Abstract
Concerns have been raised regarding the long-term impacts of early life exposure to the ubiquitous environmental contaminant bisphenol A (BPA) on brain organization. Because BPA has been reported to affect estrogen signaling, and steroid hormones play a critical role in brain sexual differentiation, there is also concern that BPA exposure could alter neural sex differences. Here, we examine the impact of subchronic exposure from gestation to adulthood to oral doses of BPA below the current no-observed-adverse-effect level (NOAEL) of 5 mg/kg body weight (bw)/day on estrogen receptor (ESR) expression in sexually dimorphic brain regions of prepubertal and adult female rats. The dams were gavaged daily with vehicle (0.3% carboxymethylcellulose), 2.5, 25, 260, or 2700 μg BPA/kg bw/day, or 0.5 or 5.0 μg ethinyl estradiol (EE)/kg bw/day from gestational day 6 until labor began. Offspring were then gavaged directly from the day after birth until the day before scheduled sacrifice on postnatal days 21 or 90. Using in situ hybridization, one or more BPA doses produced significant decreases in Esr1 expression in the juvenile female rat anteroventral periventricular nucleus (AVPV) of the hypothalamus and significant decreases in Esr2 expression in the adult female rat AVPV and medial preoptic area (MPOA), relative to vehicle controls. BPA did not simply reproduce EE effects, indicating that BPA is not acting solely as an estrogen mimic. The possible consequences of long-term changes in hypothalamic ESR expression resulting from subchronic low dose BPA exposure on neuroendocrine effects are discussed and being addressed in ongoing, related work.
Keywords: brain, endocrine disruptor, endocrine disruption, hypothalamus, development, subchronic exposure, sexually dimorphic, ethinyl estradiol, bisphenol A
Abbreviations
- 3V
third ventricle
- AC
anterior commissure
- ANOVA
analysis of variance
- ARC
arcuate hypothalamic nucleus
- rARC
rostral arcuate nucleus
- cARC
caudal arcuate nucleus
- AVPV
anteroventral periventricular nucleus
- bw
body weight
- CA1
CA1 region of the hippocampus
- CC
corpus callosum
- CM
central medial thalamic nucleus
- CPu
Caudate Putamen
- BPA
bisphenol A
- EDC
endocrine disrupting compound
- EE
ethinyl estradiol
- ERα
estrogen receptor alpha
- ERβ
estrogen receptor beta
- ESR
estrogen receptor
- Esr1
estrogen receptor alpha gene
- Esr2
estrogen receptor beta gene
- GD
gestational day
- ic
internal capsule
- ISHH
in situ hybridization histochemistry
- LOAEL
lowest-observed-adverse-effect level
- LV
lateral ventricle
- MBH
mediobasal hypothalamus
- MPOA
medial preoptic area
- NCTR
National Center for Toxicological Research
- NOAEL
no-observed-adverse-effect level
- PND
postnatal day
- POA
preoptic area
- ROI
region of interest
- SD
Sprague Dawley
- VMN
ventromedial hypothalamic nucleus
- VMNvl
ventrolateral division of the ventromedial hypothalamic nucleus
- cVMNvl
caudal ventrolateral division of the ventromedial hypothalamic nucleus
- rVMNvl
rostral ventrolateral division of the ventromedial hypothalamic nucleus
- WHO
World Health Organization
Exposure to bisphenol A (BPA), a common component of epoxy resins and polycarbonate plastics, is nearly ubiquitous with >90% of the United States population having traces of BPA in their urine (Calafat et al., 2008; Vandenberg et al., 2010). Exposure occurs primarily by consuming food and beverage products (estimated by the World Health Organization (WHO) to be in the range of 0.01–0.05 μg/kg bw/day for adults and 0.02–0.12 μg/kg bw/day for children) into which BPA has leached from the plastic or the resin-based coatings lining the interior of the container (Calafat, 2011; FAO/WHO, 2011). Dermal contact with thermal receipts and inhalation of airborne particles are additional suspected sources of exposure (Biedermann et al., 2010; Cooper et al., 2011; FAO/WHO, 2011; Geens et al., 2011, 2012). In their 2008 evaluation of developmental and reproductive effects of BPA exposure, the National Toxicology Program (NTP) concluded that there was “some concern for effects on the brain and behavior” (Shelby, 2008). In a 2010 statement, the FDA indicated that they shared these concerns, although the current FDA assessment is that “BPA is safe at the very low levels that occur in some foods” (http://www.fda.gov/newsevents/publichealthfocus/ucm064437.htm, updated March 2013). Here, we tested the hypothesis that subchronic low dose BPA exposure alters estrogen receptor (ESR) expression in the female rat hypothalamus.
Sexually dimorphic brain ESR distribution and activity fundamentally contribute to the organization of steroid hormone-directed morphological and functional sex differences in the developing brain (McCarthy, 2008; Rissman, 2008; Schwarz and McCarthy, 2008). Sex-specific neural organization is requisite for physiological and behavioral sex differences that emerge later in life (De Vries, 2004; Simerly, 2002). Studies in a variety of species, including rats and mice, have shown that early life exposure to BPA perturbs the organization of numerous estrogen-sensitive neural endpoints including the sexual differentiation of hypothalamic subnuclei seminal to sex-specific reproductive physiology and behavior (Kundakovic et al., 2013; Patisaul et al., 2012; Wolstenholme et al., 2012) (and reviewed in Rosenfeld, 2012; Wolstenholme et al., 2011). The classical, estrogenic mode of action for BPA has been challenged because it has relatively low binding affinities for nuclear ESRs (10,000- to 100,000-fold lower than estradiol) (Andersen et al., 1999; Barkhem et al., 1998; Kuiper et al., 1998). Disruption of ESR expression, particularly in regions with pronounced sex differences, may be an alternative mechanism by which BPA alters ESR-dependent sex-specific brain organization. This may occur via epigenetic changes (Kundakovic et al., 2013; Nugent et al., 2010) or alternative mechanisms which ultimately result in altered ESR expression (La Rosa et al., 2014; Wright et al., 2010). Understanding the specific molecular and cellular mechanisms through which BPA can alter the developing brain will help address how neural effects in rodents might be predictive of similar effects in humans.
It is well established that even transient sex differences in gene expression during critical windows of brain development can cause permanent differences in brain structure and, consequently, neuroendocrine physiology and behavior (Cooke et al., 1998; De Vries, 2004; McCarthy, 2008; Morris et al., 2004; Simerly, 2002). In a related prior study, we showed that prenatal exposure to 2.5 and/or 25 μg/kg bw/day BPA (via orogastric gavage to the dam) increases the distribution and density of estrogen receptor alpha (Esr1) and estrogen receptor beta (Esr2) gene expression in the mediobasal hypothalamus and amygdala of newborn rats (Cao et al., 2013). We and others have also demonstrated that neonatal BPA exposure at dosages ranging from 50 μg/kg bw/day to 50 mg/kg bw/day (via subcutaneous injection to the pup) can alter Esr1 and Esr2 gene expression and ESR1 immunoreactivity in the anterior hypothalamus of peripubertal female rats (Adewale et al., 2011; Cao et al., 2012, 2014; Kundakovic et al., 2013; Monje et al., 2007, 2010), suggesting that perturbed ESR mRNA levels may persist across the lifespan (direction and magnitude of the effect is region and age-specific). This hypothesis is supported by the observation that oral exposure to BPA in late adolescence (40 μg/kg bw/day) can also result in altered hypothalamic ESR1 immunoreactivity in adult rats of both sexes (Ceccarelli et al., 2007). Establishing how ESR expression patterns are changed across adolescence and into adulthood was a primary goal of the present study. Additionally, although human exposure is low and chronic, rather than confined to one critical period, most prior studies examining the neural impacts of BPA have confined exposure to a specific critical window of development. To better model human exposure in the current study, BPA was administered orally and subchronically within a range that included doses below the 5 mg/kg/day NOAEL established from guideline toxicological studies. These studies, therefore, provide critical information regarding potential effects of lifetime BPA exposure at doses that meet the NTP's definition of “low dose” (Melnick et al., 2002).
We have previously generated a detailed profile of sexually dimorphic expression pattern of Esr1 and Esr2 across several hypothalamic subregions of the prepubertal rat (Cao and Patisaul, 2011). Nuclei showing pronounced sex differences were selected as the regions of interest (ROIs) for the present study. Hypothalamic ROIs included the anteroventral periventricular nucleus (AVPV) and medial preoptic area (MPOA). Both are structurally and functionally sexually dimorphic and essential for initiating ovulation in females and coordinating gonadotropin releasing hormone (GnRH) activity in both sexes. Work from our group and others have examined BPA-related effects on ESR content and gene expression in the AVPV and MPOA in peripubertal and adult rodents (Adewale et al., 2011; Monje et al., 2007; Patisaul, 2013; Patisaul et al., 2006; Rubin et al., 2006); data which enhance their relevance as focal areas for the present studies. Areas of interest in the mediobasal hypothalamus included the ventrolateral division of the ventromedial hypothalamic nucleus (VMNvl) and the arcuate nucleus (ARC). These regions contribute to a wide range of neuroendocrine activities including growth, feeding, and reproductive behavior. Collectively, these regions were also assessed in our prior studies (Adewale et al., 2011) including the related study (using different animals but derived from the same National Center for Toxicological Research (NCTR) colony), where ESR expression was quantified on PND 1 following gestational exposure to the two lowest BPA doses used for the present studies (2.5 and 25 μg/kg bw/day) (Cao et al., 2013).
To maximize the potential to detect effects with human relevance and minimize interexperimental inconsistencies likely resulting from experimental design differences (e.g., exposure duration, dose, route of administration) and species differences in neural structure and responsivity to steroid hormones in early development (Bonthuis et al., 2010), study design related recommendations for BPA research have been published by several groups (Goodman et al., 2006; Hengstler et al., 2011; Hunt et al., 2009; Richter et al., 2007). These include minimization of xenoestrogen exposure, statistical control for litter effects, oral dosing over a wide and closely spaced dose range which include doses below the NOAEL and, in situations where BPA is thought to act as a weak estrogen, the inclusion of a concurrent reference estrogen. All of these recommendations were incorporated into the current study. Two of the four BPA doses used (2.5 and 25 μg/kg bw/day) are well below the current reference dose (tolerable daily intake) of 50 μg/kg bw/day (Chapin et al., 2008; NTP, 1982), and the two highest doses (260 and 2700 μg/kg bw/day) are below the lowest-observed-adverse-effect level (LOAEL) of 50 mg/kg bw/day. Two doses of ethinyl estradiol (EE) were included as the reference estrogen because gestational estrogen exposure is well recognized to induce region-specific hypothalamic masculinization in female rats (McCarthy, 2008; Simerly, 2002). A group of vehicle-exposed male conspecifics was included to specifically test for BPA-related impacts on sex-specific ESR expression. The animals used for the present studies were siblings of animals used for a larger, more comprehensive toxicity study, the experimental design details and results of which were published previously (Churchwell et al., 2014; Delclos et al., 2014). Those studies did not contain neural endpoints thus the results from the present study are novel in that they yield insight into the mechanisms by which BPA exposure impacts the sex-specific organization of hypothalamic subnuclei over a wide range of doses (2.5–2700 μg/kg bw/day).
MATERIALS AND METHODS
Animal care, BPA and EE exposure, brain collection, and section preparation
All animals were obtained from an Association for Assessment and Accreditation of Laboratory Animal Care (AALAC)-accredited National Center for Toxicological Research (NCTR) facility and all procedures were approved by the NCTR Laboratory Animal Care and Use Committee. The animals used in the current study were part of a larger study, conducted in compliance with good laboratory practices (GLP); the full description of animal care and dosing procedures can be found in Churchwell et al. (2014) and Delclos et al. (2014). Briefly, weanling male and female Sprague Dawley (SD) rats, obtained from the NCTR breeding colony, were maintained on a soy- and alfalfa-free diet (Test Diets 5K96 irradiated pellets; Purina Mills, Richmond, IN), and extracts of all housing materials (polysulfone caging (Ancare Corp., Bellmore, NY), hardwood chip bedding (P.J. Murphy, Montville, NJ), silicone water bottle stoppers (Plasticoid Co., Elkton, MD), and glass drinking water bottles) were screened to quantify BPA levels in leachates (Delclos et al., 2014). None of the materials had BPA levels above the average analytical blanks. Diet extracts were also assayed for BPA and did show BPA levels above those in analytical blanks in all six lots of diet used in the study. The mean BPA level in the diets used in the study was 2.6 ± 0.8 (standard deviation) ppb. Based on food intake measurements, the calculated ingested dose of BPA from the diet was ∼0.25 μg/kg bw/day, that is, ∼10-fold lower than the lowest dose used in the study (Delclos et al., 2014).
Two weeks prior to mating, female breeders were randomized to exposure groups stratified by body weight to give approximately equivalent body weights by exposure group. Male breeders were assigned such that breeding between siblings or first cousins did not occur. Females were checked daily for the presence of an in situ copulation plug or sperm in the vaginal smear. The day when an in situ plug or a sperm-positive smear was found was considered gestation day (GD) 0, at which point the male was removed and euthanized. Breeding occurred in four rounds spaced 3 weeks apart.
Daily gavage dosing of the pregnant dams began on GD 6 and continued until the onset of labor. BPA (TCI America, Portland, OR; lot no. 111909/AOHOK (air-milled)) and EE (Sigma-Aldrich, St Louis, MO; lot no. 028K1411) doses were prepared in the vehicle, 0.3% carboxymethylcellulose (CMC; Sigma-Aldrich; catalogue no. C5013, lot no. 048K0023) in water and administered in 5 ml/kg bw using a modified Hamilton Microlab 500 series pump system (Lewis et al., 2010). The main study used seven levels of BPA with half-log spacing between 2.5 and 2700 μg BPA/kg bw/day, two high doses of BPA (100,000 and 300,000 μg/kg bw/day), two doses of EE (0.5 and 5.0 μg/kg bw/day), a vehicle control, and a naïve control. Separate pump systems were used for vehicle control, BPA, and EE groups with each day's dosing conducted from low to high dose. Due to the specific interest in the potential for low dose effects, the labor intensity of the procedures employed, and the hypothesis that results observed in our prior, related study (conducted in different animals unrelated to those in the present study but derived from the same NCTR SD rat colony) may persist across the life span (Cao et al., 2013), exposure groups selected for analysis of the ESR expression levels in brain were vehicle, 2.5 μg BPA/kg bw/day (BPA 2.5), 25 μg BPA/kg bw/day (BPA 25), 260 μg BPA/kg bw/day (BPA 260), 2700 μg BPA/kg bw/day (BPA 2700), 0.5 μg EE/kg bw/day (EE 0.5), and 5.0 μg EE/kg bw/day (EE 5). The range of BPA groups was chosen to analyze effects of exposure both above and below the EPA reference dose of 50 μg BPA/kg bw/day. Detailed information regarding internal dosimetry is available in Churchwell et al. (2014). Notably, and despite substantial efforts to minimize the environmental levels of BPA in the animal rooms, the predominant phase II metabolite, BPA-glucuronide (BPA-G), was detected in the serum of naïve (not included in the present study) and vehicle control animals (LOD 0.4–0.6 nM (∼0.15–0.25 ng/ml); detected levels varied from ∼2 to 40 times the LOD), in both 21- and 80-day-old animals. Although the measured levels of BPA-G are consistent with unintentional exposure of control animals to BPA at levels approximating the lowest dose of BPA in the study (2.5 μg/kg bw/day) (Churchwell et al., 2014), the exact source of the environmental BPA is unconfirmed. This caveat must be considered in evaluating the apparent effects at the lowest BPA dose level.
Neither dams nor pups were dosed on the day of birth (PND 0). On PND 1, litters were culled to a maximum of five pups per sex per litter (and a minimum of three pups per sex per litter), and daily weighing and direct gavage of the pups began. Dosing stopped on the day before scheduled sacrifice (PND 21 or 90 ± 5). Pups were individually housed after weaning (PND 21; a practice consistent with the FDA guidance in place at the time the experiment was initiated) in the same conditions as their parents. The only males used for this study were vehicle exposed males. BPA and EE exposed animals were all females (one per litter). Adult females (PND 90 ± 5) were sacrificed on the predicted day of estrus to minimize the influence of variable endogenous sex steroid hormone levels on ESR expression levels. All cycling females were sacrificed on estrus, with the exception of five animals, three in the low and two in the high EE groups, which were in an intermediate stage (estrus/diestrus). As described in Delclos et al. (2014), a high proportion of the EE animals were not cycling normally. All animals were sacrificed by CO2 asphyxiation, the brains removed and rapidly frozen on a flat block of dry ice, and stored at −80°C until shipping on dry ice to North Carolina State University (NCSU). There were 18–23 litters per exposure group in the main study and no more than one pup per sex per litter was evaluated. For the present study, 10 brains per exposure group (up to one per sex per litter) were randomly selected at NCTR prior to shipment to NCSU. Although there were no same sex littermates evaluated, there were male and female vehicle control littermates evaluated (four at PND 21 and five at PND 90). All tissues were coded prior to shipping and all work at NCSU was done blinded to sex and exposure groups. The brains were cryosectioned (Leica CM1900, Nussloch, Germany) into four serial sets of 20 μm coronal sections, mounted on Superfrost plus slides (Fisher Scientific, Pittsburgh, PA), and stored at −80°C until in situ hybridization histochemistry (ISHH) processing.
In situ hybridization histochemistry
Riboprobe-based ISHH was performed using probes designed for similar studies, and procedures are described in detail elsewhere (Cao et al., 2013, 2014; Cao and Patisaul, 2011, 2013). ISHH was performed in eight total batches, one for each time point (PND 21 and PND 90), gene (Esr1 and Esr2), and region of interest (ROI; anterior and mediobasal hypothalamus) to minimize interbatch variance. Immediately following ISHH, the slides were dried and exposed to Kodak Biomax MR X-ray film (Eastman Kodak, Rochester, NY). A 14C autoradiographic microscale (Amersham Life Sciences, Arlington Heights, IL) was included to generate a standard curve for the optical density calculations. Exposure time was 11 days for Esr1 (all regions) and 17 days for Esr2 (all regions).
Image analysis and film quantification
ROIs for both the juvenile and adult rats were identified using the same criteria and landmarks extensively described in our prior papers using similar approaches (Cao et al., 2012, 2014; Cao and Patisaul, 2011) with the guidance of a brain atlas (Paxinos and Watson, 2007) and (as needed) our in-house library of NISSL stained sections spanning a range of ages in both sexes. Figure 1 and Supplementary figure 1 depict several of the landmarks used for section selection. ROIs in the anterior hypothalamus were the AVPV and MPOA (for additional information on landmark identification using different techniques see Herbison, 2008; Losa et al., 2010; Patisaul et al., 2006, and the rostral and caudal portions of the VMNvl and ARC (rVMNvl, rARC, cVMNvl, cARC) in the mediobasal hypothalamus). Intensity of ESR signal on the autoradiograms was quantified using the digital densitometry application of the MCID Core Image software program (InterFocus Imaging Ltd, Cambridge, UK) using routine procedures (Cao et al., 2013; Patisaul et al., 1999). All analyses were done blinded to sex and exposure groups.
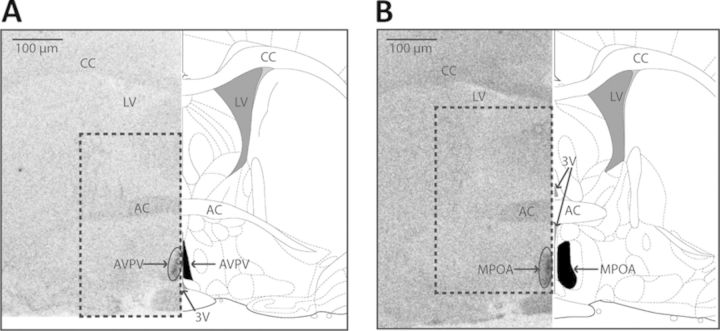
Fig. 1.
(A and B) Depiction of primary landmarks and regions of interest on the autoradiograms (left panel) and corresponding rat atlas images modified from Paxinos and Watson (2007) (right panel). (A) AVPV and surrounding landmarks. (B) MPOA and surrounding landmarks. The AVPV and MPOA are encircled on the autoradiograph image, and shaded in black on the atlas image (corresponding to bregma −0.12 mm for the AVPV and bregma −0.36 mm for the MPOA). The dotted box encapsulates the region depicted in the images presented in Figures 2A, 3A, and 4A. (3V = third ventricle, AC = anterior commissure, CC = corpus callosum, LV = lateral ventricle.)
ROI densities and background levels were measured from three anatomically matched sections per animal. The resulting values for each brain section after background subtraction were then averaged to obtain a representative measurement (for that ROI) for each animal. Densities were then converted to nCi/g tissue equivalents using a best-fit curve (cubic spline), derived from the autoradiographic 14C microscales included with each ISHH experiment. For all measurements, signal was within curve limits. All measurements were obtained by two investigators, both blind to exposure groups, to ensure repeatability in densitometry. The data sets were in high concordance with each other, and thus averaged to obtain final values for each gene and ROI.
Statistics
Data analysis was performed using published guidelines established for assessing low-dose endocrine disruptor data (Haseman et al., 2001). Within each exposure group, no same-sex litter mates were included, so potential litter effects did not need to be statistically accounted for within sex. In the vehicle control litters, four male/female litter mates were used at PND 21 and five were used at PND 90. Potential sibling effects were not taken into account in the analysis because all comparisons regarding impacts of BPA and EE exposure were made within females. Any derivation from the n = 10 animals/sex/group received resulted from an insufficient number of quantifiable sections in the ROI and the removal of statistically significant outliers via studentized residual (PND 21: two less samples in the AVPV, two in the rVMN, and one in the cVMN of the ESR1 batch; one in the cVMN of the ESR2 batch; PND 90: two outliers in the AVPV, one in the rVMN, and one in the rARC of the ESR1 batch; one in the MPOA and six in the rVMN of the ESR2 batch). Exclusion did not affect study outcome. The study had multiple, related but independent hypotheses embedded in the design, which necessitated a statistical approach where each hypothesis was independently considered, and included only the relevant groups for addressing that specific hypothesis. The primary goal was to establish if BPA impacts ESR expression in the female hypothalamus. Vehicle males were used for the purposes of establishing if any BPA-related effects in females resulted in the loss of expected sex difference in expression. The two EE groups were used for the purposes of determining if statistically significant BPA-related effects were consistent with an “estrogenic” effect. Thus, it was first established with t-tests (pooled variance) if sex differences between the vehicle male and female controls were present, this is represented with a thin line across graphs, where sex differences were found at the male vehicle expression level, in order to visually compare differences in expression levels to the male vehicle group (Figs. 2–4 and Supplementary figs. 2–4). This confirmed assay sensitivity to known sex differences in ESR expression. Next, a one-way ANOVA was run to compare the female vehicle and four BPA exposure groups, followed by a Holm-Sidak post hoc test to establish whether any of the BPA exposure groups differed from the female vehicle group. The EE groups were not included in this ANOVA because inclusion of known “positive controls” biases the analysis toward a statistically significant outcome (Haseman et al., 2001). If no significance was found by one-way ANOVA, no further statistical testing was conducted. BPA groups found to differ significantly from the female vehicle group were then compared via t-test to each EE group and the male vehicle group to establish if the effect was consistent with estrogenic activity and/or masculinization. Because the effects of EE on gene expression were considered informative, but a separate experiment, the effect of EE exposure on ESR expression was then considered by comparing the two EE groups to the vehicle controls by a Dunnett's post hoc test (Haseman et al., 2001). All analyses were two-tailed and results were considered significantly different when p ≤ 0.05.
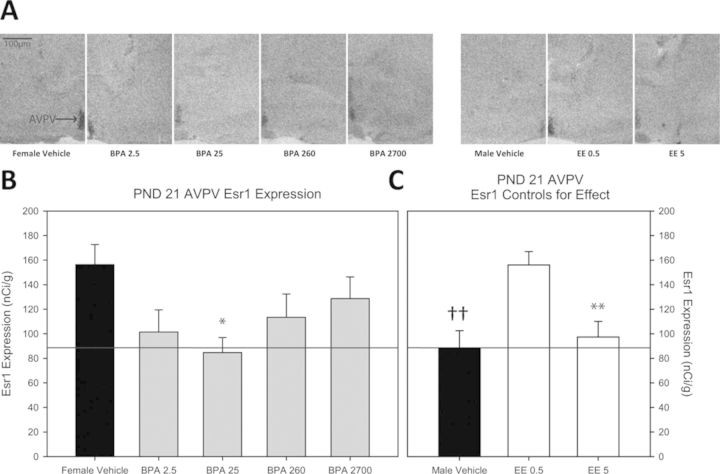
Fig. 2.
(A) Representative autoradiograph images of Esr1 expression in the AVPV (indicated by arrow in female vehicle image) of each exposure group. (B) Impact of BPA exposure on Esr1 expression in the PND 21 female AVPV. Only the BPA 25 group significantly differed from the female vehicle group. The positive control groups are depicted in (C). Esr1 expression was significantly lower in the male vehicle group compared with the female vehicle group and this sex difference is indicated by the thin line traversing across panels (B) and (C). Expression in the BPA 25 group was reduced to male-typical levels. Among the EE groups, Esr1 expression was only significantly reduced in the EE 5 group compared with female vehicle controls (n = 9–10 per group; *p ≤ 0.05, **p ≤ 0.01 compared with female vehicle group; sex differences denoted with ††p ≤ 0.01; graphs depict mean ± SEM).
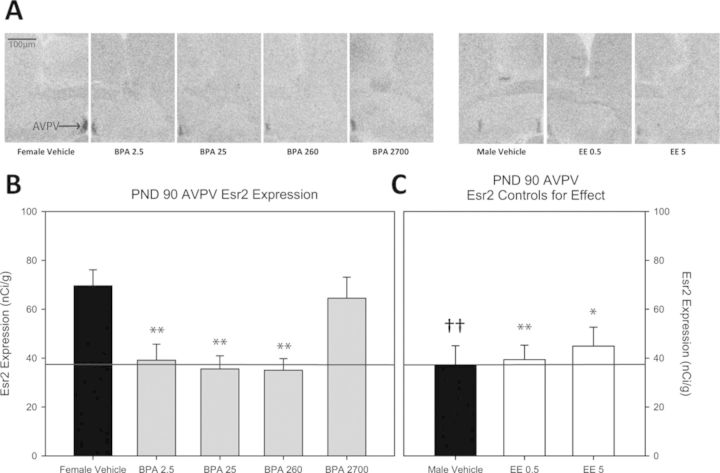
Fig. 3.
(A) Representative autoradiograph images of Esr2 in the AVPV (indicated by arrow in female vehicle image) of each exposure group. (B) Impact of BPA exposure on Esr2 expression in the PND 90 female AVPV. Esr2 expression was significantly lower in all of the BPA exposed groups except the BPA 2700 group compared with the female vehicle controls. The positive control groups are depicted in (C). Esr2 expression was significantly lower in the male vehicle group compared with the female vehicle group and this sex difference is indicated by the thin line traversing across panels (B) and (C). Expression the three significantly impacted BPA groups was reduced to male-typical levels. Among the EE groups, Esr2 expression was significantly reduced in both the EE 0.5 and EE 5 groups compared with female vehicle controls, with levels approximating those seen in the male vehicle group (n = 10 for all groups; *p ≤ 0.05, **p ≤ 0.01 compared with female vehicle group; sex differences denoted with ††p ≤ 0.01; graph depicts mean ± SEM).
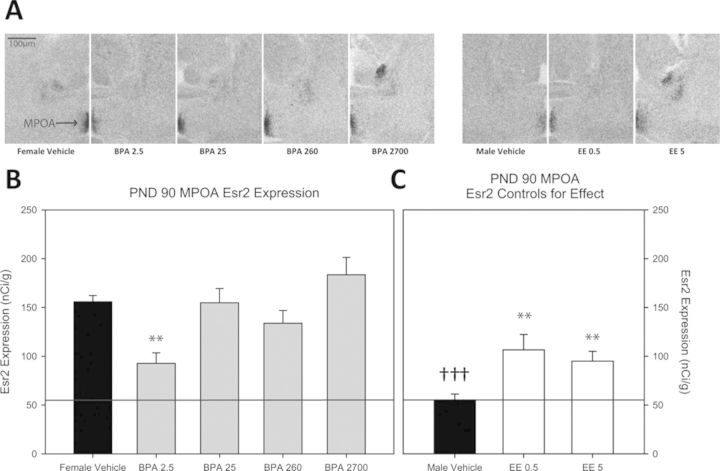
Fig. 4.
(A) Representative autoradiograph images of Esr2 expression in MPOA (indicated by arrow in female vehicle image) of each exposure group. (B) Impact of BPA exposure on Esr2 expression in the PND 90 female MPOA. Expression was significantly reduced in the BPA 2.5 group compared with the female vehicle controls. The positive control groups are depicted in (C). Esr2 expression was significantly lower in the male vehicle group compared with the female vehicle group and this sex difference is indicated by the thin line traversing across panels (B) and (C). Among the EE groups, Esr2 expression was significantly reduced in both the EE 0.5 and EE 5 groups compared with female vehicle controls. Although the magnitude of decreased Esr2 expression was approximately equivalent among the BPA 2.5, EE 0.5, and EE 5 groups, expression was higher than typical for unexposed males, so the sex difference in Esr2 expression was only partially abrogated (n = 8–10 per group; **p ≤ 0.01; sex differences denoted with †††p ≤ 0.001; graph depicts mean ± SEM).
RESULTS
PND 21 Esr1 and Esr2 Expression in the POA
Esr1
Representative autoradiograph images for each experimental group and their expression levels are found in Figure 2A and an image of larger area and identified landmarks is found in Figure 1. AVPV Esr1 expression in the female vehicle group (mean ± SEM = 156.315 ± 13.937 nCi/g) was significantly higher than the male vehicle group (88.591 ± 16.291 nCi/g; p ≤ 0.006; Figs. 2B and 2C) and masculinized by EE, but only at the highest dose (97.360 ± 12.727 nCi/g; p ≤ 0.004; Fig. 2C). Of the BPA exposed groups, expression was only significantly altered in the BPA 25 exposure group (84.713 ± 12.236 nCi/g; p ≤ 0.02) and not significantly different from the male controls, indicative of masculinization (Fig. 2B and 2C). In the MPOA, a sex difference in expression was observed as anticipated (357.820 ± 37.588 nCi/g, females and 227.643 ± 23.539 nCi/g, males; p ≤ 0.02) but no significant effects of BPA or EE exposure were found (Supplementary figs. 2A and 2B).
Esr2
For both the AVPV and MPOA, female expression (107.206 ± 18.497 nCi/g, AVPV and 123.847 ± 21.193 nCi/g, MPOA) was significantly higher than the male expression (45.104 ± 8.772 nCi/g, AVPV and 62.249 ± 9.2 nCi/g, MPOA; p ≤ 0.01 and p ≤ 0.02, respectively), but no effect of BPA or EE exposure was identified (Supplementary fig. 3).
PND 21 Esr1 and Esr2 Expression in the MBH
Esr1
Esr1 expression was significantly higher in the female rVMNvl (202.376 ± 15.522 nCi/g) compared with the male rVMNvl (107.774 ± 11.779 nCi/g; p ≤ 0.001), but no sex difference was observed in the cVMNvl (Supplementary figs. 2C and 2D). Female Esr1 levels were masculinized by EE in both the rVMNvl (EE 5, 106.767 ± 6.88 nCi/g) and the cVMNvl (female vehicle, 174.171 ± 17.032 nCi/g and EE 5, 115.820 ± 12.951 nCi/g), but only at the highest dose (Supplementary figs. 2D and 2F; p ≤ 0.001 and p ≤ 0.038, respectively). No significant effects of BPA exposure were found in either subregion of the VMNvl (Supplementary figs. 2C and 2E). In the ARC (rARC and cARC), Esr1 expression was not sexually dimorphic, and no significant effects of BPA or EE exposure were observed (Supplementary figs. 2G–J).
Esr2
Expression of Esr2 in the VMNvl was not sexually dimorphic, and no significant effects of BPA or EE exposure were observed (Supplementary figs. 3E–H). As expected, no appreciable Esr2 expression was found in the ARC in any of the experimental groups (Cao and Patisaul, 2011).
PND 90 Esr1 and Esr2 Expression in the POA
Esr1
Esr1 expression was sexually dimorphic in the MPOA (326.819 ± 32.923 nCi/g, females and 191.175 ± 25.743 nCi/g, males) (p ≤ 0.007), but not the AVPV (Supplementary figs. 4A–D). Both doses of EE masculinized MPOA Esr1 expression (EE 0.5, 181.872 ± 16.702 nCi/g; p ≤ 0.006 and EE 5, 208.376 ± 23.827 nCi/g; p ≤ 0.001), but neither dose had a significant effect on AVPV Esr1 expression (Supplementary figs. 4A–D). No significant effects of BPA exposure were found in either the MPOA or AVPV (Supplementary figs. 4A and 4C).
Esr2
Representative autoradiograph images for each experimental group and their expression levels are found in Figures 3A and 4A and an image of larger area and identified landmarks is found in Figure 1. AVPV Esr2 expression was significantly higher in females (69.582 ± 6.563 nCi/g, females and 37.251 ± 7.835 nCi/g, males; p ≤ 0.005) and significantly reduced in the EE groups (EE 0.5, 39.383 ± 5.904 nCi/g; p ≤ 0.008 and EE 5, 44.904 ± 7.762 nCi/g; p ≤ 0.03), indicative of masculinization (Figs. 3B and 3C). Compared with vehicle conspecifics, Esr2 expression was significantly lower in the BPA 2.5, BPA 25, and BPA 260 groups (39.143 ± 6.608 nCi/g; p ≤ 0.005, 35.556 ± 5.385 nCi/g; p ≤ 0.005, and 35.034 ± 4.724 nCi/g; p ≤ 0.02, respectively). Expression levels in these BPA-exposure groups did not significantly vary from the male vehicle group, indicative of masculinization (Figs. 3B and 3C). In the MPOA, Esr2 expression was significantly higher in females (155.919 ± 6.304 nCi/g, females and 55.217 ± 6.059 nCi/g, males; p ≤ 0.001) and significantly reduced by both doses of EE (EE 0.5, 106.555 ± 15.734 nCi/g; p ≤ 0.009 and EE 5, 95.025 ± 10.034 nCi/g; p ≤ 0.002) (Figs. 4B and 4C). Among the BPA exposure groups, expression was only significantly altered in the BPA 2.5 exposure group (95.715 ± 10.781 nCi/g; p ≤ 0.01) with levels comparable to the EE groups but significantly higher than the male group (Figs. 4B and 4C; p ≤ 0.01).
PND 90 Esr1 and Esr2 Expression in the MBH
Esr1
Esr1 expression was significantly higher in the female rVMNvl (256.379 ± 37.774 nCi/g, females and 123.115 ± 15.468 nCi/g, males; p ≤ 0.005) and cVMNvl (276.734 ± 46.437 nCi/g, females and 126.289 ± 14.712 nCi/g, males; p ≤ 0.013), but not masculinized by either dose of EE (Supplementary figs. 4E–H). No significant effects of BPA exposure were found in either VMNvl subregion (Supplementary figs. 4E and 4G). In the ARC, female expression of Esr1 was significantly higher than male expression in the rARC (199.561 ± 21.284 nCi/g, females and 132.069 ± 13.568 nCi/g, males; p ≤ 0.02), but not the cARC, and no significant effects of EE or BPA were identified in either subregion (Supplementary figs. 4I–L).
Esr2
In the VMN, no significant sex differences were identified and no significant effects of EE or BPA were observed (Supplementary fig. 5). Esr2 expression was absent in the ARC, as previously reported (Cao and Patisaul, 2011).
DISCUSSION
The present study represents the most comprehensive and region-specific evaluation of BPA and EE exposure effects on ESR expression levels in limbic subnuclei of the female rat at weaning and early adulthood. These results suggest that subchronic, low dose BPA or EE exposure can induce age- and region-specific effects on hypothalamic ESR expression in female rats. Expression changes induced by BPA and EE were primarily confined to the anterior hypothalamus, an estrogen-sensitive region required for ovulation and aspects of female reproductive behavior (Semaan and Kauffman, 2010; Simerly, 2002). AVPV Esr1 expression was reduced on PND 21 (at 25 μg BPA/kg bw/day), but not PND 90, and Esr2 expression was reduced in both the AVPV (2.5, 25, and 260 μg BPA/kg bw/day) and MPOA (2.5 μg BPA/kg bw/day) at PND 90. Significant effects of BPA were observed only at doses of 260 μg/kg bw/day and lower, demonstrating that consistent and statistically significant effects of subchronic BPA exposure on ESR expression can manifest at doses equivalent to, or below, the current oral reference dose of 50 μg/kg bw per day.
A notable caveat, as described in Delclos et al. (2014) and Churchwell et al. (2014), is that BPA-G was found in the serum of naïve (not included in the present study) and vehicle control animals littermates to the ones used in the current study at levels that were statistically indistinguishable from the 2.5 μg BPA/kg bw/day exposure group. Although the source of the background BPA exposure in the naïve and vehicle groups remains undetermined, the presence of BPA-G in negative control serum potentially confounds the interpretation of the changes observed in the lowest (2.5 μg BPA/kg bw/day) BPA exposure group, but not those in higher dose groups. In the parent toxicity study (from which the animals for the present study were obtained), Delclos et al. concluded that “Our interpretation of the results of the present study is that BPA in the “low dose” region from 2.5 to 2700 μg/kg bw/day did not produce effects in the evaluated endpoints that differ from normal background biological variation.” No neural endpoints were included in those evaluated endpoints, thus the present data suggest that neural effects within that low dose range are plausible. This assertion is supported by prior data from similar studies using different animals (Cao et al., 2013) and different routes of administration (Cao et al., 2012, 2014).
Ongoing studies in the CLARITY-BPA research program (Schug et al., 2013), which incorporate a similar (2.5–2500 μg BPA/kg bw/day) dose range, assessment of BPA-G serum levels, and combine behavior, neuroanatomical, and molecular endpoints, should provide resolution regarding confirmation of ESR expression changes and the functional significance of observed ESR expression changes. This important follow-up study will be able to examine the potential linkage of the transcriptional changes observed here to neuroendocrine and behavioral outcomes. Recent behavioral studies in this rat model using 2.5 and 25 μg BPA/kg bw/day have failed to detect significant behavioral effects in juveniles or adults (Ferguson et al., 2011, accepted), but a range of behavioral effects have been reported by other groups (reviewed in Wolstenholme et al., 2011; Rochester, 2013). In addition, the main study from which the animals were obtained, detected effects on the female reproductive tract only at very high doses of BPA (100,000 and 300,000 μg/kg bw/day; Delclos et al., 2014). The present data suggest, however, that reproductive effects reported in numerous prior studies, including altered ovarian morphology, estrus cyclicity, and subfertility (Calhoun et al., 2014; Souter et al., 2013 and reviewed in FAO/WHO, 2011; Rochester, 2013) may be attributable to changes in ESR content within the anterior hypothalamus.
Inclusion of a wide range of BPA doses, ranging from 2.5 to 2700 μg/kg bw/day (with 10-fold spacing between consecutive doses) allowed the characterization of the dose-response and assessment of impacts below the current NOAEL. The shape of the dose-response relationships for the BPA-related effects differed among the regions examined. For example, AVPV Esr2 levels were modulated by three BPA doses at PND 90, including the lowest dose group, and the magnitude of the effect did not appreciably differ between doses. At PND 21, AVPV Esr1 levels were modulated by 25 μg BPA/kg bw/day only. Inclusion of the intermediate and high BPA dose groups available from the main study (Delclos et al., 2014) may have provided additional resolution of dose-response relationships, but the data presented here reveal the potential for significant effects in the low BPA dose range. As noted elsewhere, this is being further investigated in this same model.
These experiments serve as an important follow-up to our prior, related study showing that limbic ESR expression just after birth (PND 1) is altered by gestational exposure to BPA (2.5 and 25 μg/kg bw/day) (Cao et al., 2013). For that study, the animals were derived from the same colony of NCTR SD rats but bred, housed, dosed, and handled in a separate building so they are not siblings or otherwise related to the animals in the present study. Although no direct measurements of serum BPA or BPA-G were made in the Cao et al. (2013) study, our unpublished data suggest that the unintentional exposure to BPA in the present study is related to the use of very high BPA doses (100 and 300 mg/kg bw/day, Delclos et al., 2014). The highest dose in the Cao et al. (2013) study was 25 μg BPA/kg bw/day; hence the low dose range expression effects reported in those animals are not believed to be confounded by unintentional exposure to BPA. One of the primary hypotheses tested in the present study was if the ESR expression changes observed on PND 1 persist across the lifespan. Although ESR expression was found to be altered at puberty and early adulthood, the impacted brain regions and the direction of BPA-related ESR expression changes reported here differ from those observed in the complementary study examining neonates. In the PND 1 animals, BPA-related ESR expression level differences were observed in the MBH, rather than the anterior hypothalamus. Additionally, in the neonates, BPA was found to increase Esr1 and Esr2 expression, an effect which is directionally opposite to the expression changes reported here for older animals. Age at assessment and exposure duration likely primarily account for these differences. Importantly, endogenous steroid hormone levels are higher in males than females on PND 1, a difference which fundamentally contributes to brain sexual differentiation (McCarthy, 2008; Simerly, 2002), and thus may also confer sex and age-related differences in BPA sensitivity (FAO/WHO, 2011). Notably, our prior study (Cao et al., 2013) found a significant impact of gavage on PND 1 ESR expression levels, suggesting a critical interaction between gestational BPA exposure and prenatal stress on brain ESR expression. Although the main study from which the animals used in the present study were obtained (Delclos et al., 2014) did include a naïve control group, the PND 1 results were not available at the time that the present study was designed. Thus, unfortunately the naïve control group was not incorporated to address the separate issue of gavage-related effects on ESR expression.
The ESR subtype impacted by BPA differed on PNDs 21 and 90, suggesting that Esr1 and Esr2 may be differentially sensitive to BPA depending on gonadal state. In the prepubertal animals, when endogenous estrogen levels are naturally low, Esr1 was found to be decreased while, in the adults, when circulating estrogen levels are elevated, Esr2 expression was reduced. Consequently, the sexually dimorphic pattern of Esr1 and Esr2 expression normally seen in the AVPV and MPOA was lost in BPA-exposed animals, specifically, AVPV Esr1 expression in the BPA 25 group at PND 21, AVPV Esr2 expression in the BPA 2.5, 25, and 260 groups at PND 90, and MPOA Esr2 expression in the BPA 2.5 group at PND 90. These age- and region-specific results are not entirely unexpected because ESR expression naturally varies with age in limbic nuclei, particularly those which are morphologically and functionally sexually dimorphic, including the hypothalamic regions investigated here (Cao and Patisaul, 2011; Chakraborty et al., 2003a,b; Ikeda et al., 2003; Walker et al., 2012; Wilson et al., 2002). This temporal variability in ESR expression is crucial for organizing and maintaining sex differences across the life span; thus disruption, even a temporary disruption, during critical windows of development could alter sex-specific brain structure and function (De Vries, 2004). The present data suggest that perturbation of region- and age-specific ESR expression may underlie previously reported BPA-related morphological and ESR protein level differences in sexually dimorphic brain regions such as the sexually dimorphic nucleus (SDN) and the AVPV (Adewale et al., 2011; Cao et al., 2014; He et al., 2012; McCaffrey et al., 2013; Patisaul et al., 2006, 2007; Rubin et al., 2006; Viberg et al., 2011).
Although there are well recognized species differences in terms of how and where the brain is sexually dimorphic, steroid hormones are known to be fundamental to the orchestration and maintenance of these differences (Bonthuis et al., 2010; Swaab, 2007; Wallen, 2005). Reports published by the NTP, WHO, and others have expressed concerns that BPA exposure, at levels below the current NOAEL, might alter sex-specific neural organization and thereby pose a risk to developing fetuses, infants, and young children (Chapin et al., 2008; Gontier-Latonnelle et al., 2007; Rochester, 2013; Shelby, 2008; Shelnutt et al., 2013), although a direct demonstration of such effects in this model has not yet been assessed. Further work is ongoing (Schug et al., 2013) to address these concerns. In humans, the period encompassing the rodent perinatal period is believed to occur in mid to late gestation (Abbott et al., 2008; Aksglaede et al., 2006; Selevan et al., 2000; Simerly, 2002); thus, the rat perinatal “critical window” is likely to be entirely prenatal in humans. Additionally, although estrogen derived from local aromatization of testicular androgens is well known to be required for masculinization in the rat brain, the role estrogen plays in the sex-specific organization of the human brain remains unclear (Giedd et al., 1997; Herman et al., 2000; Swaab, 2007). Thus, it is difficult to predict with certainty how disruption of ESR expression in the human brain may manifest.
The male vehicle group was specifically included to ensure that expected sex differences in sexually dimorphic ESR expression were present in vehicle control animals, and to evaluate BPA-related changes on these differences. The observed sex differences reported here at PND 21 are largely consistent with prior work characterizing brain ESR distribution (Cao and Patisaul, 2011). Importantly, Esr1 and Esr2 expression was higher in the female MPOA, as expected. On PND 21, AVPV Esr1 expression was observed to be significantly higher in females than in males, but previous work from our lab using Long Evans rats found no statistically significant sex difference on PND 19 (Cao and Patisaul, 2011), suggesting that the magnitude of this difference is enhanced at weaning or differs by strain. Our data are consistent with the findings of Kuhnemann et al. which reported that, in Wistar rats, sex differences in ESR binding persist from PNDs 28–49 in the POA (Kuhnemann et al., 1994). Similarly, it has also been reported that Esr1 expression is greater in SD females than in males at PND 14, an observation that is consistent with our findings in the prepubertal animals (Orikasa et al., 2002). Overall, in the present study, the sex differences abrogated by BPA were primarily restricted to the preoptic area.
Inclusion of the two EE exposure groups served to (1) confirm that these animals are sensitive to estrogen-induced masculinization and (2) establish if any BPA-related effects were consistent with an estrogenic mode of action. Our study examining ESR expression in PND 1 animals of the same SD strain, found no significant evidence that EE, at 5 and 10 μg/kg bw/day, masculinizes female Esr1 and Esr2 expression in either the anterior or the mediobasal hypothalamus (Cao et al., 2013). However, littermates examined at PND 21 showed a significant increase in the volume of the female sexually dimorphic nucleus of the preoptic area (SDN-POA), although the magnitude of the change did not reach male-typical levels at either dose (He et al., 2012). A study from a different lab group using Long Evans rats investigated the impact of EE exposure ranging from 0.05 to 50 μg/kg bw/day on a variety of neurobehavioral endpoints and suggested that exposure in the 5–50 μg/kg bw/day range is the most effective to masculinize reproductive behaviors and advance female pubertal onset. No endpoints were affected by the 0.5 μg/kg bw/day dose of EE (Ryan et al., 2010); however, neonatal exposure in that study was lactational rather than direct as in the present study. In the current study, exposure to EE decreased ESR expression in regions where expression was sexually dimorphic and affected by BPA exposure, suggesting that BPA has masculinizing effects in these regions. At PND 21, masculinization of Esr1 expression by EE was found in the female AVPV, but only at the higher dose (5 μg/kg bw/day). At PND 90, masculinization of Esr2 expression by EE in the AVPV and MPOA was achieved with both exposures of EE (0.5 and 5 μg/kg bw/day). Collectively, the data suggest that the minimally effective exposure level for inducing hypothalamic masculinization of ESR expression by EE in SD rats (when administered by gavage) is at or above 0.5 μg/kg bw, though sensitivity may vary with age and route of administration.
Although BPA has a 10,000-fold lower binding affinity for ESRs, in some cases where ESR expression was significantly altered by BPA and EE, the effect occurred at a lower relative dose of BPA than EE. This observation is consistent with what we previously reported in PND 1 animals from a companion study (Cao et al., 2013) and suggests that, although BPA has historically been characterized as a weak estrogen, it may act through alternative pathway(s) to perturb ESR expression. Possibilities other than classic estrogen signaling include activity through membrane receptors (including GPR30 (Ge et al., 2014; Thomas and Dong, 2006)), epigenetic changes, or interactions with other steroid hormone receptors (Gentilcore et al., 2013; La Rosa et al., 2014; Wolstenholme et al., 2011). For example, emerging evidence suggests that BPA binds to estrogen related receptor gamma (ERRγ), a nuclear receptor whose natural ligand is not known and is thought to play a role in the differentiation and maturation of the fetal brain (Hermans-Borgmeyer et al., 2000; Lorke et al., 2000; Matsushima et al., 2007; Takayanagi et al., 2006). Although the present studies were not designed to specifically examine the specific mechanisms by which BPA impacts cell-specific ESR expression, subsequent studies should address this data gap.
The BPA-related decreases in ESR expression reported here could reflect either a decrease in cellular levels of Esr1 and Esr2 or reduced numbers of cells transcribing Esr1 and Esr2. A change in cell number would suggest that permanent, organizational changes within the impacted brain region have occurred (McCarthy, 2008). Because BPA-related effects on ESR expression differed with age, this appears to be unlikely, but compensation for cell loss via increased expression in existing cells cannot be ruled out (De Vries, 2004). Presumably, decreased ESR expression is indicative of reduced ESR protein levels; a relationship established in prior studies (Monje et al., 2007).
CONCLUSIONS
The data provided here contribute novel information regarding mechanisms by which subchronic, low dose exposure to BPA may influence sex-specific brain development. Effects of BPA on hypothalamic ESR expression were primarily observed at exposure levels less than the current oral reference dose for BPA of 50 μg/kg bw/day, but not at the highest dose (2700 μg/kg bw/day) employed. The functional significance of perturbed hypothalamic ESR expression during prepuberty and early adulthood remains to be definitively established, but the data presented here suggest the potential for apical effects on neuroendocrine systems below the currently established NOAEL of 5 mg/kg bw/day. Ongoing studies will further address the relationships between transcriptional changes in the brain and physiological/behavioral effects (Schug et al., 2013).
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org.
FUNDING
The brain region receptor measurements were supportedby the National Institutes of Health [R01-16001-04S1 to H.B.P.]. The animal treatment and tissue collection were conducted at NCTR under the auspices of the National Toxicology Program (NTP Study # C10034) and funded by an Interagency agreement (IAG) between the Food and Drug Administration (FDA) and the National Institute of Environmental Health Sciences (NIEHS)/National Institutes of Health [FDA IAG: 224-12-0003; NIEHS IAG: AES12013].
Acknowledgments
The authors thank Kelly McCaffrey for her support in completion of in situ hybridizations and Karina Todd, Natalie Mabrey, Stephanie Leyrer, and Nicole Russ for their assistance in brain sectioning. The authors also thank Brian Horman and Sheryl Arambula for their critical reading of the manuscript and David Aylor and David Reif for their consultative statistical expertise. The authors thank the NCTR Animal Care and Pathology staffs for animal maintenance and treatment and brain collection and Kathy Carroll for assistance in the planning and oversight of the study.
Disclaimer: This document has been reviewed in accordance with the United States Food and Drug Administration (FDA) procedures and approved for publication. Approval does not signify that the contents necessarily reflect the position or opinions of the FDA nor does mention of trade names or commercial products constitute endorsement or recommendation for use. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the FDA.
REFERENCES
- Abbott D. H., Zhou R., Bird I. M., Dumesic D. A., Conley A. J. Fetal programming of adrenal androgen excess: lessons from a nonhuman primate model of polycystic ovary syndrome. Endocr. Dev. 2008;13:145–158. doi: 10.1159/000134831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adewale H. B., Todd K. L., Mickens J. A., Patisaul H. B. The impact of neonatal bisphenol-A exposure on sexually dimorphic hypothalamic nuclei in the female rat. Neurotoxicology. 2011;32:38–49. doi: 10.1016/j.neuro.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksglaede L., Juul A., Leffers H., Skakkebaek N. E., Andersson A. M. The sensitivity of the child to sex steroids: possible impact of exogenous estrogens. Hum. Reprod. Update. 2006;12:341–349. doi: 10.1093/humupd/dml018. [DOI] [PubMed] [Google Scholar]
- Andersen H. R., Andersson A. M., Arnold S. F., Autrup H., Barfoed M., Beresford N. A., Bjerregaard P., Christiansen L. B., Gissel B., Hummel R., et al. Comparison of short-term estrogenicity tests for identification of hormone-disrupting chemicals. Environ. Health Perspect. 1999;107(Suppl. 1):89–108. doi: 10.1289/ehp.99107s189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkhem T., Carlsson B., Nilsson Y., Enmark E., Gustafsson J., Nilsson S. Differential response of estrogen receptor alpha and estrogen receptor beta to partial estrogen agonists/antagonists. Mol. Pharmacol. 1998;54:105–112. doi: 10.1124/mol.54.1.105. [DOI] [PubMed] [Google Scholar]
- Biedermann S., Tschudin P., Grob K. Transfer of bisphenol A from thermal printer paper to the skin. Anal. Bioanal. Chem. 2010;398:571–576. doi: 10.1007/s00216-010-3936-9. [DOI] [PubMed] [Google Scholar]
- Bonthuis P. J., Cox K. H., Searcy B. T., Kumar P., Tobet S., Rissman E. F. Of mice and rats: key species variations in the sexual differentiation of brain and behavior. Front. Neuroendocrinol. 2010;31:341–358. doi: 10.1016/j.yfrne.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat A. M. Background paper on BPA biomonitoring and biomarker studies. Ottawa, Canada: 2011. In: FAO/WHO Expert Meeting on Bisphenol A. (BPA) [Google Scholar]
- Calafat A. M., Ye X., Wong L. Y., Reidy J. A., Needham L. L. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ. Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun K. C., Padilla-Banks E., Jefferson W. N., Liu L., Gerrish K. E., Young S. L., Wood C. E., Hunt P. A., Vandevoort C. A., Williams C. J. Bisphenol a exposure alters developmental gene expression in the fetal rhesus macaque uterus. PLoS One. 2014;9:e85894. doi: 10.1371/journal.pone.0085894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Joyner L., Mickens J. A., Leyrer S. M., Patisaul H. B. Sex-specific Esr2 mRNA expression in the rat hypothalamus and amygdala is altered by neonatal bisphenol A exposure. Reproduction. 2014;147:537–554. doi: 10.1530/REP-13-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Mickens J. A., McCaffrey K. A., Leyrer S. M., Patisaul H. B. Neonatal Bisphenol A exposure alters sexually dimorphic gene expression in the postnatal rat hypothalamus. Neurotoxicology. 2012;33:23–36. doi: 10.1016/j.neuro.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Patisaul H. B. Sexually dimorphic expression of hypothalamic estrogen receptors alpha and beta and kiss1 in neonatal male and female rats. J. Comp. Neurol. 2011;519:2954–2977. doi: 10.1002/cne.22648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Patisaul H. B. Sex specific expression of estrogen receptors alpha and beta and kiss1 in the postnatal rat amygdala. J. Comp. Neurol. 2013;521:465–478. doi: 10.1002/cne.23185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Rebuli M. E., Rogers J., Todd K. L., Leyrer S. M., Ferguson S. A., Patisaul H. B. Prenatal bisphenol a exposure alters sex-specific estrogen receptor expression in the neonatal rat hypothalamus and amygdala. Toxicol. Sci. 2013;133:157–173. doi: 10.1093/toxsci/kft035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli I., Della Seta D., Fiorenzani P., Farabollini F., Aloisi A. M. Estrogenic chemicals at puberty change ERalpha in the hypothalamus of male and female rats. Neurotoxicol. Teratol. 2007;29:108–115. doi: 10.1016/j.ntt.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Chakraborty T. R., Hof P. R., Ng L., Gore A. C. Stereologic analysis of estrogen receptor alpha (ER alpha) expression in rat hypothalamus and its regulation by aging and estrogen. J. Comp. Neurol. 2003a;466:409–421. doi: 10.1002/cne.10906. [DOI] [PubMed] [Google Scholar]
- Chakraborty T. R., Ng L., Gore A. C. Age-related changes in estrogen receptor beta in rat hypothalamus: a quantitative analysis. Endocrinology. 2003b;144:4164–4171. doi: 10.1210/en.2003-0052. [DOI] [PubMed] [Google Scholar]
- Chapin R. E., Adams J., Boekelheide K., Gray L. E., Jr, Hayward S. W., Lees P. S., McIntyre B. S., Portier K. M., Schnorr T. M., Selevan S. G., et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Res. B Dev. Reprod. Toxicol. 2008;83:157–395. doi: 10.1002/bdrb.20147. [DOI] [PubMed] [Google Scholar]
- Churchwell M. I., Camacho L., Vanlandingham M. N., Twaddle N. C. E. S., Delclos K. B., Fisher J. W., Doerge D. R. Comparison of lifestage-dependent internal dosimetry for bisphenol A, ethinyl estradiol, a reference estrogen, and endogenous estradiol to test an estrogenic model of action in Sprague-Dawley rats. Toxicol. Sci. 2014;139:4–20. doi: 10.1093/toxsci/kfu021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke B., Hegstrom C. D., Villeneuve L. S., Breedlove S. M. Sexual differentiation of the vertebrate brain: principles and mechanisms. Front. Neuroendocrinol. 1998;19:323–362. doi: 10.1006/frne.1998.0171. [DOI] [PubMed] [Google Scholar]
- Cooper J. E., Kendig E. L., Belcher S. M. Assessment of bisphenol A released from reusable plastic, aluminium and stainless steel water bottles. Chemosphere. 2011;85:943–947. doi: 10.1016/j.chemosphere.2011.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delclos K. B., Camacho L., Lewis S. M., Vanlandingham M. N., Latendresse J. R., Olson G. R., Davis K. J., Patton R. E., Gamboa da Costa G., Woodling K. A., et al. Toxicity evaluation of bisphenol A administration by gavage to Sprague-Dawley rats from gestation day 6 through postnatal day 90. Toxicol. Sci. 2014;139:174–197. doi: 10.1093/toxsci/kfu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO/WHO. Toxicological and Health Aspects of Bisphenol A: Report of Joint FAO/WHO Expert Meeting and Report of Stakeholder Meeting on Bisphenol A. World Health Organization; 2011. [Google Scholar]
- Ferguson S. A., Law C. D., Jr, Abshire J. S. Developmental treatment with bisphenol A or ethinyl estradiol causes few alterations on early preweaning measures. Toxicol. Sci. 2011;124:149–160. doi: 10.1093/toxsci/kfr201. [DOI] [PubMed] [Google Scholar]
- Ferguson S. A., Law C. D., Kissling G. E. Developmental treatment with ethinyl estradiol, but not bisphenol A, causes alterations in sexually dimorphic behaviors in male and female Sprague-Dawley. Tox. Sci. doi: 10.1093/toxsci/kfu077. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge L. C., Chen Z. J., Liu H. Y., Zhang K. S., Liu H., Huang H. B., Zhang G., Wong C. K., Giesy J. P., Du J., et al. Involvement of activating ERK1/2 through G protein coupled receptor 30 and estrogen receptor alpha/beta in low doses of bisphenol A promoting growth of Sertoli TM4 cells. Toxicol. Lett. 2014;226:81–89. doi: 10.1016/j.toxlet.2014.01.035. [DOI] [PubMed] [Google Scholar]
- Geens T., Aerts D., Berthot C., Bourguignon J. P., Goeyens L., Lecomte P., Maghuin-Rogister G., Pironnet A. M., Pussemier L., Scippo M. L., et al. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem. Toxicol. 2012;50:3725–3740. doi: 10.1016/j.fct.2012.07.059. [DOI] [PubMed] [Google Scholar]
- Geens T., Goeyens L., Covaci A. Are potential sources for human exposure to bisphenol-A overlooked? Int. J. Hyg. Environ. Health. 2011;214:339–347. doi: 10.1016/j.ijheh.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Gentilcore D., Porreca I., Rizzo F., Ganbaatar E., Carchia E., Mallardo M., De Felice M., Ambrosino C. Bisphenol A interferes with thyroid specific gene expression. Toxicology. 2013;304:21–31. doi: 10.1016/j.tox.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Giedd J. N., Castellanos F. X., Rajapakse J. C., Vaituzis A. C., Rapoport J. L. Sexual dimorphism of the developing human brain. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1997;21:1185–1201. doi: 10.1016/s0278-5846(97)00158-9. [DOI] [PubMed] [Google Scholar]
- Gontier-Latonnelle K., Cravedi J. P., Laurentie M., Perdu E., Lamothe V., Le Menn F., Bennetau-Pelissero C. Disposition of genistein in rainbow trout (Oncorhynchus mykiss) and siberian sturgeon (Acipenser baeri) Gen. Comp. Endocrinol. 2007;150:298–308. doi: 10.1016/j.ygcen.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Goodman J. E., McConnell E. E., Sipes I. G., Witorsch R. J., Slayton T. M., Yu C. J., Lewis A. S., Rhomberg L. R. An updated weight of the evidence evaluation of reproductive and developmental effects of low doses of bisphenol A. Crit. Rev. Toxicol. 2006;36:387–457. doi: 10.1080/10408440600758317. [DOI] [PubMed] [Google Scholar]
- Haseman J. K., Bailer A. J., Kodell R. L., Morris R., Portier K. Statistical issues in the analysis of low-dose endocrine disruptor data. Toxicol. Sci. 2001;61:201–210. doi: 10.1093/toxsci/61.2.201. [DOI] [PubMed] [Google Scholar]
- Hengstler J. G., Foth H., Gebel T., Kramer P. J., Lilienblum W., Schweinfurth H., Volkel W., Wollin K. M., Gundert-Remy U. Critical evaluation of key evidence on the human health hazards of exposure to bisphenol A. Crit. Rev. Toxicol. 2011;41:263–291. doi: 10.3109/10408444.2011.558487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z., Paule M. G., Ferguson S. A. Low oral doses of bisphenol A increase volume of the sexually dimorphic nucleus of the preoptic area in male, but not female, rats at postnatal day 21. Neurotoxicol. Teratol. 2012;34:331–337. doi: 10.1016/j.ntt.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Herbison A. E. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V) Brain Res. Rev. 2008;57:277–287. doi: 10.1016/j.brainresrev.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman R. A., Jones B., Mann D. R., Wallen K. Timing of prenatal androgen exposure: anatomical and endocrine effects on juvenile male and female rhesus monkeys. Horm. Behav. 2000;38:52–66. doi: 10.1006/hbeh.2000.1608. [DOI] [PubMed] [Google Scholar]
- Hermans-Borgmeyer I., Susens U., Borgmeyer U. Developmental expression of the estrogen receptor-related receptor gamma in the nervous system during mouse embryogenesis. Mech. Dev. 2000;97:197–199. doi: 10.1016/s0925-4773(00)00422-6. [DOI] [PubMed] [Google Scholar]
- Hunt P. A., Susiarjo M., Rubio C., Hassold T. J. The bisphenol A experience: a primer for the analysis of environmental effects on mammalian reproduction. Biol. Reprod. 2009;81:807–813. doi: 10.1095/biolreprod.109.077008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y., Nagai A., Ikeda M. A., Hayashi S. Sexually dimorphic and estrogen-dependent expression of estrogen receptor beta in the ventromedial hypothalamus during rat postnatal development. Endocrinology. 2003;144:5098–5104. doi: 10.1210/en.2003-0267. [DOI] [PubMed] [Google Scholar]
- Kuhnemann S., Brown T. J., Hochberg R. B., MacLusky N. J. Sex differences in the development of estrogen receptors in the rat brain. Horm. Behav. 1994;28:483–491. doi: 10.1006/hbeh.1994.1046. [DOI] [PubMed] [Google Scholar]
- Kuiper G. G., Lemmen J. G., Carlsson B., Corton J. C., Safe S. H., van der Saag P. T., van der Burg B., Gustafsson J. A. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Kundakovic M., Gudsnuk K., Franks B., Madrid J., Miller R. L., Perera F. P., Champagne F. A. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc. Natl. Acad. Sci. U.S.A. 2013;110:9956–9961. doi: 10.1073/pnas.1214056110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S. M., Lee F. W., Ali A. A., Allaben W. T., Weis C. C., Leakey J. E. Modifying a displacement pump for oral gavage dosing of solution and suspension preparations to adult and neonatal mice. Lab Anim. 2010;39:149–154. doi: 10.1038/laban0510-149. [DOI] [PubMed] [Google Scholar]
- Lorke D. E., Susens U., Borgmeyer U., Hermans-Borgmeyer I. Differential expression of the estrogen receptor-related receptor gamma in the mouse brain. Brain Res. Mol. Brain Res. 2000;77:277–280. doi: 10.1016/s0169-328x(00)00063-2. [DOI] [PubMed] [Google Scholar]
- Losa S. M., Todd K. L., Sullivan A. W., Cao J., Mickens J. A., Patisaul H. B. Neonatal exposure to genistein adversely impacts the ontogeny of hypothalamic kisspeptin signaling pathways and ovarian development in the peripubertal female rat. Reprod. Toxicol. 2010;31:280–289. doi: 10.1016/j.reprotox.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima A., Kakuta Y., Teramoto T., Koshiba T., Liu X., Okada H., Tokunaga T., Kawabata S., Kimura M., Shimohigashi Y. Structural evidence for endocrine disruptor bisphenol A binding to human nuclear receptor ERR gamma. J. Biochem. 2007;142:517–524. doi: 10.1093/jb/mvm158. [DOI] [PubMed] [Google Scholar]
- McCaffrey K. A., Jones B., Mabrey N., Weiss B., Swan S. H., Patisaul H. B. Sex specific impact of perinatal bisphenol A (BPA) exposure over a range of orally administered doses on rat hypothalamic sexual differentiation. Neurotoxicology. 2013;36:55–62. doi: 10.1016/j.neuro.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy M. M. Estradiol and the developing brain. Physiol. Rev. 2008;88:91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick R., Lucier G., Wolfe M., Hall R., Stancel G., Prins G., Gallo M., Reuhl K., Ho S. M., Brown T., et al. Summary of the National Toxicology Program's report of the endocrine disruptors low-dose peer review. Environ. Health Perspect. 2002;110:427–431. doi: 10.1289/ehp.02110427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje L., Varayoud J., Luque E. H., Ramos J. G. Neonatal exposure to bisphenol A modifies the abundance of estrogen receptor alpha transcripts with alternative 5′-untranslated regions in the female rat preoptic area. J. Endocrinol. 2007;194:201–212. doi: 10.1677/JOE-07-0014. [DOI] [PubMed] [Google Scholar]
- Monje L., Varayoud J., Munoz-de-Toro M., Luque E. H., Ramos J. G. Exposure of neonatal female rats to bisphenol A disrupts hypothalamic LHRH pre-mRNA processing and estrogen receptor alpha expression in nuclei controlling estrous cyclicity. Reprod. Toxicol. 2010;30:625–634. doi: 10.1016/j.reprotox.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Morris J. A., Jordan C. L., Breedlove S. M. Sexual differentiation of the vertebrate nervous system. Nat. Neurosci. 2004;7:1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- NTP. Carcinogenesis bioassay of bisphenol A (CAS No. 80-05-7) in F344 rats and B6C3F1 mice (feed study) Natl. Toxicol. Program Tech. Rep. Ser. 1982;215:1–116. [PubMed] [Google Scholar]
- Nugent B. M., Schwarz J. M., McCarthy M. M. Hormonally mediated epigenetic changes to steroid receptors in the developing brain: Implications for sexual differentiation. Horm. Behav. 2010;59:338–344. doi: 10.1016/j.yhbeh.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orikasa C., Kondo Y., Hayashi S., McEwen B. S., Sakuma Y. Sexually dimorphic expression of estrogen receptor beta in the anteroventral periventricular nucleus of the rat preoptic area: implication in luteinizing hormone surge. Proc. Natl. Acad. Sci. U.S.A. 2002;99:3306–3311. doi: 10.1073/pnas.052707299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul H. B. Effects of environmental endocrine disruptors and phytoestrogens on the kisspeptin system. Adv. Exp. Med. Biol. 2013;784:455–479. doi: 10.1007/978-1-4614-6199-9_21. [DOI] [PubMed] [Google Scholar]
- Patisaul H. B., Fortino A. E., Polston E. K. Neonatal genistein or bisphenol-A exposure alters sexual differentiation of the AVPV. Neurotoxicol. Teratol. 2006;28:111–118. doi: 10.1016/j.ntt.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Patisaul H. B., Fortino A. E., Polston E. K. Differential disruption of nuclear volume and neuronal phenotype in the preoptic area by neonatal exposure to genistein and bisphenol-A. Neurotoxicology. 2007;28:1–12. doi: 10.1016/j.neuro.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Patisaul H. B., Sullivan A. W., Radford M. E., Walker D. M., Adewale H. B., Winnik B., Coughlin J. L., Buckley B., Gore A. C. Anxiogenic effects of developmental bisphenol a exposure are associated with gene expression changes in the juvenile rat amygdala and mitigated by soy. PLoS One. 2012;7:e43890. doi: 10.1371/journal.pone.0043890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul H. B., Whitten P. L., Young L. Regulation of estrogen receptor beta mRNA in the brain: opposite effects of 17β-estradiol and the phytoestrogen, coumestrol. Brain Res. Mol. Brain Res. 1999;67:165–171. doi: 10.1016/s0169-328x(99)00058-3. [DOI] [PubMed] [Google Scholar]
- Paxinos G., Watson C. The Rat Brain in Stereotaxic Coordinates. 6th ed. London: Academic Press; 2007. [Google Scholar]
- Richter C. A., Birnbaum L. S., Farabollini F., Newbold R. R., Rubin B. S., Talsness C. E., Vandenbergh J. G., Walser-Kuntz D. R., vom Saal F. S. In vivo effects of bisphenol A in laboratory rodent studies. Reprod. Toxicol. 2007;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman E. F. Roles of oestrogen receptors alpha and beta in behavioural neuroendocrinology: beyond Yin/Yang. J. Neuroendocrinol. 2008;20:873–879. doi: 10.1111/j.1365-2826.2008.01738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochester J. R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013;42:132–155. doi: 10.1016/j.reprotox.2013.08.008. [DOI] [PubMed] [Google Scholar]
- La Rosa P., Pellegrini M., Totta P., Acconcia F., Marino M. Xenoestrogens alter estrogen receptor (ER) alpha intracellular levels. PLoS One. 2014;9:e88961. doi: 10.1371/journal.pone.0088961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld C. S. Effects of maternal diet and exposure to bisphenol A on sexually dimorphic responses in conceptuses and offspring. Reprod. Domest. Anim. 2012;47(Suppl. 4):23–30. doi: 10.1111/j.1439-0531.2012.02051.x. [DOI] [PubMed] [Google Scholar]
- Rubin B. S., Lenkowski J. R., Schaeberle C. M., Vandenberg L. N., Ronsheim P. M., Soto A. M. Evidence of altered brain sexual differentiation in mice exposed perinatally to low, environmentally relevant levels of bisphenol A. Endocrinology. 2006;147:3681–3691. doi: 10.1210/en.2006-0189. [DOI] [PubMed] [Google Scholar]
- Ryan B. C., Hotchkiss A. K., Crofton K. M., Gray L. E., Jr In utero and lactational exposure to bisphenol A, in contrast to ethinyl estradiol, does not alter sexually dimorphic behavior, puberty, fertility, and anatomy of female LE rats. Toxicol. Sci. 2010;114:133–148. doi: 10.1093/toxsci/kfp266. [DOI] [PubMed] [Google Scholar]
- Schug T. T., Heindel J. J., Camacho L., Delclos K. B., Howard P., Johnson A. F., Aungst J., Keefe D., Newbold R., Walker N. J., et al. A new approach to synergize academic and guideline-compliant research: the CLARITY-BPA research program. Reprod. Toxicol. 2013;40:35–40. doi: 10.1016/j.reprotox.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Schwarz J. M., McCarthy M. M. Cellular mechanisms of estradiol-mediated masculinization of the brain. J. Steroid Biochem. Mol. Biol. 2008;109:300–306. doi: 10.1016/j.jsbmb.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selevan S. G., Kimmel C. A., Mendola P. Identifying critical windows of exposure for children's health. Environ. Health Perspect. 2000;108(Suppl. 3):451–455. doi: 10.1289/ehp.00108s3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semaan S. J., Kauffman A. S. Sexual differentiation and development of forebrain reproductive circuits. Curr. Opin. Neurobiol. 2010;20:424–431. doi: 10.1016/j.conb.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelby M. D. NTP-CERHR monograph on the potential human reproductive and developmental effects of bisphenol A. NTP CERHR MON. 2008;22(v):vii–ix. 1–64 passim. [PubMed] [Google Scholar]
- Shelnutt S., Kind J., Allaben W. Bisphenol A: Update on newly developed data and how they address NTP's 2008 finding of “Some Concern”. Food Chem. Toxicol. 2013;57:284–295. doi: 10.1016/j.fct.2013.03.027. [DOI] [PubMed] [Google Scholar]
- Simerly R. B. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu. Rev. Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- Souter I., Smith K. W., Dimitriadis I., Ehrlich S., Williams P. L., Calafat A. M., Hauser R. The association of bisphenol-A urinary concentrations with antral follicle counts and other measures of ovarian reserve in women undergoing infertility treatments. Reprod. Toxicol. 2013;42:224–231. doi: 10.1016/j.reprotox.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaab D. F. Sexual differentiation of the brain and behavior. Best Pract. Res. Clin. Endocrinol. Metab. 2007;21:431–444. doi: 10.1016/j.beem.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Takayanagi S., Tokunaga T., Liu X., Okada H., Matsushima A., Shimohigashi Y. Endocrine disruptor bisphenol A strongly binds to human estrogen-related receptor gamma (ERRgamma) with high constitutive activity. Toxicol. Lett. 2006;167:95–105. doi: 10.1016/j.toxlet.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Thomas P., Dong J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of endocrine disruption. J. Steroid Biochem. Mol. Biol. 2006;102:175–179. doi: 10.1016/j.jsbmb.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Vandenberg L. N., Chahoud I., Heindel J. J., Padmanabhan V., Paumgartten F. J., Schoenfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ. Health Perspect. 2010;118:1055–1070. doi: 10.1289/ehp.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viberg H., Fredriksson A., Buratovic S., Eriksson P. Dose-dependent behavioral disturbances after a single neonatal Bisphenol A dose. Toxicology. 2011;290:187–194. doi: 10.1016/j.tox.2011.09.006. [DOI] [PubMed] [Google Scholar]
- De Vries G. J. Minireview: Sex differences in adult and developing brains: Compensation, compensation, compensation. Endocrinology. 2004;145:1063–1068. doi: 10.1210/en.2003-1504. [DOI] [PubMed] [Google Scholar]
- Walker D. M., Kirson D., Perez L. F., Gore A. C. Molecular profiling of postnatal development of the hypothalamus in female and male rats. Biol. Reprod. 2012;87:129. doi: 10.1095/biolreprod.112.102798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallen K. Hormonal influences on sexually differentiated behavior in nonhuman primates. Front. Neuroendocrinol. 2005;26:7–26. doi: 10.1016/j.yfrne.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Wilson M. E., Rosewell K. L., Kashon M. L., Shughrue P. J., Merchenthaler I., Wise P. M. Age differentially influences estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) gene expression in specific regions of the rat brain. Mech. Ageing Dev. 2002;123:593–601. doi: 10.1016/s0047-6374(01)00406-7. [DOI] [PubMed] [Google Scholar]
- Wolstenholme J. T., Edwards M., Shetty S. R., Gatewood J. D., Taylor J. A., Rissman E. F., Connelly J. J. Gestational exposure to bisphenol A produces transgenerational changes in behaviors and gene expression. Endocrinology. 2012;153:3828–2838. doi: 10.1210/en.2012-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme J. T., Rissman E. F., Connelly J. J. The role of Bisphenol A in shaping the brain, epigenome and behavior. Horm. Behav. 2011;59:296–305. doi: 10.1016/j.yhbeh.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C. L., Schwarz J. S., Dean S. L., McCarthy M. M. Cellular mechanisms of estradiol-mediated sexual differentiation of the brain. Trends Endocrinol. Metab. 2010;21:553–561. doi: 10.1016/j.tem.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]