored by exogenous estradiol treatment (Gray et al., 1989; Morgan and Pfaff, 2001, 2002; Rivera and Eckel, 2005; Ruiz de Elvira et al., 1992). Adult ovariectomy, however, removes the potential activating effects of endogenous estrogens. In the current study, not only were all females gonadally intact, but serum estradiol levels were comparable in all treatment groups. Thus, the decreased running wheel activity levels may be due to an EE2-induced alteration in hormonal organizational effects. The estrogen receptor alpha in the medial preoptic area seems crucially responsible for estrogen-induced increases in running wheel activity (Fahrbach et al., 1985; Hertrampf et al., 2008; Ogawa et al., 2003; Spiteri et al., 2012). However, estrogen receptor alpha expression in the medial preoptic area of PND 1 female siblings of these EE2-treated subjects here was not significantly different from that of vehicle control females (Cao et al., 2013). Thus, it may be that the continued direct EE2 treatment on PNDs 1–21 contributed largely to the potential organizational alterations which then led to the decreased running wheel activity.
EE2-treated females were more aggressive toward stimulus males during tests of sexual behavior. That aggression was typically expressed as biting and pouncing on top of the male. Those pounces were not misdirected mountings attempted by the female to the male. Similarly, adult female offspring of dams treated with DES during gestation and via lactation performed more rejection behaviors, such as kicking or fighting, when tested with a sexually experienced male (Kubo et al., 2003). Developmental EE2 treatment also caused increased male-directed aggression during sexual behavior tests (Della Seta et al., 2008). Increased agonistic behaviors during resident intruder tests were exhibited by adult female rats that had received an injection of estradiol benzoate at birth (Berretti et al., 2014). Although lordosis postures were rare in our assessment paradigm, none of the EE2-treated females exhibited a lordosis posture during any of the five test sessions. Likewise, EE2 treatment (at doses >5.0 μg/kg/day) from GD 7 through lactational day 18 or an injection of estradiol benzoate at birth decreased the lordosis behavior of adult female offspring (Berretti et al., 2014; Ryan et al., 2010).
Developmental EE2 treatment had few effects on male offspring. Males of both EE2-treated groups experienced significant delays of 4–7 days in testes descent and 7–25 days in preputial separation. As adults, serum testosterone levels of males of both EE2-treated groups were only 32–35% those of vehicle control males. Despite the magnitude of that decrease, there were few behavioral alterations. Pinning frequencies of EE2-treated males during play behavior assessments were somewhat higher than those of vehicle control males, but saccharin- and sodium-flavored solution intake as well as running wheel activity were well within the range of vehicle control males. Because circulating androgen levels are critical for some of these behaviors, the apparent lack of treatment effects was somewhat surprising. For example, the necessity of normal circulating levels of testosterone or estradiol for sex-typical running wheel activity is clearly evident from the effects caused by adult or preweaning castration and/or exogenous replacement (Hagenauer et al., 2011; Ibebunjo et al., 2011; Jackson et al., 2011; Rivera and Eckel, 2005). However, the minimum circulating levels necessary for normal male-like running wheel activity are not clear. Circulating levels that are 54%, but not 13%, of normal endogenous levels can maintain normal wheel running activity levels of male mice held under constant light or dark photoperiods (Butler et al., 2012). Although low, perhaps the testosterone levels of the EE2-treated males of the current study were sufficient to maintain normal wheel running activity. Further, there was no neuroanatomical evidence of demasculinization or feminization in PND 21 siblings of the male subjects assessed here. In contrast, males treated with 10 μg/kg/day EE2 exhibited a significant increase in SDN-POA volume at PND 21 (He et al., 2012). We previously reported that developmental treatment with 10–38 μg/kg/day of dietary EE2 caused increased sodium-flavored solution intake at adulthood in males and females (Ferguson et al., 2003), a finding not replicated here. However, in our previous study, that EE2 treatment also decreased postweaning and adult body weights of both sexes, effects not seen here. Saccharin-flavored intake was not altered in either sex, a finding similar to that reported by McGivern and Henschel (1990) for adult male and female rats treated with estradiol benzoate on PNDs 2 and 3. The relatively few significant alterations in EE2-treated males suggests that they may be less sensitive to developmental EE2 treatment than females, a hypothesis that has been presented by Ryan et al. (Howdeshell et al., 2008; Ryan et al., 2010 and see Figure 11 in Ryan et al., 2010).
To summarize, developmental EE2 treatment affected physical, physiological, and behavioral endpoints. Although males were affected, females treated with EE2 appeared more severely affected. Those treatment groups were specifically included to demonstrate sensitivity of this rat model to a reference estrogen; however, there is a need for such data by regulatory agencies (see evidence presented in Gray et al., 2010). Further, although the EE2 doses here were high enough to cause malformations, such doses are within the range of those that produce adverse effects in adult women (see argument presented in Gray et al., 2010). Nevertheless, the main focus of these studies was to ascertain potential alterations caused by developmental BPA treatment. In the endpoints measured here, BPA treatment produced no significant effects, despite the significantly increased SDN-POA volume in PND 21 males of the 2.5 and 25.0 BPA groups (He et al., 2012), siblings of the subjects described here. Our previous descriptions of significant EE2, but few BPA, effects on pre- and postweaning behaviors (Ferguson et al., 2011, 2012) add to the growing body of literature indicating lack of effect at these orally administered BPA doses.
FUNDING
National Center for Toxicological Research/Food and Drug Administration (Protocol no. P00706 to S.A.F.); National Institutes of Health (Project no. Z01ES45003 to G.E.K., in part).
Acknowledgments
The authors are grateful for the technical expertise provided by the animal care staff of the Priority One Corporation. We especially are indebted to expert statistician Mr Robert Paul Felton of the Division of Bioinformatics and Biostatistics/NCTR for his careful analyses and to Ms Melody Smith for her excellent scoring of the play behavior videos. We appreciate the thorough review of this manuscript by Jean Harry of NIEHS and the thoughtful comments by expert statistician Mr Brett Thorn of the Division of Bioinformatics and Biostatistics/NCTR.
Disclaimer: This document has been reviewed in accordance with United States Food and Drug Administration (FDA) policy and approved for publication. Approval does not signify that the contents necessarily reflect the position or opinions of the FDA nor does mention of trade names or commercial products constitute endorsement or recommendation for use. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the FDA.
REFERENCES
- Ashby J., Lefevre P. A. The weanling male rat as an assay for endocrine disruption: Preliminary observations. Regul. Toxicol. Pharmacol. 1997;26:330–337. doi: 10.1006/rtph.1997.1177. [DOI] [PubMed] [Google Scholar]
- Ashby J., Tinwell H., Lefevre P. A., Odum J., Paton D., Millward S. W., Tittensor S., Brooks A. N. Normal sexual development of rats exposed to butyl benzyl phthalate from conception to weaning. Regul. Toxicol. Pharmacol. 1997;26:102–118. doi: 10.1006/rtph.1997.1159. [DOI] [PubMed] [Google Scholar]
- Beatty W. W., Dodge A. M., Traylor K. L., Meaney M. J. Temporal boundary of the sensitive period for hormonal organization of social play in juvenile rats. Physiol. Behav. 1981;26:241–243. doi: 10.1016/0031-9384(81)90017-2. [DOI] [PubMed] [Google Scholar]
- Beronius A., Johansson N., Ruden C., Hanberg A. The influence of study design and sex-differences on results from developmental neurotoxicity studies of bisphenol A, implications for toxicity testing. Toxicology. 2013;311:13–26. doi: 10.1016/j.tox.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Beronius A., Ruden C., Hakansson H., Hanberg A. Risk to all or none? A comparative analysis of controversies in the health risk assessment of bisphenol A. Reprod. Toxicol. 2010;29:132–146. doi: 10.1016/j.reprotox.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Berretti R., Santoru F., Locci A., Sogliano C., Calza A., Choleris E., Porcu P., Concas A. Neonatal exposure to estradiol decreases hypothalamic allopregnanolone concentrations and alters agonistic and sexual but not affective behavior in adult female rats. Horm. Behav. 2014;65:142–153. doi: 10.1016/j.yhbeh.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Boctor S. Y., Ferguson S. A. Altered adult locomotor activity in rats from phencyclidine treatment on postnatal days 7, 9 and 11, but not repeated ketamine treatment on postnatal day 7. Neurotoxicology. 2010;31:42–54. doi: 10.1016/j.neuro.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Bushnik T., Haines D., Levallois P., Levesque J., Van Oostdam J., Viau C. Lead and bisphenol A concentrations in the Canadian population. Health Rep. 2010;21:7–18. [PubMed] [Google Scholar]
- Butler M. P., Karatsoreos I. N., LeSauter J., Silver R. Dose-dependent effects of androgens on the circadian timing system and its response to light. Endocrinology. 2012;153:2344–2352. doi: 10.1210/en.2011-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buynitsky T., Mostofsky D. I. Restraint stress in biobehavioral research: Recent developments. Neurosci. Biobehav. Rev. 2009;33:1089–1098. doi: 10.1016/j.neubiorev.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Calafat A. M., Ye X., Wong L. Y., Reidy J. A., Needham L. L. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ. Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Rebuli M. E., Rogers J., Todd K. L., Leyrer S. M., Ferguson S. A., Patisaul H. B. Prenatal bisphenol a exposure alters sex-specific estrogen receptor expression in the neonatal rat hypothalamus and amygdala. Toxicol. Sci. 2013;133:157–173. doi: 10.1093/toxsci/kft035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delclos K. B., Camacho L., Lewis S., Vanlandingham M., Chidambaram M., Churchwell M., Davis K., Doerge D., Gamboa da Costa G., Juliar B., et al. Effects of Bisphenol A (BPA) and ethinyl estradiol (EE2) in Sprague-Dawley rats exposed orally from GD 6 through PND 90. The Toxicologist (published by Society of Toxicology). Abstract #1719. San Francisco, CA: 2012. p. 371. [Google Scholar]
- Delclos K. B., Camacho L., Lewis S. M., Vanlandingham M. M., Latendresse J. R., Olson G. R., Davis K. J., Patton R. E., da Costa G. G., Woodling K. A., et al. Toxicity evaluation of bisphenol A administered by gavage to Sprague Dawley rats from gestation day 6 through postnatal day 90. Toxicol. Sci. 2014;139:174–197. doi: 10.1093/toxsci/kfu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delclos K. B., Weis C. C., Bucci T. J., Olson G., Mellick P., Sadovova N., Latendresse J. R., Thorn B., Newbold R. R. Overlapping but distinct effects of genistein and ethinyl estradiol (EE(2)) in female Sprague-Dawley rats in multigenerational reproductive and chronic toxicity studies. Reprod. Toxicol. 2009;27:117–132. doi: 10.1016/j.reprotox.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Seta D., Farabollini F., Dessi-Fulgheri F., Fusani L. Environmental-like exposure to low levels of estrogen affects sexual behavior and physiology of female rats. Endocrinology. 2008;149:5592–5598. doi: 10.1210/en.2008-0113. [DOI] [PubMed] [Google Scholar]
- Dessi-Fulgheri F., Porrini S., Farabollini F. Effects of perinatal exposure to bisphenol A on play behavior of female and male juvenile rats. Environ. Health Perspect. 2002;110(Suppl. 3):403–407. doi: 10.1289/ehp.110-1241190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz Weinstein S., Villafane J. J., Juliano N., Bowman R. E. Adolescent exposure to Bisphenol-A increases anxiety and sucrose preference but impairs spatial memory in rats independent of sex. Brain Res. 2013;1529:56–65. doi: 10.1016/j.brainres.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Dixon D. P., Ackert A. M., Eckel L. A. Development of, and recovery from, activity-based anorexia in female rats. Physiol. Behav. 2003;80:273–279. doi: 10.1016/j.physbeh.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Donohoe T. P., Stevens R. Effects of ovariectomy, estrogen treatment and CI-628 on food intake and body weight in female rats treated neonatally with gonadal hormones. Physiol. Behav. 1983;31:325–329. doi: 10.1016/0031-9384(83)90196-8. [DOI] [PubMed] [Google Scholar]
- Eckel L. A., Houpt T. A., Geary N. Spontaneous meal patterns in female rats with and without access to running wheels. Physiol. Behav. 2000;70:397–405. doi: 10.1016/s0031-9384(00)00278-x. [DOI] [PubMed] [Google Scholar]
- Fahrbach S. E., Meisel R. L., Pfaff D. W. Preoptic implants of estradiol increase wheel running but not the open field activity of female rats. Physiol. Behav. 1985;35:985–992. doi: 10.1016/0031-9384(85)90270-7. [DOI] [PubMed] [Google Scholar]
- Ferguson S. A., Boctor S. Y. Cocaine responsiveness or anhedonia in rats treated with methylphenidate during adolescence. Neurotoxicol. Teratol. 2010;32:432–442. doi: 10.1016/j.ntt.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Ferguson S. A., Cada A. M. Developmental treatment with difluoromethylornithine has few effects on behavior or body weight in Sprague-Dawley rats. Neurotoxicol. Teratol. 2004;26:83–93. doi: 10.1016/j.ntt.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Ferguson S. A., Cisneros F. J., Hanig J. P., Berry K. J. Oral treatment with ACCUTANE((R)) does not increase measures of anhedonia or depression in rats. Neurotoxicol. Teratol. 2007;29:642–651. doi: 10.1016/j.ntt.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Ferguson S. A., Delclos K. B., Newbold R. R., Flynn K. M. Dietary ethinyl estradiol exposure during development causes increased voluntary sodium intake and mild maternal and offspring toxicity in rats. Neurotoxicol. Teratol. 2003;25:491–501. doi: 10.1016/s0892-0362(03)00015-1. [DOI] [PubMed] [Google Scholar]
- Ferguson S. A., Law C. D., Abshire J. S. Developmental treatment with bisphenol A causes few alterations on measures of postweaning activity and learning. Neurotoxicol. Teratol. 2012;34:598–606. doi: 10.1016/j.ntt.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Ferguson S. A., Law C. D., Jr, Abshire J. S. Developmental treatment with bisphenol A or ethinyl estradiol causes few alterations on early preweaning measures. Toxicol. Sci. 2011;124:149–160. doi: 10.1093/toxsci/kfr201. [DOI] [PubMed] [Google Scholar]
- Ferguson S. A., Racey F. D., Paule M. G., Holson R. R. Behavioral effects of methylazoxymethanol-induced micrencephaly. Behav. Neurosci. 1993;107:1067–1076. doi: 10.1037//0735-7044.107.6.1067. [DOI] [PubMed] [Google Scholar]
- Flynn K. M., Delclos K. B., Newbold R. R., Ferguson S. A. Behavioral responses of rats exposed to long-term dietary vinclozolin. J. Agric. Food Chem. 2001;49:1658–1665. doi: 10.1021/jf0008893. [DOI] [PubMed] [Google Scholar]
- Flynn K. M., Delclos K. B., Newbold R. R., Ferguson S. A. Long term dietary methoxychlor exposure in rats increases sodium solution consumption but has few effects on other sexually dimorphic behaviors. Food Chem. Toxicol. 2005;43:1345–1354. doi: 10.1016/j.fct.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Flynn K. M., Ferguson S. A., Delclos K. B., Newbold R. R. Effects of genistein exposure on sexually dimorphic behaviors in rats. Toxicol. Sci. 2000;55:311–319. doi: 10.1093/toxsci/55.2.311. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration. Exposure to Bisphenol A for infants, toddlers and adults from the consumption of infant formula, toddler food and adult (canned) food [FDA-2010-N-0100–0001] 2009 Available at: http://www.regulations.gov/#!documentDetail;D=FDA-2010-N-0100-0009. [Google Scholar]
- Gans S. E., McClintock M. K. Individual differences among female rats in the timing of the preovulatory LH surge are predicted by lordosis reflex intensity. Horm. Behav. 1993;27:403–417. doi: 10.1006/hbeh.1993.1030. [DOI] [PubMed] [Google Scholar]
- Gans S. E., Stamper J. L., Butler T., McClintock M. K. Endocrine basis for two types of individual differences in lordosis reflex intensity. Horm. Behav. 1995;29:367–391. doi: 10.1006/hbeh.1995.1026. [DOI] [PubMed] [Google Scholar]
- Geens T., Aerts D., Berthot C., Bourguignon J. P., Goeyens L., Lecomte P., Maghuin-Rogister G., Pironnet A. M., Pussemier L., Scippo M. L., et al. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem. Toxicol. 2012;50:3725–3740. doi: 10.1016/j.fct.2012.07.059. [DOI] [PubMed] [Google Scholar]
- Girard D. M., Sager D. B. The use of Markov chains to detect subtle variation in reproductive cycling. Biometrics. 1987;43:225–234. [PubMed] [Google Scholar]
- Gorski R. A. Sexual differentiation of the brain: A model for drug-induced alterations of the reproductive system. Environ. Health Perspect. 1986;70:163–175. doi: 10.1289/ehp.8670163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray L. E., Jr, Bryan B., Hotchkiss A. K., Crofton K. M. Rebuttal of “flawed experimental design reveals the need for guidelines requiring appropriate positive controls in endocrine disruption research” by vom Saal. Toxicol. Sci. 2010;115:614–620. doi: 10.1093/toxsci/kfq073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray L. E., Jr, Ostby J., Ferrell J., Rehnberg G., Linder R., Cooper R., Goldman J., Slott V., Laskey J. A dose-response analysis of methoxychlor-induced alterations of reproductive development and function in the rat. Fundam. Appl. Toxicol. 1989;12:92–108. doi: 10.1016/0272-0590(89)90065-1. [DOI] [PubMed] [Google Scholar]
- Hagenauer M. H., King A. F., Possidente B., McGinnis M. Y., Lumia A. R., Peckham E. M., Lee T. M. Changes in circadian rhythms during puberty in Rattus norvegicus: Developmental time course and gonadal dependency. Horm. Behav. 2011;60:46–57. doi: 10.1016/j.yhbeh.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseman J. K., Bailer A. J., Kodell R. L., Morris R., Portier K. Statistical issues in the analysis of low-dose endocrine disruptor data. Toxicol. Sci. 2001;61:201–210. doi: 10.1093/toxsci/61.2.201. [DOI] [PubMed] [Google Scholar]
- He Z., Paule M. G., Ferguson S. A. Low oral doses of bisphenol A increase volume of the sexually dimorphic nucleus of the preoptic area in male, but not female, rats at postnatal day 21. Neurotoxicol. Teratol. 2012;34:331–337. doi: 10.1016/j.ntt.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Henry E. C., Miller R. K., Baggs R. B. Direct fetal injections of diethylstilbestrol and 17 beta-estradiol: A method for investigating their teratogenicity. Teratology. 1984;29:297–304. doi: 10.1002/tera.1420290216. [DOI] [PubMed] [Google Scholar]
- Hertrampf T., Seibel J., Laudenbach U., Fritzemeier K. H., Diel P. Analysis of the effects of oestrogen receptor alpha (ERalpha)- and ERbeta-selective ligands given in combination to ovariectomized rats. Br. J. Pharmacol. 2008;153:1432–1437. doi: 10.1038/sj.bjp.0707664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman C. S., Westin T. M., Miner H. M., Johnson P. L., Summers C. H., Renner K. J. GABAergic drugs alter hypothalamic serotonin release and lordosis in estrogen-primed rats. Brain Res. 2002;946:96–103. doi: 10.1016/s0006-8993(02)02867-6. [DOI] [PubMed] [Google Scholar]
- Holson R. R., Freshwater L., Maurissen J. P., Moser V. C., Phang W. Statistical issues and techniques appropriate for developmental neurotoxicity testing: A report from the ILSI Research Foundation/Risk Science Institute expert working group on neurodevelopmental endpoints. Neurotoxicol. Teratol. 2008;30:326–348. doi: 10.1016/j.ntt.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Howdeshell K. L., Furr J., Lambright C. R., Wilson V. S., Ryan B. C., Gray L. E., Jr Gestational and lactational exposure to ethinyl estradiol, but not bisphenol A, decreases androgen-dependent reproductive organ weights and epididymal sperm abundance in the male long evans hooded rat. Toxicol. Sci. 2008;102:371–382. doi: 10.1093/toxsci/kfm306. [DOI] [PubMed] [Google Scholar]
- Hunt P. A., Susiarjo M., Rubio C., Hassold T. J. The bisphenol A experience: A primer for the analysis of environmental effects on mammalian reproduction. Biol. Reprod. 2009;81:807–813. doi: 10.1095/biolreprod.109.077008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibebunjo C., Eash J. K., Li C., Ma Q., Glass D. J. Voluntary running, skeletal muscle gene expression, and signaling inversely regulated by orchidectomy and testosterone replacement. Am. J. Physiol. Endocrinol. Metab. 2011;300:E327–E3240. doi: 10.1152/ajpendo.00402.2010. [DOI] [PubMed] [Google Scholar]
- Jackson K. C., Wohlers L. M., Valencia A. P., Cilenti M., Borengasser S. J., Thyfault J. P., Spangenburg E. E. Wheel running prevents the accumulation of monounsaturated fatty acids in the liver of ovariectomized mice by attenuating changes in SCD-1 content. Appl. Physiol. Nutr. Metab. 2011;36:798–810. doi: 10.1139/h11-099. [DOI] [PubMed] [Google Scholar]
- Kalra P. S., Kalra S. P. Circadian periodicities of serum androgens, progesterone, gonadotropins and luteinizing hormone-releasing hormone in male rats: The effects of hypothalamic deafferentation, castration and adrenalectomy. Endocrinology. 1977;101:1821–1827. doi: 10.1210/endo-101-6-1821. [DOI] [PubMed] [Google Scholar]
- Kim Y. S., Harry G. J., Kang H. S., Goulding D., Wine R. N., Kissling G. E., Liao G., Jetten A. M. Altered cerebellar development in nuclear receptor TAK1/TR4 null mice is associated with deficits in GLAST(+) glia, alterations in social behavior, motor learning, startle reactivity, and microglia. Cerebellum. 2010;9:310–323. doi: 10.1007/s12311-010-0163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo K., Arai O., Omura M., Watanabe R., Ogata R., Aou S. Low dose effects of bisphenol A on sexual differentiation of the brain and behavior in rats. Neurosci. Res. 2003;45:345–356. doi: 10.1016/s0168-0102(02)00251-1. [DOI] [PubMed] [Google Scholar]
- Kwon S., Stedman D. B., Elswick B. A., Cattley R. C., Welsch F. Pubertal development and reproductive functions of Crl:CD BR Sprague-Dawley rats exposed to bisphenol A during prenatal and postnatal development. Toxicol. Sci. 2000;55:399–406. doi: 10.1093/toxsci/55.2.399. [DOI] [PubMed] [Google Scholar]
- Lenz K. M., McCarthy M. M. Organized for sex-steroid hormones and the developing hypothalamus. Eur. J. Neurosci. 2010;32:2096–2104. doi: 10.1111/j.1460-9568.2010.07511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. R., Faber K. A., Ayyash L., Hughes C. L., Jr The effect of prenatal exposure to the phytoestrogen genistein on sexual differentiation in rats. Proc. Soc. Exp. Biol. Med. 1995;208:60–66. doi: 10.3181/00379727-208-43832. [DOI] [PubMed] [Google Scholar]
- Li A. A., Baum M. J., McIntosh L. J., Day M., Liu F., Gray L. E., Jr Building a scientific framework for studying hormonal effects on behavior and on the development of the sexually dimorphic nervous system. Neurotoxicology. 2008;29:504–519. doi: 10.1016/j.neuro.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Maccari S., Morley-Fletcher S. Effects of prenatal restraint stress on the hypothalamus-pituitary-adrenal axis and related behavioural and neurobiological alterations. Psychoneuroendocrinology. 2007;32(Suppl. 1):S10–S15. doi: 10.1016/j.psyneuen.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Marler M. R., Gehrman P., Martin J. L., Ancoli-Israel S. The sigmoidally transformed cosine curve: A mathematical model for circadian rhythms with symmetric non-sinusoidal shapes. Stat. Med. 2006;25:3893–3904. doi: 10.1002/sim.2466. [DOI] [PubMed] [Google Scholar]
- McGivern R. F., Henschel D. M. Interaction of naltrexone with postnatal administration of testosterone and estrogen on neurobehavioral sexual differentiation in rats. Horm. Behav. 1990;24:20–39. doi: 10.1016/0018-506x(90)90024-r. [DOI] [PubMed] [Google Scholar]
- Meaney M. J. The sexual differentiation of social play. Psychiatr. Dev. 1989;7:247–261. [PubMed] [Google Scholar]
- Meaney M. J., McEwen B. S. Testosterone implants into the amygdala during the neonatal period masculinize the social play of juvenile female rats. Brain Res. 1986;398:324–328. doi: 10.1016/0006-8993(86)91492-7. [DOI] [PubMed] [Google Scholar]
- Meaney M. J., Stewart J. Neonatal-androgens influence the social play of prepubescent rats. Horm. Behav. 1981;15:197–213. doi: 10.1016/0018-506x(81)90028-3. [DOI] [PubMed] [Google Scholar]
- Mock E. J., Norton H. W., Frankel A. I. Daily rhythmicity of serum testosterone concentration in the male laboratory rat. Endocrinology. 1978;103:1111–1121. doi: 10.1210/endo-103-4-1111. [DOI] [PubMed] [Google Scholar]
- Morgan M. A., Pfaff D. W. Effects of estrogen on activity and fear-related behaviors in mice. Horm. Behav. 2001;40:472–482. doi: 10.1006/hbeh.2001.1716. [DOI] [PubMed] [Google Scholar]
- Morgan M. A., Pfaff D. W. Estrogen's effects on activity, anxiety, and fear in two mouse strains. Behav. Brain Res. 2002;132:85–93. doi: 10.1016/s0166-4328(01)00398-9. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. Washington D.C.: National Academy Press; 1996. [Google Scholar]
- National Toxicology Program. NTP-CERHR monograph on the potential human reproductive and developmental effects of Bisphenol A. Center for the Evaluation of Risks to Human Reproduction, National Toxicology Program, U.S. Department of Health and Human Services; 2008. [Google Scholar]
- Naule L., Picot M., Martini M., Parmentier C., Hardin-Pouzet H., Keller M., Franceschini I., Mhaouty-Kodja S. Neuroendocrine and behavioral effects of maternal exposure to oral bisphenol A in female mice. J. Endocrinol. 2014;220:375–388. doi: 10.1530/JOE-13-0607. [DOI] [PubMed] [Google Scholar]
- Nikaido Y., Danbara N., Tsujita-Kyutoku M., Yuri T., Uehara N., Tsubura A. Effects of prepubertal exposure to xenoestrogen on development of estrogen target organs in female CD-1 mice. In Vivo. 2005;19:487–494. [PubMed] [Google Scholar]
- Nikaido Y., Yoshizawa K., Danbara N., Tsujita-Kyutoku M., Yuri T., Uehara N., Tsubura A. Effects of maternal xenoestrogen exposure on development of the reproductive tract and mammary gland in female CD-1 mouse offspring. Reprod. Toxicol. 2004;18:803–811. doi: 10.1016/j.reprotox.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Odum J., Lefevre P. A., Tinwell H., Van Miller J. P., Joiner R. L., Chapin R. E., Wallis N. T., Ashby J. Comparison of the developmental and reproductive toxicity of diethylstilbestrol administered to rats in utero, lactationally, preweaning, or postweaning. Toxicol. Sci. 2002;68:147–163. doi: 10.1093/toxsci/68.1.147. [DOI] [PubMed] [Google Scholar]
- Ogawa S., Chan J., Gustafsson J. A., Korach K. S., Pfaff D. W. Estrogen increases locomotor activity in mice through estrogen receptor alpha: Specificity for the type of activity. Endocrinology. 2003;144:230–239. doi: 10.1210/en.2002-220519. [DOI] [PubMed] [Google Scholar]
- Olesen K. M., Jessen H. M., Auger C. J., Auger A. P. Dopaminergic activation of estrogen receptors in neonatal brain alters progestin receptor expression and juvenile social play behavior. Endocrinology. 2005;146:3705–3712. doi: 10.1210/en.2005-0498. [DOI] [PubMed] [Google Scholar]
- Porrini S., Belloni V., Della Seta D., Farabollini F., Giannelli G., Dessi-Fulgheri F. Early exposure to a low dose of bisphenol A affects socio-sexual behavior of juvenile female rats. Brain Res. Bull. 2005;65:261–266. doi: 10.1016/j.brainresbull.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Richter C. A., Birnbaum L. S., Farabollini F., Newbold R. R., Rubin B. S., Talsness C. E., Vandenbergh J. G., Walser-Kuntz D. R., vom Saal F. S. In vivo effects of bisphenol A in laboratory rodent studies. Reprod. Toxicol. 2007;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera H. M., Eckel L. A. The anorectic effect of fenfluramine is increased by estradiol treatment in ovariectomized rats. Physiol. Behav. 2005;86:331–337. doi: 10.1016/j.physbeh.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Rothschild T. C., Calhoon R. E., Boylan E. S. Effects of diethylstilbestrol exposure in utero on the genital tracts of female ACI rats. Exp. Mol. Pathol. 1988;48:59–76. doi: 10.1016/0014-4800(88)90046-9. [DOI] [PubMed] [Google Scholar]
- Ruiz de Elvira M. C., Persaud R., Coen C. W. Use of running wheels regulates the effects of the ovaries on circadian rhythms. Physiol. Behav. 1992;52:277–284. doi: 10.1016/0031-9384(92)90271-3. [DOI] [PubMed] [Google Scholar]
- Ryan B. C. Developmental exposure to environmental extrogens alters adult behavior in female rodents. Zoology. Dissertation. Raleigh: North Carolina State University; 2005. p. 213. [Google Scholar]
- Ryan B. C., Hotchkiss A. K., Crofton K. M., Gray L. E., Jr In utero and lactational exposure to bisphenol A, in contrast to ethinyl estradiol, does not alter sexually dimorphic behavior, puberty, fertility, and anatomy of female LE rats. Toxicol. Sci. 2010;114:133–148. doi: 10.1093/toxsci/kfp266. [DOI] [PubMed] [Google Scholar]
- Sawaki M., Noda S., Muroi T., Mitoma H., Takakura S., Sakamoto S., Yamasaki K. Evaluation of an in utero through lactational exposure protocol for detection of estrogenic effects of ethinyl estradiol on the offspring of rats: Preliminary trial. Reprod. Toxicol. 2003a;17:335–343. doi: 10.1016/s0890-6238(03)00005-4. [DOI] [PubMed] [Google Scholar]
- Sawaki M., Noda S., Muroi T., Mitoma H., Takakura S., Sakamoto S., Yamasaki K. In utero through lactational exposure to ethinyl estradiol induces cleft phallus and delayed ovarian dysfunction in the offspring. Toxicol. Sci. 2003b;75:402–411. doi: 10.1093/toxsci/kfg180. [DOI] [PubMed] [Google Scholar]
- Shelnutt S., Kind J., Allaben W. Bisphenol A: Update on newly developed data and how they address NTP's 2008 finding of “Some Concern”. Food Chem. Toxicol. 2013;57C:284–295. doi: 10.1016/j.fct.2013.03.027. [DOI] [PubMed] [Google Scholar]
- Shiorta M., Kawashima J., Nakamura T., Ogawa Y., Kamiie J., Yasuno K., Shirota K., Yoshida M. Delayed effects of single neonatal subcutaneous exposure of low-dose 17alpha-ethynylestradiol on reproductive function in female rats. J. Toxicol. Sci. 2012;37:681–690. doi: 10.2131/jts.37.681. [DOI] [PubMed] [Google Scholar]
- Spiteri T., Ogawa S., Musatov S., Pfaff D. W., Agmo A. The role of the estrogen receptor alpha in the medial preoptic area in sexual incentive motivation, proceptivity and receptivity, anxiety, and wheel running in female rats. Behav. Brain Res. 2012;230:11–20. doi: 10.1016/j.bbr.2012.01.048. [DOI] [PubMed] [Google Scholar]
- Steiner M., Katz R. J., Carroll B. J. Detailed analysis of estrous-related changes in wheel running and self-stimulation. Physiol. Behav. 1982;28:201–204. doi: 10.1016/0031-9384(82)90127-5. [DOI] [PubMed] [Google Scholar]
- Tonjes R., Docke F., Dorner G. Effects of neonatal intracerebral implantation of sex steroids on sexual behaviour, social play behaviour and gonadotrophin secretion. Exp. Clin. Endocrinol. 1987;90:257–263. doi: 10.1055/s-0029-1210699. [DOI] [PubMed] [Google Scholar]
- Weinstock M. Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Prog. Neurobiol. 2001;65:427–451. doi: 10.1016/s0301-0082(01)00018-1. [DOI] [PubMed] [Google Scholar]
- Willoughby K. N., Sarkar A. J., Boyadjieva N. I., Sarkar D. K. Neonatally administered tert-octylphenol affects onset of puberty and reproductive development in female rats. Endocrine. 2005;26:161–168. doi: 10.1385/ENDO:26:2:161. [DOI] [PubMed] [Google Scholar]
- Wolstenholme J. T., Taylor J. A., Shetty S. R., Edwards M., Connelly J. J., Rissman E. F. Gestational exposure to low dose bisphenol A alters social behavior in juvenile mice. PLoS One. 2011;6:e25448. doi: 10.1371/journal.pone.0025448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Tan L., Himi T., Sadamatsu M., Tsutsumi S., Akaike M., Kato N. Changed preference for sweet taste in adulthood induced by perinatal exposure to bisphenol A-A probable link to overweight and obesity. Neurotoxicol. Teratol. 2011;33:458–463. doi: 10.1016/j.ntt.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Shirai M., Sugita K., Nagai N., Miura Y., Mogi R., Yamamoto K., Tamura A., Arishima K. Effects of maternal exposure to diethylstilbestrol on the development of the reproductive system and thyroid function in male and female rat offspring. J. Toxicol. Sci. 2003;28:385–394. doi: 10.2131/jts.28.385. [DOI] [PubMed] [Google Scholar]