Abstract
Early phase drug development relies on primary human hepatocytes for studies of drug metabolism, cytotoxicity, and drug-drug interactions. However, primary human hepatocytes rapidly lose metabolic functions ex vivo and are refractory to expansion in culture and thus are limited in quantity. Hepatocytes derived from human pluripotent stem cells (either embryonic stem (ES) or induced pluripotent stem (iPS) cells), have the potential to overcome many of the limitations of primary human hepatocytes, but to date the use of human pluripotent stem cell-derived hepatocytes has been limited by poor enzyme inducibility and immature metabolic function. Here, we present a simple suspension culture of aggregates of ES cell-derived hepatocytes that compared to conventional monolayer adherent culture significantly increases induction of CYP 1A2 by omeprazole and 3A4 by rifampicin. Using liquid chromatography-tandem mass spectrometry, we further show that ES cell-derived hepatocytes in aggregate culture convert omeprazole and rifampicin to their human-specific metabolites. We also show that these cells convert acetaminophen (APAP) to its cytotoxic metabolite (N-acetyl-p-benzoquinone imine (NAPQI)), although they fail to perform APAP glucuronidation. In summary, we show that human pluripotent stem cell-derived hepatocytes in aggregate culture display improved enzymatic inducibility and metabolic function and is a promising step toward a simple, scalable system, but nonetheless will require further improvements to completely replace primary human hepatocytes in drug development.
Keywords: Drug testing, Toxicity testing, Stem cells, Hepatocytes, ES cells, iPS cells
Primary human hepatocytes are used in drug development to determine how a drug candidate is metabolized, how it may interact with other drugs, and whether the drug or its metabolites are toxic. Although primary human hepatocytes are the gold standard for such studies, they cannot be expanded in culture and still maintain metabolic function, and they are limited in number. Moreover, available primary human hepatocytes are often of poor quality because they are sourced from discarded grafts. Thus, there is a compelling need to develop a scalable, consistent, genetically defined source of metabolically competent human hepatocytes for drug discovery and development.
Human embryonic stem (ES) cell or human induced pluripotent stem (iPS) cell-derived hepatocytes have the potential to provide a scalable, genetically defined, consistent source of hepatocytes. Although there are already protocols published for the differentiation of human pluripotent stem cells down the hepatic lineage (Agarwal et al., 2008; Basma et al., 2009; Cai et al., 2007; Chen et al., 2012; Hay et al., 2008; Song et al., 2009), the xenobiotic inducibility and metabolic function of the resulting cells has generally been poor and inadequate for routine drug testing (Hengstler et al., 2005; Navarro-Alvarez et al., 2009; Snykers et al., 2009; Soto-Gutierrez et al., 2008). Here, we show that suspension culture of aggregates of ES cell-derived hepatocytes vastly improves xenobiotic induction of cytochrome P450 (CYP) genes. We further demonstrate that ES cell-derived hepatocytes in suspension culture perform xenobiotic metabolism and are able to convert acetaminophen to its hepatotoxic metabolite. In conclusion, we discuss the current value of aggregate culture of ES cell-derived hepatocytes and the improvements still required for broader applications in drug development.
MATERIALS AND METHODS
Hepatocyte differentiation
Modifying a previous protocol (Cai et al., 2007), H1 human embryonic stem cells (ES cells) (Thomson et al., 1998) cultured in E8 medium (Chen et al., 2011) were differentiated to hepatocytes. At ∼50% confluency, the ES cells were treated with 0.5 mg/ml albumin fraction V (Sigma) and 100 ng/ml activin A (Sigma) for 3 days. During the second and third days, 0.1 and 1.0% insulin-transferrin-selenium (Sigma) were added, respectively. From day 4 to day 8, the cells were treated with 30 ng/ml FGF4 (R&D Systems) along with 20 ng/ml BMP2. From day 9 to day 13, 20 ng/ml HGF (R&D Systems) were added to the medium, and finally cells were treated for 5 days with 10 ng/ml Oncostatin M (R&D Systems) and 0.1μM dexamethasone (Sigma) (Fig. 1A). Throughout differentiation, hepatocyte growth medium (Promocell) containing 10 ng/ml epidermal growth factor, 5 μg/ml insulin, 0.5 μg/ml hydrocortisone, 10 μg/ml holo-transferrin, 250 μg/ml ascorbic acid and 3.75 mg/ml bovine serum albumin-fatty acid free that has been reported for culturing pluripotent stem cell-derived hepatocytes (Krueger et al., 2013) was used and the cells were cultured on matrigel coated plates. On day 18, they were gently dissociated to single cells with TrypLE (Life Technologies) and cultured in ultralow attachment 12-well cell culture plates (Sigma) in 1 ml of basal hepatocyte growth medium in a nutator (TCS Scientific Corporation) at 24 rpm to allow the cells to form aggregates in suspension.
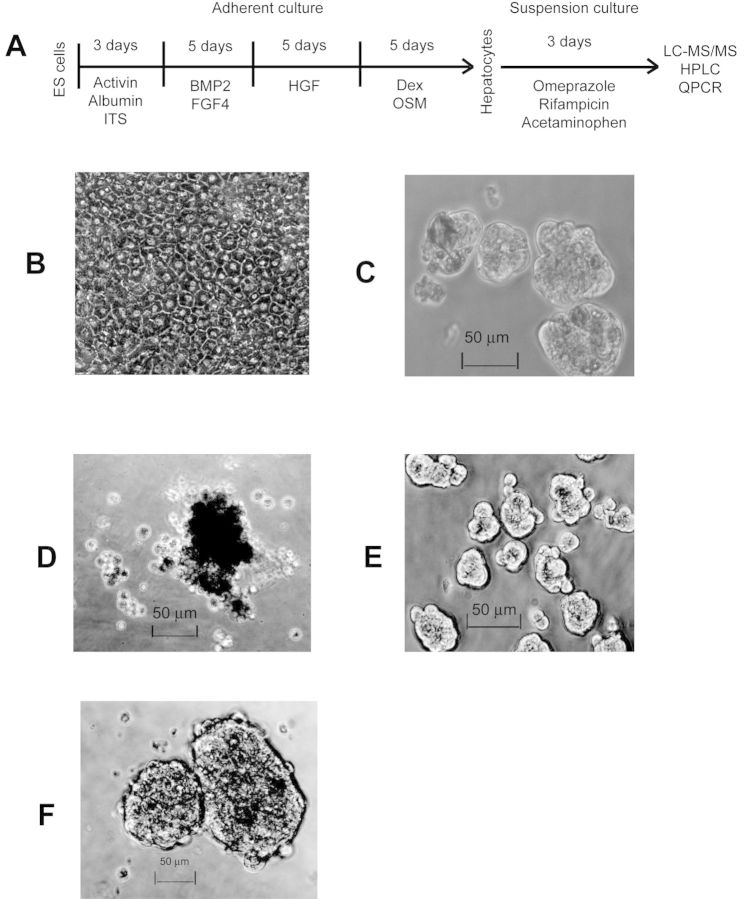
Fig. 1.
Differentiation and experimental scheme for functional characterization of human embryonic stem cell (ES cell)-derived hepatocytes: (A) protocol for hepatocyte differentiation from ES cells, drug induction, metabolism, and toxicity testing; (B) ES cell-derived hepatocytes in adherent culture. Suspension culture (for 72 h) of: (C) ES cell-derived hepatocytes, (D) primary hepatocytes, (E) HepG2 cells, and (F) 293 cells.
Human hepatocytes
Cryopreserved induction qualified primary human hepatocytes were purchased from Life Technologies. Primary human hepatocytes 1–5, used in induction and metabolism experiments, were from batches Hu1232, Hu8084, Hu8119, Hu1442, and Hu1192, respectively. Primary human hepatocytes 1 and 2 were cultured as adherent cells whereas hepatocytes 3–5 were cultured in suspension. Primary human hepatocytes from batch Hu1442 were used for the acetaminophen toxicity study.
Drug testing
ES cell-derived hepatocytes were treated with omeprazole (Sigma) or rifampicin (Sigma) for 72 h both in adherent culture and after they were put in suspension culture. Primary human hepatocytes were cultured both as adherent cells on matrigel coated plates or in ultralow attachment cell culture plates (Sigma) on a nutator in hepatocyte growth medium (Promocell). HepG2s were cultured both as adherent cells and in suspension in Dulbecco's Modified Eagle's Medium (DMEM)/F12 with 10% FBS in ultralow attachment cell culture plates (Sigma) on a nutator at 24 rpm. Two hundred and ninety-three cells were cultured as adherent cells or in suspension as above in DMEM containing 10% Fetal Bovine Serum (FBS), 0.1mM Minimum Essential Medium (MEM) nonessential amino acids (NEAA), and 2mM l-glutamine. After 72 h, culture media were harvested for metabolite quantification and cells were harvested for gene expression measurements.
Gene expression measurements
Total RNA from all cells were isolated with Trizol (Invitrogen) and treated with DNase I. Gene expression measurements were done by RNA-Seq and QPCR. RNA-Seq was performed on Illumina sequencer with TruSeq protocol and kit and results expressed in transcripts per million (TPM). RNA-Seq gene expression profiles of uncultured primary human hepatocytes, HepG2 cells, H1 ES cells, ES cell-derived hepatocytes in adherent, and suspension cultures are given in Supplementary table 1. Adherent ES cell-derived hepatocytes were harvested for RNA-Seq after completion of differentiation on day 18. Suspension culture was performed for another 72 h postdifferentiation before harvesting. QPCR was performed on ViiA7 QPCR system (Applied Biosystems) with SYBR green. Expression values measured by QPCR were normalized to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) expression and are given in relative quantity (RQ) with error bars representing RQ min and RQ max derived from standard error calculated with ViiA7 software on the Applied Biosystems QPCR system. Primers used were: GAPDH (forward: TCAACGACCACTTTGTCAAGCT, reverse: CCATGAGGTCCACCACCCT), CYP1A2 (forward: GCCCGGCCCACAATTAA, reverse: GCTAATGGGTGCAGGGTTTC), CYP3A4 (forward: CCTGGCCTACATGGTTGAAAC, reverse: CGAGTCCACCATGCCTAGCT).
Drug metabolism
Metabolites of omeprazole and rifampicin secreted into the media were quantified by liquid chromatography-tandem mass spectrometry (LC-MS/MS) by Covance laboratories (Madison, WI). Calibration standards, QCs, and study samples were analyzed for compound concentrations following extraction with methanol and subsequent centrifugation. Analysis was performed using an LC-MS/MS system comprised of a Shimadzu HPLC (Kyoto, Japan) and an API 4000 mass spectrometer (Applied Biosystems, Foster City, CA). Chromatographic retention and separation of the analytes and internal standards were achieved using a Phenomenex Luna C18 (2.0 × 50 mm, 5 μm) analytical column in conjunction with gradient conditions using mobile phases A (25mM ammonium acetate in water with 0.1% formic acid) and B (0.1% formic acid in 75:25 (vol/vol) acetonitrile:methanol). Mass spectrometric detection of the analytes was accomplished using ESI positive ionization mode. Analyte responses were measured by multiple reaction monitoring (MRM) of transitions unique to each analyte. The concentration of each compound in the study samples was determined by interpolation from the calibration curve. Samples with compound concentrations exceeding the upper limit of quantitation were diluted. Metabolites were also measured in (1) media where parent compounds were freshly added and from media that was incubated for 72 h after addition of parent compounds in the absence of cells and (2) in media used in cell culture (but where no compounds were added) to ascertain that experimental samples were above background.
Toxicity testing
Acetaminophen (Sigma) was added to medium at different concentrations and cell survival was measured by dye exclusion (Trypan blue, Sigma) on an automated cell counter (Countess, Invitrogen). All cells were cultured as described before. Cellular media were collected and subjected to high-performance liquid chromatography (HPLC) analysis for the quantification of acetaminophen (APAP) and its conjugates and metabolites. Five hundred microliters of media were added to 50 μl perchloric acid and vortexed for 1 min to precipitate proteins. Samples were then centrifuged at 13,000 × g for 10 min at 4°C and the supernatant was filtered through a 0.2-μm nylon filter.
For quantification, the samples were injected onto an Agilent 1100 HPLC consisting of a binary pump, autosampler (4°C), column thermostat 35°C, diode array detector, and fraction collector (Agilent, Palo Alto, CA). Samples (50 μl) were separated by a Varian Microsorb column (5 μm × 250 mm, C18), and peaks detected at 254 nm. A gradient elution was used consisting of mobile phase A: 4% methanol and 0.1% formic acid, and mobile phase B: acetonitrile 0.1% formic acid. 0–4 min 100% A; 4–5 min 0–4.7% B; 5–13 min 4.7% B; 13–15 min 4.7–15% B; 15–20 min 15% B; 20–22 min 15–0% B to separate metabolites which were identified by coelution with known standards. Metabolite standards APAP-glucuronide, APAP-NAC, APAP-sulfate, and APAP-cysteine were a generous gift of McNeil laboratories; APAP-GSH was a generous gift of Sydney Nelson (University of Washington). A standard sample made up of APAP, APAP-glucuronide, APAP-NAC, APAP-cysteine, and APAP-sulfate was injected several times each day to ensure accuracy of peak identification and stability of samples in the autosampler. Peak integration (area under the curve) was calculated in Chemstation (Agilent) and relative concentrations were determined from a reference curve of the parent compound, APAP.
RESULTS
Aggregate Suspension Culture of ES Cell-Derived Hepatocytes Improves CYP Induction by Xenobiotics
We differentiated H1 human ES cells to hepatocytes (Fig. 1A) that exhibited morphology similar to primary human hepatocytes in conventional adherent culture (Fig. 1B). To test whether aggregate suspension culture (Fig. 1C) improves metabolic functions, ES cell-derived hepatocytes in adherent and suspension cultures were treated with omeprazole (100μM) or rifampicin (10μM), two common inducers of CYP genes (Guidance for Industry, Drug Interaction Studies—Study Design, Data Analysis, Implications for Dosing, and Labeling Recommendations, CDER, U.S. F.D.A., 2012). After 72 h of drug treatment, we collected the cells for RNA isolation and saved the media for LC-MS/MS analysis. CYP mRNA levels were measured by quantitative PCR and normalized to GAPDH. Fold-induction was calculated by comparing with control untreated cells in respective culture conditions. CYP3A4 mRNA induction by rifampicin was almost absent in human ES cell-derived hepatocytes cultured as adherent monolayers (Fig. 2A). However, CYP3A4 mRNA induction by rifampicin in suspension was close to 10-fold, matching the highest induction seen among our primary human hepatocytes.
Fig. 2.
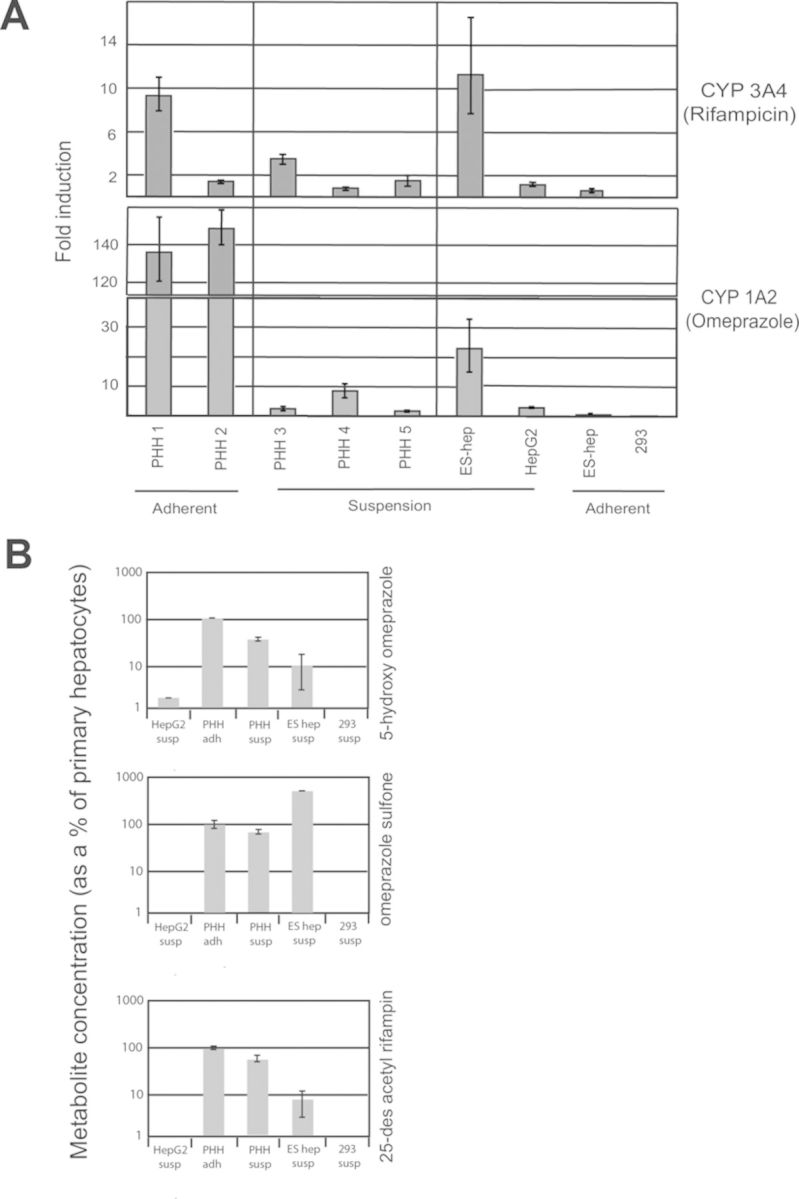
ES cell-derived hepatocytes in aggregate suspension culture show improved induction and metabolize xenobiotics: (A) CYP1A2 and 3A4 induction by two prototypical inducers, omeprazole, and rifampicin, is significantly increased in suspension culture of ES cell-derived hepatocytes compared with adherent culture, where it is almost absent. HepG2 cells, even in suspension fail to show much induction, whereas primary human hepatocytes (PHHs) display variability between culture conditions. Gene expressions were measured by QPCR, normalized to GAPDH and expressed in RQ (relative quantity), with error bars representing RQ min and RQ max derived from standard error. Fold-induction was calculated by comparing experimental samples against control untreated samples. (B) Metabolism of the two above drugs as quantified by the amount of metabolites secreted into the media/cell by LC-MS/MS. Adherent cultured primary human hepatocytes have been set to 100%. ES cell-derived hepatocytes in aggregate suspension metabolize the two drugs to their human-specific metabolites whereas HepG2 cells generates only 5-hydroxy omeprazole above background levels. Metabolism is totally absent in negative control 293 cells.
Similarly, CYP1A2 mRNA induction by omeprazole was also almost absent in adherent cultures, but increased to 23-fold in suspension culture, although it remained much lower than the ∼150-fold induction observed in primary hepatocytes. Moreover, there was very little or no upregulation in basal mRNA levels of these two enzymes between adherent and suspension cultured cells which remained very low compared with primary hepatocytes (Supplementary table 1). HepG2 cells, a hepatoma cell line sometimes used for drug and toxicity testing, demonstrated minimal induction, even in suspension culture where they readily formed aggregates (Fig. 1E). Primary human hepatocytes in suspension clumped together but failed to form tight aggregates (Fig. 1D) (and subsequently died) and displayed greater induction when cultured as adherent cells. Control 293 cells (Fig. 1F), an embryonic kidney cell line, failed to show any induction (Fig. 2A).
ES Cell-Derived Hepatocytes in Aggregate Suspension Culture Metabolize Omeprazole and Rifampicin
We measured secreted metabolites in media saved from the previous experiment. In humans, omeprazole is metabolized primarily to omeprazole sulfone and 5-hydroxy omeprazole, whereas rifampicin is metabolized primarily to 25-desacetyl rifampicin. LC-MS/MS was performed first to ascertain that ES cell-derived hepatocytes were able to metabolize the two drugs to their human specific metabolites, and then to quantify the amount of the metabolites produced. We measured metabolism of omeprazole and rifampicin from two separate differentiation batches of ES-cell derived hepatocytes in suspension culture, five batches of primary human hepatocytes derived from different donors (batches 1 and 2 in adherent culture, batches 3–5 in suspension), and HepG2 and control 293 cells (both in suspension). Metabolism was quantified as the amount of metabolites secreted into the media per cell and displayed as percentage of positive control primary human hepatocytes in adherent culture (Fig. 2B). We found little variation in metabolic activity among different lots of primary cells in each culture condition, but interestingly, as in induction studies, adherent cultured primary cells performed better than suspension cells, showing higher metabolism for both compounds tested and hence were used as the positive standard. Although there was intrabatch variation in drug metabolism levels by the ES cell-derived hepatocytes, both batches were competent in metabolizing both drugs. On average, ES cell-derived hepatocytes generated 10% of 5-hydroxy omeprazole produced by adherent primary human hepatocytes, and production of omeprazole sulfone was about 5-fold higher than adherent primary hepatocytes (Fig. 2B). ES cell-derived hepatocytes produced ∼10% of 25-desacetyl rifampicin produced by adherent primary human hepatocytes. HepG2 cells generated ∼2.5% of 5-hydroxy omeprazole produced by adherent primary human hepatocytes and failed to generate omeprazole sulfone or 25-desacetyl rifampicin above background levels. Negative control 293 cells showed no metabolic activity toward omeprazole or rifampicin. (Fig. 2B).
ES Cell-Derived Hepatocytes Show Toxicity to Acetaminophen in Aggregate Suspension Culture
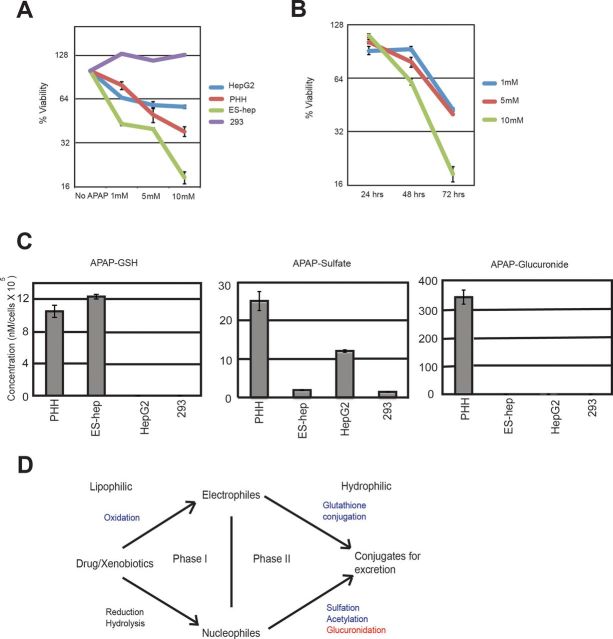
Acetaminophen is an extensively studied hepatotoxicant that is only toxic after hepatocytes metabolize it to its reactive metabolite, N-acetyl-p-benzoquinone imine (NAPQI). Thus, APAP would only be toxic to ES cell-derived hepatocytes if they were sufficiently metabolically active. We added acetaminophen to cell culture media at concentrations of 1, 5, and 10mM, and measured cell death/survival by dye exclusion at 24, 48, and 72 h, and saved the media for later analysis. Cell survival measured at 72 h correlated with APAP concentration for ES cell-derived hepatocytes, primary human hepatocytes and HepG2 cells, whereas 293 cells failed to show any toxic response to APAP (Fig. 3A). ES cell-derived hepatocytes exhibited the greatest sensitivity to APAP followed by primary human hepatocytes, with HepG2 cells demonstrating only minimal cell death. For ES cell-derived hepatocytes, there was also a correlation between cell survival and exposure time to acetaminophen (Fig. 3B). In this experiment, ES cell-derived hepatocytes were in suspension culture and other cell types were in adherent culture.
Fig. 3.
ES cell-derived hepatocytes display hepatotoxic response to acetaminophen through reactive metabolite formation at levels similar to primary human hepatocytes. Cell viability (cell survival as percent of untreated control cells) was measured after addition of acetaminophen to medium by dye exclusion. (A) At 72 h, ES cell-derived hepatocytes, primary human hepatocytes (PHHs), and HepG2 cells show toxicity response to acetaminophen (APAP) in a concentration dependent manner, whereas control 293 do not. (B) ES cell-derived hepatocytes survival is correlated to APAP exposure time. (C) Formation of acetaminophen metabolites and conjugates by ES cell-derived hepatocytes along with control cells. (D) Cartoon illustrating metabolic pathways active (green) and inactive (red) in ES cell-derived hepatocytes.
We next performed HPLC to ascertain the presence of APAP cytotoxic metabolites in the cell culture media. APAP is primarily cleared in vivo by formation of APAP-sulfate and APAP-glucuronide (Chen et al., 2008). High doses saturate these pathways and lead to increase formation of the minor oxidative hepatotoxic metabolite, NAPQI. NAPQI has a very short half-life (Mannery et al., 2010) and is reduced back to APAP or conjugated to glutathione, forming the APAP-glutathione (APAP-GSH) conjugate. Figure 3C shows that ES cell-derived hepatocytes produced APAP-GSH in the medium, suggesting that the cell death we observed occurs through NAPQI. APAP-GSH formation was also observed in primary human hepatocytes, but not in HepG2s, or 293 cells. APAP-sulfate, one of the two major metabolites of APAP, was detected in ES cell-derived hepatocytes, PHH, HepG2s, and 293 cells (Fig. 3C). However, significant levels of APAP-glucuronide were produced by primary human hepatocytes, but not by human ES cell-derived hepatocytes (Fig. 3C). Global gene expression by sequencing (Table 1, Supplementary table 1) revealed the UDP glucuronosyltransferases (UGTs) family of genes to be poorly expressed in ES cell-derived hepatocytes. Although suspension cultured cells expressed increased mRNA levels of three of the four major acetaminophen glucuronidating enzymes (Mutlib et al., 2006) (UGTs 1A1, 1A6 and 2B15, UGT1A9 absent in both cases), they failed to approach levels expressed in primary human hepatocytes (Table 1).
TabLE 1. Genes Differentially Expressed Between Adherent and Suspension Cultured ES-Hepatocytes.
| Gene | H1 ES cells | HepG2 | Primary hepatocytesa | Adherent ES hepatocytes | Suspension ES hepatocytes | log2 fold changeb |
|---|---|---|---|---|---|---|
| SERPINA1 | 0.00 | 3439.17 | 6166.76 | 0.00 | 439.31 | 8.78 |
| KRT8 | 389.03 | 311.59 | 294.54 | 0.00 | 5048.34 | 12.30 |
| G6PC | 0.40 | 66.31 | 130.73 | 0.02 | 55.05 | 5.78 |
| HSD17B13 | 1.55 | 1.18 | 219.10 | 0.81 | 36.38 | 4.37 |
| CYP1A1 | 0.00 | 6.22 | 4.25 | 2.75 | 37.74 | 3.37 |
| FN1 | 8.22 | 350.13 | 332.62 | 1755.14 | 21879.27 | 3.64 |
| SGK1 | 5.87 | 30.73 | 130.25 | 0.95 | 112.21 | 5.86 |
| SGK2 | 1.29 | 7.53 | 13.56 | 2.56 | 21.87 | 2.68 |
| C3 | 5.21 | 421.10 | 1898.51 | 92.27 | 677.13 | 2.86 |
| PRODH2 | 0.00 | 83.72 | 76.54 | 1.90 | 11.05 | 2.05 |
| SLC30A1 | 1.31 | 11.10 | 21.74 | 0.24 | 42.54 | 5.13 |
| SLC25A3 | 513.95 | 417.24 | 126.74 | 0.00 | 26.14 | 4.76 |
| SLC25A4 | 18.25 | 11.65 | 12.09 | 0.23 | 27.28 | 4.52 |
| SLC16A1 | 35.12 | 82.12 | 31.13 | 1.13 | 10.87 | 2.48 |
| SLC25A18 | 0.63 | 0.23 | 48.63 | 1.07 | 6.11 | 1.78 |
| SLC22A3 | 0.00 | 8.48 | 12.54 | 0.72 | 62.09 | 5.20 |
| SLC6A13 | 0.00 | 0.37 | 12.77 | 0.77 | 4.53 | 1.64 |
| SLC25A18 | 0.63 | 0.23 | 48.63 | 1.07 | 6.11 | 1.78 |
| UGT1A1 | 0.00 | 0.56 | 114.66 | 0.00 | 3.25 | 2.09 |
| UGT1A6 | 0.00 | 0.08 | 264.35 | 1.57 | 5.18 | 1.27 |
| UGT1A9 | 0.00 | 0.00 | 56.83 | 0.00 | 0.00 | 0.00 |
| UGT2B15 | 0.77 | 250.68 | 309.52 | 6.30 | 10.64 | 0.67 |
| ALB | 0.00 | 46062.04 | 86272.40 | 247.52 | 63.72 | −1.94 |
| ABCB1 | 0.11 | 19.20 | 10.57 | 9.62 | 0.03 | −3.37 |
| AFP | 0.68 | 9412.51 | 21.27 | 11413.50 | 3786.49 | −1.59 |
Note. Gene expression values are in transcripts per million (TPM).
a Average of primary human hepatocyte batches 1–5 are reported.
b A pseudocount of 1 is added to the numerator and denominator before calculating log2((tpm x)/(tpm y)).
Suspension Culture of ES Cell-Derived Hepatocytes Upregulates Drug Transporters
We looked at genes that were upregulated significantly (>5-fold) in aggregate suspension culture compared to adherent culture of ES cell-derived hepatocytes to understand the mechanism behind increased induction of CYP450 genes. The genes whose transcriptional levels increased (Supplementary table 1, partial list is given in Table 1) ranged from structural genes like Krt8 (keratin 8), a cytoskeletal protein, to genes involved in liver function such as CYP1A1, G6PC (glucose-6-phosphatase, catalytic subunit), HSD17B13 (hydroxysteroid (17-β) dehydrogenase 13), FN1 (fibronectin 1), SGK1, 2 (serum/glucocorticoid regulated kinases 1, 2), C3 (complement component 3), and PRODH2 (proline dehydrogenase (oxidase) 2). But most importantly, a large number of genes belonging to the solute carrier family (SLC) involved in active drug transport were upregulated (SLC30A1, SLC25A3, SLC25A4, SLC16A1, SLC25A18, SLC22A3, SLC6A13, SLC25A18), majority of them to levels equivalent to primary human hepatocytes or higher. Interestingly, ABCB1, a gene that encodes permeability glycoprotein 1 (P-gp), an efflux transporter, whose lowered expression could increase intracellular xenobiotic levels, showed downregulation in suspension. SLCO1B1 and 1B3, responsible for transport of rifampicin (Development & Approval Process (Drugs), U.S. F.D.A., 2011), were not significantly upregulated in suspension. Albumin level was significantly lower in ES cell-derived hepatocytes (<250-fold of that of primary hepatocytes) and was not elevated in suspension, actually showing a slight decrease, but interestingly, alpha-feto protein, a fetal gene, was considerably downregulated.
DISCUSSION
Hypothesizing that three dimensional cell-cell signaling present in vivo might be critical in promoting the maturity and functionality lacking in monolayer cultures, we performed a suspension culture of ES cell-derived hepatocytes that allowed three dimensional cell-cell interactions in aggregates. In contrast to previous reports showing that ES cell-derived hepatocytes respond poorly to xenobiotics (Ma et al., 2013; Takayama et al., 2012a,b), we demonstrate that suspension culture of ES cell-derived hepatocytes greatly upregulates CYP 1A2 and 3A4 induction by two prototypical inducers, omeprazole, and rifampicin, respectively.
Induction of CYP 3A4 by rifampicin has previously been shown to be dependent on cell density and cell-cell contact in primary liver cells (Hamilton et al., 2001) and cell-cell contact has previously been credited with more broadly enhancing the metabolic function of hepatocytes (reviewed in detail in LeCluyse et al., 2012). We were somewhat surprised that primary human hepatocytes failed to form aggregates in suspension, but this could be due to cell stress during isolation from ischemia times, cryopreservation, and thawing as well as the harsh proteolytic enzymatic treatments used in their isolation which damages cell membrane, cell surface receptors, cell junctions, and disrupts intercellular contacts (Cervenková et al., 2001; Soldatow et al., 2013) thereby inhibiting their aggregation. At any rate, primary hepatocytes in monolayers cultures, with limited cell-cell contact, exhibited better induction than primary liver cells in single cell suspensions, with essentially no cell-cell contact. Primary cells also performed better metabolism in adherent culture, possibly due to higher basal levels of enzymes retained in cultures with higher cell-cell contact (Hamilton et al., 2001). We also found that aggregate culture of human ES cell-derived hepatocytes supported the metabolism of omeprazole, rifampicin, and acetaminophen. Aggregate culture though failed to increase basal level of these enzymes, which remained significantly lower in ES cell-derived hepatocytes compared with primary hepatocytes, at least at the mRNA level. Although competent in carrying out many reactions, we found that human ES cell-derived hepatocytes cultured in suspension failed to support the measurable glucuronidation of acetaminophen (Fig. 3D), and we found mRNAS for UDP UGTs, the enzymes needed for this reaction, were present at very low levels in suspension cultured ES cell-derived hepatocytes compared with primary hepatocytes (Table 1, Supplementary table 1). Absence of glucuronidation and low rate of sulfation in ES cell-derived hepatocytes could have led to generation of higher amounts of NAPQI resulting in greater cell death. UGT expression is associated with later hepatocyte maturity (Strassburg et al., 2002), indicating that the current aggregate culture does not yet support complete maturation. Some limited glucuronidation by ES/iPS cell-derived hepatocytes has been reported (Duan et al., 2010; Ma et al., 2013), but at an order of magnitude less and at a slower rate than primary human hepatocytes. The cells in these reports were cultured for a longer time period and thus it is possible that more prolonged culture of our cells in suspension could induce glucuronidation. In a previous report, two hepatocyte-specific transcription factors, FOXA2 and HNF1A, were used to drive ES/iPS cell hepatocyte differentiation, and the resulting cells were cultured on nanopillar plates to promote close 3D cell-cell contact (Takayama et al., 2013). This micro-3D system improved transcription of several hepatic genes, improved drug metabolism, and increased secretion of urea. Importantly, the authors also showed the 3D constructs to be stable and to survive for a long time, giving them an advantage over primary hepatocytes cultured as monolayers. The aggregate suspension culture approach reported here is similar in that it promotes 3D interactions between hepatocytes and improves metabolic function, but its simplicity and scalability should make it more applicable for assays where a larger amount of material is needed.
We also performed transcriptional profiling of adherent and suspension cultured ES cell-derived hepatocytes to understand molecular mechanisms involved in increase of CYP gene induction. We found that a number of drug transporters of the SLC to be upregulated in aggregate suspension culture. Plasma membrane transporters are essential to drug uptake and efflux (Marin, 2012), with the SLC of genes involved primarily in influx of drugs (Frans and Russel, 2010). It is likely that enhanced expression of SLCs seen in suspension helps make xenobiotics available to the hepatocytes, thus resulting in the observed increase in induction. Takayama et al. (2013) also reported upregulation of SLC and ABC (ATP-binding cassette) transporters in 3D microculture. We did not find significant changes in ABC transporters.
Here, we have presented a suspension culture system of ES cell-derived hepatocytes as aggregates that show more mature metabolic functions. Despite this improvement, these cells still fall short of primary cells in their basal expression levels of phase I and phase II enzymes. For example, low levels of UGTs resulted in lack of glucuronidation by ES cell-derived hepatocytes in our study. ES cell-derived hepatocytes thus need further improvement in their level of differentiation to increase expression levels of genes and enzymes involved in various pathways related to drug discovery and also requires interrogation with a broader range of compounds, along with detailed kinetic studies before use as a tool in drug development. In conclusion, aggregate culture system of ES cell-derived hepatocytes leads to better metabolic functions over conventional monolayer culture and is a step toward providing a simple scalable system for use in the future for drug development.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/lookup/suppl/doi:10.1093/toxsci/kfu069/-/DC1.
FUNDING
National Institutes of Health (1UO1ES017166-01 to B.R., T32ES007015 to B.J.); Morgridge Institute for Research and the University of Wisconsin Foundation.
Acknowledgments
We thank Krista Eastman for editorial assistance. Conflict of interest: J.A.T. is a founder, stock owner, consultant, and board member of Cellular Dynamics International (CDI). He also serves as a scientific advisor and has financial interests in Tactics II Stem Cell Ventures.
REFERENCES
- Agarwal S., Holton K. L., Lanza R. Efficient differentiation of functional hepatocytes from human embryonic stem cells. Stem Cells. 2008;26:1117–1127. doi: 10.1634/stemcells.2007-1102. [DOI] [PubMed] [Google Scholar]
- Basma H., Soto-Gutiérrez A., Yannam G. R., Liu L., Ito R., Yamamoto T., Ellis E., Carson S. D., Sato S., Chen Y., et al. Differentiation and transplantation of human embryonic stem cell-derived hepatocytes. Gastroenterology. 2009;136:990–999. doi: 10.1053/j.gastro.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J., Zhao Y., Liu Y., Ye F., Song Z., Qin H., Meng S., Chen Y., Zhou R., Song X., et al. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology. 2007;45:1229–1239. doi: 10.1002/hep.21582. [DOI] [PubMed] [Google Scholar]
- Cervenková K., Belejova M., Veselý J., Chmela Z., Rypka M., Ulrichová J., Modrianský M., Maurel P. Cell suspensions, cell cultures, and tissue slices-important metabolic in vitro systems. Biomed. Pap. 2001;145:57–60. doi: 10.5507/bp.2001.012. [DOI] [PubMed] [Google Scholar]
- Chen G., Gulbranson D. R., Hou Z., Bolin J. M., Ruotti V., Probasco M. D., Smuga-Otto K., Howden S. E., Diol N. R., Propson N. E., et al. Nat. Methods. 2011;8:424–429. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Krausz K. W., Idle J. R., Gonzalez F. J. Identification of novel toxicity-associated metabolites by metabolomics and mass isotopomer analysis of acetaminophen metabolism in wild-type and Cyp2e1-null mice. J. Biol. Chem. 2008;283:4543–4559. doi: 10.1074/jbc.M706299200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. F., Tseng C. Y., Wang H. W., Kuo H. C., Yang V. W., Lee O. K. Rapid generation of mature hepatocyte-like cells from human induced pluripotent stem cells by an efficient three-step protocol. Hepatology. 2012;55:1193–1203. doi: 10.1002/hep.24790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Development & Approval Process (Drugs) Drug Development and Drug Interactions: Table of Substrates, Inhibitors and Inducers. U.S. F.D.A. 2011.
- Duan Y., Ma X., Zou W., Wang C., Bahbahan I. S., Ahuja T. P., Tolstikov V., Zern M. A. Differentiation and characterization of metabolically functioning hepatocytes from human embryonic stem cells. Stem Cells. 2010;28:674–86. doi: 10.1002/stem.315. [DOI] [PubMed] [Google Scholar]
- Russel F.G.M. Enzyme- and Transporter-Based Drug-Drug Interactions Progress and Future Challenges. New York: Springer; 2010. Transporters: Importance in Drug Absorption, Distribution, and Removal; pp. 27–49. [Google Scholar]
- Guidance for Industry, Drug Interaction Studies—Study Design, Data Analysis, Implications for Dosing, and Labeling Recommendations. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER) 2012.
- Hamilton G. A., Jolley S. L., Gilbert D., Coon D. J., Barros S., LeCluyse E. L. Regulation of cell morphology and cytochrome P450 expression in human hepatocytes by extracellular matrix and cell-cell interactions. Cell Tissue Res. 2001;306:85–99. doi: 10.1007/s004410100429. [DOI] [PubMed] [Google Scholar]
- Hay D. C., Zhao D., Fletcher J., Hewitt Z. A., McLean D., Urruticoechea-Uriguen A., Black J. R., Elcombe C., Ross J. A., Wolf R., et al. Efficient differentiation of hepatocytes from human embryonic stem cells exhibiting markers recapitulating liver development in vivo. Stem Cells. 2008;26:894–902. doi: 10.1634/stemcells.2007-0718. [DOI] [PubMed] [Google Scholar]
- Hengstler J. G., Brulport M., Schormann W., Bauer A., Hermes M., Nussler A. K., Fandrich F., Ruhnke M., Ungefroren H., Griffin L., et al. Generation of human hepatocytes by stem cell technology: Definition of the hepatocyte. Expert Opin. Drug Metab. Toxicol. 2005;1:61–74. doi: 10.1517/17425255.1.1.61. [DOI] [PubMed] [Google Scholar]
- Krueger W. H., Tanasijevic B., Barber V., Flamier A., Gu X., Manautou J., Rasmussen T. P. Cholesterol-secreting and statin-responsive hepatocytes from human ES and iPS cells to model hepatic involvement in cardiovascular health. PLoS One. 2013;8:e67296. doi: 10.1371/journal.pone.0067296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeCluyse E. L., Witek R. P., Andersen M. E., Powers M. J. Organotypic liver culture models: Meeting current challenges in toxicity testing. Crit. Rev. Toxicol. 2012;42:501–548. doi: 10.3109/10408444.2012.682115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Duan Y., Tschudy-Seney B., Roll G., Behbahan I. S., Ahuja T. P., Tolstikov V., Wang C., McGee J., Khoobyari S., et al. Highly efficient differentiation of functional hepatocytes from human induced pluripotent stem cells. Stem Cells Transl. Med. 2013;2:409–419. doi: 10.5966/sctm.2012-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannery Y. O., Ziegler T. R., Park Y., Jones D. P. Acetaminophen elimination half-life in humans is unaffected by short-term consumption of sulfur amino acid-free diet. J. Pharmacol. Exp. Ther. 2010;333:948–953. doi: 10.1124/jpet.110.166439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin J. J. Plasma membrane transporters in modern liver pharmacology. Scientifica. 2012;2012:428139. doi: 10.6064/2012/428139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlib A. E., Goosen T. C., Bauman J. N., Williams J. A., Kulkarni S., Kostrubsky S. Kinetics of acetaminophen glucuronidation by UDP-glucuronosyltransferases 1A1, 1A6, 1A9 and 2B15. Potential implications in acetaminophen-induced hepatotoxicity. Chem. Res. Toxicol. 2006;19:701–709. doi: 10.1021/tx050317i. [DOI] [PubMed] [Google Scholar]
- Navarro-Alvarez N., Soto-Gutierrez A., Kobayashi N. Stem cell research and therapy for liver disease. Curr. Stem Cell Res. Ther. 2009;4:141–146. doi: 10.2174/157488809788167418. [DOI] [PubMed] [Google Scholar]
- Snykers S., De Kock J., Rogiers V., Vanhaecke T. In vitro differentiation of embryonic and adult stem cells into hepatocytes: State of the art. Stem Cells. 2009;27:577–605. doi: 10.1634/stemcells.2008-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldatow V. Y., Lecluyse E. L., Griffith L. G., Rusyn I. In vitro models for liver toxicity testing. Toxicol. Res. (Camb.) 2013;2:23–39. doi: 10.1039/C2TX20051A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z., Cai J., Liu Y., Zhao D., Yong J., Duo S., Song X., Guo Y., Zhao Y., Qin H., et al. Efficient generation of hepatocyte-like cells from human induced pluripotent stem cells. Cell Res. 2009;19:1233–1242. doi: 10.1038/cr.2009.107. [DOI] [PubMed] [Google Scholar]
- Soto-Gutierrez A., Basma H., Navarro-Alvarez N., Uygun B. E., Yarmush M. L., Kobayashi N., Fox I. J. Differentiating stem cells into liver. Biotechnol. Genet. Eng. Rev. 2008;25:149–163. doi: 10.5661/bger-25-149. [DOI] [PubMed] [Google Scholar]
- Strassburg C. P., Strassburg A., Kneip S., Barut A., Tukey R. H., Rodeck B., Manns M. P. Developmental aspects of human hepatic drug glucuronidation in young children and adults. Gut. 2002;50:259–265. doi: 10.1136/gut.50.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K., Inamura M., Kawabata K., Katayama K., Higuchi M., Tashiro K., Nonaka A., Sakurai F., Hayakawa T., Furue M. K., et al. Efficient generation of functional hepatocytes from human embryonic stem cells and induced pluripotent stem cells by HNF4α transduction. Mol. Ther. 2012b;20:127–137. doi: 10.1038/mt.2011.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K., Inamura M., Kawabata K., Sugawara M., Kikuchi K., Higuchi M., Nagamoto Y., Watanabe H., Tashiro K., Sakurai F., et al. Generation of metabolically functioning hepatocytes from human pluripotent stem cells by FOXA2 and HNF1α transduction. J. Hepatol. 2012a;57:628–636. doi: 10.1016/j.jhep.2012.04.038. [DOI] [PubMed] [Google Scholar]
- Takayama K., Kawabata K., Nagamoto Y., Kishimoto K., Tashiro K., Sakurai F., Tachibana M., Kanda K, Hayakawa T., Furue M. K., et al. 3D spheroid culture of hESC/hiPSC-derived hepatocyte-like cells for drug toxicity testing. Biomaterials. 2013;34:1781–1789. doi: 10.1016/j.biomaterials.2012.11.029. [DOI] [PubMed] [Google Scholar]
- Thomson J. A., Itskovitz-Eldor J., Shapiro S. S., Waknitz M. A, Swiergiel J. J, Marshall V. S., Jones J. M. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]