First-line Xpert testing on 1, 2, or 3 respiratory specimens significantly decreased airborne isolation duration compared with the 3-smear microscopy strategy in the United States. The 2-specimen Xpert strategy was optimal, capturing all tuberculosis cases while minimizing airborne isolation.
Keywords: Xpert, tuberculosis, infection control, United States
Abstract
Background. In the United States, individuals with presumptive pulmonary tuberculosis are placed in airborne infection isolation (AII) and assessed by smear microscopy on 3 respiratory specimens collected 8–24 hours apart. Xpert MTB/RIF assay (Xpert) on 1, 2, or 3 specimens may be more efficient for determining AII discontinuation.
Methods. This single-center, observational cohort study of inpatients with presumptive pulmonary tuberculosis enrolled adults with 1 or more sputum specimens submitted for smear microscopy. Smear microscopy and Xpert were performed on each sputum specimen. Clinicians were blinded to Xpert results. The primary endpoint was AII duration. Secondary endpoints were laboratory processing time, strategy-based tuberculosis detection, and sensitivity and specificity.
Results. Among 207 subjects, the median AII duration was 68.0 hours (interquartile range [IQR], 47.1–97.5) for smear microscopy compared with 20.8 hours (IQR, 16.8–32.0) for the 1-specimen Xpert, 41.2 hours (IQR, 26.6–54.8) for the 2-specimen Xpert, and 54.0 hours (IQR, 43.3–80.0) for the 3-specimen Xpert strategies (P ≤ .004). Median laboratory processing time for smear microscopy was 2.5 times as long as Xpert (P < .001). The 2- and 3-specimen Xpert and smear microscopy strategies captured all 6 tuberculosis cases. The 1-specimen Xpert strategy missed 1 case. No difference was observed between smear microscopy and Xpert in sensitivity or specificity for detection of Mycobacterium tuberculosis.
Conclusions. Xpert-based strategies significantly reduced AII duration compared with the smear-based strategy. The 2-specimen Xpert strategy was most efficient in minimizing AII time while identifying all tuberculosis cases among individuals with presumptive tuberculosis in this low-burden setting.
Tuberculosis continues to be an important public health problem in the United States. Following several healthcare-associated tuberculosis outbreaks in the 1980s, the Centers for Disease Control and Prevention (CDC) set a goal of tuberculosis elimination in the United States, defined as <1 case per million population [1]. Despite declining incidence over the last 20 years, tuberculosis has not been eliminated in the United States; in 2012 there were 9945 new cases (incidence rate, 3.2 cases per 100 000 population) [2].
The current CDC guidelines for prevention of Mycobacterium tuberculosis transmission in the healthcare setting recommend a 3-level hierarchy of control measures: administrative controls aimed at reducing exposure to individuals with presumptive pulmonary tuberculosis; environmental controls by placing individuals with presumptive tuberculosis in airborne infection isolation (AII), ideally using single-patient, negative-pressure ventilation rooms; and respiratory protection controls through use of disposable N95 respirators by healthcare workers [3]. Patients must produce 3 respiratory specimens 8–24 hours apart, with at least 1 early morning specimen, for smear microscopy for acid-fast bacilli (AFB) and M. tuberculosis culture [3]. Smear microscopy has been effective in reducing healthcare-associated tuberculosis transmission [2, 3], but it has important health system limitations. Smear microscopy has relatively poor sensitivity for diagnosing active tuberculosis [4, 5], especially in persons infected with human immunodeficiency virus (HIV) [6]. Specimen processing is labor-intensive, requiring experienced personnel to avoid errors [7, 8], and is therefore typically performed in batches once daily [9].
The Xpert MTB/RIF assay (Xpert, Cepheid, Sunnyvale, California), an automated, rapid nucleic acid amplification test (NAAT) endorsed by the World Health Organization in 2010 [10] and authorized for marketing by the US Food and Drug Administration in 2013 [11], can expedite the diagnostic process given its sensitive and specific ability to diagnose tuberculosis within 2 hours with minimal operator training [12, 13]. We aimed to determine whether implementing Xpert as a first-line diagnostic test on 1, 2, or 3 specimen(s) reduces AII duration while optimally identifying tuberculosis cases compared with the 3-smear microscopy strategy.
METHODS
Study Setting
The University of North Carolina (UNC) Hospitals is an 830-bed tertiary medical center providing care to 200–300 patients with presumptive tuberculosis annually, of whom an average of 8 are diagnosed with pulmonary tuberculosis. At UNC Hospitals, individuals with presumptive tuberculosis are required to be placed in AII and provide 3 sputum specimens obtained at least 8–24 hours apart. In addition to routine smear microscopy and culture, Xpert was performed on sputum specimens. Because Xpert was approved for research use only in the United States at time of the study, only smear microscopy results were communicated to physicians.
Study Population
From March 2012 through July 2013, we enrolled consecutive inpatient adults (aged ≥18 years) for whom at least 1 sputum specimen was submitted for AFB smear microscopy and culture. Subjects determined not to have presumptive pulmonary tuberculosis through medical record review were excluded from the analysis. Patients with cystic fibrosis were not enrolled due to their high rates of nontuberculous mycobacteria (NTM) and low rates of M. tuberculosis [14].
Laboratory Methods
The UNC McLendon Clinical Laboratories performed all laboratory testing. When the respiratory specimen volume was ≥5 mL, 1 mL of the unprocessed specimen was removed for the study Xpert assay (G4 cartridges) and the remaining specimen (≥4 mL) was processed for routine smear microscopy and culture. When the respiratory specimen volume was <5 mL, sterile saline (5 mL) was added prior to extracting the 1 mL for the study Xpert assay; the remainder (>4 mL) was again used for smear microscopy and culture. Bronchoalveolar lavage (BAL) and tracheal aspirate specimens were processed for smear microscopy and culture but not for Xpert due to limitations in the resources needed for prospectively identifying specimens only from inpatients with presumptive tuberculosis.
AFB smear microscopy was performed using auramine-rhodamine fluorescent stain once daily, Monday through Friday. Processing batches for smear microscopy and culture consisted of specimens received in the laboratory by 8 am on weekdays. Liquid culture (BACTEC MGIT 960 System, Becton Dickinson, Sparks, Maryland) was monitored continually via automation for 6 weeks and solid culture (Lowenstein-Jensen medium, Remel, Lenexa, Kansas) was monitored weekly for 8 weeks. To simulate the expected future weekday implementation of Xpert for clinical purposes at UNC Hospitals, Xpert was performed according to manufacturer instructions twice daily directly on sputum specimens at 9 am and 5 pm on weekdays. Although it is expected that Xpert could routinely be performed on weekends, this was not possible due to study resource constraints. Invalid Xpert M. tuberculosis results were repeated once.
Data Collection
Data were collected from the UNC Hospitals laboratory information system and electronic medical record database, and the Xpert device electronic records.
AII Strategy Definitions
We examined 4 AII discontinuation strategies: the smear microscopy–based strategy, and 3 Xpert-based strategies requiring 1, 2, or 3 Xpert results for AII discontinuation. The AII duration for each strategy was determined from available data. AII initiation time was defined as the electronic AII order time. If this was unavailable, the first smear microscopy order time, as placed by a physician, was used. When the first specimen was collected in an outpatient clinic or the emergency department prior to the admission, the AII initiation time was defined as 1 minute prior to the laboratory specimen receipt time.
Laboratory specimen receipt time was defined as the electronically recorded specimen arrival time at the laboratory. Specimen receipt times were recorded for all successfully collected specimens. For failed sputum inductions, the electronically documented sputum induction time was used as the specimen receipt time, if available. Specimens rejected by the laboratory prior to processing were excluded from the analysis.
Smear microscopy result time was defined as the time when the smear microscopy result was electronically recorded. Xpert result time was captured from the Xpert software output. If an Xpert result was initially invalid, the repeat result time was used. Xpert result times were not available for BAL or tracheal aspirates and respiratory specimens that were inadvertently not processed by Xpert. In these instances, the missing Xpert result time for specimens received on a weekday before 8 am or between 8 am and 5 pm was replaced with the median morning or afternoon weekday Xpert result time as observed in the study. To simulate future routine weekend implementation of Xpert, a 2:33 pm Xpert result time was used for all specimens received between 5 pm on Friday and 12 pm on Sunday, reflecting daily weekend processing at 12 pm and a median Xpert laboratory processing time of 2 hours 33 minutes as observed in the study.
Laboratory processing time was defined as the difference between specimen receipt time and result time. Failed inductions could not be assigned a laboratory processing time.
The AII discontinuation time for the smear-based strategy was defined as the smear microscopy result time for the third specimen. A failed sputum induction was considered an attempted specimen collection because it is considered equivalent to 1 negative smear microscopy result. When a smear microscopy result time was not available for the third specimen, the electronic AII discontinuation order time was used. When the AII discontinuation order was also not available, the time stamp of the clinician progress note documenting AII discontinuation was used. For subjects discharged prior to the third smear result, or prior to an AII discontinuation order, the hospital discharge order time was used. The AII discontinuation time for the Xpert-based strategies could not be observed, as all AII decisions were based solely on smear microscopy and Xpert results were not communicated to physicians. AII discontinuation time for the 1-, 2-, and 3-specimen Xpert strategies was estimated as the time from AII initiation to Xpert result for the first, second, and third successfully collected specimen, respectively. Subjects with false-positive Xpert results were assumed to remain in AII; therefore, the AII discontinuation time was the hospital discharge order time.
The AII duration was defined as the difference between the AII initiation and discontinuation times for 4 AII strategies: smear microscopy, and Xpert on 1, 2, and 3 specimen(s). Subjects diagnosed with tuberculosis were excluded from the AII time analyses as they remained in AII after tuberculosis diagnosis.
Statistical Analyses
Standard descriptive statistics were used to characterize the cohort. Sensitivity and specificity and their 95% confidence intervals (CIs) of smear microscopy and Xpert for detection of M. tuberculosis were calculated using growth of M. tuberculosis in liquid or solid culture as the reference standard. Contaminated cultures and Xpert results that were invalid on repeat testing were excluded. The time between AII initiation and first, second, and third specimen collection was described graphically using Kaplan-Meier curves. Laboratory processing time was calculated for each smear and Xpert test performed and compared visually using Kaplan-Meier curves and statistically by the log-rank test. Finally, we compared the AII duration of the smear-based and the 3 Xpert-based AII discontinuation strategies by constructing Kaplan-Meier curves and by the log-rank test. All statistical analyses were performed using Stata software version 10.0 (StataCorp, College Station, Texas).
Ethics Statement
The UNC Institutional Review Board approved the study. Written informed consent was waived because the Notice of Privacy Practices consent, which is signed by patients upon admission, covers the study procedures and outlines the use and disclosure of health information for research purposes. Study-related risk was minimal, as routine laboratory and hospital procedures were performed.
RESULTS
Study Population
Between March 2012 and July 2013, 246 hospitalized subjects had at least 1 sputum specimen submitted for smear microscopy. Thirty-nine subjects were excluded based on lack of concern for pulmonary tuberculosis in the medical record. NTM were recovered from 10 of the 39 excluded subjects; no specimens grew M. tuberculosis. Of the 207 subjects included in the analysis, 3 patients were hospitalized and eligible twice during the study period; each counted as 2 subjects in the analyses. No subjects died in AII.
The median subject age was 51 years, and 36% were female; approximately one-quarter were HIV infected, 37% were African American, 37% were white, and 16% were Hispanic (Table 1). The majority (79%) presented with cough, which was the predominant tuberculosis symptom documented, and 74% had a chest radiograph compatible with active pulmonary tuberculosis.
Table 1.
Demographic, Clinical, and Management Characteristics (n = 207)
| Characteristic | No. (%) |
|---|---|
| Age at enrollment, y, median (IQR) | 51 (39–63) |
| Sex | |
| Female | 74 (35.8) |
| Male | 133 (64.3) |
| Race/ethnicity | |
| African American | 77 (37.2) |
| White | 77 (37.2) |
| Hispanic | 34 (16.4) |
| Asian | 7 (3.4) |
| Other | 12 (5.8) |
| HIV status | |
| Negative | 127 (61.4) |
| Positive | 49 (23.7) |
| Unknown | 31 (15.0) |
| Symptoms | |
| Fever | 91 (44.0) |
| Chills | 49 (23.7) |
| Sweats | 52 (25.1) |
| Weight loss | 61 (29.5) |
| Dyspnea | 103 (49.8) |
| Cough | 163 (78.7) |
| Productive | 124 (76.1) |
| Hemoptysis | 39 (31.5) |
| Radiographic imaging | |
| Chest radiograph compatible with TBa | 150 (73.9) |
| Chest CT compatible with TBa | 133 (96.4) |
| Latent TB testing performed | |
| TSTb | 84 (40.6) |
| IGRAb | 27 (13.0) |
| No test for latent TB | 102 (49.3) |
Abbreviations: CT, computed tomography; HIV, human immunodeficiency virus; IGRA, interferon-γ release assay; IQR, interquartile range; TB, tuberculosis; TST, tuberculin skin testing.
a Two hundred three and 138 subjects had chest radiography and chest CT performed, respectively.
b Six subjects had both TST and IGRA performed.
Respiratory Specimens
A total of 546 respiratory specimens were successfully collected from the 207 study subjects: 511 induced or expectorated sputum, 28 BAL, and 7 tracheal aspirates. Most subjects (n = 153 [74%]) had 3 successfully collected specimens prior to AII discharge, 33 subjects (16%) had 2 specimens, and 21 (10%) had 1 specimen. All 546 respiratory specimens were processed for smear microscopy and culture. Xpert was performed on 505 of the 511 (99%) sputum specimens. In addition, 6 failed sputum induction times were documented, and the laboratory rejected 2 specimens due to insufficient quantity and inaccurate labeling.
Microbiological Diagnoses
Of the 207 subjects included in the analysis, 6 (3%) were diagnosed with active tuberculosis based on 1 or more cultures positive for M. tuberculosis (Table 2). In all 6, smear and Xpert were positive on 1 or more respiratory specimen(s). Xpert correctly categorized rifampicin resistance on the M. tuberculosis isolates from all 6 subjects—5 without resistance mutations detected, and 1 with rifampicin resistance detected. One subject with a history of treatment for pulmonary tuberculosis 18 months prior to hospitalization had results that were Xpert positive but smear and culture negative. Another subject showed smear-positive but Xpert- and culture-negative results. The proportion of specimens with an initial invalid Xpert M. tuberculosis result was 4% (20/505), with 3 of 20 invalid on repeat Xpert. Culture contamination occurred in <1% (3/546) of samples. NTM were recovered from 40 specimens in 22 subjects. All were Xpert negative; 5 specimens in 3 of these 22 subjects were smear positive.
Table 2.
Type, Distribution, and Result of Respiratory Specimens Positive on Smear Microscopy or Xpert MTB/RIF Assay (n = 11)
| Subject No. | Specimen | Smear Microscopy | Xpert | Culture |
|---|---|---|---|---|
| 004 | S S S | + + + | + + + | TB TB TB |
| 010 | S S S | + + + | + + + | TB TB TB |
| 020 | S S | + + | – – | NTM NTM |
| 036 | S S S | – + + | – + + | – TB TB |
| 109 | F F S | F F – | F F + | F F – |
| 115a | S S S | + – + | + – + | TB – TB |
| 161b | S S B | I + + | + + N | TB TB TB |
| 201b | B S S | + + I | N – – | NTM NTM NTM |
| 208 | S S S | + – – | – – – | NTM NTM NTM |
| 219 | S S | + – | + – | TB TB |
| 222 | T S | – + | N – | – – |
Abbreviations: +, positive result; –, negative result; B, bronchoalveolar lavage specimen (not run for Xpert testing); F, failed sputum induction (not run for smear microscopy, Xpert, or culture); I, indeterminate result; N, not performed; NTM, nontuberculous mycobacteria; S, sputum specimen (induced or expectorated); T, tracheal aspirate specimen (not run for Xpert testing); TB, Mycobacterium tuberculosis; Xpert, Xpert MTB/RIF assay.
a Subject 115 demonstrated rifampicin resistance on Xpert and was subsequently diagnosed with multidrug-resistant TB based on culture.
b Indeterminate smear microscopy result indicates 1–2 acid-fast bacilli per 300 fields. The laboratory protocol considers this result nonnegative and suggests repeat specimen collection.
Sensitivity and Specificity
Using the total number of specimens submitted, the sensitivity of smear microscopy for detection of M. tuberculosis (n = 543) was 93.3% (95% CI, 68%–100%) and specificity was 98.9% (95% CI, 98%–100%). For Xpert, the sensitivity (n = 499) was 92.9% (95% CI, 66%–100%) and specificity was 99.8% (95% CI, 99%–100%).
Specimen Collection and Laboratory Processing
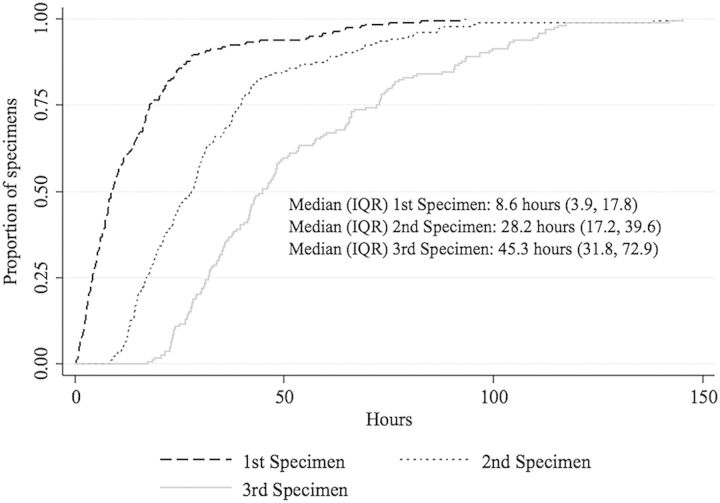
The median time from AII initiation to specimen arrival at the laboratory was 8.6 hours (interquartile range [IQR], 3.9–17.8) for the first specimen, 28.2 hours (IQR, 17.2–39.6) for the second, and 45.3 hours (IQR, 31.8–72.9) for the third (Figure 1). The initial respiratory specimen was obtained prior to admission in 5 (2.4%) subjects.
Figure 1.
Kaplan-Meier curve displaying time from airborne infection isolation initiation to laboratory receipt of the first (n = 201), second (n = 185), and third (n = 166) respiratory specimen(s). Failed sputum inductions were used to determine specimen order regardless of whether an induction time was available. Failed sputum inductions contributed time data to this Kaplan-Meier curve only when the induction time was available (n = 6). Abbreviation: IQR, interquartile range.
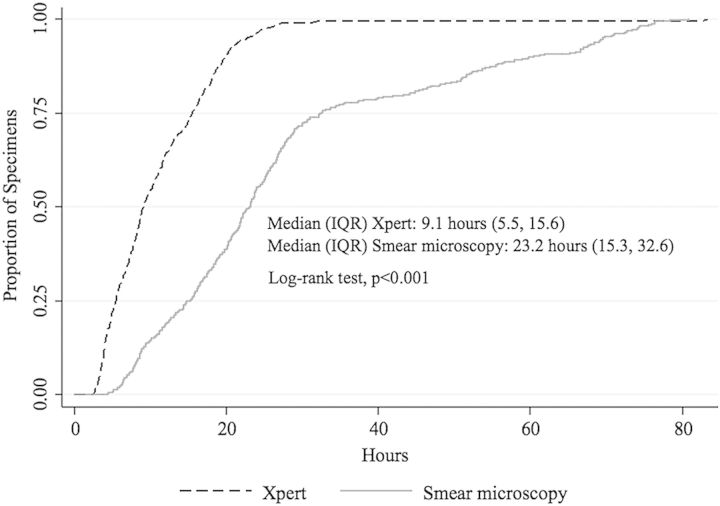
The median laboratory processing time for smear microscopy was 2.5 times as long as Xpert (23.2 hours [IQR, 15.3–32.6] vs 9.1 hours [IQR, 5.5–15.6]; log-rank test P < .001; Figure 2). A 4-day stock-out of Xpert cartridges occurred during the study, which affected 6 specimens from 5 subjects. The median laboratory processing duration of Xpert for these specimens was 100 hours.
Figure 2.
Kaplan-Meier curve comparing Xpert MTB/RIF assay and smear microscopy laboratory processing time for successfully collected respiratory specimens (n = 546). Abbreviation: IQR, interquartile range.
AII Duration
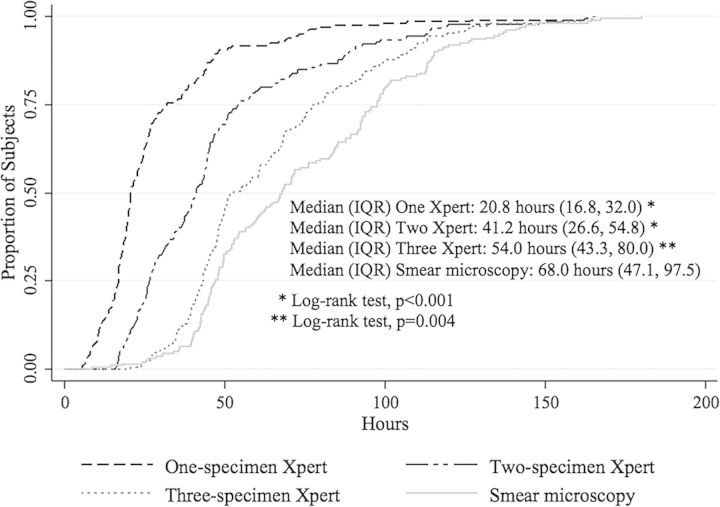
When using smear microscopy for AII discontinuation decision making, the median AII duration among 201 individuals hospitalized for presumptive pulmonary tuberculosis but not diagnosed with active tuberculosis was 68.0 hours (IQR, 47.1–97.5). The median AII duration for the Xpert AII discontinuation strategies was 20.8 hours (IQR, 16.8–32.0) for a single Xpert strategy (n = 201), 41.2 hours (IQR, 26.6–54.8) for a 2-specimen strategy (n = 180), and 54.0 hours (IQR, 43.3–80.0) for a 3-specimen strategy (n = 148) (Figure 3). The median AII duration was longer when using the smear-based strategy for decision making compared with any of the 3 Xpert strategies (all log-rank tests P ≤ .004).
Figure 3.
Kaplan-Meier curve comparing airborne infection isolation duration for the Xpert MTB/RIF assay strategies on 1 specimen (n = 201), 2 specimens (n = 180), and 3 specimens (n = 148) to the smear microscopy–based strategy (n = 201). Abbreviation: IQR, interquartile range.
DISCUSSION
In this observational cohort study, we found that all 3 Xpert-based strategies significantly reduced AII duration compared with the smear microscopy–based strategy. The 3-specimen strategy was less efficient than the 2-specimen Xpert strategy, as the median AII duration was longer (54.0 vs 41.2 hours), without any gain in tuberculosis case detection. Although faster (20.8 vs 41.2 hours), the 1-specimen Xpert strategy was less effective in our population, as it would have missed 1 case of tuberculosis. Thus, having 2 specimens tested by Xpert was the most efficient strategy to determine AII discontinuation in our cohort.
In October 2013, the CDC published interim practical considerations for incorporation of Xpert into diagnostic algorithms and infection control [15]. They state that for active tuberculosis evaluation and AII decision making, 3 sputum specimens should be collected 8–24 hours apart and tested by smear microscopy, a NAAT (including Xpert), or a combination of the 2 strategies [15]. Our data directly address these considerations by demonstrating that 3 specimens may not be needed and Xpert testing on 2 specimens may be the most efficient AII discontinuation strategy. Although smear microscopy was redundant for AII decision making, it remains necessary for NTM detection. Additionally, AFB culture continues to be critical for drug susceptibility testing and public health surveillance.
Only one other study addresses the impact of different tuberculosis diagnosis strategies on the duration of AII in the United States. Based on a retrospective cohort analysis of patients with presumptive pulmonary tuberculosis from Georgia, Hawaii, Maryland, and Massachusetts, Marks et al demonstrated that the use of the Amplified MTD NAAT (Gen-Probe, San Diego, California) decreased respiratory isolation duration for patients with smear-positive/NAAT-negative/culture-negative specimens [16]. Our prospective study extends these findings to the Xpert assay, the most common NAAT for rapid tuberculosis diagnosis, with >4 million assays procured worldwide by mid-2013, and as a first-line test to all people hospitalized for presumptive pulmonary tuberculosis [17].
Although the primary importance of AII is preventing healthcare-associated transmission of M. tuberculosis, reducing AII time is a beneficial secondary endpoint from a health system, health economic, and patient care perspective. AII rooms are a scarce resource in many institutions, and efficiently utilizing the available supply through rapid turnover is critical [18]. Prolonged AII occupancy drives increased institutional healthcare costs through consumption of AII-associated resources, such as AII room engineering controls and N95 respirators [19]. Perhaps most important, although the relationship remains unsettled [20], data suggest that isolated hospitalized patients may experience more preventable adverse events, have less care documented, and express greater dissatisfaction with their care [21–23].
While we report important findings, our study has several limitations. We did not directly observe AII initiation and discontinuation but rather estimated these based on electronic order entry and test result times, respectively. Although we may underestimate AII duration, as AII discontinuation occurs less efficiently than the moment test results are available [18], our design allows an objective comparison of the AII strategies given that clinicians were blinded to Xpert results. Our study was not powered to determine the sensitivity and specificity of the Xpert assay for detection of M. tuberculosis. We found no smear-negative tuberculosis, which is unexpected because it represents 37% of all US tuberculosis cases [24]. However, a recent study from Canada described high Xpert M. tuberculosis specificity (99.8%) but low sensitivity (46%) among predominantly asymptomatic outpatients evaluated for immigration screening where 72% of cases were smear negative [25]. Thus, our findings may not be generalizable to low-burden settings where smear-negative tuberculosis is more prevalent. In our cohort, 2 specimens were appropriate for discontinuing AII when using either Xpert or smear microscopy. However, 3 smears are recommended in the United States, with the third smear increasing sensitivity by 2%–5% [26]. Studies powered to determine the incremental specimen yield with Xpert in low-burden settings are needed. We made assumptions about Xpert implementation in the United States. Due to the minimal operator training requirements, we assumed that Xpert could routinely be performed twice daily during weekdays and once daily during weekends. Although this may have biased our result in favor of Xpert, laboratories could process specimens more frequently than assumed in this study. Our resources limited Xpert processing to sputum specimens only; however, based on published reports, we assumed Xpert is efficacious when performed on BAL specimens in our analysis [27–29]. Finally, we assessed Xpert effectiveness but not cost-effectiveness. Choi et al reported that Xpert was cost-effective in conjunction with smear microscopy in the United States, but they did not assess the cost-effectiveness of Xpert to replace the current smear microscopy–based approach for AII discontinuation [30].
In conclusion, we demonstrate that routine first-line Xpert testing of respiratory specimens from patients in AII has potential to significantly decrease AII time for individuals hospitalized without active tuberculosis in the United States. Because Xpert is now authorized for marketing in the United States [11], additional prospective studies in low-burden settings will be important to confirm our observation that a 2-specimen Xpert strategy minimizes AII duration while diagnosing all tuberculosis cases among hospitalized individuals with presumptive tuberculosis.
Notes
Acknowledgments. The authors thank David Weber for study design and manuscript preparation; David Murdoch for study design; Kevin Alby for laboratory and study database technical assistance; Brant Stalzer for study database design; Andrea Biddle for protocol development and study design; Steven Cole for sample size determination; Emily Sickbert-Bennett for hospital epidemiology technical assistance; and the Clinical Molecular and Mycobacteriology Laboratories of UNC Health Care for their efforts in processing all respiratory specimens in this study.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases [grant number T32 AI007001 to CKL] and the Fogarty International Center, the National Cancer Institute, the National Heart, Lung, and Blood Institute, the NIH Office of the Director Office of Research on Womens Health and the NIH Office of the Director Office of AIDS Research [grant number R25 TW009340 to CKL]. Cepheid provided G4 cartridges at no cost.
Disclaimer. Cepheid had no role in the design, conduct, or analysis of the study nor the decision to submit this work for publication.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Dowdle WR. A strategic plan for the elimination of tuberculosis in the United States. MMWR Morb Mortal Wkly Rep. 1989;38(suppl 3):1–25. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Atlanta, GA: US Department of Health and Human Services,; 2013. Reported tuberculosis in the United States, 2012. [Google Scholar]
- 3.Jensen PA, Lambert LA, Iademarco MF, Ridzon R. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005;54:1–141. [PubMed] [Google Scholar]
- 4.Steingart KR, Henry M, Ng V, et al. Fluorescence versus conventional sputum smear microscopy for tuberculosis: a systematic review. Lancet Infect Dis. 2006;6:570–81. doi: 10.1016/S1473-3099(06)70578-3. [DOI] [PubMed] [Google Scholar]
- 5.Mathew P, Kuo YH, Vazirani B, Eng RH, Weinstein MP. Are three sputum acid-fast bacillus smears necessary for discontinuing tuberculosis isolation? J Clin Microbiol. 2002;40:3482–4. doi: 10.1128/JCM.40.9.3482-3484.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Getahun H, Harrington M, O'Brien R, Nunn P. Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: informing urgent policy changes. Lancet. 2007;369:2042–9. doi: 10.1016/S0140-6736(07)60284-0. [DOI] [PubMed] [Google Scholar]
- 7.Steingart KR, Ramsay A, Pai M. Optimizing sputum smear microscopy for the diagnosis of pulmonary tuberculosis. Expert Rev Anti Infect Ther. 2007;5:327–31. doi: 10.1586/14787210.5.3.327. [DOI] [PubMed] [Google Scholar]
- 8.Diagnostic Standards and Classification of Tuberculosis in Adults and Children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med. 2000;161(4 pt 1):1376–95. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]
- 9.Watterson SA, Drobniewski FA. Modern laboratory diagnosis of mycobacterial infections. J Clin Pathol. 2000;53:727–32. doi: 10.1136/jcp.53.10.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Geneva, Switzerland: WHO; 2011. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF system. Policy statement. [PubMed] [Google Scholar]
- 11.US Food and Drug Administration. Silver Spring, MD: FDA; 2013. FDA permits marketing of first U.S. test labeled for simultaneous detection of tuberculosis bacteria and resistance to the antibiotic rifampin. [PubMed] [Google Scholar]
- 12.Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005–15. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steingart KR, Sohn H, Schiller I, et al. Xpert(R) MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2013;1:CD009593. doi: 10.1002/14651858.CD009593.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valenza G, Tappe D, Turnwald D, et al. Prevalence and antimicrobial susceptibility of microorganisms isolated from sputa of patients with cystic fibrosis. J Cyst Fibros. 2008;7:123–7. doi: 10.1016/j.jcf.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Availability of an assay for detecting Mycobacterium tuberculosis, including rifampin-resistant strains, and considerations for its use—United States, 2013. MMWR Morb Mortal Wkly Rep. 2013;62:821–4. [PMC free article] [PubMed] [Google Scholar]
- 16.Marks SM, Cronin W, Venkatappa T, et al. The health-system benefits and cost-effectiveness of using Mycobacterium tuberculosis direct nucleic acid amplification testing to diagnose tuberculosis disease in the United States. Clin Infect Dis. 2013;57:532–42. doi: 10.1093/cid/cit336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. WHO monitoring of Xpert MTB/RIF roll-out. Available at: http://www.who.int/tb/laboratory/mtbrifrollout/en/ Accessed February 2014. [Google Scholar]
- 18.Thomas BS, Bello EF, Seto TB. Prevalence and predictors of compliance with discontinuation of airborne isolation in patients with suspected pulmonary tuberculosis. Infect Control Hosp Epidemiol. 2013;34:967–72. doi: 10.1086/671732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leonard MK, Egan KB, Kourbatova E, et al. Increased efficiency in evaluating patients with suspected tuberculosis by use of a dedicated airborne infection isolation unit. Am J Infect Control. 2006;34:69–72. doi: 10.1016/j.ajic.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Harris AD, Pineles L, Belton B, et al. Universal glove and gown use and acquisition of antibiotic-resistant bacteria in the ICU: a randomized trial. JAMA. 2013;310:1571–80. doi: 10.1001/jama.2013.277815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stelfox HT, Bates DW, Redelmeier DA. Safety of patients isolated for infection control. JAMA. 2003;290:1899–905. doi: 10.1001/jama.290.14.1899. [DOI] [PubMed] [Google Scholar]
- 22.Abad C, Fearday A, Safdar N. Adverse effects of isolation in hospitalised patients: a systematic review. J Hosp Infect. 2010;76:97–102. doi: 10.1016/j.jhin.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vinski J, Bertin M, Sun Z, et al. Impact of isolation on hospital consumer assessment of healthcare providers and systems scores: is isolation isolating? Infect Control Hosp Epidemiol. 2012;33:513–6. doi: 10.1086/665314. [DOI] [PubMed] [Google Scholar]
- 24.Shah NS, Cavanaugh JS, Pratt R, et al. Epidemiology of smear-negative pulmonary tuberculosis in the United States, 1993–2008. Int J Tuberc Lung Dis. 2012;16:1234–40. doi: 10.5588/ijtld.11.0794. [DOI] [PubMed] [Google Scholar]
- 25.Sohn H, Aero AD, Menzies D, et al. Xpert MTB/RIF testing in a low tuberculosis incidence, high-resource setting: limitations in accuracy and clinical impact. Clin Infect Dis. 2014;58:970–6. doi: 10.1093/cid/ciu022. [DOI] [PubMed] [Google Scholar]
- 26.Mase SR, Ramsay A, Ng V, et al. Yield of serial sputum specimen examinations in the diagnosis of pulmonary tuberculosis: a systematic review. Int J Tuberc Lung Dis. 2007;11:485–95. [PubMed] [Google Scholar]
- 27.Lee HY, Seong MW, Park SS, et al. Diagnostic accuracy of Xpert(R) MTB/RIF on bronchoscopy specimens in patients with suspected pulmonary tuberculosis. Int J Tuberc Lung Dis. 2013;17:917–21. doi: 10.5588/ijtld.12.0885. [DOI] [PubMed] [Google Scholar]
- 28.Theron G, Peter J, Meldau R, et al. Accuracy and impact of Xpert MTB/RIF for the diagnosis of smear-negative or sputum-scarce tuberculosis using bronchoalveolar lavage fluid. Thorax. 2013;68:1043–51. doi: 10.1136/thoraxjnl-2013-203485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller MB, Popowitch EB, Backlund MG, Ager EP. Performance of Xpert MTB/RIF RUO assay and IS6110 real-time PCR for Mycobacterium tuberculosis detection in clinical samples. J Clin Microbiol. 2011;49:3458–62. doi: 10.1128/JCM.05212-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi HW, Miele K, Dowdy D, Shah M. Cost-effectiveness of Xpert(R) MTB/RIF for diagnosing pulmonary tuberculosis in the United States. Int J Tuberc Lung Dis. 2013;17:1328–35. doi: 10.5588/ijtld.13.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]