Abstract
Background. The United States is experiencing a pertussis resurgence that resulted in a 60-year high of 48 000 cases in 2012. The majority of hospitalizations and deaths occur in infants too young to be vaccinated. Neonatal and maternal vaccination have been proposed to protect newborns until the first vaccination, currently recommended at 2 months of age. These interventions result in elevated anti–Bordetella pertussis titers, but there have been no studies demonstrating that these measures confer protection.
Methods. Baboons were vaccinated with acellular pertussis vaccine at 2 days of age or at 2 and 28 days of age. To model maternal vaccination, adult female baboons primed with acellular pertussis vaccine were boosted in the third trimester of pregnancy. Neonatally vaccinated infants, infants born to vaccinated mothers, and naive infants born to unvaccinated mothers were infected with B. pertussis at 5 weeks of age.
Results. Naive infant baboons developed severe disease when challenged with B. pertussis at 5 weeks of age. Baboons receiving acellular pertussis vaccine and infants born to mothers vaccinated at the beginning of their third trimester were protected.
Conclusions. Our results demonstrate that neonatal vaccination and maternal vaccination confer protection in the baboon model and support further study of these strategies for protection of newborns from pertussis.
Keywords: pertussis, maternal vaccination, neonatal vaccination, baboon, animal models
Pertussis rates in the United States have been steadily rising over the last 30 years despite nationwide vaccination coverage in children in excess of 95% [1]. With >27 000 reported cases in the United States in 2010 and >48 000 reported cases in 2012, the United States is experiencing levels of pertussis not observed since the 1950s [1]. The highest incidence of disease is in infants too young to have completed primary vaccinations, and the vast majority of severe pertussis cases requiring hospitalization and fatal cases occur in infants <3 months of age [2, 3].
In humans, infection with Bordetella pertussis results in a wide spectrum of clinical manifestations, depending on the age and immune status of the host, ranging from mild respiratory symptoms to a severe cough illness accompanied by the hallmark inspiratory whoop and posttussive emesis [4]. In infants <3 months of age, onset is typically not alarming and begins with coryza and no or minimal fever, followed by the development of cough [5, 6]. Cough in very young infants is often not recognized as such but occurs in paroxysms and often leads to apnea and hypoxia. In infants with pertussis requiring admission to intensive care units, mortality rates can approach 50%–70%, and common complications include bacterial pneumonia, substantial leukocytosis, and pulmonary hypertension [6–9]. Complete blood counts are increasingly being used to identify severe pertussis cases because substantial leukocytosis (>50 000 white blood cells/μL) is associated with increased mortality rates [10]. It is postulated that the high leukocyte mass can trigger thrombi formation, restrict blood flow, and exacerbate the development of pulmonary hypertension [11].
Several strategies have been proposed to reduce the incidence of pertussis in infants during the first few months of life. These include adolescent pertussis booster vaccination, cocooning, neonatal vaccination, and maternal vaccination. A marked increase in pertussis incidence in 11–18-year-old individuals at the beginning of the 21st century led to the introduction of a tetanus, reduced-dose diphtheria, reduced-dose acellular pertussis (Tdap) adolescent booster vaccine in the United States. It was postulated that this vaccination strategy would reduce the circulation of Bordetella pertussis in the population and thereby reduce infant exposure. However, although Tdap successfully decreased pertussis incidence among recipients aged 11–18 years, there was no impact on disease incidence among infants [12]. Similarly, cocooning was proposed as a mechanism to reduce infant exposure to B. pertussis by vaccinating parents and siblings of newborns, as well as other frequent contacts. The rationale for cocooning is based on the evidence that infants most often acquire pertussis from a parent or other family member [13–15]. However, in an evaluation of the effectiveness of cocooning in 4 Houston hospitals, no significant benefit was observed [16]. Also, while cocooning programs have achieved moderate vaccination coverage among postpartum mothers, there has been limited success in vaccinating fathers or other family members, leading to the conclusion that this approach would be difficult to implement [17, 18]. Additionally, we recently showed that baboons vaccinated with DTaP (a diphtheria, tetanus and acellular pertussis vaccine formulation in which higher doses of each antigen are present) are not protected from B. pertussis infection and can transmit pertussis to naive cage mates, suggesting that cocooning, even if fully implemented, may not optimally protect infants [19]. The apparent failures to reduce infant pertussis by vaccinating contacts likely suggest that newborns need anti–B. pertussis antibodies, to be protected for the first few months of life. Immunization at birth (ie, neonatal vaccination) has been proposed, based on the demonstration that newborns are able to mount antibody responses to acellular pertussis vaccination [20, 21]. Alternatively, vaccinating women in the third trimester of pregnancy has been proposed and is now recommended by the US Advisory Committee on Immunization Practices (ACIP) [22, 23]. Several studies demonstrated efficient transplacental transfer of anti–B. pertussis antibodies, supporting the potential of this approach [24–26]. While both neonatal and maternal vaccination strategies induce elevated anti–B. pertussis antibody titers in newborns, the lack of a serological correlate of protection presents a significant challenge in demonstrating the clinical efficacy of either approach.
We developed a baboon model of pertussis that accurately reproduces severe clinical pertussis, including heavy respiratory colonization, leukocytosis, prolonged cough illness, and transmission from infected to naive animals [27–29]. In addition to providing an excellent model of pertussis, the baboon has proven to be a relevant model for reproductive studies, since their reproductive cycles are year round, and they form a single discoid placentation that is very similar to that in humans [30, 31]. In addition, it has been documented that baboons possess the same 4 immunoglobulin G (IgG) subclasses as humans [32], and that transplacental transfer of IgG from mother to fetus occurs as in humans [33, 34]. These considerations support the use of the baboon model of pertussis vaccination and infection for studying the effectiveness of neonatal and maternal vaccination. This model provides a unique opportunity to determine if maternal and/or neonatal vaccination confers protection to very young infant primates. Newborn baboons vaccinated at 2 days of age or at 2 days and 28 days of age with licensed DTaP vaccines were protected from a robust B. pertussis challenge at 5–6 weeks of age. Protection was also observed in 5–6-week-old animals born to DTaP-primed mothers that were boosted at the beginning of their third trimester. These results demonstrate that neonatal vaccination and maternal vaccination confer protection in the baboon model and, for the first time, provide proof of concept for these strategies in a primate model.
MATERIALS AND METHODS
Ethics Statement
Animal procedures were performed in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International in accordance with protocols approved by the University of Oklahoma Health Sciences Center Animal Care and Use Committee and the principles outlined in the Guide for the Care and Use of Laboratory Animals by the Institute for Laboratory Animal Resources, National Research Council.
Bacterial Strains and Media
B. pertussis strain D420 was provided by the Centers for Disease Control and Prevention. Bordet-Gengou (BG) agar plates were prepared with BG agar (Becton-Dickinson, Sparks, MD) containing 1% proteose peptone (Becton-Dickinson) and 15% defibrinated sheep blood. Regan-Lowe plates were prepared from Regan-Lowe Charcoal Agar Base (Becton-Dickinson) with 10% defibrinated sheep blood and 40 µg/mL cephalexin.
Vaccination of Baboons
Maternal Vaccination Studies
Female baboons of reproductive age were vaccinated 3 times with the full human dose of DTaP on a 0-, 2-, and 4-month schedule between pregnancies. Equal numbers of the animals were vaccinated with Daptacel (Sanofi Pasteur, Toronto, Canada) and with Infanrix (GlaxoSmithKline, Rixensart, Belgium). Animals in the breeding program are closely monitored allowing for accurate identification of the start of pregnancy. The vaccinated baboons became pregnant 2–6 months following their third priming dose (average time to pregnancy, 4 months). Pregnant baboons were boosted with a human dose of the same vaccine they were primed with at the beginning of their third trimester of pregnancy (day 120 of gestation).
Neonatal Vaccination Studies
Two baboons were vaccinated once, at 2 days of age, and 2 baboons were vaccinated twice, at 2 and 28 days of age. In each group, 1 animal received the full human dose of Daptacel (Sanofi Pasteur), and 1 animal received the full human dose of Infanrix (GlaxoSmithKline, Rixensart, Belgium).
Infection of Baboons
Neonatally vaccinated infant baboons, infant baboons born to vaccinated and boosted mothers, and naive baboons born to unvaccinated mothers were challenged at 5–6 weeks of age. Inocula were prepared to a concentration of 108 bacteria/mL as previously described [35]. Baboons were anesthetized with 10–15 mg/kg ketamine administered intramuscularly. The pharynx was swabbed with 2% lidocaine solution, and animals were intubated using a 2–3-mm (inner diameter) endotracheal tube to deliver 0.5 mL of the inoculum to the proximal trachea. In addition, a 22-gauge, 3.2-cm Teflon intravenous catheter (Abbocath) was used to deliver 0.25 mL of inoculum to both nasal cavities. Animals were placed in a sitting position for 3 minutes, returned to their cages, and observed until they recovered from anesthesia.
Evaluation of Animals
Baboons were anesthetized with ketamine. Whole-blood specimens were evaluated for the number of circulating white blood cells, by complete blood count. Each nasal cavity was flushed with 0.5 mL of phosphate-buffered saline (PBS), using a 22-gauge/3.2-cm intravenous catheter. The recovered nasopharyngeal wash samples from both nares were combined, and 100 µL of the recovered sample was divided, serially diluted in PBS, and plated onto Regan-Lowe plates. Colony-forming units were enumerated after incubation at 37°C for 4–5 days. Infection with B. pertussis was confirmed by examining colony morphology and hemolysis on BG blood agar plates and polymerase chain reaction amplification of IS481, a genomic insertion site that is specific for B. pertussis [4].
Detection of Serum Antibodies to B. pertussis Antigens
Nunc Maxisorp 96-well plates were coated overnight with 0.2 µg/mL pertussis toxin (PT), 0.5 µg/mL filamentous hemagglutinin (FHA), or 2 µg/mL pertactin (PRN; List Biologicals, Campbell, CA) as described previously [28, 36]. Serum IgG for each antigen was measured as described previously, using horseradish peroxidase–conjugated goat anti-monkey IgG polyclonal secondary antibody (AbD Serotec [catalog code AAI42P], Raleigh, NC) [28]. For each plate, a standard curve was constructed by serial dilution of the World Health Organization international standard pertussis antiserum (NIBSC, Hertfordshire, England). This curve was used to assign international units for PT, FHA, and PRN to each serum sample on the same plate.
Statistical Analysis
All data are reported as mean values ± standard error of the mean. Statistical analyses were performed by analysis of variance with a post hoc t test, using JMP (version 9) software (SAS Institute, Cary, NC). Antibody data were normalized by log transformation before analysis.
RESULTS
Human clinical studies have provided evidence that maternal and neonatal vaccination are immunogenic and safe, but it is not practical for clinical studies to enroll enough participants to provide data on protection. The development of the baboon model of pertussis provided the opportunity to test the ability of neonatal vaccination and maternal vaccination to provide protection from exposure to pertussis, in controlled studies, in a very relevant animal model.
To evaluate the protection conferred by neonatal vaccination, 4 animals were vaccinated with US-licensed DTaP vaccines. Two animals were vaccinated at 2 days of age, and 2 were vaccinated at 2 and 28 days of age. The level of circulating serum antibodies in each of the 4 infant baboons and 3 age-matched unvaccinated baboons was determined for pertussis vaccine antigens by enzyme-linked immunosorbent assay (Figure 1A). These results demonstrated that all 4 animals responded to vaccination, with relatively high levels of circulating antibodies detected for PT, PRN, and FHA. The 4 vaccinated animals and 3 unvaccinated control animals were challenged at 5 weeks of age with 108 colony-forming units of B. pertussis strain D420, divided between the trachea and nasal cavity. The unvaccinated animals developed serious disease following inoculation. All 3 were heavily colonized, as determined by the number of colony-forming units recovered from nasopharyngeal washes (Figures 2A and 3A). All 3 animals were also observed coughing by day 5 after inoculation and exhibited substantial leukocytosis consistent with severe B. pertussis infection (Figure 2B and 3B). Despite a significant cough illness, 1 baboon remained alert and active and continued to intake formula and water throughout its infection. This baboon was closely monitored and allowed to progress through the full course of the infection before clearing B. pertussis naturally approximately 5 weeks after inoculation. The second unvaccinated baboon was also alert and was responsive through day 5 after inoculation, but it was unexpectedly found dead on the morning of day 6 after inoculation. Necropsy revealed that this animal died from pulmonary edema and hemorrhage related to bacterial pneumonia. The third unvaccinated baboon appeared extremely ill and failed to eat or drink, beginning day 5, and had a white blood cell count of 97 000 cells/μL. Antibiotic treatment was initiated, and the baboon was fed double-strength formula through an oral-gastric tube daily for 5 days and then intermittently over an additional 5 days. By day 23 after inoculation, this baboon had returned to normal food consumption and normal activity. All 4 vaccinated infant baboons were heavily colonized, but in stark contrast to the unvaccinated animals, they were free of pertussis symptoms (Figure 2A and 2B). The white blood cell counts of the vaccinated animals never rose significantly above the prechallenge levels (Figure 2B). These animals exhibited no observable signs of illness and had no reduction in physical activity. This result clearly demonstrated that although vaccination with the licensed acellular vaccines had little impact on bacterial colonization of the airway, even a single vaccine dose, delivered on day 2 of life, conferred protection to infant baboons following a robust B. pertussis challenge delivered at 5 weeks of age.
Figure 1.

Maternal vaccination and neonatal vaccination both induce high levels of serum anti-pertussis immunoglobulin G (IgG) in infant baboons. Serum samples were collected just before challenge, at 5–6 weeks of age. IgG antibody to the 3 vaccine antigens present in both vaccines: pertussis toxin (PT), pertactin (PRN), and filamentous hemagglutinin (FHA) were measured by enzyme-linked immunosorbent assay. A, For neonatal vaccination studies, sera were collected from unvaccinated baboons, and baboons vaccinated with tetanus, diphtheria, acellular pertussis vaccine (DTaP) once (at 2 days of age) or twice (at 2 and 28 days of age; n = 2 per group). B, For maternal vaccination studies, sera were collected from DTaP-vaccinated mothers after delivery (n = 5) and from offspring of DTaP-vaccinated (n = 7) or unvaccinated (n = 2) mothers. For each sample, international units (IU) were calculated by comparison to the World Health Organization pertussis antiserum. **P < .001, *P < .01, versus unvaccinated infants. Abbreviation: B. pertussis, Bordetella pertussis.
Figure 2.
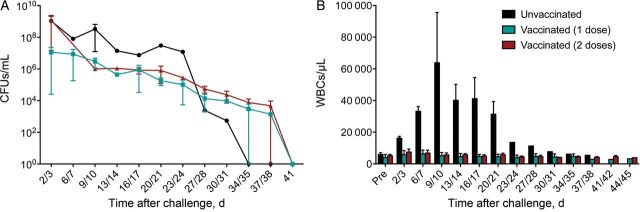
Neonatal vaccination prevents leukocytosis but not colonization. Unvaccinated baboons and baboons vaccinated with tetanus, diphtheria, acellular pertussis once (at 2 days of age) or twice (at 2 and 28 days of age) were directly challenged with Bordetella pertussis at 5–6 weeks of age (n = 2 per group). A, Colonization was monitored by quantifying B. pertussis colony-forming units (CFUs) per milliliter in biweekly nasopharyngeal washes, with a limit of detection of 10 CFUs/mL. B, The mean circulating white blood cell (WBC) counts before and after challenge are shown for each group of animals (n = 2 per group). **P < .01 versus preinfection values from the same group.
Figure 3.
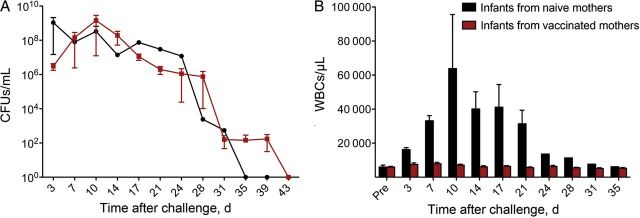
Maternal vaccination prevents leukocytosis but not colonization. Infant baboons were born to mothers that were vaccinated with the tetanus, diphtheria, acellular pertussis priming series and boosted during the third trimester (n = 7) or to unvaccinated mothers (n = 2). All animals were directly challenged with Bordetella pertussis at 5–6 weeks of age. A, Colonization was monitored by quantifying B. pertussis colony-forming units (CFUs) per milliliter in biweekly nasopharyngeal washes, with a limit of detection of 10 CFUs/mL. B, The mean circulating white blood cell (WBC) counts before and after challenge are shown for each group of animals (n = 2 per group). **P < .01 versus preinfection values from the same group.
To evaluate the protection conferred by maternal vaccination, it was first necessary to generate a pool of adult female baboons primed by DTaP vaccination. This was accomplished by vaccinating adult female baboons with full human doses of the US-licensed acellular pertussis vaccines on a 0-, 2-, and 4-month schedule between pregnancies. A total of 8 adult female baboons were vaccinated. Seven subsequently became pregnant and delivered full-term infants. The time between receipt of the last priming dose of vaccine and initiation of pregnancy ranged from 2 to 6 months, with a mean interval of 4 months. Each of these mothers was boosted with a full human dose of US-licensed DTaP vaccine at the beginning of the third trimester of pregnancy. All 7 infant baboons born to vaccinated mothers were challenged at 5 weeks of age with 108 colony-forming units of B. pertussis strain D420, divided between the trachea and nasal cavity. The timing of the infections of these animals was interspersed with the infections of the infant baboons vaccinated for the neonatal vaccination study and the unvaccinated animals from that study so that the same 3 unvaccinated animals could function as controls for both groups. As described above, all 3 unvaccinated animals experienced severe pertussis symptoms. All 7 infant baboons born to vaccinated mothers were heavily colonized, but in contrast to the unvaccinated age-matched infant baboons, they were free of pertussis signs (Figure 3A and 3B). The white blood cell counts of the infant baboons born to vaccinated mothers never rose significantly above the prechallenge levels (Figure 3B), and the animals did not exhibit observable signs of illness and had no reduction in physical activity. This result clearly demonstrated that maternal vaccination, at the beginning of the third trimester of pregnancy, conferred protection to infant baboons following a robust B. pertussis challenge at 5 weeks of age.
DISCUSSION
The US-licensed pertussis vaccine schedule begins at 2 months of age, leaving younger children vulnerable to infection and most likely to experience severe disease. In response to the increase in infant mortality due to pertussis infection, the ACIP recommended in 2011 that Tdap be administered to all pregnant woman between 27 and 36 weeks of gestation [22]. In 2012, the ACIP further recommended Tdap booster vaccination with every pregnancy [23]. Several studies have demonstrated efficient transplacental transfer of anti–B. pertussis antibodies and have demonstrated high titers in 1-month-old infants born to mothers vaccinated in their third trimester [24–26]. Neonatal vaccination is another strategy that is being proposed to shorten the window of vulnerability for infants to severe pertussis. Studies have shown that DTaP vaccination of newborn humans results in elevated anti–B. pertussis antibody titers [20, 21]. Although it is considered likely that both vaccination strategies will confer protection to the infant, no direct evidence is currently available. Past and currently ongoing clinical studies for maternal and neonatal vaccination are designed to investigate safety and immunogenicity but are not sufficiently large to provide protection data. It is likely that surveillance data following the implementation of maternal vaccination programs will provide protection data if acceptance of the vaccine by pregnant women reaches a sufficiently high level. As a complement to these studies, the baboon model of pertussis allows us to evaluate the ability of vaccination to protect neonates against severe disease following direct challenge with B. pertussis. In the present study, we used this model to address whether neonatal or maternal acellular pertussis vaccination confers protection to infant baboons.
For the maternal vaccination study, we challenged 3 infants born to unvaccinated mothers and 7 infant baboons born to mothers that were boosted with DTaP during pregnancy. The infants born to unvaccinated mothers experienced severe pertussis characterized by high leukocytosis and cough illness. Two of the 3 animals had reduced physical activity, and 1 animal died of complications from pneumonia. Meanwhile, the infants born to mothers who were boosted with DTaP during pregnancy possessed anti–B. pertussis serum antibodies and were fully protected from all signs of pertussis disease (Figure 3B). This study clearly represents a best-case scenario in which mothers became pregnant and were boosted relatively soon after completing a priming vaccine series. We also used DTaP in our studies instead of the corresponding reduced-dose Tdap vaccines. However, the use of a best-case approach does not diminish our results demonstrating that in the baboon model of pertussis, maternal vaccination with the acellular pertussis vaccines results in efficient transplacental transfer of IgG to the infant and protection of that infant from exposure to pertussis at 5 weeks following birth.
For the neonatal vaccination study, 2 animals were vaccinated with a single dose of DTaP, at 2 days of age. An additional 2 animals were vaccinated twice, at 2 and 28 days of age. Our results demonstrate that a single neonatal vaccination resulted in increased anti–B. pertussis antibody titers and full protection from severe pertussis symptoms following direct challenge with B. pertussis at 5 weeks of age. This demonstration of protection in the baboon model provides increased confidence that, if implemented, neonatal vaccination may provide protection to infants from severe pertussis earlier than the protection provided by the current licensed vaccine schedule.
Although maternal vaccination and neonatal vaccination both confer anti–B. pertussis antibodies to newborns, it remains controversial whether these vaccination strategies will interfere with future vaccinations during infancy. Some studies have suggested that circulating maternal anti–B. pertussis antibodies blunt the B. pertussis–specific antibody responses after administration of DTaP to infants of mothers vaccinated with Tdap during pregnancy [26, 37]. This could potentially result in an increase in the occurrence of pertussis in older infants. In addition, some evidence suggests that a birth dose of DTaP may suppress the immune response to subsequent DTaP doses and suppress responses to Haemophilus influenza type B and hepatitis B virus vaccines [38, 39].
We recently reported that vaccination of baboons with licensed DTaP vaccines protects animals from disease symptoms but fails to prevent infection or transmission [19]. These findings may explain the pertussis resurgence and highlight the long-term need to develop a vaccination strategy that achieves optimal herd immunity by preventing B. pertussis colonization. Those results were mirrored in this study. Maternal vaccination and neonatal vaccination protected infants from disease symptoms but did not prevent or shorten the duration of infection. However, it is important to bear in mind that the goal of maternal and neonatal vaccination is to protect infants from the respiratory distress and leukocytosis associated with severe pertussis. Therefore, the infection and asymptomatic carriage observed in these studies should not preclude the consideration of these strategies in the near term. Since only immunoglobulin is transferred from the mother to the infant, our results indicate that antibodies alone are sufficient to confer protection against pertussis symptoms. Inhibition of leukocytosis likely occurs through neutralization of PT, a toxin that interferes with leukocyte extravasation by blocking chemokine receptor signaling [4]. The mechanism by which the acellular pertussis vaccine prevents coughing despite heavy bacterial colonization is not known but deserves further attention.
Although our data suggest that both maternal and neonatal acellular pertussis vaccination would be effective at reducing the incidence of pertussis among infants, maternal vaccination provides the added advantages of protecting the mother, as well as providing antibodies to the infant from birth. For neonatal vaccination, it is important to consider that there is a delay between vaccination and the induction of protective immunity. So although neonatal vaccination is likely to shorten the window of vulnerability, infants will remain vulnerable in the initial weeks following the birth dose of vaccine. For these and other reasons, maternal vaccination has recently gained favor in the United States and other countries [40]. Numerous clinical studies have documented the safety of maternal vaccination for the mother and the infant and have demonstrated that maternal vaccination results in elevated B. pertussis antibody titers in infants. Our results in the baboon model provide a proof of concept in a primate model that maternal vaccination may confer protection against severe pertussis during the first months of life.
Notes
Acknowledgments. We thank Dr John Dennis, Dr Jill Ascher, Lewis Shankle, Perry Altland, and Ernest Madison, for technical assistance; and Dr Drusilla Burns and Dr Scott Stibitz, for critical reading of the manuscript.
Financial support. This work was supported by the Food and Drug Administration and the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH; interagency agreement Y1-AI-1727-01); and the National Center For Research Resources, NIH (grants P40RR012317 and 5R24RR016556-10 to the Oklahoma Baboon Research Resource).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Epidemiology and prevention of vaccine-preventable diseases. 12th ed. Atlanta: Centers for Disease Control and Prevention; 2011. Pertussis; pp. 215–32. [Google Scholar]
- 2.Haberling DL, Holman RC, Paddock CD, Murphy TV. Infant and maternal risk factors for pertussis-related infant mortality in the United States, 1999 to 2004. Pediat Infect Dis J. 2009;28:194–8. doi: 10.1097/INF.0b013e31818c9032. [DOI] [PubMed] [Google Scholar]
- 3.Winter K, Harriman K, Zipprich J, et al. California pertussis epidemic, 2010. J Pediatr. 2012;161:1091–6. doi: 10.1016/j.jpeds.2012.05.041. [DOI] [PubMed] [Google Scholar]
- 4.Mattoo S, Cherry JD. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev. 2005;18:326–82. doi: 10.1128/CMR.18.2.326-382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McIntyre P, Wood N. Pertussis in early infancy: disease burden and preventive strategies. Curr Opin Infect Dis. 2009;22:215–23. doi: 10.1097/QCO.0b013e32832b3540. [DOI] [PubMed] [Google Scholar]
- 6.Namachivayam P, Shimizu K, Butt W. Pertussis: severe clinical presentation in pediatric intensive care and its relation to outcome. Pediatr Crit Care Med. 2007;8:207–11. doi: 10.1097/01.PCC.0000265499.50592.37. [DOI] [PubMed] [Google Scholar]
- 7.Paddock CD, Sanden GN, Cherry JD, et al. Pathology and pathogenesis of fatal Bordetella pertussis infection in infants. Clin Infect Dis. 2008;47:328–38. doi: 10.1086/589753. [DOI] [PubMed] [Google Scholar]
- 8.Smith C, Vyas H. Early infantile pertussis; increasingly prevalent and potentially fatal. Eur J Pediatr. 2000;159:898–900. doi: 10.1007/PL00008365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halasa NB, Barr FE, Johnson JE, Edwards KM. Fatal pulmonary hypertension associated with pertussis in infants: does extracorporeal membrane oxygenation have a role? Pediatrics. 2003;112:1274–8. doi: 10.1542/peds.112.6.1274. [DOI] [PubMed] [Google Scholar]
- 10.Mikelova LK, Halperin SA, Scheifele D, et al. Predictors of death in infants hospitalized with pertussis: a case-control study of 16 pertussis deaths in Canada. J Pediatr. 2003;143:576–81. doi: 10.1067/S0022-3476(03)00365-2. [DOI] [PubMed] [Google Scholar]
- 11.Ulloa-Gutierrez R, Boza R, Carvajal-Riggioni D, Baltodano A. Pertussis: should we improve intensive care management or vaccination strategies? Exp Rev Vacines. 2011;10:49–53. doi: 10.1586/erv.10.156. [DOI] [PubMed] [Google Scholar]
- 12.Skoff TH, Cohn AC, Clark TA, Messonnier NE, Martin SW. Early Impact of the US Tdap vaccination program on pertussis trends. Arch Pediatr Adolesc Med. 2012;166:344–9. doi: 10.1001/archpediatrics.2011.1093. [DOI] [PubMed] [Google Scholar]
- 13.Bisgard KM, Pascual FB, Ehresmann KR, et al. Infant pertussis: who was the source? Pediatr Infect Dis J. 2004;23:985–9. doi: 10.1097/01.inf.0000145263.37198.2b. [DOI] [PubMed] [Google Scholar]
- 14.Kowalzik F, Barbosa AP, Fernandes VR, et al. Prospective multinational study of pertussis infection in hospitalized infants and their household contacts. Pediatr Infect Dis J. 2007;26:238–42. doi: 10.1097/01.inf.0000256750.07118.ee. [DOI] [PubMed] [Google Scholar]
- 15.Wendelboe AM, Hudgens MG, Poole C, Van Rie A. Estimating the role of casual contact from the community in transmission of Bordetella pertussis to young infants. Emerg Themes Epidemiol. 2007;4:15. doi: 10.1186/1742-7622-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castagnini LA, Healy CM, Rench MA, Wootton SH, Munoz FM, Baker CJ. Impact of maternal postpartum tetanus and diphtheria toxoids and acellular pertussis immunization on infant pertussis infection. Clin Infect Dis. 2012;54:78–84. doi: 10.1093/cid/cir765. [DOI] [PubMed] [Google Scholar]
- 17.Healy CM, Rench MA, Baker CJ. Implementation of cocooning against pertussis in a high-risk population. Clin Infect Dis. 2011;52:157–62. doi: 10.1093/cid/ciq001. [DOI] [PubMed] [Google Scholar]
- 18.Healy CM, Rench MA, Castagnini LA, Baker CJ. Pertussis immunization in a high-risk postpartum population. Vaccine. 2009;27:5599–602. doi: 10.1016/j.vaccine.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 19.Warfel JM, Zimmerman LI, Merkel TJ. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc Natl Acad Sci U S A. 2014;111:787–92. doi: 10.1073/pnas.1314688110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belloni C, De Silvestri A, Tinelli C, et al. Immunogenicity of a three-component acellular pertussis vaccine administered at birth. Pediatrics. 2003;111:1042–5. doi: 10.1542/peds.111.5.1042. [DOI] [PubMed] [Google Scholar]
- 21.Wood N, McIntyre P, Marshall H, Roberton D. Acellular pertussis vaccine at birth and one month induces antibody responses by two months of age. Pediatr Infect Dis J. 2010;29:209–15. doi: 10.1097/INF.0b013e3181bc98d5. [DOI] [PubMed] [Google Scholar]
- 22.CDC. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Tdap) in pregnant women and persons who have or anticipate having close contact with an infant aged <12 months—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1424–6. [PubMed] [Google Scholar]
- 23.CDC. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep. 2013;62:131–5. [PMC free article] [PubMed] [Google Scholar]
- 24.Gall SA, Myers J, Pichichero M. Maternal immunization with tetanus-diphtheria-pertussis vaccine: effect on maternal and neonatal serum antibody levels. Am J Obstet Gynecol. 2011;204:334 e1–5. doi: 10.1016/j.ajog.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 25.Leuridan E, Hens N, Peeters N, de Witte L, Van der Meeren O, Van Damme P. Effect of a prepregnancy pertussis booster dose on maternal antibody titers in young infants. Pediatr Infect Dis J. 2011;30:608–10. doi: 10.1097/INF.0b013e3182093814. [DOI] [PubMed] [Google Scholar]
- 26.Van Savage J, Decker MD, Edwards KM, Sell SH, Karzon DT. Natural history of pertussis antibody in the infant and effect on vaccine response. J Infect Dis. 1990;161:487–92. doi: 10.1093/infdis/161.3.487. [DOI] [PubMed] [Google Scholar]
- 27.Warfel JM, Beren J, Merkel TJ. Airborne transmission of Bordetella pertussis. J Infect Dis. 2012;206:902–6. doi: 10.1093/infdis/jis443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warfel JM, Beren J, Kelly VK, Lee G, Merkel TJ. Nonhuman primate model of pertussis. Infect Immun. 2012;80:1530–6. doi: 10.1128/IAI.06310-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Warfel JM, Merkel TJ. Bordetella pertussis infection induces a mucosal IL-17 response and long-lived Th17 and Th1 immune memory cells in nonhuman primates. Mucosal Immunol. 2013;6:787–96. doi: 10.1038/mi.2012.117. [DOI] [PubMed] [Google Scholar]
- 30.Carter AM. Animal models of human placentation--a review. Placenta. 2007;28(Suppl A):S41–7. doi: 10.1016/j.placenta.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Luckett WP. Comparative development and evolution of the placenta in primates. Contrib Primatol. 1974;3:142–234. [PubMed] [Google Scholar]
- 32.Shearer MH, Dark RD, Chodosh J, Kennedy RC. Comparison and characterization of immunoglobulin G subclasses among primate species. Clin Diagn Lab Immunol. 1999;6:953–8. doi: 10.1128/cdli.6.6.953-958.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Payton ME, d'Offay JM, Prado ME, et al. Comparative transmission of multiple herpesviruses and simian virus 40 in a baboon breeding colony. Comp Med. 2004;54:695–704. [PubMed] [Google Scholar]
- 34.Wolf RF, Eberle R, White GL. Generation of a specific-pathogen-free baboon colony. J Am Assoc Lab Anim Sci. 2010;49:814–20. [PMC free article] [PubMed] [Google Scholar]
- 35.Warfel JM, Beren J, Kelly VK, Lee G, Merkel TJ. A non-human primate model of pertussis. Infect Immun. 2012;80:1530–6. doi: 10.1128/IAI.06310-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meade BD, Deforest A, Edwards KM, et al. Description and evaluation of serologic assays used in a multicenter trial of acellular pertussis vaccines. Pediatrics. 1995;96:570–5. [PubMed] [Google Scholar]
- 37.Englund JA, Anderson EL, Reed GF, et al. The effect of maternal antibody on the serologic response and the incidence of adverse reactions after primary immunization with acellular and whole-cell pertussis vaccines combined with diphtheria and tetanus toxoids. Pediatrics. 1995;96:580–4. [PubMed] [Google Scholar]
- 38.Halasa NB, O'Shea A, Shi JR, LaFleur BJ, Edwards KM. Poor immune responses to a birth dose of diphtheria, tetanus, and acellular pertussis vaccine. J Pediatr. 2008;153:327–32. doi: 10.1016/j.jpeds.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knuf M, Schmitt HJ, Wolter J, et al. Neonatal vaccination with an acellular pertussis vaccine accelerates the acquisition of pertussis antibodies in infants. J Pediatr. 2008;152:655–60. doi: 10.1016/j.jpeds.2007.09.034. 660 e1. [DOI] [PubMed] [Google Scholar]
- 40.Libster R, Edwards KM. Re-emergence of pertussis: what are the solutions? Exp Rev of Vaccines. 2012;11:1331–46. doi: 10.1586/erv.12.118. [DOI] [PubMed] [Google Scholar]


