Abstract
Benzo[a]pyrene (BaP) is a polycyclic aromatic hydrocarbon (PAH) that has been implicated in modulating aromatase enzyme function with the potential to interrupt normal reproductive function. The aim of this study was to use a fish model, Fundulus heteroclitus, to assess whether BaP exposure adversely impacts reproduction. Adult fish were exposed to waterborne BaP nominal concentrations of (0, 1, or 10 μg/l) for 28 days. Males and females were combined for the second half of the exposure (days 14–28) in order to quantitate egg production and fertilization success. Egg fertilization and subsequent hatching success of F1 embryos was significantly decreased by the high dose of BaP. In males, both gonad weight and plasma testosterone concentrations were significantly reduced compared to controls by 10 μg/l BaP. Histopathological examination of testes including spermatogonia, spermatocyte and spermatid cyst areas, percentage of cysts per phase, and area of spermatozoa per seminiferous tubule were not significantly affected. Other biomarkers, including male liver weight, liver vitellogenin (vtg) mRNA expression and sperm concentrations, were also not affected. In females, estradiol concentrations were significantly reduced after BaP exposure, but egg production, gonad weight, liver weight, vtg expression and oocyte maturation were not altered. Steroid concentrations in Fundulus larvae from exposed parents at 1 and 3 weeks posthatch were not significantly changed. BaP exposure at these environmentally relevant concentrations caused negative alterations particularly in male fish to both biochemical and phenotypic biomarkers associated with reproduction and multigenerational embryo survival.
Keywords: benzo[a]pyrene, reproduction, steroid concentrations, Fundulus
The steroid biosynthesis pathway and its involvement with the hypothalamo-pituitary-gonad (HPG) axis is one of the key factors involved in maintaining proper function of reproduction and development. The multitude of enzymes working in concert is very similar among vertebrate animals (Lohr and Hammerschmidt, 2011; Payne and Hales, 2004). Steroid production is stimulated by upstream peptide hormones (Zohar et al., 2010), and gonadotropins are synthesized in the hypothalamus to stimulate the pituitary gland to produce luteinizing and follicle stimulating hormones (LH/FSH) (Shimizu et al., 2008). They subsequently initiate a cascade of events in steroidogenic tissues that leads to cholesterol being converted into downstream steroids including testosterone and estradiol. Aromatase is one of the primary downstream enzymes responsible for converting androgens into estrogens (Simpson, 2003). In fish, two different genes encoding aromatase CYP19A1 and CYP19A2 are localized in the gonads and brain, respectively (Callard and Tchoudakova, 1997).
Negative effects on teleost reproduction have resulted from changes in steroidogenic enzyme expression and hormone concentrations (Munkittrick et al., 1991; Rempel and Schlenk, 2008). Environmental endocrine disrupting chemicals (EDCs) are a primary cause in destabilizing the HPG axis of organisms residing in aquatic habitats. One such class of EDCs includes polycyclic aromatic hydrocarbons (PAHs) that result from the incomplete combustion of carbon. PAHs have many natural and anthropogenic sources which include forest fires, volcanic eruptions, tobacco smoke, and car exhaust (Maisto et al., 2006; Neal et al., 2008; Olivella et al., 2006; van Metre et al., 2000).
Benzo[a]pyrene (BaP) is a PAH that is known for causing cancer and is an aryl hydrocarbon receptor ligand that stimulates the expression of the CYP1 family of P450 monooxygenases. The same enzymes also biotransform BaP into several metabolites (Scornaienchi et al., 2010), one of which is considered the ultimate carcinogen, due to the formation of a stable epoxide (Van et al., 1985). More recently, BaP has been recognized as a reproductive and developmental toxicant. Reproductive impairment has occurred in wild populations of fish, including Fundulus, residing in PAH-contaminated environments (Collier et al., 1998; Nicolas, 1999; Pait and Nelson 2009). In BaP-injected croaker (Micropogonias undulates), ovarian growth and steroidogenesis were decreased (Thomas, 1990). BaP also affects fish aromatase (CYP19) expression, although the reported effects vary depending on study design (Hoffman and Oris, 2006; Patel et al., 2006). Through the use of in situ hybridization, cellular expression of CYP19A2 in the brain was significantly reduced in both Fundulus larvae and adults by BaP (Dong et al., 2008).
The following study utilized Fundulus heteroclitus in a short-term waterborne exposure in order to assess if BaP's published effects on aromatase modulation would translate into reproductive deficits. The experiment was adapted from previously described studies (Bosker et al., 2010; MacLatchy et al., 2003; Peters et al., 2007) in order to quickly observe potential impacts of BaP exposure to reproductive and steroidogenic endpoints.
MATERIALS AND METHODS
Fish care
A parental population of F. heteroclitus was collected from an uncontaminated site at the Newport River near Beaufort Inlet, NC. They were raised under the University Institutional Animal Care and Use Committee (IACUC) approved conditions. Sexually mature fish were bred and kept in salt water (20–25 ppt). The temperature was maintained at 20–25°C with a 14:10 light-dark cycle in the summer versus a 10:14 light-dark cycle in the winter. Adult fish were fed twice daily with tropical flake fish food (Tetramin, Tetra Werke, Germany) and live brine shrimp. First generation offspring from wild parents were used for the studies described here.
Adult BaP exposure
For 14 or 28 days adult male and female Fundulus were exposed to the following treatments: control (300 μl ethanol = solvent carrier), BaP 1 μg/l or BaP 10 μg/l. For the first 14 days, males and females were kept in separate 30-l tanks with six fish per tank and five tanks per treatment group. Half of the fish were dissected on day 14 for the liver, gonad, sperm, and blood. The fish were euthanized with buffered MS-222, and their weights and lengths recorded. Blood was collected using a 10 μl microcapillary tube (Drummond Scientific) after cutting off the caudal fin. Plasma was separated by centrifuging the blood at 2400 × g for 12 min at 4°C. Plasma samples were stored at −80°C for steroid extractions. For days 14–28 of the exposure, the sexes were combined with six fish per tank and six tanks per treatment group. Spawning and reproductive success were measured by collecting eggs every other day from days 16 to 28. Following collection, embryos were kept in control water. The total number of eggs was counted and fertilization success was calculated by dividing the number of fertilized eggs by the total number of eggs. Hatching success was measured on days 15–18 postfertilization. Unhatched Fundulus embryos were aerated for the same amount of time per group each day. The number of hatched larvae was recorded immediately following aeration. Hatching percentage was calculated for each parental tank by (cumulative number of hatched larvae/total number of eggs collected × 100%). Additionally, eggs were raised until 1 and 3 weeks posthatch when larvae were flash frozen, and stored at −80°C for further steroid extraction and analysis. The remaining adults were dissected on day 28 as described above. To achieve six tanks per treatment, two separate 28-day exposures were needed (June and September 2010). Exposure conditions for both exposures were 21–24°C, 14:10 light-dark period, and 23–24 ppt salinity. Steroid concentration and sperm concentration data were collected only from the September exposure. Fish were kept in the tanks for at least 1 week for acclimatization prior to the start of the exposure, and tanks were routinely checked for elevated ammonia. During the BaP exposure, water was changed every 24 h (90% static water renewal) and tanks were subsequently dosed at approximately the same time each day. Water samples (100–200 ml) were collected to confirm nominal concentrations of BaP using gas chromatography/mass spectrometry in selected ion monitoring mode (Wang et al., 2006).
Sperm counts
In order to quantify the number of sperm, ∼0.01 g of male gonad was homogenized in 50 μl of 18 ppt salinity H2O for 1 min. Then 1 μl of the homogenate was diluted in 99 μl of 18 ppt H2O/trypan blue mix and the solution was vortexed and loaded onto both chambers of a Neubauer hemacytometer and incubated at 26°C for 10 min. Sperm were counted according to WHO laboratory manual (2010) for the examination and processing of human semen fifth edition at ×400 magnification (Olympus BX40). In brief, sperm from the central grid (number 5) were counted until a total of at least 200 spermatozoa were observed in a complete row. Counts were repeated in the second chamber. The sum and difference of the two numbers were calculated and an acceptability value of the difference was determined. If the difference was acceptable, the concentration was calculated. However, if the difference was too high, two new dilutions were prepared and counted. First, number sperm/μl were calculated by the following equation: (N/n) × (1/0.02) × 100, where N = number of sperm, n = number of rows counted, 1:0.02 = the 0.02 μl volume per row of the hemacytometer, and 100 = 1:99 dilution used prior to loading the hemacytometer. Next, the tissue concentration was calculated by: (number of sperm/μl) × (50 μl/g gonad), where 50 μl is volume in which the gonad was homogenized and g is the gonad weight homogenized.
Sex steroid extraction and quantification
Plasma samples were thawed on ice and pooled from two or three fish of the same sex. The ether extraction protocol used for adult Fundulus was adapted from a previous study (MacLatchy et al., 2005). Briefly, pooled samples were added to 100 μl of DI H2O in a glass test tube. Then 5 ml of ether was added and each test tube was vortexed for three times of 15-s intervals. Samples were then incubated at room temperature for 10 min. The glass tubes were placed in an acetone/dry ice bath for 1 min and subsequently swirled to ensure that the white precipitate would stick to the bottom. The rest of the clear solution was decanted into a 20-ml scintillation vial. The addition of 5 ml ether and subsequent steps were repeated two more times. The scintillation vials were kept uncapped and allowed to evaporate overnight in the fume hood. Once completely dry, 1 ml of phosgel buffer (40.5mM Na2HPO4, 9.3mM NaH2PO4-H2O, 0.1% gelatin, 0.25mM thimersol) was added and the steroids were allowed to reconstitute into the buffer for 20 min at room temperature. The samples were stored at −20°C until analyzed by radioimmunoassay as previously described (Dube and MacLatchy, 2001; MacLatchy et al., 2005).
The methanol extraction procedure used for whole larvae homogenates was also adapted from a previous study (Morthorst et al., 2013). Five larvae were pooled together in a 1.5-ml epitube. The sample was homogenized for 1 min in 50 μl of homogenizing buffer consisting of PBS and 1mM EDTA using a pellet pestle. Following homogenization, 200 μl methanol was added and samples were vortexed for 1 min. They were stored at 4°C for 1 h with intermittent vortexing every 15 min. Following the incubation period, the tubes were centrifuged at 3000 × g for 5 min. They were frozen on dry ice and the supernatant was transferred into 7 ml glass tubes. The addition of methanol and subsequent steps were repeated two more times. Samples were stored at −80°C until evaporated with a gentle stream of nitrogen gas. Once completely dry, samples were reconstituted with 1 ml of 50mM acetate buffer, incubated at room temperature for 30 min and stored at −20°C until ready for solid-phase extractions (SPE DSC-18, 100 mg; Sigma-Aldrich). The larvae samples were passed through the mini columns according to manufacturer's instructions for reversed phase sorbents. Ethyl acetate (1% methanol) was used to collect steroids and the solution was dried under a gentle stream of nitrogen gas. Larvae samples were reconstituted in 210 μl of EIA buffer. Estradiol and testosterone were analyzed with enzyme immunoassay and a HTS 7000 Bioassay Reader (Perkin Elmer) according to manufacturer's instructions (Cayman Chemical, Ann Arbor, MI).
Vitellogenin mRNA expression
Fundulus livers were stored at −80°C in 1 ml RNA later (Ambion, Austin, TX). Tissues were homogenized in 1 ml RNAzol RT (Molecular Research Center, Cincinnati, OH) and extracted by the manufacturer's protocol. The total RNA was further cleaned by using an RNeasy kit (Qiagen) with a DNAse I digestion. The RNA concentration was determined with a Nanodrop 2000 (ThermoScientific). RNA was stored at −80°C until reverse transcribed. RNA was reverse transcribed, and vitellogenin (vtg) expression was analyzed through real-time PCR with an ABI 7500 Sequence Detection System (Applied Biosystems). Detailed methods have previously been described (Wang et al., 2006) including melt curve analyses and nontemplate controls (Cq > 39). Slopes and amplification efficiencies were determined for both vtg and 18S and were not statistically different (slopes = −4.1 and −3.7, respectively; p = 0.105; amplification efficiencies = 75 and 86%, respectively). Briefly, samples were normalized to the reference gene 18S rRNA (average ± SEM Cq values for 18S were 13.4 ± 0.09). Although statistics were performed on 2−ΔCq, data is presented as fold induction compared with controls. Primers specific to vtg were synthesized by Invitrogen (Carlsbad, CA). Forward: 5′-GAGGATCTGTGCTGATGCAGTTGTG-3′; reverse: 5′-GGGTAGAAGGCAGTCTTTCCC-3′. Use of these primers has been described previously with Fundulus (Garcia-Reyero et al., 2004). 18S rRNA primers were: forward: 5′-TGGTTAATTCCGATAACGAACGA-3′; reverse: 5′-CGCCACTTGTCCCTCTAAGAA-3′).
Gonad histology
Gonads were fixed in 20 ml of 4% (wt/vol) paraformaldehyde overnight, and then processed by dehydration through increasing concentrations of ethanol. Tissues were subsequently rinsed with Clearify (American Master Tech Scientific, Lodi, CA) and were embedded in molten paraffin (paraplast embedding media paraplast X-tra, Sigma, St Louis, MO). Gonads were sectioned at 5 μm thickness using a microtome (Olympus Cut4055, Olympus American, San Jose, CA) and subsequently stained with hematoxylin and eosin (HE) for ovary/testis staging.
The developing oocytes were divided into three different categories that include previtellogenic (stages 1a–1c), vitellogenic (stage 2), and mature (stage 3). The size based divisions have previously been described (Dong and Willett, 2008; Fang et al., 2010; Wallace and Selman, 1985) and number of oocytes at certain stages were normalized to area of the ovary being examined (n = 3 tanks/treatment for 2 weeks (1–2 fish/tank) and 5–6 tanks/treatment for 4 weeks (1–3 fish/tank)).
For testes, the area of spermatogenic cysts was measured using ImageJ and each cyst was categorized as spermatogonia (SG), spermatocyte (SC), spermatid (ST), or spermatozoa (S) (van der Ven et al., 2003). Three regions within one section (n = 4–5 tanks per treatment for 4 weeks) were analyzed at ×40 magnification with an average of 79 cysts per gonad (44–101 cysts/gonad) scored for immature spermatocysts and an average of 61 seminiferous tubules per gonad (40–116 seminiferous tubules/gonad) for spermatozoa. Percentage of cysts per stage was calculated by dividing number of cysts in each stage by the total number of cysts analyzed. Fullness of seminiferous tubules was calculated by dividing the area of mature spermatozoa by the area of the seminiferous tubule. All gonad scoring was done blind to treatment.
Statistics
Results were analyzed by GraphPad Prism 5.0 and presented as mean ± SEM. In this study, n represents the number of tanks per treatment by averaging the values of each fish per tank. Statistical differences between treatment groups were determined using unpaired t-test or one-way ANOVA followed by Tukey's posttest. Nonparametric data were analyzed by Kruskal-Wallis followed by Dunn's posttest. Gonad and liver weight relative to body weight were first analyzed using analysis of covariance (ANCOVA). Assumptions were not met for ANCOVA, so fish weight was analyzed using one-way ANOVA. Gonad staging was analyzed by arcsin transforming percentages followed by ANOVA. For all statistical comparisons, significance was determined by p < 0.05.
RESULTS
Water Concentrations and Survival
The actual water mean and standard error concentrations for the 1 and 10 μg/l BaP exposure in June were 1.59 ± 0.41 and 17.1 ± 3, and for September they were 0.67 ± 0.14 and 7.5 ± 1.4 μg/l, respectively. Female adults had 100% survival for all treatment groups. Males exhibited 90% survival in the controls and 100% for low and high dose BaP.
Steroid Concentrations
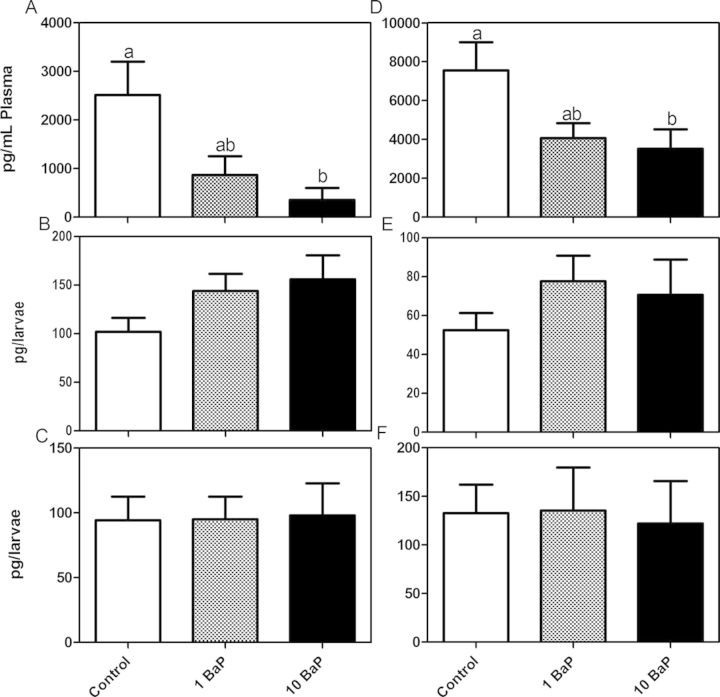
Steroid concentrations determined at both the 2- and 4-week times were not significantly different so data were pooled. Control male testosterone and female estrogen concentrations were 2510 ± 691 and 7540 ± 1450 pg/ml plasma, respectively (Figs. 1A and 1D). Testosterone in the males was significantly reduced by high dose BaP to 355 ± 242 pg/ml plasma. Estradiol concentrations were also significantly reduced to 3510 ± 1010 pg/ml plasma. Fundulus larvae at 1 and 3 weeks posthatch did not exhibit any significant changes to testosterone (Figs. 1B and 1C) and estradiol (Figs. 1E and 1F) concentrations when whole larvae were homogenized.
Fig. 1.
Testosterone (A–C) and estradiol (D–F) concentrations (mean ± SEM). In the adult Fundulus, the nominal 10 μg/l BaP treatment significantly reduced circulating testosterone concentrations in the males (A) (Kruskal-Wallis, p < 0.05, n = 7 tanks/treatment, 2–3 males/sample; bars with the same letter are not significantly different) and estradiol concentrations in the female (D) (ANOVA, p < 0.05, n = 7 tanks/treatment, 2–3 females/sample). Whole body steroid concentrations of larvae (n = 5–6/treatment, five larvae pooled per sample) at 1 week posthatch (WPH) (B, E) and 3 weeks posthatch (C, F) were not altered by either 1 or 10 μg/l BaP treatment.
Body, Liver, and Gonad Weights
Because there were no significant differences in fish weights among treatment groups (data not shown), changes in gonad and liver weight were analyzed using one-way ANOVA. Males did not experience any changes in gonad weight after 2 weeks exposure, but exhibited a significant decrease after 4 weeks in the high treatment group (n = 5 tanks at 2 weeks with 2–3 fish/tank; six tanks at 4 weeks with 3 fish/tank; Figure 2A and Supplementary fig. 1A shown as GSI (gonad somatic index)). There were no changes in female gonad weight at either time point among treatment groups, but female controls did have a significantly higher gonad weight at the 4-week time point compared with the 2-week controls (Student's t-test; Fig. 2B and Supplementary fig. 1B). The liver size for both sexes was not significantly altered from the exposure (Supplementary fig. 2 shown as (hepatosomatic index)).
Fig. 2.
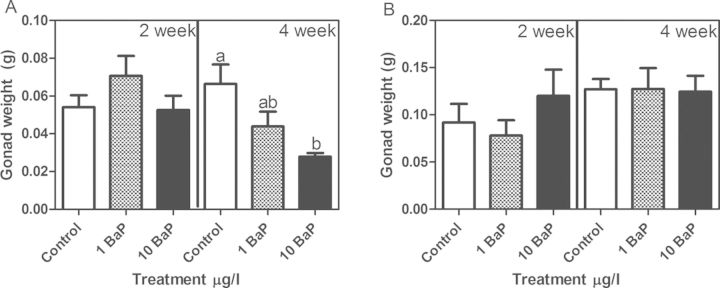
Male (A) and female (B) gonad weight (mean ± SEM). In males, no significant changes were observed in testis weight after 2 weeks exposure to BaP, however, 10 μg/l BaP treatment for 4 weeks significantly reduced testis weight. In the females, gonad weight was not altered at either of the time points or BaP doses (ANOVA, p < 0.05, n = 5 tanks at 2 weeks with 2–3 fish/tank; and n = 6 tanks at 4 weeks with 3 fish/tank; bars with the same letter are not statistically different).
Fertilization Success, Egg Production, and Hatching Success
From days 14 to 28 of the exposure, sexes were combined and eggs were collected every other day. Although total egg production was not significantly altered (Fig. 3A), fertilization success was significantly lower in the high dose BaP treatment group (Fig. 3B). Total egg production averaged 287 ± 24.4, 270 ± 47.3, and 398 ± 78.6 per tank whereas fertilization success averaged 91 ± 1.0, 72 ± 9.3, and 71 ± 5.9% for control, low, and high dose BaP, respectively. Hatching success was measured at days 15–18 postfertilization (dpf) (Supplementary fig. 3). By 18 dpf, 93.4 ± 1.1% of F1 control embryos had hatched whereas only 79.8 ± 5.7% of F1s from the high dose BaP-exposed parents had hatched (p = 0.024, one-tailed, two sample t-test).
Fig. 3.
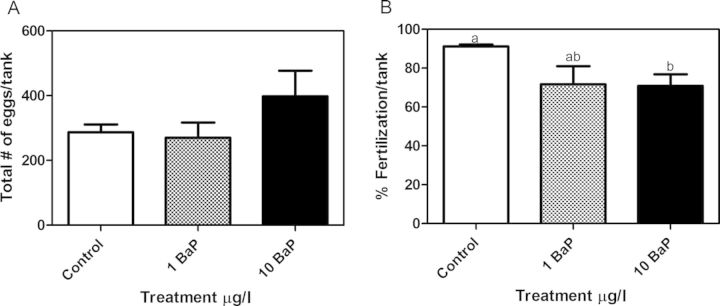
Total eggs produced (A) and percentage fertilization success (B) per tank during days 14–28 of adult exposure (mean ± SEM). Waterborne BaP exposure significantly reduced percentage of eggs fertilized at 10 μg/l BaP (n = 6 tanks [total egg production], n = 5 tanks [percentage fertilization success], 2-sample t-test, p < 0.05, 3 males + 3 females/tank; bars with the same letter are not statistically different; ANOVA did not show significant changes between treatment groups). Total egg counts were not significantly altered by BaP exposure.
Gonad Morphology and Sperm Concentrations
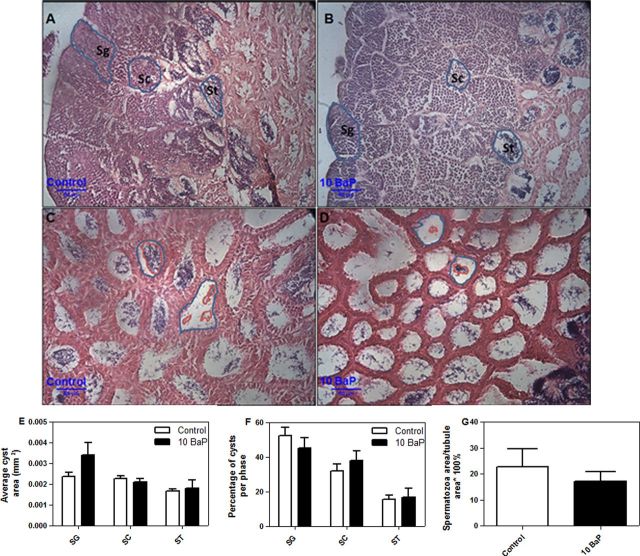
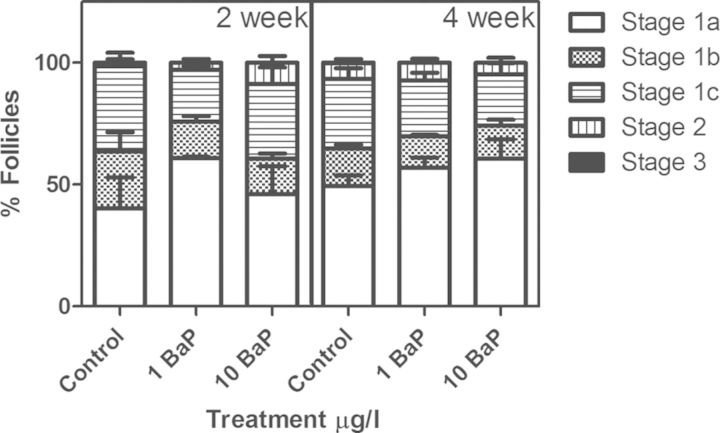
In testes, cyst area, percentage of cysts per phase, and percentage fullness of seminiferous tubules were measured. The average cyst area of spermatogonia was nonsignificantly increased following high dose BaP exposure (0.0034 mm2 compared with control 0.0024 mm2) (Fig. 4E) when analyzed by tank (n = 4–5). The average areas of spermatocyte and spermatid cysts and seminiferous tubules were not significantly affected by high dose BaP treatment (Fig. 4E). Likewise percentage of immature cysts per phase (Fig. 4F) were not altered compared with control. High dose BaP treatment did not significantly affect the amount of mature spermatozoa in the seminiferous tubules (% fullness) (Fig. 4G). Similarly, no significant differences in the number of sperm produced in each treatment were found during either sampling period (Supplementary fig. 4). Treatment and exposure time did not alter the proportions of differently staged oocytes (Fig. 5).
Fig. 4.
Testis morphology of Fundulus (mean ± SEM). Immature spermatogenic cysts in control (A) and high dose BaP-treated (B) males, testis morphology of mature sperm in control (C) and high dose BaP-treated (D) males. The average cyst size (E) of immature SG = spermatogonia, SC = spermatocyte, ST = spermatid and percentage of immature cysts per phase (F) were not altered compared to control (t-test, p < 0.05, n = 4–5 tanks). High dose BaP treatment did not significantly affect the amount of mature spermatozoa in the seminiferous tubules (percentage fullness) (G). The percentages were calculated as (number of cysts within a stage/total cysts observed) × 100% and (area of spermatozoa/area of tubule) × 100% (n = 4–5 tanks /treatment group at 4 weeks).
Fig. 5.
Percentage of different developmental stages of follicles represented in female ovaries (mean ± SEM). The percentages were calculated as (number of follicles at certain stage/total number) × 100%. No significant differences were found (n = 3 tanks/treatment analyzed at 2 weeks with 1–2 fish/tank and 6 tanks/treatment at 4 weeks with 2–3 fish/tank). Follicle stages 1a–1c represent the previtellogenic phase. Stages 2 and 3 are considered vitellogenic and mature follicle stages, respectively.
Vitellogenin Expression
Quantitative RT/RT-PCR was used to quantitate vtg mRNA expression in male and female liver. In males, vtg mRNA expression was very low and highly variable (Fig. 6A). When both males and females from all treatment groups were normalized to the 2-week control males, females expressed at least 3000-fold more vtg expression (Supplementary fig. 5). In the females, expression was high but also highly variable. After 4 weeks of high dose BaP exposure, females had significantly higher vtg mRNA expression compared with low dose BaP, but not compared with controls (Fig. 6B).
Fig. 6.
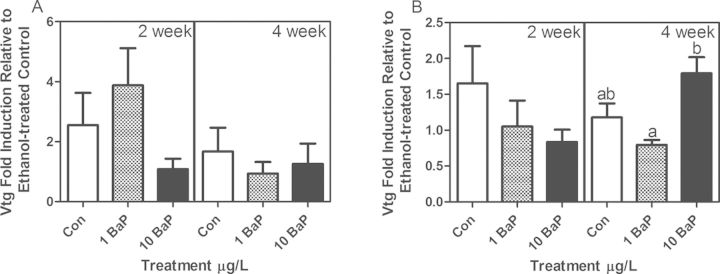
Male (A) and female (B) liver vtg mRNA expression measured by qRT/RT-PCR (mean ± SEM). Fold induction is expressed relative to controls within each time point after normalization to 18S rRNA by the 2−ΔΔCq method. After 4 weeks of exposure, female fish from 10 μg/l BaP had a significant induction of vtg compared with 1 μg/l BaP but not compared with controls (ANOVA on 2−ΔCq, p < 0.05; n = 6 tanks with 3 fish/tank at 4 weeks; n = 5 tanks with 2–3 fish/tank at 2 weeks; bars with the same letters are not significantly different).
DISCUSSION
Short-term reproductive bioassays are used to quantitate the effects of EDCs on fish reproduction and endocrine-related biomarkers. In Fundulus these studies have been developed and optimized using primarily 17α-ethynylestradiol as the stressor (Blewett et al., 2013; Bosker et al., 2009, 2010; Doyle et al., 2013; MacLatchy et al., 2003). Yet there is evidence both from wild fish populations (Bugel et al., 2010; Pait and Nelson, 2009) and mammalian studies that PAHs, including BaP, also adversely impact reproductive potential. For example, pregnant women who smoke or are exposed to second-hand smoke have increased concentrations of BaP in their follicular fluid (Neal et al., 2008). Furthermore, this PAH exposure has been correlated with increased difficulty in conceiving, increased rates of miscarriage, and developmental abnormalities in their offspring (Lee et al., 2011; Neal et al., 2008; Wu et al., 2010). As reported here, in Fundulus exposed to nominally 10 μg/l (11.9 ± 2.02 μg/l, actual) waterborne BaP, there was a >20% decrease in the fertilization success and a 14% decrease in F1 hatching success/survival.
The most significant concern associated with decreased reproductive success from EDC environmental exposure is that there could be population level impacts. Precedent for potential population crashes was provided by whole lake experiments wherein fathead minnow populations crashed following chronic low dose ethynylestradiol exposure (Kidd et al., 2007). Although it is more difficult to scale laboratory-based studies to population effects, a study with wild salmon used a projection matrix model in order to show that a 10% change in reproductive fecundity parameters could lead up to a 64% decline in baseline population percentages after a simulated 20 years of toxic impact (Spromberg and Meador, 2005). Ankley et al. (2008) projected that a 50% decrease in fathead minnow plasma estradiol concentrations would correlate with a 92% decrease in population size after 5 years. The high BaP exposure caused a 53% reduction in Fundulus female estradiol concentrations so the potential for population impact from PAHs is conceivable. The environmentally relevant concentrations used in this study bracket the pure water solubility of BaP (4 μg/l) (Mackay and Shiu, 1977), but fish from contaminated locations would be expected to be exposed to potentially higher concentrations from sediment (Kimbrough and Dickhut, 2006).
In this study, fertilization success but not egg production was significantly decreased, thereby suggesting potential male sensitivity. In fact, male gonad weight and plasma testosterone concentrations were decreased by 38 and 86%, respectively. Fundulus collected from Newark Bay (a heavily contaminated bay) also had significantly decreased GSIs compared with Fundulus collected from Tuckerton (a less contaminated site) but no qualitative testis histopathological alterations were observed (Bugel et al., 2010). In our study, when histopathological analysis of testes was done, the high dose BaP exposure caused a nonsignificant increase (44.7%) in spermatogonia cyst size compared to control (result was significant when analyzed by fish (n = 8 individuals)). To our knowledge, this has not been previously reported but is a result that should be investigated with higher statistical power, because it may suggest a delay in proliferation of spermatogonia. However, neither the cyst size of the other phases (spermatocytes, spermatids, and spermatozoa) nor the percentage of cysts per phase was significantly affected. A higher percentage of spermatogonia has been reported following 17β-estradiol and 17α-ethinylestradiol exposure (van der Ven et al., 2003; van den Belt et al., 2002; Weber et al., 2003) in zebrafish, but cyst size was not significantly altered. Similarly, fathead minnows exposed to aromatase inhibitors and antiandrogens, such as prochloraz and fenarimol, experienced a significant increase in the percentage of spermatogonia (Ankley et al., 2005). In the BaP exposed Fundulus, despite the impacts on male reproductive parameters mentioned, sperm counts were not significantly decreased, which agreed with no significant observed reduction in the fullness of seminiferous tubules. However, we did not measure sperm fitness or motility. To better predict fertility, measuring a combination of sperm parameters (motility, count, morphology, and volume) has been suggested (Mangeladorf et al., 2003; Wang et al., 1988). In mammals, there is precedent for the sensitivity of male fertility to BaP. Sperm counts and sperm motility were decreased and testicular malformations increased in multiple generations of mice following a F0 6-week, daily oral BaP exposure (Mohamed et al., 2010).
Vitellogenin expression, especially its induction in male fish, is a classic biomarker of exposure to environmental estrogens (Hutchinson et al., 2006; Jones et al., 2013). Although many studies measure circulating vtg protein expression, a quantitative PCR approach of liver mRNA expression was used. Using this method, Fundulus males exposed to estradiol had 10 times higher expression compared with control females whereas nonylphenol was a weak inducer (Garcia-Reyero et al., 2004). In our study, BaP did not cause a dose-dependent effect on vtg mRNA expression in either males or females, though as would be expected, vtg expression was increased in reproductively active females (4 weeks vs. 2 weeks).
In females, despite a significant reduction in estradiol concentrations, egg production was not significantly affected. Conservation of egg production capability despite exposure to high concentrations of EDCs may be a Fundulus-specific phenomenon. Doyle et al. (2013) note that in many freshwater fish laboratory models, relatively low doses of ethynylestradiol decrease egg production, and Fundulus are resistant possibly because of their ability to maintain lipid transport into the ovary. Fundulus also preferentially accumulate ethynylestradiol in the liver (where lipophilic toxicants are metabolized), the gall bladder (where metabolized toxicants enter bile), and the gut (where bile is received) in higher proportions than other tissues, indicating efficiency in metabolism and clearance (Blewett et al., 2013). These mechanisms may explain Fundulus's insensitivity to estrogenic contaminants and how Fundulus populations can survive in estrogenic environments (e.g., adjacent to Superfund sites) (Bello et al., 2001; Greytak et al., 2005; Meyer and DiGiulio, 2003; Prince and Cooper, 1995). In contrast to Fundulus ovo-insensitivity, in mice, BaP causes ovotoxicities including increased primordial follicle activation, developing follicle atresia, and effects on membrane fluidity that impair fertility; all toxicities that are consistent with PAH-exposure related premature ovarian failure and infertility in humans (Sobinoff et al., 2012).
The 53% decrease in circulating estradiol concentrations is consistent with the reduction in brain aromatase mRNA expression found in both F. heteroclitus larvae and adults (Dong et al., 2008) and ovarian aromatase enzyme activity after waterborne exposure to BaP (Patel et al., 2006). The reduction in testosterone concentrations in the adult males was perplexing because the reduction in aromatase expression would hypothetically lead to an increase in circulating androgens. A study in rats also showed a significant decrease in testosterone after BaP exposure and provided a plausible mechanism for the change. Through acetylation-mediated epigenetic control, the expression of the steroid acute regulatory protein (StAR) was significantly reduced (Liang et al., 2012). StAR is an important protein upstream in the steroid biosynthesis pathway (Manna et al., 2003) responsible for transporting cholesterol from the outer to the inner mitochondrial membrane where another P450 side chain cleavage enzyme converts cholesterol to the obligate steroid precursor pregnenolone. To our knowledge, BaP's effects on StAR expression have not been studied in Fundulus and additional insight into these upstream enzymes and steroids will contribute to better understanding of mechanisms related to the reproductive and developmental toxicities associated with PAH exposure.
The recognition of BaP as an endocrine disruptor is generally considered secondary to its established carcinogenicity. This study clearly indicates that BaP has multiple effects on classic markers of endocrine function in Fundulus. Yet when conducting bioassay testing, it is important to distinguish biomarkers of exposure that provide potential insight into mechanism and true adverse effect measures that may be population relevant (Hutchinson et al., 2006). Importantly, in this study, BaP exposure caused both a decrease in fertilization success as well as the multigenerational impact on F1 hatching success. BaP had multigenerational impacts on F1 and F2 zebrafish development (Corrales et al., 2014) and fathead minnows F2 embryos had decreased survival, but potential mechanisms for the multigenerational toxicity were not explored (White et al., 1999). Our study indicates that although BaP caused a >50% decrease in female circulating estrogen concentration, this depression had no significant effect on fecundity or gonad morphology. In contrast, BaP effects on male reproductive endpoints (e.g., gonad weight and decreased circulating testosterone) were more susceptible to change and could be related to the decreased fertilization success in the subsequent generation.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institute of Environmental Health Sciences (R03ES018962).
Supplementary Material
Acknowledgments
Dr. Patricia McClellan-Green (NC State University) provided wild Fundulus that were the parents of the animals used in this study. We would like to thank Dr. Xiefan Fang for technical assistance with the PCR reactions, Hallie Freyaldenhoven for assistance with exposure and ovary scoring, Adam Hawkins for help with gonad histology sectioning, Dr. Asok Dasmahapatra for methodological input for scoring testes, Nathalia Ribeiro dos Santos for assistance in scoring the testes, Mallory White for assistance with steroid extractions, Dr. Jone Corrales for assistance with statistics, and the rest of the Willett lab for help with the exposures and fish care.
REFERENCES
- Ankley G. T., Miller D. H., Jensen K. M., Villeneuve D. L., Martinovic D. Relationship of plasma sex steroid concentrations in female fathead minnows to reproductive success and population status. Aquat. Toxicol. 2008;88:69–74. doi: 10.1016/j.aquatox.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Ankley G. T., Jensen K. M., Durhan E. J., Makynen E. A., Butterworth B. C., Kahl M. D., Villeneuve D. L., Linnum A., Gray L. E., Cardon M., et al. Effects of two fungicides with multiple modes of action on reproductive endocrine function in the fathead minnow (Pimephales promelas) Toxicol. Sci. 2005;86:300–308. doi: 10.1093/toxsci/kfi202. [DOI] [PubMed] [Google Scholar]
- Bello S. M., Franks D. G., Stegeman J. J., Hahn M. E. Acquired resistance to Ah receptor agonists in a population of Atlantic killifish (Fundulus heteroclitus) inhabiting a marine superfund site: In vivo and in vitro studies on the inducibility of xenobiotic metabolizing enzymes. Toxicol. Sci. 2001;60:77–91. doi: 10.1093/toxsci/60.1.77. [DOI] [PubMed] [Google Scholar]
- Blewett T. A., Robertson L. M., MacLatchy D. L., Wood C. M. Impact of environmental oxygen, exercise, salinity, and metabolic rate on the uptake and tissue-specific distribution of 17alpha-ethynylestradiol in the euryhaline teleost Fundulus heteroclitus. Aquat. Toxicol. 2013;138–139C:43–51. doi: 10.1016/j.aquatox.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Bosker T., Hewitt L. M., Munkittrick K. R., MacLatchy D. L. Validation of a refined short-term adult fish reproductive test with improved power for mummichog (Fundulus heteroclitus) to test complex effluents. Ecotoxicol. Environ. Saf. 2010;73:1596–1601. doi: 10.1016/j.ecoenv.2010.07.037. [DOI] [PubMed] [Google Scholar]
- Bosker T., Munkittrick K. R., MacLatchy D. L. Challenges in current adult fish laboratory reproductive tests: Suggestions for refinement using a mummichog (Fundulus heteroclitus) case study. Environ. Toxicol. Chem. 2009;28:2386–2396. doi: 10.1897/09-032.1. [DOI] [PubMed] [Google Scholar]
- Bugel S. M., White L. A., Cooper K. R. Impaired reproductive health of killifish (Fundulus heteroclitus) inhabiting Newark Bay, NJ, a chronically contaminated estuary. Aquat. Toxicol. 2010;96:182–193. doi: 10.1016/j.aquatox.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Callard G. V., Tchoudakova A. Evolutionary and functional significance of two CYP19 genes differentially expressed in brain and ovary of goldfish. J. Steroid Biochem. Mol. Biol. 1997;61:387–392. doi: 10.1016/s0960-0760(97)80037-4. [DOI] [PubMed] [Google Scholar]
- Collier T. K., Johnson L. L., Stehr C. M., Myers M. S., Stein J. E. A comprehensive assessment of the impacts of contaminants on fish from an urban waterway. Mar. Environ. Res. 1998;46:243–247. [Google Scholar]
- Corrales J., Thornton C., White M., Willett K.L. Multigenerational effects of benzo[a]pyrene exposure on survival and developmental deformities in zebrafish larvae. Aquat. Toxicol. 2014;148:16–26. doi: 10.1016/j.aquatox.2013.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W., Wang L., Thornton C., Scheffler B. E., Willett K. L. Benzo(a)pyrene decreases brain and ovarian aromatase mRNA expression in Fundulus heteroclitus. Aquat. Toxicol. 2008;88:289–300. doi: 10.1016/j.aquatox.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W., Willett K. L. Local expression of CYP19A1 and CYP19A2 in developing and adult killifish (Fundulus heteroclitus) Gen. Comp. Endocrinol. 2008;155:307–317. doi: 10.1016/j.ygcen.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle M. A., Bosker T., Martyniuk C. J., MacLatchy D. L., Munkittrick K. R. The effects of 17-alpha-ethinylestradiol (EE2) on molecular signaling cascades in mummichog (Fundulus heteroclitus) Aquat. Toxicol. 2013;134–135:34–46. doi: 10.1016/j.aquatox.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Dube M. G., MacLatchy D. L. Identification and treatment of a waste stream at a bleached-kraft pulp mill that depresses a sex steroid in the mummichog (Fundulus heteroclitus) Environ. Toxicol. Chem. 2001;20:985–995. doi: 10.1897/1551-5028(2001)020<0985:iatoaw>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Fang X., Dong W., Thornton C., Willett K. L. Benzo[a]pyrene effects on glycine N-methyltransferase mRNA expression and enzyme activity in Fundulus heteroclitus embryos. Aquat. Toxicol. 2010;98:130–138. doi: 10.1016/j.aquatox.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Reyero N., Raldua D., Quiros L., Llaveria G., Cerda J., Barcelo D., Grimalt J. O., Pina B. Use of vitellogenin mRNA as a biomarker for endocrine disruption in feral and cultured fish. Anal. Bioanal. Chem. 2004;378:670–675. doi: 10.1007/s00216-003-2295-1. [DOI] [PubMed] [Google Scholar]
- Greytak S. R., Champlin D., Callard G. V. Isolation and characterization of two cytochrome P450 aromatase forms in killifish (Fundulus heteroclitus): Differential expression in fish from polluted and unpolluted environments. Aquat. Toxicol. 2005;71:371–389. doi: 10.1016/j.aquatox.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Hoffman J. L., Oris J. T. Altered gene expression: A mechanism for reproductive toxicity in zebrafish exposed to benzo[a]pyrene. Aquat. Toxicol. 2006;78:332–340. doi: 10.1016/j.aquatox.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Hutchinson T. H., Ankley G. T., Segner H., Tyler C. R. Screening and testing for endocrine disruption in fish-biomarkers as “signposts,” not “traffic lights,” in risk assessment. Environ. Health Perspect. 2006;114:106–114. doi: 10.1289/ehp.8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. D., Tremblay L. A., De Coen W., Giesy J. P. Vitellogenin as a biomarker for environmental estrogens. Australas. J. Ecotoxicol. 2013;6:45–58. [Google Scholar]
- Kidd K. A., Blanchfield P. J., Mills K. H., Palace V. P., Evans R. E., Lazorchak J. M., Flick R. W. Collapse of a fish population after exposure to a synthetic estrogen. Proc. Natl. Acad. Sci. U.S.A. 2007;104:8897–8901. doi: 10.1073/pnas.0609568104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrough K. L., Dickhut R. M. Assessment of polycyclic aromatic hydrocarbon input to urban wetlands in relation to adjacent land use. Mar. Pollut. Bull. 2006;52:1355–1363. doi: 10.1016/j.marpolbul.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Lee B. E., Hong Y. C., Park H., Ha M., Kim J. H., Chang N., Roh Y. M., Kim B. N., Kim Y., Oh S. Y., et al. Secondhand smoke exposure during pregnancy and infantile neurodevelopment. Environ. Res. 2011;111:539–544. doi: 10.1016/j.envres.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Liang J., Zhu H., Li C., Ding Y., Zhou Z., Wu Q. Neonatal exposure to benzo[a]pyrene decreases the levels of serum testosterone and histone H3K14 acetylation of the StAR promoter in the testes of SD rats. Toxicology. 2012;302:285–291. doi: 10.1016/j.tox.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Lohr H., Hammerschmidt M. Zebrafish in endocrine systems: Recent advances and implications for human disease. Annu. Rev. Physiol. 2011;73:183–211. doi: 10.1146/annurev-physiol-012110-142320. [DOI] [PubMed] [Google Scholar]
- Mackay D., Shiu W. Y. Aqueous solubility of polynuclear aromatic hydrocarbons. J. Chem. Eng. Data. 1977;22:399–402. [Google Scholar]
- MacLatchy D. L., Courtenay S. C., Rice C. D., Van Der Kraak G. J. Development of a short-term reproductive endocrine bioassay using steroid hormone and vitellogenin end points in the estuarine mummichog (Fundulus heteroclitus) Environ. Toxicol. Chem. 2003;22:996–1008. [PubMed] [Google Scholar]
- MacLatchy D. L., Gormley K. L., Ibey R. E. M., Sharpe R. L., Shaughnessy K. S., Courtenay S. C., Dube M. G., Van Der Kraak G. J. A short-term mummichog (Fundulus heteroclitus) bioassay to assess endocrine responses to hormone-active compounds and mixtures. Tech. Aquat. Toxicol. 2005;2:55–92. [Google Scholar]
- Maisto G., De Nicola F., Iovieno P., Prati M. V., Alfani A. PAHs and trace elements in volcanic, urban, and natural soils. Geoderma. 2006;136:20–27. [Google Scholar]
- Mangeladorf I., Buschmann J., Orthen B. Some aspects relating to the evaluation of the effects of chemicals on male fertility. Regul. Toxicol. Pharmacol. 2003;37:356–369. doi: 10.1016/s0273-2300(03)00026-6. [DOI] [PubMed] [Google Scholar]
- Manna P. R., Wang X. J., Stocco D. M. Involvement of multiple transcription factors in the regulation of steroidogenic acute regulatory protein gene expression. Steroids. 2003;68:1125–1134. doi: 10.1016/j.steroids.2003.07.009. [DOI] [PubMed] [Google Scholar]
- Meyer J. N., DiGiulio R. T. Heritable adaptation and fitness costs in killifish (Fundulus heteroclitus) inhabiting a polluted estuary. Ecol. Adapt. 2003;13:490–503. [Google Scholar]
- Mohamed E., Song W. H., Oh S. A., Park Y. J., You Y. A., Lee S., choi J. Y., Kim Y. J., Jo I., Pang M. G. The transgenerational impact of benzo(a)pyrene on murine male fertility. Hum. Reprod. 2010;25:2427–2433. doi: 10.1093/humrep/deq205. [DOI] [PubMed] [Google Scholar]
- Morthorst J. E., Lister A., Bjerregaard P., Van Der K. G. Ibuprofen reduces zebrafish PGE(2) levels but steroid hormone levels and reproductive parameters are not affected. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2013;157:251–257. doi: 10.1016/j.cbpc.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Munkittrick K. R., Portt C. B., Van Der Kraak G. J., Smith I. R., Rokosh D. A. Impact of bleached kraft mill effluent on population characteristics, liver MFO activity, and serum steroid levels of a Lake Superior white sucker (Catostomus commersoni) population. Can. J. Fish. Aquat. Sci. 1991;48:1371–1380. [Google Scholar]
- Neal M. S., Zhu J., Foster W. G. Quantification of benzo[a]pyrene and other PAHs in the serum and follicular fluid of smokers versus non-smokers. Reprod. Toxicol. 2008;25:100–106. doi: 10.1016/j.reprotox.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Nicolas J. M. Vitellogenesis in fish and the effects of polycyclic aromatic hydrocarbon contaminants. Aquat. Toxicol. 1999;45:77–90. [Google Scholar]
- Olivella M. A., Ribalta T. G., de Febrer A. R., Mollet J. M., de Las Heras F. X. Distribution of polycyclic aromatic hydrocarbons in riverine waters after Mediterranean forest fires. Sci. Total Environ. 2006;355:156–166. doi: 10.1016/j.scitotenv.2005.02.033. [DOI] [PubMed] [Google Scholar]
- Pait A. S., Nelson J. O. A survey of indicators for reproductive endocrine disruption in Fundulus heteroclitus (killifish) at selected sites in the Chesapeake Bay. Mar. Environ. Res. 2009;68:170–177. doi: 10.1016/j.marenvres.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Patel M. R., Scheffler B. E., Wang L., Willett K. L. Effects of benzo(a)pyrene exposure on killifish (Fundulus heteroclitus) aromatase activities and mRNA. Aquat. Toxicol. 2006;77:267–278. doi: 10.1016/j.aquatox.2005.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne A. H., Hales D. B. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 2004;25:947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- Peters R. E., Courtenay S. C., Cagampan S., Hewitt M. L., MacLatchy D. L. Effects on reproductive potential and endocrine status in the mummichog (Fundulus heteroclitus) after exposure to 17alpha-ethynylestradiol in a short-term reproductive bioassay. Aquat. Toxicol. 2007;85:154–166. doi: 10.1016/j.aquatox.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Prince R., Cooper K. Comparisons of the effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on chemically impacted and nonimpacted subpopulations of Fundulus heteroclitus. I. TCDD toxicity. Environ. Toxicol. Chem. 1995;14:579–587. [Google Scholar]
- Rempel M. A., Schlenk D. Effects of environmental estrogens and antiandrogens on endocrine function, gene regulation, and health in fish. Int. Rev. Cell. Mol. Biol. 2008;267:207–252. doi: 10.1016/S1937-6448(08)00605-9. [DOI] [PubMed] [Google Scholar]
- Scornaienchi M. L., Thornton C., Willett K. L., Wilson J. Y. Functional differences in the cytochrome P450 1 family enzymes from zebrafish (Danio rerio) using heterologously expressed proteins. Arch. Biochem. Biophys. 2010;502:17–22. doi: 10.1016/j.abb.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu A., Hamaguchi M., Ito H., Ohkubo M., Udagawa M., Fujii K., Kobayashi T., Nakamura M. Appearances and chronological changes of mummichog Fundulus heteroclitus FSH cells and LH cells during ontogeny, sexual differentiation, and gonadal development. Gen. Comp. Endocrinol. 2008;156:312–322. doi: 10.1016/j.ygcen.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Simpson E. R. Sources of estrogen and their importance. J. Steroid Biochem. Mol. Biol. 2003;86:225–230. doi: 10.1016/s0960-0760(03)00360-1. [DOI] [PubMed] [Google Scholar]
- Sobinoff A. P., Pye V., Nixon B., Roman S. D., McLaughlin E. A. Jumping the gun: Smoking constituent BaP causes premature primordial follicle activation and impairs oocyte fusibility through oxidative stress. Toxicol. Appl. Pharmacol. 2012;260:70–80. doi: 10.1016/j.taap.2012.01.028. [DOI] [PubMed] [Google Scholar]
- Spromberg J. A., Meador J. P. Relating results of chronic toxicity responses to population-level effects: Modeling effects on wild Chinook salmon populations. Integr. Environ. Assess. Manag. 2005;1:9–21. doi: 10.1897/ieam_2004a-005.1. [DOI] [PubMed] [Google Scholar]
- Thomas P. Teleost model for studying the effects of chemicals on female reproductive endocrine function. J. Exper. Zool. Suppl. 1990;4:126–128. doi: 10.1002/jez.1402560421. [DOI] [PubMed] [Google Scholar]
- van den Belt K., Wester P. W., van der Ven L., Verheyen R., Witters H. Effects of ethynylestradiol on the reproductive physiology in zebrafish (Danio rerio): Time dependency and reversibility. Environ. Toxicol. Chem. 2002;21:767–775. [PubMed] [Google Scholar]
- van der Ven L., Wester P. W., Vos J. G. Histopathology as a tool for the evaluation of endocrine disruption in zebrafish (Danio rerio) Environ. Toxicol. Chem. 2003;22:908–913. [PubMed] [Google Scholar]
- van Metre P. C., Mahler B. J., Furlong E. T. Urban sprawl leaves its PAH signature. Environ. Sci. Technol. 2000;34:4064–4070. [Google Scholar]
- Van C. J., Gielen J. E., Nebert D. W. Benzo[a]pyrene metabolism in mouse liver. Association of both 7,8-epoxidation and covalent binding of a metabolite of the 7,8-diol with the Ah locus. Biochem. Pharmacol. 1985;34:1821–1826. doi: 10.1016/0006-2952(85)90655-0. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Selman K. Major protein changes during vitellogenesis and maturation of Fundulus oocytes. Dev. Biol. 1985;110:492–498. doi: 10.1016/0012-1606(85)90106-x. [DOI] [PubMed] [Google Scholar]
- Wang C., Chan S. Y., Ng M., So W. W., Tsoi W. L., Lo T., Leung A. Diagnostic value of sperm function tests and routine semen analyses in fertile and infertile men. J. Androl. 1988;9:384–389. doi: 10.1002/j.1939-4640.1988.tb01070.x. [DOI] [PubMed] [Google Scholar]
- Wang L., Scheffler B. E., Willett K. L. CYP1C1 messenger RNA expression is inducible by benzo[a]pyrene in Fundulus heteroclitus embryos and adults. Toxicol. Sci. 2006;93:331–340. doi: 10.1093/toxsci/kfl072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber L. P., Hill R. L., Janz D. M. Developmental estrogenic exposure in zebrafish (Danio rerio). II Histological evaluation of gametogenesis and organ toxicity. Aquat. Toxicol. 2003;63:431–446. doi: 10.1016/s0166-445x(02)00208-4. [DOI] [PubMed] [Google Scholar]
- White P. A., Robitaille S., Rasmussen J. B. Heritable reproductive effects of benzo[a]pyrene on the fathead minnow (Pimephales promelas) Environ. Toxicol. Chem. 1999;18:1843–1847. [Google Scholar]
- Wu J., Hou H., Ritz B., Chen Y. Exposure to polycyclic aromatic hydrocarbons and missed abortion in early pregnancy in a Chinese population. Sci. Total Environ. 2010;408:2312–2318. doi: 10.1016/j.scitotenv.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohar Y., Munoz-Cueto J. A., Elizur A., Kah O. Neuroendocrinology of reproduction in teleost fish. Gen. Comp. Endocrinol. 2010;165:438–455. doi: 10.1016/j.ygcen.2009.04.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.