IL-21 plus CpG enhances APC function and cytotoxic activity in B-CLL B cells
Keywords: cross-presentation, cytotoxic B cells, immunogenization, leukemia vaccine, toll-like receptor agonist
Abstract
CpG oligodeoxynucleotides (CpG) and IL-21 are two promising agents for the treatment of B-cell chronic lymphocytic leukemia (B-CLL). Recently, we reported that the combination of CpG and IL-21 (CpG/IL-21) can induce granzyme B (GrB)-dependent apoptosis in B-CLL cells. Here, we demonstrate that treatment of B-CLL cells with CpG and IL-21 results in the development of antigen-presenting cell (APC)-like cells with cytotoxic features. These properties eventually give rise to B-CLL cell apoptosis, independently of their cytogenetic phenotype, whereas normal B-cell survival is not negatively affected by CpG/IL-21. APC- and CTL-typical molecules found to be up-regulated in CpG/IL-21-stimulated B-CLL cells include GrB, perforin, T-bet, monokine-induced by IFN-γ and IFN-γ-inducible protein 10 (IP-10), as well as molecules important for cell adhesion, antigen cross-presentation and costimulation. Also induced are molecules involved in GrB induction, trafficking and processing, whereas the GrB inhibitor Serpin B9 [formerly proteinase inhibitor-9 (PI-9)] is down-modulated by CpG/IL-21. In conclusion, CpG/IL-21-stimulated B-CLL cells acquire features that are reminiscent of killer dendritic cells, and which result in enhanced immunogenicity, cytotoxicity and apoptosis. Our results provide novel insights into the aberrant immune state of B-CLL cells and may establish a basis for the development of an innovative cellular vaccination approach in B-CLL.
Introduction
B-cell chronic lymphocytic leukemia (B-CLL) is the most common leukemia in the western world (1). It is characterized by accumulation of CD5+ neoplastic B cells that show varying proliferative activity and survival (2, 3), resulting in heterogeneous patient prognosis and survival. There is evidence that leukemia-specific T cells are present in B-CLL patients (4), and that they can be induced by either hematopoietic stem cell transplantation (5) or by vaccination with leukemia antigen-loaded dendritic cells (DC) (6, 7). Nevertheless, immunogenicity of B-CLL cells is generally low and different strategies for their enhancement have been tested (8, 9). Two novel candidates with strong immunogenizing potential in B-CLL are IL-21 and CpG oligodeoxynucleotides (CpG). While their effects on B-CLL cells as single agents have been extensively evaluated in vitro (8, 10–12) and in vivo (13, 14), detailed information on their combined effects is incomplete.
The IL-2 family cytokine IL-21 exhibits pleiotropic effects on various immune cells including B cells, NK cells and CTL (15). In B cells, it can induce either activation or apoptosis, depending on additional stimuli such as CD40 ligation and BCR or TLR triggering (16). In B-CLL, IL-21 was shown to mediate both direct induction of B-cell lymphoma 2 interacting mediator of cell death (BIM)-dependent apoptosis and enhancement of antibody-dependent cytotoxicity (17). CpG on the other hand are synthetically produced danger signals that mimic the immunological effects of unmethylated microbial DNA. They are recognized by TLR-9, which is expressed on normal and malignant B cells (10). The most prominent effects of CpG on B-CLL cells are the development of an immunogenic phenotype (8) and the induction of apoptosis (10, 11), suggesting CpG may exhibit direct and indirect antileukemic effects in vivo. Clinical studies have, at least in part, confirmed this hypothesis (13). However, the observed effects were inconsistent and appeared to be influenced by the presence of certain cytogenetic aberrations (11). We therefore hypothesized that combinations of CpG with other immunomodulatory molecules such as IL-21 may enhance their apoptosis-inducing and immunogenizing effects.
Recently, we demonstrated that the combination of CpG and IL-21 (CpG/IL-21) triggers B-CLL cells to express the cytotoxic serine protease granzyme B (GrB) (12). Moreover, CpG/IL-21-stimulated GrB+ B-CLL cells were able to induce GrB-dependent apoptosis in bystander B-CLL cells. In the present study, we investigated in more detail the impact of CpG/IL-21 on the differentiation of B-CLL cells as compared with healthy B cells. Here, we show that although CpG/IL-21 induces GrB and GrB-associated molecules in both healthy B cells and B-CLL cells, apoptosis is triggered only in B-CLL cells, regardless of their cytogenetic phenotype, but not in normal B cells. This difference may be due to the fact that B-CLL cell expression of the GrB inhibitor Serpin B9 is down-modulated by CpG/IL-21, whereas a variety of molecules involved in immunological cross-talk are up-regulated.
In summary, our results indicate that stimulation of B-CLL cells, but not healthy B cells with CpG/IL-21 induces two seemingly opposing differentiation pathways allowing B-CLL cells to acquire cytotoxic features and features of antigen-presenting cells (APC), and eventually resulting in an immunogenic phenotype, fratricidal cytotoxicity and apoptosis. Our study provides information and outlook on a novel immunogenization approach in B-CLL based on the use of CpG and IL-21.
Methods
Human subjects and cell culture
The present study was approved by the institutional review boards at both Ulm University (Ulm, Germany) and the University of Iowa (Iowa City, IA, USA). Table 1 shows the clinical profile of a group of patients studied at the University of Iowa. Peripheral blood from healthy volunteers was used as a control. After obtaining informed consent, peripheral blood from donors was collected and PBMC immediately isolated by Ficoll density gradient centrifugation. Cells were lysed to remove RBCs and finally re-suspended in AIM-V medium (Gibco BRL, Grand Island, NY, USA). For some experiments, B cells from healthy individuals and from cord blood donors were purified (>99.5% purity) using negative selection kits (Miltenyi Biotech, Bergisch Gladbach, Germany). If not stated otherwise, B cells were plated on round-bottom 96-well plates at 1×106 cells ml−1, 200 μl per well, at 37°C and a 5% CO2 atmosphere in the presence of various reagents as indicated.
Table 1.
Clinical profile of patients examined in this study
| Sample number | Age | Sex | Survival of untreated B-CLL cells at day 4 (%) | Survival of treated B-CLL cells at day 4 (%) | Non-B-CLL cells (%) | WBC (1000mm−3) | Rai stage | LDH (U l−1) | Cytogenetics | CD5 expression | Time since most recent treatment | Most recent therapeutic regimen |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 74 | M | 54 | 12 | 35 | 29.4 | 0 | 190 | Normal karyotype | + | Untreated | Untreated |
| 2 | 72 | M | 59 | 27 | 32 | 32.3 | 0 | 187 | 13q14.3 del | + | Untreated | Untreated |
| 3 | 65 | M | 93 | 39 | 36 | 15.9 | 0 | n.a. | 13q14.3 del | + | Untreated | Untreated |
| 4 | 50 | F | 82 | 7 | 23 | 59.8 | 0 | 196 | 13q14.3 del | + | Untreated | Untreated |
| 6 | 82 | M | 40 | 10 | 10 | 53.3 | 1 | 217 | 13q14.3 del | + | Untreated | Untreated |
| 5 | 76 | F | 36 | 7 | 12 | 14.4 | 0 | 162 | n.a. | + | Untreated | Untreated |
| 7 | 78 | F | >95 | 35 | 11 | 113.5 | 0 | 312 | 13q14.3 del, 11q22.3 del | + | 4 months | Fludarabine, cytoxan |
| 8 | 55 | M | 22 | 1 | 43 | 12 | 2 | 131 | 17p13.1 del, trisomy 12 | + | Untreated | Untreated |
| 9 | 55 | F | 67 | 38 | 39 | 29.6 | 3 | 334 | 17p13.1 del, 13q14.3 del | + | Untreated | Untreated |
| 10 | 75 | M | 26 | 56 | 11 | 66.6 | 1 | 182 | 17p13.1 del | − | Untreated | Untreated |
| 11 | 72 | F | 24 | 45 | 9 | 29.2 | 4 | 190 | 17p13.1 del | − | 11 months | Fludarabine, cytoxan |
LDH, lactate dehydrogenase; n.a., not available; WBC, white blood cell.
Reagents for functional assays
Human recombinant IL-21 (50ng ml−1) was purchased from BioSource (Camarillo, CA, USA), the CpG ODN 2006 (2.5 μg ml−1) were obtained from Coley Pharmaceutical Group (Wellesley, MA, USA). For polyclonal BCR stimulation, affinity purified rabbit F(ab′)2 against human IgA+IgG+IgM (H+L) was used at 6.5 μg ml−1 (anti-BCR; Jackson ImmunoResearch Laboratories, West Grove, PA, USA). The JAK inhibitor pyridone 6 (1 μM) was purchased from Calbiochem (San Diego, CA, USA), and IFN-γ (100U ml−1) from PBL Interferon Source (Piscataway, NC, USA). For CD5 antibody-binding experiments, mouse anti-human CD5 mAb (clone MF7-14–5, azide free) and an appropriate control mAb (clone W3/25, azide free) were used (Serotec, Raleigh, NC, USA).
Flow cytometry
For flow cytometric detection of apoptosis, B cells were incubated for 4 days in the presence of IL-2, IL-21 and CpG. Then, cells were stained with Annexin V (BD Biosciences, Heidelberg, Germany) for 15min at room temperature. Propidium iodide was added immediately before FACS analysis. For intracellular GrB staining, cells were stimulated for 16h with IL-21, anti-BCR, CpG and pyridone 6 (P6) as indicated. After adding brefeldin A (1 μg ml−1) for 4h, cells were harvested and stained as described previously (18). PE-labeled antibodies to human GrB (clone GB12) and appropriate isotype controls were purchased from Invitrogen (Carlsbad, CA, USA). For phenotypic analysis of cell surface markers, cells were stimulated with IL-21 and CpG and stained with FITC-, PE-, PE-Cy5-, PE-Cy7- or allophycocyanin-labeled antibodies to CD5, CD10, CD19, CD27, CD40, CD54, CD58, CD70, CD80, CD86, CD154, CD222, CXCR3 and MHC I (Becton Dickinson Biosciences, Heidelberg, Germany) as described recently (18). Flow cytometry was conducted on a FACScan or a FACSCalibur (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA). Data were analyzed using FlowJo software (version 8.7.1; Tree Star, Stanford, CA, USA).
CFSE staining and proliferation assay
For coculture experiments, T cells from healthy individuals were isolated using the CD3+ T-cell isolation kit II (Miltenyi Biotech). T cells were labeled with 5- (and 6-) carboxyfluorescein diacetate succinimidyl ester (CFSE) (Life technologies, Carlsbad, CA, USA) for 10min at a concentration of 2.5 μM as described before. Then T cells were cocultured with allogeneic B-CLL cells at a B-CLL:T cells ratio of 2:1 (1×105:5×104 cells) in the presence of CpG (2.5 μg ml−1), IL-21 (50ng ml−1) or both as indicated. After 7 days, cells were harvested, stained for CD3 and CD8 and analyzed by FACS.
MIG ELISpot
Monokine-induced by IFN-γ (MIG) is also known as CXCL9. The Human CXCL9/MIG Development Module was purchased from R&D Systems (Wiesbaden-Nordenstadt, Germany). Polyvinylidene fluoride-bottomed 96-well plates were from Millipore (Bedford, MA, USA). The ELISpot was performed according to the manufacturer’s instructions. Briefly, plates were incubated overnight with 100 μl of diluted capture antibody per well. Blocking was performed with 1% BSA, 5% sucrose in PBS for 2h. Purified B cells from healthy donors or patients with B-CLL were plated at 1×105 cells per 100 µl per well in AIM-V medium with various reagents as indicated and cultured for 16h. Detection antibody was added and incubated overnight. After incubation, color was developed with streptavidin–alkaline phosphatase and BCIP/NBT buffer. Plates were analyzed and dots counted on an Immunospot Series 1 Analyzer using Immunospot 3 software, both from CTL Cellular Technology (Cleveland, OH, USA).
RT–PCR
B cells from healthy donors and patients with B-CLL were magnetically purified to 99.5% and plated in AIM-V medium for 3 days in the presence or absence of IL-21, CpG or anti-BCR. Cells were harvested, counted and mRNA was isolated using RNAqueous - 4PCR kit (Ambion, Austin, TX, USA). Then, cDNA was prepared using SuperSript III reverse Transcriptase kit (Invitrogen) and RT–PCR performed in a Flexigene thermocycler (Techne, Burlington, NJ, USA) using Platinum Taq DNA Polymerase High Fidelity (Invitrogen). Primers were purchased from Integrated DNA Technologies (Coralville, IA, USA) and were designed as summarized below (Table 2). After reverse transcription, products were run along with the housekeeping gene RPL-32 on 2% agarose gels.
Table 2.
DNA primers used in this study
| Gene | Sequence (5′ to 3′) |
|---|---|
| Granzyme B (218bp) | F: CCATCCAGCCTATAATCCTA |
| R: CCTGCACTGTCATCTTCACCT | |
| Perforin (146bp) | F: TGGAGTGCCGCTTCTACAGTT |
| R: GTGGGTGCCGTAGTTGGAGAT | |
| Serglycin (336bp) | F: GCTACTCAAATGCAGTCGGCTTGT |
| R: ATTTCCGTTAGGAAGCCACTCCCA | |
| Cathepsin C (298bp) | F: TGCTCGGTTATGGGACCACAAGAA |
| R: ACACATTCTCAGAGGCAGTTCCCA | |
| MIG (179bp) | F: ACCAACCAAGGGACTATCCACCTA |
| R: TGGCTGACCTGTTTCTCCCACTTT | |
| IP-10 (151bp) | F: GTACGCTGTACCTGCATCAGCATT |
| R: CTGGATTCAGACATCTCTTCTCACCC | |
| EOMES (187bp) | F: ATGAGCCCTCAAAGACCCAGACTT |
| R: AAGAAGGATTGAACGCCGTACCGA | |
| T-bet (114bp) | F: TGACCCAGATGATTGTGCTCCAGT |
| R: AGATATGCGTGTTGGAAGCTTGC | |
| NFATc4 (421bp) | F: TCAGAAGACACGGCGGACTTCC |
| R: TGAACATCTGTAGGGTCAGTGG | |
| RPL-32 (210bp) | F: GCGTAACTGGCGGAAACCCA |
| R: TTGTGAGCGATCTCGGCACA |
F, forward primer; R, reverse primer.
Western immunoblot
Purified B cells from healthy donors or patients with B-CLL were incubated for 16h (for GrB and Serpin B9) or 15/20 min (for JAK and STAT) at 2×106 cells per ml per well with IL-21, anti-BCR, CpG or a combination of these reagents. Cells were lysed and western immunoblot performed as recently described (18). Primary antibodies against pJAK1, pSTAT3 and β-Actin were from Cell Signaling Technology Inc. (Danvers, MA, USA). The antibody against Serpin B9 (mAb clone 7D8) was kindly provided by Vivien Sutton (Peter MacCallum Cancer Centre, Melbourne, Australia). Antibodies against pJAK3 were purchased from Santa Cruz (Santa Cruz, CA, USA), against pSTAT1 from Biosource (Nivelle, Belgium). As secondary antibodies, rabbit anti-goat IgG from Sigma-Aldrich Laborchemikalien GmbH (Seelze, Germany), goat anti-rabbit IgG and goat anti-mouse IgG from GE Healthcare (Munich, Germany) were used.
Statistics
Data are expressed as means ± SEM. To determine statistical differences in the means of two data columns, the paired Student’s t-test was used as appropriate. A P value of <0.05 was considered statistically significant.
Results
The combination of CpG and IL-21 induces apoptosis in CD5+ B-CLL cells, but not in CD5− B cells
Previously, we demonstrated that stimulation of B cells from B-CLL patients results in differentiation of such cells into GrB-producing cytotoxic cells capable of inducing GrB-dependent apoptosis in bystander B-CLL cells (12). GrB production is not limited to B-CLL cells, but also occurs in other B-cell subsets. Recently, we found that CD5+ B cells, but not CD5− B cells from patients with systemic lupus erythematosus (SLE) constitutively express GrB and undergo apoptosis in response to CpG/IL-21 (19). On the other hand, both CpG (10) and IL-21 (20) can support B-cell survival, so that additional factors may determine whether stimulation of B cells with CpG/IL-21 results in B-cell apoptosis or survival.
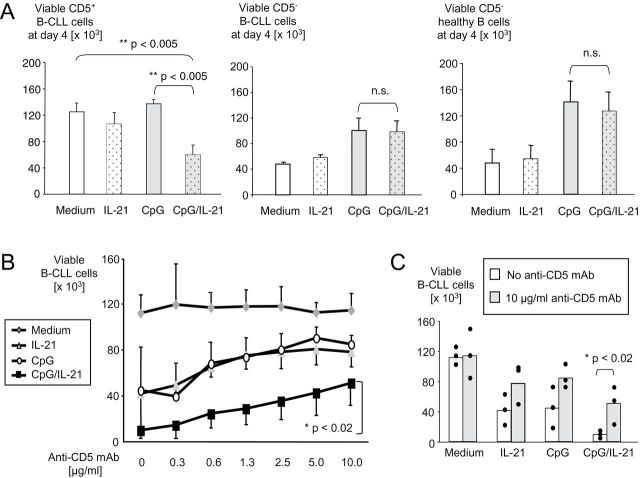
The combination CpG/IL-21 strongly induces apoptosis in CD5+ B-CLL cells after 4 days incubation (Fig. 1A, left panel and Supplementary Figure 1, available at International Immunology Online, upper panel). Importantly, this effect appears to occur independently of B-CLL cell cytogenetics (Table 1), a finding not observed with CpG alone (12). In contrast to CD5+ B-CLL cells, CD5− healthy B cells are not killed by CpG/IL-21. Instead, CpG protects B cells from apoptosis (Fig. 1A, right panel). Interestingly, when we tested two cases of a lymphoproliferative disease with accumulation of CD5− leukemic B cells, CpG/IL-21 did not induce apoptosis in these cells either (Fig. 1A, middle panel; Supplementary Figure 1A, available at International Immunology Online, lower panel and Table 1).
Fig. 1.
CpG and IL-21 induce apoptosis in CD5+ B-CLL cells, but not in CD5− B cells. (A) PBMC from five subjects with CD5+ B-CLL (left panel), two subjects with CD5− B-CLL (middle panel) and four healthy subjects (right panel) were isolated and cultured for 4 days in the presence of CpG, IL-21 or both. Cells were harvested, stained with antibodies to CD19, CD5, annexin V and propidium iodide (PI) and analyzed by flow cytometry. Shown are mean percentages of viable B cells determined by annexin V/PI staining. Error bars indicate SEM for left and right panels and standard deviation for middle panel. (B and C) B-CLL cells from three different donors with high cellularity (>80% CD19+CD5+ B-CLL cells) were incubated with IL-21, CpG or a combination of both, in the presence of increasing concentrations of a monoclonal anti-CD5 antibody. Viability of B-CLL cells was determined by FACS, using annexin V/PI. Line graphs (B) and bar graphs (C) show absolute numbers of viable B-CLL cells on day 4. Error bars indicate SEM from three independent experiments.
On the basis of these observations, we hypothesized that CD5 may be involved in the apoptotic response after stimulation of B-CLL cells with CpG/IL-21. We therefore repeated our experiments in the presence of increasing concentrations of a CD5-binding antibody. These experiments demonstrated that the proapoptotic effect of CpG/IL-21 was partially inhibited by antibody binding to CD5 in a dose-dependent manner (Fig. 1B). Importantly, this CD5-dependent protection from apoptosis was observed only in malignant CD5+ B-CLL cells, but not in CD5+ cord blood B cells, where the CD5 antibody even seemed to induce apoptosis by itself (Supplementary Figure 2, available at International Immunology Online).
IL-21 and CpG induce GrB in B-CLL cells, but GrB levels are significantly lower in B-CLL cells than in healthy B cells
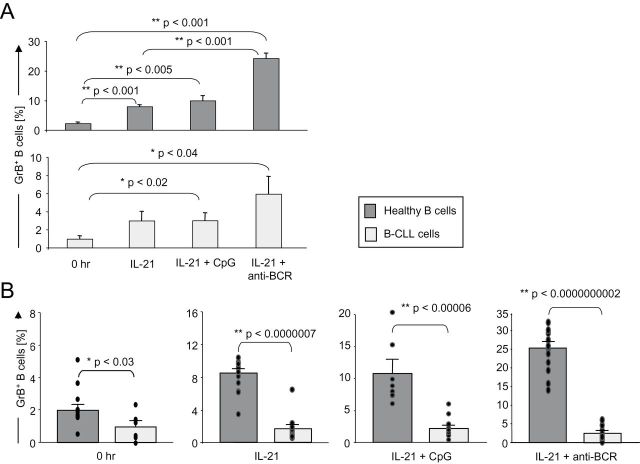
Given our previous findings that CpG/IL-21-induced apoptosis in B-CLL cells is mediated by GrB (12), we directly compared the potential of B-CLL cells versus healthy B cells to produce GrB. As demonstrated above, CpG/IL-21 strongly induced apoptosis in B-CLL, but not in healthy B cells (Fig. 1), suggesting B-CLL cells may produce higher levels of GrB as compared with healthy B cells. As expected, IL-21 induced GrB in both healthy B and B-CLL cells, an effect enhanced by BCR stimulation (anti-BCR) (Fig. 2A and Supplementary Figure 3, available at International Immunology Online). Contrary to what we expected, however, we found that healthy B cells produced significantly more GrB after stimulation with IL-21 than B-CLL cells did (Fig. 2B and Supplementary Figure 3, available at International Immunology Online).
Fig. 2.
GrB expression is higher in normal B cells compared with B-CLL cells. (A and B) Healthy B cells or B-CLL cells were isolated from peripheral blood and either immediately stained for GrB (0h) or cultured for 16h in the presence of IL-21, CpG, anti-BCR and combinations. Brefeldin A was added for four more hours, cells were stained for intracellular GrB and analyzed by FACS. (A) Bar graphs show average percentages of GrB-expressing healthy B cells (n = 10) compared with B-CLL cells (n = 4). Error bars indicate SEM. (B) In a second experiment with a different B-CLL cohort, we directly compared normal B cells and B-CLL cells. Bar graphs show average percentages of GrB-producing healthy B cells (n = 10) and B-CLL cells (n = 6). Error bars indicate SEM, dots indicate individual donors.
GrB production in B-CLL cells requires activation of STAT, but not JAK members
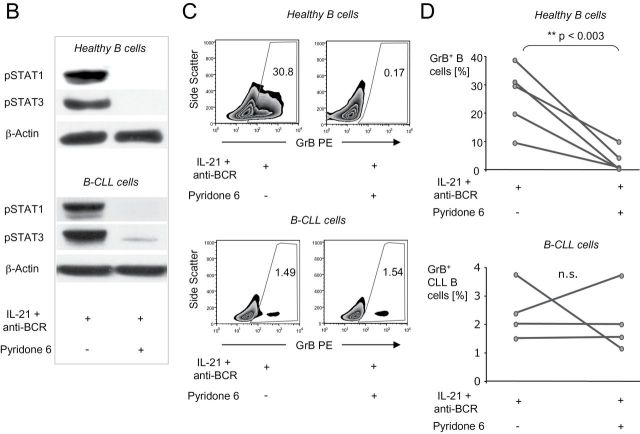
In healthy B cells, induction of GrB secretion after stimulation with IL-21 and anti-BCR requires JAK1 and JAK3 phosphorylation, followed by subsequent STAT1 and STAT3 activation (18). We therefore wished to compare the involvement of these signaling molecules in healthy B cells versus B-CLL cells after stimulation with CpG/IL-21. Stimulation with IL-21 and either anti-BCR or CpG clearly induced phosphorylated JAK1 in healthy B cells (Fig. 3A, upper panel), whereas no relevant expression of this molecule was detected in B-CLL cells under the same conditions (Fig. 3A, lower panel). Nevertheless, both phosphorylated STAT1 and STAT3 were found in healthy B cells and in B-CLL cells after activation with IL-21 (Fig. 3A). Our findings were further supported by the observation that the pan-JAK inhibitor P6 suppressed STAT1 and STAT3 phosphorylation in both healthy B cells and B-CLL cells (Fig. 3B) and was able to suppress GrB expression in IL-21/anti-BCR-stimulated healthy B cells, but not in IL-21/anti-BCR-stimulated B-CLL cells (Fig. 3C and D).
Fig. 3.
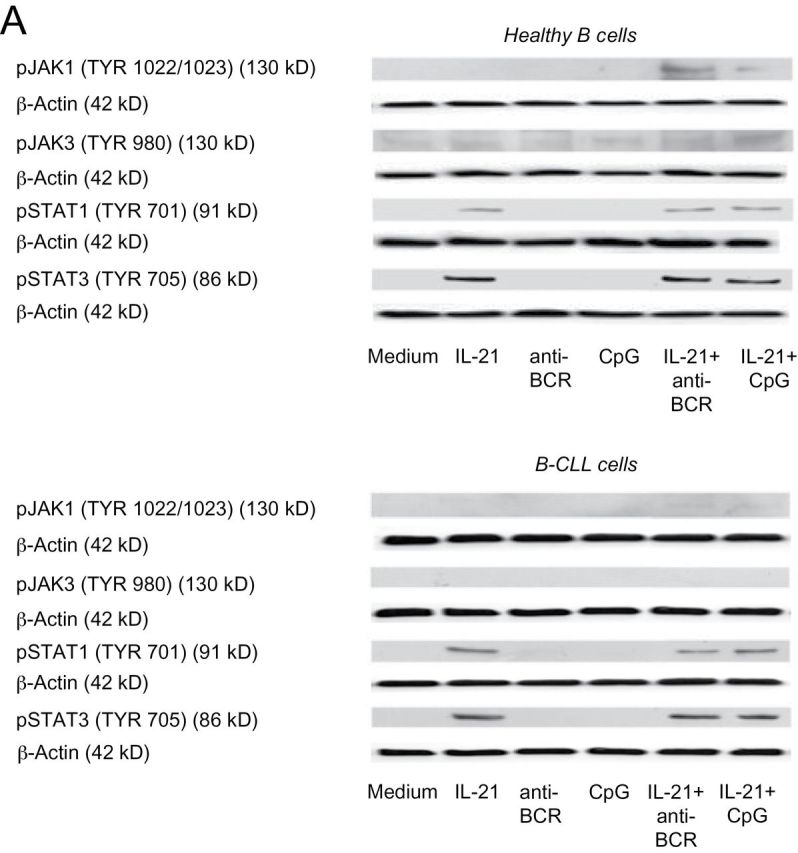
GrB production in B cells requires activation of members of the JAK/STAT pathway. (A) Purified B cells from healthy donors or B-CLL patients (>99.5%) were cultured for 15min with IL-21, anti-BCR or CpG. Analysis of pJAK1, pJAK3, pSTAT1 and pSTAT3 was performed by western immunoblotting, each membrane was controlled for correct loading by stripping and restaining the membrane for β-actin (n = 3). (B) Purified healthy B cells and B-CLL cells were cultured for 20min with IL-21 and anti-BCR in the presence or absence of the JAK inhibitor P6. Analysis of pSTAT1 and pSTAT3 was performed by western immunoblotting (n = 3). (C and D) Purified normal B cells and B-CLL cells were cultured for 16h with IL-21 and anti-BCR in the presence or absence of P6. After incubation, cells were stained for intracellular GrB. Shown is GrB production of CD5+CD19+ B-CLL cells and CD19+ B cells as determined by flow cytometry. Panels in (C) illustrate representative dot plots out of five donors (healthy B cells) or four patients (B-CLL). Panels in (D) indicate percentages of GrB+ B cells with each line indicating an individual donor.
B-CLL cells can express molecules involved in GrB induction, trafficking, processing and inhibition
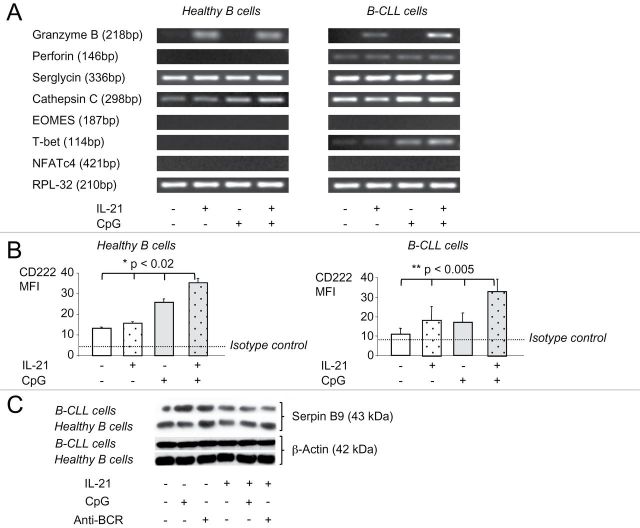
In CTL and NK cells, GrB-dependent cytotoxicity requires induction of transcription factors including eomesodermin (EOMES), nuclear factor of activated T cells (NFAT) and T-bet (21). Additional molecules such as cathepsin C, serglycin and perforin are essential to mediate processing and correct trafficking of GrB into target cells (22). Here, we show on an mRNA level that some of these molecules were expressed by B-CLL, but not by healthy B cells. Expression occurred either constitutively, e.g. for perforin, serglycin and cathepsin C, or after stimulation with CpG/IL-21, as for T-bet (Fig. 4A). Expression of GrB mRNA itself was detected after stimulation of B-CLL cells with IL-21 alone and was further enhanced by CpG (Fig. 4A). No expression of EOMES or NFAT was detected in B-CLL cells.
Fig. 4.
IL-21 and CpG induce a CTL-like transcriptional profile in B-CLL cells and up-regulate molecules involved in GrB uptake and inhibition. Purified healthy B cells or B-CLL cells were cultured with IL-21, CpG or both. (A) After 16h, cells were harvested, mRNA prepared and RT–PCR for indicated molecules performed. Data are representative for three independent experiments. (B) On day 4, B cells were analyzed by flow cytometry for the expression of CD222. Error bars indicate SEM (healthy B cells n = 3, B-CLL cells n = 9). (C) Cell lysates were prepared after 16h and expression of Serpin B9 and β-actin determined by western immunoblot. Shown is one representative blot out of three with similar results.
We hypothesized that CpG/IL-21-induced mutual cytotoxicity in B-CLL cells is in part caused by a fratricidal killing mechanism involving transfer of GrB between B-CLL cells (12). We therefore tested another molecule known to mediate GrB uptake, the mannose-6-phosphate receptor (CD222), which was strongly up-regulated on both B-CLL cells and normal B cells in response to CpG/IL-21 (Fig. 4B). Of note, an attempt to suppress CpG/IL-21-induced B-CLL cell apoptosis in the presence of a CD222 inhibitor (mannose-6-phosphate) did not show any effects (data not shown). Finally, we tested expression of Serpin B9 [formerly proteinase inhibitor-9 (PI-9)], one of the major proteins responsible for protection of cytotoxic cells from GrB (23). As shown in Fig. 4(C), both, healthy B cells and B-CLL cells showed constitutive expression of Serpin B9, which was not significantly changed by CpG/IL-21 stimulation in normal B cells, while it was mildly suppressed in B-CLL cells (Fig. 4C).
IL-21 and CpG synergistically induce MIG in B-CLL cells, but not in healthy B cells
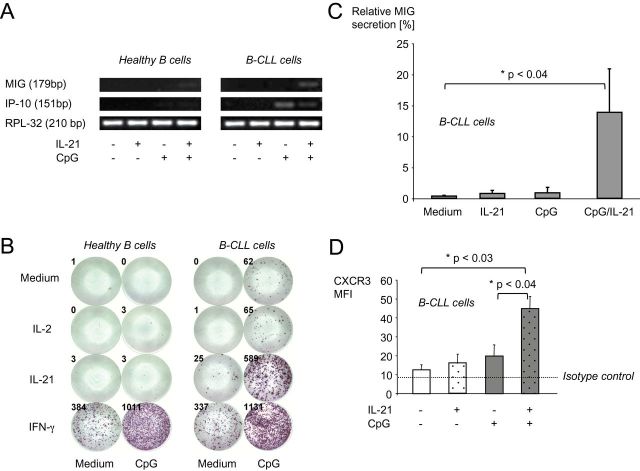
MIG is a CXC subfamily chemokine. After binding to its cognate receptor, CXCR3, it induces chemotactic cell movement along an MIG gradient. CXCR3 is present on various immune cells and was previously demonstrated to be expressed by B-CLL cells as well (24). We hypothesized that expression of MIG and its receptor may change after stimulation of B-CLL cells with CpG/IL-21, thereby helping to explain how CpG/IL-21-activated, GrB-expressing B-CLL cells may physically approach each other. We first tested mRNA levels of MIG and the related chemokine IP-10 (IFN-γ-inducible protein 10) in B-CLL and healthy B cells after CpG/IL-21 stimulation. CpG alone induced IP-10, but not MIG in B-CLL cells. In contrast, the combination CpG/IL-21 induced MIG, but not IP-10 in B-CLL cells (Fig. 5A). Neither MIG nor IP-10 was induced in healthy B cells by CpG/IL-21.
Fig. 5.
CpG and IL-21 synergistically induce MIG in B-CLL, but not in normal B cells. Purified healthy B cells or B-CLL cells (>99.5%) were incubated in the presence of IL-2, IL-21, IFN-γ and CpG as indicated. (A) Total RNA was isolated after 16h and RT–PCR for MIG and IP-10 mRNA performed (n = 3). (B) 1×105 B cells were cultured as indicated on a 96-well ELISpot plate coated with specific antibodies to MIG. After 16h, plates were developed and MIG-secreting cells counted. (C) Bar graphs illustrate average dot numbers out of three independent experiments. Error bars show SEM. (D) On day 4, B-CLL cells were analyzed by flow cytometry for the expression of the chemokine receptor CXCR3. Bar graphs represent means of three independent experiments and error bars show SEM.
Next, we tested the secretion of both chemokines by B-CLL and healthy B cells. IFN-γ served as positive control. CpG/IL-21 induced significant amounts of MIG secreted by B-CLL cells, but not by healthy B cells (Fig. 5B and C). In contrast, stimulation of B-CLL cells with single agents or with IL-2 instead of IL-21 did not trigger MIG secretion (Fig. 5B and C). IP-10 secretion was induced only by IFN-γ, but not by CpG/IL-21 (data not shown). Of note, the MIG receptor CXCR3 was strongly up-regulated in B-CLL cells by CpG/IL-21 (Fig. 5D).
Activation of B-CLL cells with CpG and IL-21 results in enhanced immunogenicity
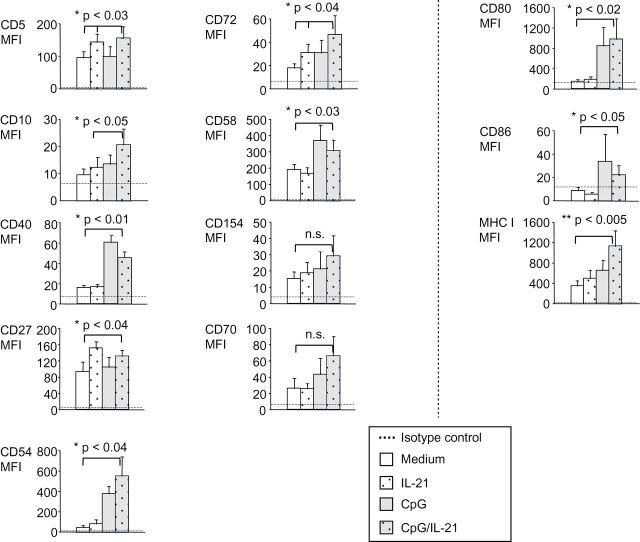
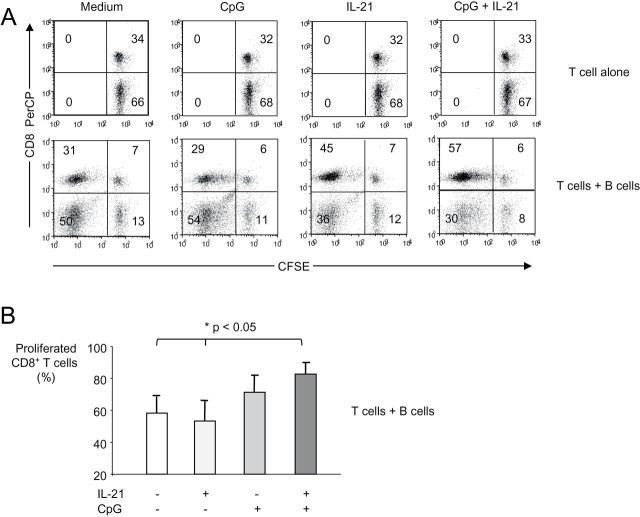
The results presented above show that CpG/IL-21 may enable B-CLL cells to attract each other and T cells. For the establishment of a tight cell contact between cells, however, further molecules may be necessary. Such molecules may involve classical cell adhesion molecules like CD54 (ICAM-1), but also receptor/ligand pairs such as CD10/CD58, CD40/CD154, CD27/CD70 and CD5/CD72. We therefore tested expression of these molecules on B-CLL cells in the presence of CpG/IL-21 and found various of them to be significantly up-regulated (Fig. 6, left two panel rows and Supplementary Figure 4, available at International Immunology Online). Furthermore, we observed enhanced expression of the antigen-presenting molecule MHC class I and the costimulatory molecules CD80 and CD86 (Fig. 6, right panel row and Supplementary Figure 4, available at International Immunology Online). To directly test the MHC class I-restricted immunogenicity of B-CLL cells, we finally performed cocultures between B-CLL cells and allogeneic T cells in the presence of CpG, IL-21 or both. These experiments demonstrated that B-CLL cells activated in the presence of both CpG and IL-21 are superior to unstimulated B-CLL cells, or B-CLL cells stimulated in the presence of CpG alone to induce allogeneic CD8+ T-cell proliferation (Fig. 7).
Fig. 6.
IL-21 and CpG induce B-CLL cell up-regulation of molecules required for cellular cross-talk. PBMC from B-CLL patients were cultured in the presence or absence of IL-21 and CpG for 4 days. Then they were harvested, stained for the markers indicated and analyzed by FACS. Error bars indicate SEM from three independent experiments for each marker.
Fig. 7.
B-CLL cells activated with IL-21 and CpG are superior to B-CLL cells stimulated with CpG alone to induce allogeneic CD8+ T-cell proliferation. B-CLL cells were purified and cocultured with carboxyfluorescein diacetate succinimidyl ester-stained allogeneic T cells at a ratio of 2:1 for 6 days in the presence of CpG, IL-21 or both reagents as indicated. Then cells were harvested, stained for CD3 and CD8 and analyzed by FACS. (A) Dot plots show CD3+ T cells from one representative experiment out of four with similar results. T cells cultured in the absence of B-CLL cells did not proliferate (top row). (B) Bar graphs show average CD8+ T-cell proliferation from four individual experiments. Error bars indicate SEM.
Discussion
A series of studies suggest immunogenization of B-cell leukemias may be a promising immunotherapeutic approach (13, 25, 26). Some of these studies revealed that the combination of immunomodulatory agents may be more effective in inducing an immunogenic phenotype than single agents (26, 27). Here, we hypothesized that combining CpG with IL-21 may be more effective in inducing immunological B-CLL cell responses than CpG or IL-21 alone. We demonstrate that CpG plus IL-21 (CpG/IL-21) effectively induces apoptosis in B-CLL cells, independently of their cytogenetic status, whereas it promotes survival and proliferation in healthy B cells. Importantly, CpG/IL-21-mediated induction of B-CLL cell death is associated with expression of GrB and further molecules involved in GrB induction, trafficking, processing and cellular uptake, as well as mild suppression of the GrB inhibitor Serpin B9. Moreover, CpG/IL-21 induces up-regulation of molecules important for immunological cross-talk with other cells such as chemokines, chemokine receptors and molecules involved in adhesion, antigen presentation and costimulation.
The fact that B-CLL cells undergo GrB-dependent apoptosis after stimulation with CpG/IL-21 (12) can be explained by similar mechanisms existing in NK cells that involve GrB leakage from cytoplasmatic granules (28, 29). However, the capacity of GrB-secreting B-CLL cells to induce GrB-mediated killing in resting bystander B-CLL cells (12) remains unclear so far. Here, we identified a series of molecules that can explain how B-CLL cells may approach and contact other cells. It is known that trafficking through cytokines and chemokines plays a critical role in the pathophysiology of B-CLL, for example by secretion of chemokines like CCL3, CCL4 and CCL22 (30). B-CLL cell-secreted chemokines can attract other cells such as monocytes, thereby creating a supportive microenvironment for their survival and proliferation. In our current study, we observed that CpG/IL-21 trigger B-CLL cells to secrete the T-cell chemokine CXCL-9 (MIG). Since B-CLL cells coexpress the cognate MIG receptor CXCR3 (24), CpG/IL-21-induced MIG secretion may enable B-CLL cells to localize and approach each other. Moreover, we found that various further receptor/ligand pairs on B-CLL cells are up-regulated after CpG/IL-21 stimulation including CD5/CD72, CD10/CD58, CD40/CD154 and CD27/CD70, suggesting that B-CLL cells cannot only establish cell contact with each other but are also able to keep this contact.
CD5 as an example appears to be involved in the fratricidal killing effect between B-CLL cells, since its absence or antibody ligation suppressed the killing. Of note, CD5+ B cells are a unique subset within the various B-cell populations and are thought to be involved in the pathogenesis of autoimmunity (31). It is therefore remarkable that we recently found CD5+ B cells from patients with SLE to constitutively express GrB and to undergo apoptosis in response to IL-21 (19). These findings, and the fact that IL-21 can ameliorate the course of autoimmunity in early phases (32), support the hypothesis that GrB expressed by IL-21-stimulated CD5+ B cells may be involved in the maintenance of B-cell homeostasis. Also noteworthy in this context are preliminary findings showing that bone marrow stromal cells are able to induce GrB in B cells, suggesting GrB may be involved in B-cell deletion processes in the bone marrow (B. Jahrsdörfer, unpublished results). Since we found that the potential of B-CLL cells to express GrB is significantly lower than that of healthy B cells, one may speculate that a putative GrB-dependent deletion mechanism is disrupted in malignant B-CLL cells. This regulatory dysfunction may contribute to the typical uncontrolled B-CLL cell accumulation and may also explain why B-CLL is frequently associated with various autoimmune phenomena (33).
Apart from mutual interaction, activation of B-CLL cells with CpG/IL-21 may also enable cross-talk with various immune cells including professional APC and CTL. Moreover, CpG/IL-21-activated B-CLL cells may themselves function as APC by expressing adhesion, costimulatory and antigen-presenting molecules. Expression of CD54, CD80 and MHC class I on B-CLL cells is of particular interest in terms of an efficient anti-tumor immune response, since these molecules are important for antigen cross-presentation. Previous studies have shown that CD80 and CD86 differentially modulate the activation of Treg and Th cells with CD80 rather favoring Th1-biased CTL and CD86 rather favoring Treg responses (34). This is in line with previous vaccination studies in B-CLL, which found that CD54 and CD80 are particularly important for induction of an efficient antileukemic CTL response (4, 27), while CD86 was found to reduce cytotoxicity of resulting CTLs (27). These considerations, and the fact that in our study CD80 was subject to stronger enhancement by CpG/IL-21 than CD86 was, suggest that CpG/IL-21-stimulated B-CLL cells may be able to induce particularly efficient Th1-biased CTL, and comparably weaker Treg responses. When considering the use of CpG/IL-21-activated B-CLL cells as APC, GrB may serve additional important roles. Very recent reports indicate that GrB is involved not only in antigen uptake and cross-presentation (35), but also in the initiation of immune responses against auto-antigens (36). Therefore, the induction of GrB in B-CLL cells may allow antigens to be optimally degraded and prepared for antigen loading and presentation on MHC class I molecules.
The above-mentioned findings may represent a remarkable parallel to professional APC such as plasmacytoid DC and IFN-α-matured monocyte-derived DC. These two APC types also express significant levels of GrB and MHC class I (37, 38), while at the same time being potent antigen cross-presenters (39, 40). Obviously, the immune system has evolved cells that use GrB and other cytotoxic molecules to unify the capabilities of killing target cells, providing certain proteases that contribute to the appropriate digestion of antigens and being able to present these antigens on MHC molecules in order to activate T lymphocytes. Such cells have meanwhile been described in both mice (41) and humans (38, 42, 43). Therefore, CpG/IL-21 appears to induce changes in B-CLL cells that are reminiscent of killer DC.
In summary, several independent points suggest that stimulation of B-CLL cells with IL-21/CpG may render these cells particularly suitable as cellular tumor vaccine (Supplementary Figure 5, available at International Immunology Online). First, immunogenization of B-CLL cells by CpG/IL-21 may allow them to directly stimulate B-CLL-specific T-cell responses (Supplementary Figure 5A, available at International Immunology Online). Furthermore, induction of GrB in B-CLL cells may generate apoptotic bodies in vivo that can support uptake, processing and presentation of tumor-specific antigens by DC (Supplementary Figure 5B, available at International Immunology Online) (44). Several studies have shown that the use of apoptotic bodies for the generation of DC-based vaccines generally allows the development of more robust immune responses than the use of tumor cell lysates, tumor cell RNA or molecularly defined antigens (6, 27, 45, 46). One reason for this may be the fact that CTL-delivered GrB induces ‘eat me’ signals on attacked tumor cells, resulting in a better uptake by DC, thereby allowing more efficient cross-presentation of tumor-specific antigens (35). Moreover, GrB may contribute to the generation of B-CLL-specific auto-antigens, as suggested by studies demonstrating a direct role of GrB in the initiation of autoimmune responses (36, 47). Finally, APC (Supplementary Figure 5C, available at International Immunology Online) and T cells (Supplementary Figure 5D, available at International Immunology Online), may be additionally stimulated by CpG (48) and IL-21 (49), resulting in even more robust and longer-lasting anti-tumor immune responses.
In conclusion, we have demonstrated that stimulation of B-CLL cells with CpG and IL-21 induces phenotypic changes that are reminiscent of APC with GrB-dependent killer functions. Future studies have to elucidate whether these findings may be translated into the development of enhanced B-CLL cell vaccines.
Supplementary data
Supplementary data are available at International Immunology Online.
Funding
Grants JA 1769/1-1 and JA 1769/1–2 from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) ; through grant SP 07/10 from the Deutsche Jose Carreras Leukämiestiftung; through grant D/08/11870 from the Deutscher Akademischer Austauschdienst (DAAD, German Academic Exchange Service) to B.J.; Deutsche Forschungsgemeinschaft (HA 6136/1-1 to M.H.); United States Public Health Service (P50 CA97274 to G.J.W.).
Supplementary Material
Acknowledgements
Author contributions: B.J. generated the hypothesis and designed the research; M.H., V.E., T.B. and S.E.B. performed experiments; M.H., V.E., T.B., S.E.B and B.J. conducted data analyses; D.F., S.S., T.S., C.T., P.N., J.A.T., H.S. and G.J.W. provided key research tools; M.H., T.B., S.E.B. and B.J. prepared the figures; M.H. and B.J. wrote the manuscript. We wish to thank H. J. Fehling from the Department of Immunology for permission to use his four-color flow cytometer and B. Böhm from the Department of Medicine II for permission to use his ELISpot reader (both Ulm University). We also wish to thank M. Waibel (Peter MacCallum Cancer Centre) for technical assistance.
Conflict of Interest statement: The authors declare no conflict of interest.
References
- 1. O’Brien S., del Giglio A., Keating M. 1995. Advances in the biology and treatment of B-cell chronic lymphocytic leukemia. Blood 85:307. [PubMed] [Google Scholar]
- 2. Jahrsdörfer B., Wooldridge J. E., Blackwell S. E., Taylor C. M., Link B. K., Weiner G. J. 2005. Good prognosis cytogenetics in B-cell chronic lymphocytic leukemia is associated in vitro with low susceptibility to apoptosis and enhanced immunogenicity. Leukemia 19:759. [DOI] [PubMed] [Google Scholar]
- 3. Longo P. G., Laurenti L., Gobessi S., et al. 2007. The Akt signaling pathway determines the different proliferative capacity of chronic lymphocytic leukemia B-cells from patients with progressive and stable disease. Leukemia 21:110. [DOI] [PubMed] [Google Scholar]
- 4. Rezvany M. R., Jeddi-Tehrani M., Rabbani H., et al. 2000. Autologous T lymphocytes may specifically recognize leukaemic B cells in patients with chronic lymphocytic leukaemia. Br. J. Haematol. 111:608. [DOI] [PubMed] [Google Scholar]
- 5. Kollgaard T., Petersen S. L., Hadrup S. R., et al. 2005. Evidence for involvement of clonally expanded CD8+ T cells in anticancer immune responses in CLL patients following nonmyeloablative conditioning and hematopoietic cell transplantation. Leukemia 19:2273. [DOI] [PubMed] [Google Scholar]
- 6. Kokhaei P., Rezvany M. R., Virving L., et al. 2003. Dendritic cells loaded with apoptotic tumour cells induce a stronger T-cell response than dendritic cell-tumour hybrids in B-CLL. Leukemia 17:894. [DOI] [PubMed] [Google Scholar]
- 7. Hus I., Roliński J., Tabarkiewicz J., et al. 2005. Allogeneic dendritic cells pulsed with tumor lysates or apoptotic bodies as immunotherapy for patients with early-stage B-cell chronic lymphocytic leukemia. Leukemia 19:1621. [DOI] [PubMed] [Google Scholar]
- 8. Jahrsdörfer B., Hartmann G., Racila E., et al. 2001. CpG DNA increases primary malignant B cell expression of costimulatory molecules and target antigens. J. Leukoc. Biol. 69:81. [PubMed] [Google Scholar]
- 9. Wierda W. G., Cantwell M. J., Woods S. J., Rassenti L. Z., Prussak C. E., Kipps T. J. 2000. CD40-ligand (CD154) gene therapy for chronic lymphocytic leukemia. Blood 96:2917. [PubMed] [Google Scholar]
- 10. Jahrsdorfer B., Mühlenhoff L., Blackwell S. E., et al. 2005. B-cell lymphomas differ in their responsiveness to CpG oligodeoxynucleotides. Clin. Cancer Res. 11:1490. [DOI] [PubMed] [Google Scholar]
- 11. Jahrsdörfer B., Wooldridge J. E., Blackwell S. E., et al. 2005. Immunostimulatory oligodeoxynucleotides induce apoptosis of B cell chronic lymphocytic leukemia cells. J. Leukoc. Biol. 77:378. [DOI] [PubMed] [Google Scholar]
- 12. Jahrsdörfer B., Blackwell S. E., Wooldridge J. E., et al. 2006. B-chronic lymphocytic leukemia cells and other B cells can produce granzyme B and gain cytotoxic potential after interleukin-21-based activation. Blood 108:2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zent C. S., Smith B. J., Ballas Z. K., et al. 2012. Phase I clinical trial of CpG oligonucleotide 7909 (PF-03512676) in patients with previously treated chronic lymphocytic leukemia. Leuk. Lymphoma 53:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kolb J. P. 2008. IL-21 as new therapy for CLL? Blood 111:4424. [DOI] [PubMed] [Google Scholar]
- 15. Parrish-Novak J., Dillon S. R., Nelson A., et al. 2000. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 408:57. [DOI] [PubMed] [Google Scholar]
- 16. Jin H., Carrio R., Yu A., Malek T. R. 2004. Distinct activation signals determine whether IL-21 induces B cell costimulation, growth arrest, or Bim-dependent apoptosis. J. Immunol. 173:657. [DOI] [PubMed] [Google Scholar]
- 17. Gowda A., Roda J., Hussain S. R., et al. 2008. IL-21 mediates apoptosis through up-regulation of the BH3 family member BIM and enhances both direct and antibody-dependent cellular cytotoxicity in primary chronic lymphocytic leukemia cells in vitro. Blood 111:4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hagn M., Schwesinger E., Ebel V., et al. 2009. Human B cells secrete granzyme B when recognizing viral antigens in the context of the acute phase cytokine IL-21. J. Immunol. 183:1838. [DOI] [PubMed] [Google Scholar]
- 19. Hagn M., Ebel V., Sontheimer K., et al. 2010. CD5+ B cells from individuals with systemic lupus erythematosus express granzyme B. Eur. J. Immunol. 40:2060. [DOI] [PubMed] [Google Scholar]
- 20. Konforte D., Simard N., Paige C. J. 2009. IL-21: an executor of B cell fate. J. Immunol. 182:1781. [DOI] [PubMed] [Google Scholar]
- 21. Glimcher L. H., Townsend M. J., Sullivan B. M., Lord G. M. 2004. Recent developments in the transcriptional regulation of cytolytic effector cells. Nat. Rev. Immunol. 4:900. [DOI] [PubMed] [Google Scholar]
- 22. Catalfamo M., Henkart P. A. 2003. Perforin and the granule exocytosis cytotoxicity pathway. Curr. Opin. Immunol. 15:522. [DOI] [PubMed] [Google Scholar]
- 23. Trapani J. A., Sutton V. R. 2003. Granzyme B: pro-apoptotic, antiviral and antitumor functions. Curr. Opin. Immunol. 15:533. [DOI] [PubMed] [Google Scholar]
- 24. Jones D., Benjamin R. J., Shahsafaei A., Dorfman D. M. 2000. The chemokine receptor CXCR3 is expressed in a subset of B-cell lymphomas and is a marker of B-cell chronic lymphocytic leukemia. Blood 95:627. [PubMed] [Google Scholar]
- 25. Van den Hove L. E., Van Gool S. W., Vandenberghe P., et al. 1997. CD40 triggering of chronic lymphocytic leukemia B cells results in efficient alloantigen presentation and cytotoxic T lymphocyte induction by up-regulation of CD80 and CD86 costimulatory molecules. Leukemia 11:572. [DOI] [PubMed] [Google Scholar]
- 26. Fabricius D., Breckerbohm L., Vollmer A., et al. 2011. Acute lymphoblastic leukemia cells treated with CpG oligodeoxynucleotides, IL-4 and CD40 ligand facilitate enhanced anti-leukemic CTL responses. Leukemia 25:1111. [DOI] [PubMed] [Google Scholar]
- 27. Krackhardt A. M., Harig S., Witzens M., Broderick R., Barrett P., Gribben J. G. 2002. T-cell responses against chronic lymphocytic leukemia cells: implications for immunotherapy. Blood 100:167. [DOI] [PubMed] [Google Scholar]
- 28. Ida H., Nakashima T., Kedersha N. L., et al. 2003. Granzyme B leakage-induced cell death: a new type of activation-induced natural killer cell death. Eur. J. Immunol. 33:3284. [DOI] [PubMed] [Google Scholar]
- 29. Gorak-Stolinska P., Truman J. P., Kemeny D. M., Noble A. 2001. Activation-induced cell death of human T-cell subsets is mediated by Fas and granzyme B but is independent of TNF-alpha. J. Leukoc. Biol. 70:756. [PubMed] [Google Scholar]
- 30. Davids M. S., Burger J. A. 2012. Cell trafficking in chronic lymphocytic leukemia. Open J. Hematol. 3:pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Youinou P., Renaudineau Y. 2007. The paradox of CD5-expressing B cells in systemic lupus erythematosus. Autoimmun. Rev. 7:149. [DOI] [PubMed] [Google Scholar]
- 32. Bubier J. A., Bennett S. M., Sproule T. J., et al. 2007. Treatment of BXSB-Yaa mice with IL-21R-Fc fusion protein minimally attenuates systemic lupus erythematosus. Ann. N. Y. Acad. Sci. 1110:590. [DOI] [PubMed] [Google Scholar]
- 33. Ghia P., Scielzo C., Frenquelli M., Muzio M., Caligaris-Cappio F. 2007. From normal to clonal B cells: Chronic lymphocytic leukemia (CLL) at the crossroad between neoplasia and autoimmunity. Autoimmun. Rev. 7:127. [DOI] [PubMed] [Google Scholar]
- 34. Zheng Y., Manzotti C. N., Liu M., Burke F., Mead K. I., Sansom D. M. 2004. CD86 and CD80 differentially modulate the suppressive function of human regulatory T cells. J. Immunol. 172:2778. [DOI] [PubMed] [Google Scholar]
- 35. Hoves S., Sutton V. R., Haynes N. M., et al. 2011. A critical role for granzymes in antigen cross-presentation through regulating phagocytosis of killed tumor cells. J. Immunol. 187:1166. [DOI] [PubMed] [Google Scholar]
- 36. Casciola-Rosen L., Andrade F., Ulanet D., Wong W. B., Rosen A. 1999. Cleavage by granzyme B is strongly predictive of autoantigen status: implications for initiation of autoimmunity. J. Exp. Med. 190:815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jahrsdörfer B., Vollmer A., Blackwell S. E., et al. 2010. Granzyme B produced by human plasmacytoid dendritic cells suppresses T-cell expansion. Blood 115:1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Korthals M., Safaian N., Kronenwett R., et al. 2007. Monocyte derived dendritic cells generated by IFN-alpha acquire mature dendritic and natural killer cell properties as shown by gene expression analysis. J. Transl. Med. 5:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Di Pucchio T., Chatterjee B., Smed-Sörensen A., et al. 2008. Direct proteasome-independent cross-presentation of viral antigen by plasmacytoid dendritic cells on major histocompatibility complex class I. Nat. Immunol. 9:551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Spadaro F., Lapenta C., Donati S., et al. 2012. IFN-α enhances cross-presentation in human dendritic cells by modulating antigen survival, endocytic routing, and processing. Blood 119:1407. [DOI] [PubMed] [Google Scholar]
- 41. Chan C. W., Crafton E., Fan H. N., et al. 2006. Interferon-producing killer dendritic cells provide a link between innate and adaptive immunity. Nat. Med. 12:207. [DOI] [PubMed] [Google Scholar]
- 42. Tel J., Smits E. L., Anguille S., Joshi R. N., Figdor C. G., de Vries I. J. 2012. Human plasmacytoid dendritic cells are equipped with antigen-presenting and tumoricidal capacities. Blood 120:3936 [DOI] [PubMed] [Google Scholar]
- 43. Stary G., Bangert C., Tauber M., Strohal R., Kopp T., Stingl G. 2007. Tumoricidal activity of TLR7/8-activated inflammatory dendritic cells. J. Exp. Med. 204:1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tesniere A., Apetoh L., Ghiringhelli F., et al. 2008. Immunogenic cancer cell death: a key-lock paradigm. Curr. Opin. Immunol. 20:504. [DOI] [PubMed] [Google Scholar]
- 45. Schnurr M., Scholz C., Rothenfusser S., et al. 2002. Apoptotic pancreatic tumor cells are superior to cell lysates in promoting cross-priming of cytotoxic T cells and activate NK and gammadelta T cells. Cancer Res. 62:2347. [PubMed] [Google Scholar]
- 46. Kokhaei P., Choudhury A., Mahdian R., et al. 2004. Apoptotic tumor cells are superior to tumor cell lysate, and tumor cell RNA in induction of autologous T cell response in B-CLL. Leukemia 18:1810. [DOI] [PubMed] [Google Scholar]
- 47. Casciola-Rosen L., Miagkov A., Nagaraju K., et al. 2008. Granzyme B: evidence for a role in the origin of myasthenia gravis. J. Neuroimmunol. 201-202:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nierkens S., den Brok M. H., Garcia Z., et al. 2011. Immune adjuvant efficacy of CpG oligonucleotide in cancer treatment is founded specifically upon TLR9 function in plasmacytoid dendritic cells. Cancer Res. 71:6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Andorsky D. J., Timmerman J. M. 2008. Interleukin-21: biology and application to cancer therapy. Expert Opin. Biol. Ther. 8:1295. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.