Abstract
Acquisition of nevirapine (NVP)–resistant human immunodeficiency virus type 1 (HIV-1) by breast-feeding infants after receipt of single-dose NVP to prevent mother-to-child transmission is not well defined. A prospective observational study of 307 infants evaluated the rate of breast milk transmission of NVP-resistant HIV and the concentrations of mutants over time. NVP resistance was detected in 9 of 24 infants (37.5%; 95% confidence interval, 18.8%–59.4%) infected via breast milk. Eight had a pure mutant HIV population at the time infection was first detected, and majority mutant populations persisted in all 6 infants with follow-up specimens. Infection of breast-feeding infants with NVP-resistant HIV resulted in mutants persisting as the dominant virus, which may indefinitely compromise treatment with NVP-based antiretroviral regimens.
Keywords: HIV-1, drug-resistance, mother-to-child transmission, nevirapine, zidovudine
The World Health Organization (WHO) reports that approximately 330 000 children became infected with human immunodeficiency virus type 1 (HIV-1) in 2011 [1], with most infections in sub-Saharan Africa from mother-to-child transmission (MTCT) and 5%–20% of these through breast milk. To minimize infant morbidity and mortality, the WHO recommends that HIV–infected mothers exclusively breast-feed their infants through 6 months of life and then continue nursing with supplemental foods.
Single-dose nevirapine (NVP) reduces MTCT in breast-feeding populations but selects NVP-resistant HIV. To simplify recommendations, further reduce MTCT, and improve maternal health, single-dose NVP is no longer recommended by the WHO [2], although its use continues in some communities.
In our prior observational study, the dynamics of NVP-resistant HIV varied by the timing of infant infection, with infants infected during the peripartum period frequently having 100% mutant virus populations that persisted over time [3]. The persistence of mutant viruses could explain the reduced efficacy of NVP-based antiretroviral treatment (ART) in children switching to NVP-based ART after viral replication was suppressed by lopinavir/ritonavir-based ART [4].
To improve our understanding of the transmission of NVP-resistant HIV via breast milk after maternal single-dose NVP, we determined the prevalence of NVP-resistant mutants among infants infected via breast milk and quantified the concentration of mutant virus over time.
METHODS
Infants at risk of acquiring NVP-resistant HIV because of maternal ingestion of single-dose NVP during the peripartum period were included in this analysis of an observational cohort study of infants in Beira, Mozambique [3]. Mothers may also have received other antiretroviral (ARV) medications, including maternal short-course zidovudine (introduced in Beira in early 2006), beginning as early as gestational week 32 if CD4+ T-cell counts were >350 cells/µL, and/or maternal zidovudine and lamivudine (introduced in Beira in early 2008) for 1 week after delivery. All infants received single-dose NVP after birth and may have received short-course zidovudine over the first week of life. ARV medications were captured by mothers’ self-reports and confirmed by review of clinical registries. Blood specimens were collected from infants on filter paper (FTA; Whatman) at birth and every 2 weeks until 8 weeks of age for detection of HIV by polymerase chain reaction (PCR). If an infant was infected with HIV by 8 weeks of age, blood was collected by venipuncture every 1–2 months through 12 months of age, at 18 months of age, and at 24 months of age. If infants were PCR negative for HIV between 0 and 8 weeks of age, they had additional blood samples collected on filter paper at 6, 12, and 18 months of age. The study was approved by the institutional review boards at Seattle Children's Hospital and the Mozambique Ministry of Health. Informed consent was obtained from all mothers prior to enrollment.
HIV infection was diagnosed by PCR amplification of HIV pol DNA [3]. Infants testing negative for HIV at 2 weeks of age who subsequently tested positive at ≥4 weeks of age were defined as “definitely” infected through breast milk. The time of infection was estimated as the midpoint between the last negative and first positive PCR test result. Infants who tested negative for HIV at birth, who did not have a specimen collected at 2 weeks of age, but who tested positive at ≥4 weeks of age were defined as “possibly” infected by breast milk.
NVP resistance was assessed in all virus-positive specimens, using an oligonucleotide ligation assay (OLA) that quantified the concentration of mutations K103N, V106M, Y181C, and G190A in HIV subtype C encoding reverse transcriptase [3]. HIV DNA was quantified [3], and HIV DNA templates from each specimen (goal, 100–200 HIV DNA templates; median, 132 templates; range, 9–874 templates) were used to generate the amplicon evaluated by OLA. This allowed detection of mutant concentrations generally as low at 2% of the HIV population, but in the specimen containing only 9 copies, the lower limit was 11% of the population. HIV pol amplification and OLA testing of HIV DNA were similar for specimens collected on filter paper or by venipuncture. Consensus sequencing of the amplicon was performed on the first PCR-confirmed HIV-positive specimen from all HIV-infected infants, using Clinical Laboratory Improvement Amendments–certified in-house methods. If resistance was detected, a specimen obtained near the end of follow-up was also sequenced.
The rate of HIV acquisition through breast milk was calculated using the Kaplan–Meier method. The timing of infection was compared between those with and those without NVP resistance, using the log-rank test. Risk factors for the presence of NVP resistance were evaluated using Fisher exact tests for dichotomous variables and Wilcoxon rank sum tests for continuous variables. Analyses were initially performed on data from all infants and were repeated after excluding data from those with “possible” infection via breast milk.
RESULTS
Between June 2005 and May 2008, 873 infants were enrolled into the study. Of these, 566 were excluded from this analysis because their mothers did not receive single-dose NVP or did receive ART before delivery (n = 268), they had HIV DNA detected at ≤2 weeks of age (n = 49), they were lost to follow-up before 4 weeks of age (n = 246), or they were missing samples (n = 3). Of the remaining 307 infants, mothers of 76 (24.8%) received short-course zidovudine therapy for a median duration of 42.5 days, and mothers of 3 also received zidovudine/lamivudine for 1 week after delivery. Three mothers started ART 4 days, 7 weeks, and 8 months after delivery; one (subject 17) transmitted HIV to her infant (Figure 1). Breast-feeding was reported by 245 of 305 mothers (80.3%) with an available response at the initial postpartum study visit. However, 5 of 60 infants (12%) whose mothers reported not breast-feeding at study visits had definite (subjects 3 and 12) or possible (subjects 20, 21, and 24) transmission via breast milk.
Figure 1.
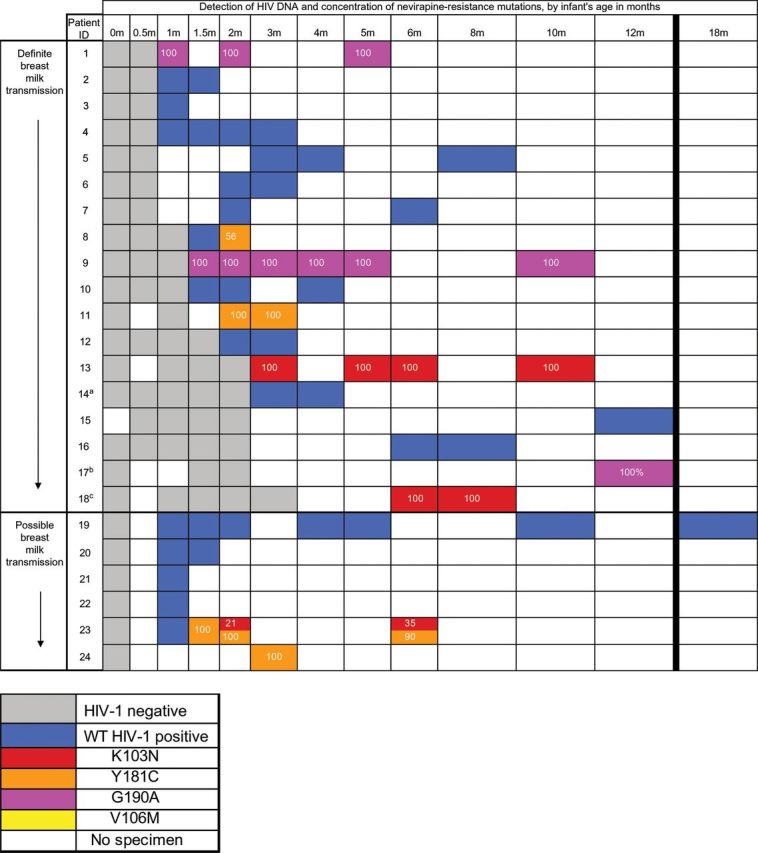
Timing of the diagnosis of human immunodeficiency virus type 1 (HIV-1) infection and the detection and concentration of NVP-resistant virus among infants infected through breast milk following single-dose NVP dosing of mothers and infants. Infants’ specimens that were positive for HIV DNA by polymerase chain reaction were tested for nevirapine resistance, using an oligonucleotide ligation assay (OLA) for detection of K103N, V106M, Y181C, and G190A; the OLA had a sensitivity to detect mutants in 2% of the population. Transmission via breast milk was considered “definite” for infants with an HIV DNA–negative PCR result at 2 weeks of age and a positive test result at ≥1 month of age. Transmission was considered “possible” for infants who tested negative for HIV DNA by PCR at birth, had no specimens obtained at 2 weeks of age available for testing, and tested positive for HIV DNA by PCR at ≥4 weeks of age. Numbers in boxes represent the proportion of the HIV population with NVP-resistant DNA. Resistance detected by OLA is shown, and additional mutations detected by consensus sequencing are as follows: aM41L, bM184V (maternal ART started at about 7 weeks after delivery, and cY188C/Y (detected at 7 months). Abbreviation: WT, wild-type virus.
Twenty-four of the 307 included infants acquired HIV infection at a median age of 1.25 months, including 18 infants with definite and 6 with possible transmission via breast milk. The Kaplan–Meier estimate of the rate of transmission via breast milk through 12 months of life, accounting for censoring, was 11.6% (95% confidence interval [CI], 7.3%–18.2%). All HIV-infected infants acquired subtype C virus, as confirmed by consensus sequencing (GenBank accession KJ395315–KJ395347). NVP resistance mutations were detected by OLA in 9 of 24 infants (37.5%; 95% CI, 18.8%–59.4%), including Y181C (in 4 [44%; 95% CI, 13.7%–78.8%]), G190A (in 3 [33%; 95% CI, 7.5%–70.1%]), and K103N (in 3 [33%; 95% CI, 7.5%–70.1%]); no infant had the V106M mutation. Among the 96 codons tested across the 24 infants, only 5 codons (in 4 infants) had indeterminate OLA results, owing to polymorphisms near the ligation site; all OLA-indeterminate codons were found to be wild type by consensus sequencing. Consensus sequencing also confirmed all NVP resistance mutations found via OLA. Eight of the 9 infants with NVP resistance at the time of diagnosis had only 1 mutant codon, confirmed by consensus sequencing, at an OLA-determined mutant concentration of 100% of the virus population. Subsequently, a lesser concentration of the K103N mutation, 21% of the virus population, was first detected in the month 2 specimen from subject 23 and persisted, at a concentration of 35% of the virus population, in the month 6 specimen. Consensus sequencing detected additional mutations not included in the OLA in 3 subjects: Y188C/Y, in the month 7 specimen but not the month 6 specimen from subject 18; M184V, in the month 12 specimen from subject 17; and M41L, in the week 14 specimen from subject 14, which appeared in the chromatogram to be a pure population of mutant HIV. Subject 14 was the only infant identified with an ARV resistance mutation (M41L) detected by consensus sequencing who did not also have virus with a NVP resistance mutation detected by OLA. The timing of HIV infection did not differ between those with and those without virus with NVP resistance mutations (P = .43, by the log-rank test), and the detection of NVP resistance mutations was not significantly related to infant or maternal ARV (Table 1) or to maternal CD4+ T-cell count (P = .63, by the Wilcoxon rank sum test). Exclusion of infants with possible transmission via breast milk did not substantially alter these results (data not shown).
Table 1.
Relationship Between Detection of Nevirapine (NVP)–Resistant Human Immunodeficiency Virus Type 1 (HIV-1) and Maternal and Infant Antiretroviral (ARV) Medication Among Infants With Definite or Possible HIV Transmission via Breast Milk
| Transmission Likelihood, Medication | Infants with NVP-Resistant HIV, Proportion (%) | Pa |
|---|---|---|
| Definite or possible (n = 24) | ||
| Maternal ARVb | ||
| None | 8/22 (36.4) | |
| ZDV | 0/1 (0) | 1.00c |
| Postpartum ARTd | 1/1 (100) | .39c |
| Infant ZDV | ||
| Yes | 6/14 (42.9) | .68 |
| No | 3/10 (30.0) | |
| Definite (n = 18) | ||
| Maternal ARVb | ||
| None | 6/16 (37.5) | |
| ZDV | 0/1 (0) | 1.00c |
| Postpartum ARTd | 1/1 (100) | .41c |
| Infant ZDV | ||
| Yes | 5/11 (45.5) | .64 |
| No | 2/7 (28.6) | |
Transmission via breast milk was considered “definite” for infants with an HIV DNA–negative PCR result at 2 weeks of age and a positive test result at ≥1 month of age. Transmission was considered “possible” for infants who tested negative for HIV DNA by PCR at birth, had no specimens obtained at 2 weeks of age available for testing, and tested positive for HIV DNA by PCR at ≥4 weeks of age.
Abbreviations: ART, antiretroviral treatment; PCR, polymerase chain reaction; ZDV, zidovudine.
a By the Fisher exact test.
b Other than single-dose NVP.
c Compared with no maternal ARV.
d This mother started ART (stavudine, lamivudine, and NVP) approximately 7 weeks after delivery, shortly before collection of the last specimen from her infant, at 8 weeks of age, that was positive for HIV DNA by PCR.
Among the 9 infants with NVP resistance, the virus population in the initial specimen from 8 (88.9%; 95% CI, 51.8%–99.7%) was 100% mutant; the remaining infant (subject 8; Figure 1) initially had a population of wild-type virus at 6 weeks of age, followed by population that was 56% mutant 2 weeks later. Among the 6 infants with 100% mutant virus population in their initial specimen and who had at least one subsequent specimen, all had virus populations that were 90%–100% mutant for the duration of their follow-up (median, 7 months; range, 3–13 months).
DISCUSSION
In this prospective observational study, over one-third of infants infected with HIV via breast milk acquired NVP-resistant viruses, and most of these had NVP resistance mutations in 100% of their virus populations that persisted for the duration of follow-up. The detection and persistence of mutant HIV in 100% of the virus population parallels the dynamics of transmitted drug-resistant virus when detected as majority populations in adults [5, 6] and infants [7–9]. The single infant with wild-type HIV at the time of diagnosis and NVP resistance in approximately 50% of the virus population 2 weeks later (subject 8) may have been coinfected with a mixture of wild-type and mutant strains or superinfected with a mutant variant shortly after initial infection. Similarly, the lesser concentrations of mutants detected during follow-up (in subjects 18 and 23) may have come from superinfection or selection of mutants. The apparently pure population of zidovudine-resistant HIV in subject 14 demonstrates that the OLA, as configured in this study, does not detect all infants with resistant HIV transmitted via breast milk.
Most infants (approximately 75%) in our study acquired HIV at or before 3 months of age, which is well within the time frame that NVP-resistant mutants selected by maternal single-dose NVP have been noted to persist in breast milk [10, 11]. Our inclusion of only a few infants who acquired HIV at later time points could explain our inability to detect a significant difference in the timing of infection among infants with and those without NVP resistance. Acquisition of NVP-resistant HIV has been reported beyond 6 months [8] and 12 months [7] of age, suggesting persistence of NVP-resistant virus in breast milk for prolonged periods. Two infants in our study (subjects 15 and 17) were first HIV positive by PCR after 6 months of age, but missing specimens made the timing of infection uncertain. One of these infants (subject 17) had virus with NVP resistance detected by OLA and lamivudine resistance detected by consensus sequencing in the first HIV-positive specimen, obtained at 12 months of age. As their mother started NVP-containing ART (stavudine, lamivudine, and NVP) shortly before the infant's last HIV–negative specimen, obtained at 8 weeks of age, it is possible that the virus transmitted to the infant was selected by a failing ART regimen.
Limitations of our study include the small number of HIV-infected infants in our analyses and the high rate of loss to follow-up, which limited our power to detect differences in the rates of NVP resistance between various subgroups. Maternal reception of ARV medications (other than single-dose NVP) was sometimes difficult to verify because of inadequate information to link to clinical charts, and adherence to ARV medications other than single-dose NVP was not assessed. Last, our OLA tested for only 4 common mutations conferring high-level NVP resistance. Although consideration of these 4 mutations identified all infants with NVP resistance in our study population (the one infant with virus containing Y188C/H, found via consensus sequencing, also had K103N detected by OLA), it may not be adequate in other populations where Y188C/H mutations predominate in late breast milk–mediated transmission [9].
In summary, a substantial proportion of infants participating in this single-dose NVP-based MTCT prevention program acquired NVP-resistant HIV apparently via breast milk. The majority of infants with NVP-resistant HIV had mutant virus populations that persisted for the duration of the study. The persistence of NVP resistance is unlike that observed in HIV–infected mothers and in utero–infected infants exposed to single-dose NVP, in which mutants decayed rapidly after delivery [3, 7]. Rather, the persistence of NVP resistance seems similar to that of virus from adults with transmitted resistance-mutations during primary infection [5, 6] and suggests that ART containing nonnucleoside reverse transcriptase inhibitors may be ineffective for the individual's lifetime. Our findings provide additional support for MTCT prevention programs to adopt ART, as recommended by the WHO [2]. While these guidelines have dramatically reduced the exposure of mothers and infants to single-dose NPV, many infants who received single-dose NVP over the past years still remain in HIV care. In addition, given the presence of resistance mutations in infants associated with maternal ART [12] and the growing transmission of drug-resistant virus in African and Asian communities [13], drug resistance testing prior to ART initiation may be the best strategy to ensure appropriate treatment in HIV–infected infants.
Notes
Acknowledgments. We thank participating women and infants, the Mozambican Ministry of Health staff, the study team, and the laboratory staff for important contributions to this study. We thank Dr. Joseph Fitzgibbon at the National Institute of Health for his support of this study.
Disclaimer. The funding agencies were not involved in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Financial support. This work was supported by the National Institutes of Health (grant R01 AI058723 to L. F. and STD/AIDS research training grant T32 AI07140 to M. A. M.) and the University of Washington CFAR (P30 AI027757 to King Holmes).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.UNAIDS. Geneva: Joint United Nations Programme on HIV/AIDS; 2012. Global Report, UNAIDS Report on the Global AIDS Epidemic: 2012. [Google Scholar]
- 2.WHO. Geneva: World Health Organization; 2013. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection; recommendations for a public health approach. [PubMed] [Google Scholar]
- 3.Micek MA, Blanco AJ, Beck IA, et al. Nevirapine resistance by timing of HIV type 1 infection in infants treated with single-dose nevirapine. Clin Infect Dis. 2010;50:1405–14. doi: 10.1086/652151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coovadia A, Abrams EJ, Stehlau R, et al. Reuse of nevirapine in exposed HIV-infected children after protease inhibitor-based viral suppression: a randomized controlled trial. JAMA. 2010;304:1082–90. doi: 10.1001/jama.2010.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Little SJ, Holte S, Routy JP, et al. Antiretroviral-drug resistance among patients recently infected with HIV. N Engl J Med. 2002;347:385–94. doi: 10.1056/NEJMoa013552. [DOI] [PubMed] [Google Scholar]
- 6.Chan KC, Galli RA, Montaner JS, Harrigan PR. Prolonged retention of drug resistance mutations and rapid disease progression in the absence of therapy after primary HIV infection. Aids. 2003;17:1256–8. doi: 10.1097/00002030-200305230-00020. [DOI] [PubMed] [Google Scholar]
- 7.Eshleman SH, Mracna M, Guay LA, et al. Selection and fading of resistance mutations in women and infants receiving nevirapine to prevent HIV-1 vertical transmission (HIVNET 012) Aids. 2001;15:1951–7. doi: 10.1097/00002030-200110190-00006. [DOI] [PubMed] [Google Scholar]
- 8.Church JD, Omer SB, Guay LA, et al. Analysis of nevirapine (NVP) resistance in Ugandan infants who were HIV infected despite receiving single-Dose (SD) NVP versus SD NVP plus daily NVP up to 6 weeks of age to prevent HIV vertical transmission. J Infect Dis. 2008;198:1075–82. doi: 10.1086/591503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moorthy A, Gupta A, Bhosale R, et al. Nevirapine resistance and breast-milk HIV transmission: effects of single and extended-dose nevirapine prophylaxis in subtype C HIV-infected infants. PLoS One. 2009;4:e4096. doi: 10.1371/journal.pone.0004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee EJ, Kantor R, Zijenah L, et al. Breast-milk shedding of drug-resistant HIV-1 subtype C in women exposed to single-dose nevirapine. J Infect Dis. 2005;192:1260–4. doi: 10.1086/444424. [DOI] [PubMed] [Google Scholar]
- 11.Gantt S, Payant R, Carlsson J, et al. Nevirapine-Resistant HIV-1 DNA in Breast Milk After Single-Dose Nevirapine With or Without Zidovudine for Prevention of Mother-to-Child Transmission. J Pediatric Infect Dis Soc. 2012;1:244–9. doi: 10.1093/jpids/pis065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeh C, Weidle PJ, Nafisa L, et al. HIV-1 drug resistance emergence among breastfeeding infants born to HIV-infected mothers during a single-arm trial of triple-antiretroviral prophylaxis for prevention of mother-to-child transmission: a secondary analysis. PLoS Med. 2011;8:e1000430. doi: 10.1371/journal.pmed.1000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta RK, Jordan MR, Sultan BJ, et al. Global trends in antiretroviral resistance in treatment-naive individuals with HIV after rollout of antiretroviral treatment in resource-limited settings: a global collaborative study and meta-regression analysis. Lancet. 2012;380:1250–8. doi: 10.1016/S0140-6736(12)61038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


