Abstract
Aims
Hypoxia induces vascular inflammation by a mechanism not fully understood. Emerging evidence implicates O-GlcNAc transferase (OGT) in inflammation. This study explored the role of OGT in hypoxia-induced vascular endothelial inflammatory response.
Methods and results
Hypoxia was either induced (1% O2 chamber) or mimicked by exposure to hypoxia-mimetic agents in cultured endothelial cells. Hypoxia increased hypoxia-inducible factor (HIF-1α) and inflammatory response (gene and protein expression of interleukin (IL)-6, IL-8, monocyte chemoattractant protein-1, and E-selectin) but, surprisingly, reduced OGT protein (not mRNA) levels. Hypoxia-mimetic CoCl2 failed to reduce OGT when proteasome inhibitors were present, suggesting proteasome involvement. Indeed, CoCl2 enhanced 26S proteasome functionality evidenced by diminished reporter (UbG76V-GFP) proteins in proteasome reporter cells, likely due to increased chymotrypsin-like activities. Mechanistically, β-TrCP1 mediated OGT degradation, since siRNA ablation of this E3 ubiquitin ligase stabilized OGT. Administration of the oxidative stress inhibitors reversed both proteasome activation and OGT degradation. Furthermore, up-regulation of OGT by stabilization, overexpression, or activation mitigated CoCl2-elicited inflammatory response. These observations were recapitulated in a mouse (C57BL/6J) model mimicking hypoxia, in which lung tissues presented higher levels of HIF-1α, proteasome activity, and inflammatory response, but lower levels of OGT (n = 5/group, hypoxia vs. normoxia, P < 0.05). However, administration of an activator of OGT (glucosamine: 1 mg/g/day, vehicle: saline, ip, 5 days) abolished the up-regulation of proteasome activity and inflammatory response (n = 5/group, the treated vs. untreated hypoxia groups, P < 0.05).
Conclusions
26S proteasome-mediated OGT reduction contributed to hypoxia-induced vascular endothelial inflammatory response. Modulation of OGT may represent a new approach to treat diseases characterized by hypoxic inflammation.
Keywords: 26S proteasomes, Hypoxia, Inflammatory response, O-GlcNAc, Oxidative stress
1. Introduction
Hypoxia, the decrease in oxygen bioavailability, is involved in a diverse range of physiological and pathophysiological processes, including development, wound healing, inflammation, vascular disease, and cancer.1 Hypoxia induces inflammation, which is intertwined at various levels.2 At molecular and cellular levels, it contributes to inflammation through the regulation of gene expression via key oxygen-sensitive transcriptional regulators such as the hypoxia-inducible factor (HIF-1α) and NF-κB, which activate immune cells of both the innate and adaptive immunity; in clinics, hypoxia promotes inflammation, which is associated with chronic inflammation in obesity,3 ischaemic injury, and loss of graft function in organ transplantation patients.2 Similarly, a causal connection of hypoxia to vascular endothelial inflammatory response is emerging and becoming increasingly appreciated, as its clinical relevance has been shown in several disease states, including obstructive sleep apnoea,4 pulmonary remodelling,5 and neuron degenerative diseases.6 Understanding the underlying mechanism will foster novel strategies for the treatment of inflammatory diseases. However, the mechanism that regulates hypoxia-mediated inflammation remains elusive.
Hypoxia increases HIF-1α protein stability by preventing its proteasome-mediated degradation.7 However, hypoxia per se reduced stability of several functional proteins through the 26S proteasome.8–14 The 26S proteasome is an essential component of the ubiquitin proteasome system responsible for regulated degradation of intracellular proteins.15 Deregulation of the proteasome causes inappropriate destruction or accumulation of specific proteins and ensuing pathological consequences.15 The role of the 26S proteasome in vascular inflammatory response is well recognized in normoxia, which operates through modulation of the NF-κB pathway,2 functions of endothelial cells16 and macrophages,17 and even generation of stressor markers.18 In contrast, the relationship between 26S proteasome functionality and hypoxia-induced vascular endothelial inflammatory response is unknown.
Post-translational modification can serve as a stress sensor in the adaptive response to a stressor (such as hypoxia).19 O-GlcNAcylation appears to be an emerging sensor of such since O-GlcNAc modification exerts protection against hypoxia-induced organ (e.g. heart) injury.20 O-GlcNAc modification (O-GlcNAcylation) is catalyzed by O-GlcNAc transferase (OGT), which promotes the attachment of O-GlcNAc to nuclear/cytoplasmic proteins.21 The substrate and activator of OGT for O-GlcNAcylation is UDP-GlcNAc, which is generated through the hexosamine biosynthetic pathway from 2 to 5% of the glucose entering a cell.22 As such, OGT is nutrient sensitive and O-GlcNAcylation of target proteins could turn a binary switch between anabolism and catabolism.23 Most importantly, recent studies have shown that OGT regulates inflammatory response in normoxia through NF-κB-dependent mechanisms involving O-GlcNAc modification.24–27 Interestingly, O-GlcNAc modification has been shown to regulate 26S proteasomes, being identified as the first endogenous inhibitor of 26S proteasomes,28 although the physiological relevance has yet to be established.29
In light of the potential connections of OGT to hypoxia, 26S proteasomes, and inflammation, we sought to explore the role of OGT in hypoxia-induced inflammation. We identified OGT as an important player in hypoxia-dependent endothelial vascular inflammatory response in both cell and mouse models.
2. Methods
2.1. Materials
The antibodies against β-actin, green fluorescent protein (GFP), or OGT were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Other antibodies used: HIF-1α from NOVUS Biologicals (Littleton, CO, USA); superoxide dismutase 1 (SOD1), β-TrCP1, and peroxidase-conjugated secondary antibodies from Cell Signaling (Danvers, MA, USA); anti-rabbit IgG conjugated to a fluorescent dye Alexa Fluor 488 and 594 from Invitrogen (Carlsbad, CA, USA). Protease inhibitor cocktail, ProLong® Gold and SlowFade® Gold Antifade Reagents, fluorogenic substrate Suc-LLVY-7-amido-4-methylcoumarin for chymotrypsin-like activity assay, and all the PCR primers from Sigma (St. Louis, MO, USA); mito-TEMPO-H (mTempol) from Enzo Life Sciences (Farmingdale, NY, USA); l-nitroarginine methyl ester (l-NAME), MG132, lactacystin, and epoxomicin from EMD Chemicals (San Diego, CA, USA); negative control-siRNA or target-specific siRNA duplex against β-TrCP1 from Santa Cruz Biotechnology. Adenoviral vectors overexpressing OGT or GFP were prepared30,31 and kindly provided by Dr Gerald Hart from Johns Hopkins University. Plasmids β-TrCP1 (Addgene plasmid 20178)32 were kindly provided by Dr Yue Xiong from Lineberger Comprehensive Cancer Center, USA; UbG76V-GFP (Addgene plasmid 11954) were prepared33 and kindly provided by Dr Nico Dantuma from Karolinska Institutet, Sweden. All other chemicals were bought from Fisher Scientific (Pittsburgh, PA, USA). Human umbilical vein endothelial cells (HUVECs) were obtained from ATCC (Manassas, VA, USA).
2.2. Cells, western blot, proteasome function assay, transfection, and imaging
The detailed methodology was included in Supplementary material online. Briefly, (i) HUVECs were cultured as reported34 except for hypoxia experiments in which cells were cultured in a 1% O2 chamber; (ii) western blot was performed as reported;34 (iii) 26S proteasome function was assessed by measuring reporter UbG76V-GFP levels and chymotrypsin-like activity;34 (iv) adenoviral transfection and siRNA infection were conducted as reported;34,35 and (v) inflammatory cytokine/chemokine/adhesion molecule gene expression analysis was performed34,36 with primers listed in Supplementary material online, Table S1. Their protein levels were quantified by using specific ELISA kits. (vi) Imaging by immunofluorescent staining was performed including tissue fixation, slide staining, and quantification as previously reported.35,37
2.3. Mice and hypoxia mimicking
Male mouse pups (C57BL/6J) were used (n = 20), which were generated from the breeding pairs provided by the Jackson Laboratory (Bar Harbor, ME, USA). The pups were subjected to an oxygen-induced retinopathy (OIR) model according to an established protocol,38 in which lung hypoxic pathology was presented.39 In this model, the pups (7 days old, ∼5 g) were exposed to hyperoxia (75% O2 chamber, 5 days) and returned to room air for 5 days where the pups experienced relative hypoxia. Some of them received treatment on the first day returning from hyperoxia (glucosamine: 1 mg/g/days, vehicle: saline; ip, 5 days). The mice were sacrificed (by CO2-induced euthanasia) for molecular and immunohistochemistry analyses. The CO2-induced euthanasia was performed by placing mice in a container exposed to a gradually increasing concentration of CO2. The mice ceased breathing within 30 s and were left in the CO2 atmosphere for a total of 5 min. The animal protocols were reviewed and approved by the University of Oklahoma Institute Animal Care and Use Committee. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health.
2.4. Statistical analysis
Data were reported as mean ± SEM. ANOVA was used to compare means of different experimental groups, and Tukey's tests were used as post hoc tests, as previously reported.34 A P-value <0.05 was considered statistically significant.
3. Results
3.1. Hypoxia or hypoxia-mimetic agents increase HIF-1α and induce endothelial inflammatory response, accompanied by reduction in levels of OGT protein
To establish a cell model for this study, we first assessed the inflammatory response of endothelial cells to hypoxia-mimetic agent cobalt. As expected, cobalt increased gene expression of IL-6, IL-8, monocyte chemoattractant protein-1 (MCP-1), and E-selectin (Figure 1A), which was associated with elevation of HIF-1α in a time- (Figure 1B) and dose- (Figure 1C) dependent manner. In contrast, a reduction of OGT protein levels was detected (Figure 1B and C), while the gene expression of OGT remained unchanged (Figure 1D). Similar results of OGT reduction were obtained when cobalt was replaced with desferrioxamine (DFO), another hypoxia-mimetic agent (Figure 1E). To confirm a true hypoxia impact, we repeated the experiments but cultured the cells in a hypoxia chamber (1% O2). Indeed, the hypoxia-exposed cells increased HIF-1α as expected but decreased OGT in a time-dependent fashion (Figure 1F), similar to those observed in hypoxia-mimetic agent-treated cells (Figure 1B, C, and E). Hence, we used cobalt in the rest of the cell studies.
Figure 1.
Hypoxia or hypoxia-mimetic agents induce endothelial inflammatory response and reduce OGT protein. The HUVECs were treated with hypoxia-mimetic agents or hypoxia. (A) Gene expression of the indicated inflammatory cytokine/chemokine/adhesion molecule (cobalt: 200 µM for up to 6 h); (B) time-dependent OGT reduction (cobalt: 200 µM up to 12 h); (C) dose-dependent reduction of OGT (cobalt: up to 300 µM for 12 h); (D) unchanged OGT mRNA levels (cobalt: 200 µM for up to 12 h); (E) time-dependent OGT reduction (DFO: 200 µM, up to 12 h); (F) time-dependent OGT reduction (hypoxia: 1% O2 up to 36 h); (B–F) reciprocal relationship between OGT and HIF-1α protein levels. All blots shown are representative of three independent experiments. The results (n = 5 for gene expression and n = 3 for western blot) were analysed by ANOVA with Tukey's tests as post hoc tests. Asterisks indicate significant difference vs. control. Cobalt, cobalt chloride; DFO, desferrioxamine; HIF-1α, hypoxia-inducible factor 1 alpha; OGT, O-GlcNAc transferase.
3.2. OGT reduction is due to decreased protein stability in cobalt-treated endothelial cells
Hypoxia-induced OGT reduction was apparently not due to alteration in gene expression (Figure 1D), alluding to altered protein stability. To confirm this, we performed a chase experiment with cycloheximide, an inhibitor of the protein translational elongation. As shown, the presence of cycloheximide reduced protein levels of OGT in a time-dependent fashion as expected (Figure 2A); when cobalt was present, however, the reduction became significantly faster (Figure 2A), suggesting that the OGT loss was due to decreased protein stability.
Figure 2.
Cobalt accelerates 26S proteasomal degradation of OGT through β-TrCP1. Western blot of HUVEC lysates: (A) cells were incubated with either vehicle or cobalt for 8 h after incubation with CHX (5 µM) for indicated time. (B) Cobalt-challenged cells were treated with DMSO (vehicle) or respective proteasome inhibitors as indicated. (C) Cobalt-treated cells were incubated with CQ (50 µM) and Z-DEVD (0.1 µM), the inhibitors of autophagy and caspase, respectively, using vehicle (cell culture medium) as the control. (D) Cells transfected either with control-siRNA or β-TrCP1-siRNA were treated with cobalt (200 µM) for 12 h. (E) Cells overexpressing either vector only or β-TrCP1 via plasmid transfection were incubated with DMSO or MG132 (0.1 µM) for 12 h. All blots shown are representative of three independent experiments. Quantifications were made based on band signal intensity of target protein normalized with those of loading control β-actin. The results (n = 3) were analysed by ANOVA with Tukey's tests as post hoc tests. Asterisks indicate significant difference vs. control. CHX, cycloheximide; Cobalt, cobalt chloride; CQ, chloroquine; DMSO, dimethyl sulfoxide; Epo, epoxomicin; Lac, lactacystin; NS, not significant vs. control; OGT, O-GlcNAc transferase; Z-DEVD, Z-Asp-Glu-Val-Asp fluoromethyl ketone (or Z-DEVD-fmk).
3.3. Cobalt induces OGT degradation through 26S proteasomes
To identify the proteolytic system responsible for OGT loss, we administrated inhibitors of various proteolytic enzymes/systems. Western blot indicated that OGT protein reduction was prevented with the presence of structurally unrelated proteasome inhibitors (Figure 2B), but not with the inhibitor of autophagy (Figure 2C, CQ) or caspase (Figure 2C, Z-DEVD). These data implicated that the OGT reduction attributed to proteasomal degradation.
3.4. E3 ubiquitin ligase β-TrCP1 mediates proteasomal degradation of OGT
E3 ubiquitin ligase is essential in substrate specificity of the 26S proteasome. To identify the E3 ligase for OGT degradation in hypoxia, we took a loss-of-function approach with siRNA. As shown, β-TrCP1-siRNA ablation significantly reduced this E3 ligase (Figure 2D); cobalt treatment diminished OGT in control-siRNA (Figure 2D) but not in β-TrCP1-siRNA-treated cells (Figure 2D). These were confirmed by a gain-of-function approach: β-TrCP1 overexpression reduced OGT (Figure 2E), which was prevented by proteasome inhibition (Figure 2E). A recent study showed that HSP90 blockade decreased OGT through another E3 ligase (CHIP).40 However, CHIP siRNA did not affect cobalt-induced OGT reduction (data not shown). Furthermore, β-TrCP1-mediated OGT reduction induced by cobalt (Figure 2D) was reproduced in cells challenged with hypoxia (see Supplementary material online, Figure S1), indicating a specific role for β-TrCP1 in OGT degradation in hypoxia.
3.5. Hypoxia elevates 26S proteasome functionality
We wondered whether hypoxia affected 26S proteasome functionality. The assessment of 26S proteasome functionality predominantly relied on protease-like activity assay until the first report of imaging 26S proteasome in living cells with a reporter system,41 which was engineered with a surrogate protein substrate (GFP) fused with a ubiquitin mutant (UbG76V).42 By taking this advantage, we could detect augmented 26S proteasome functionality in diabetic mice and cultured cells.34 Here with the same approach, we detected reduction of reporter proteins over time of cobalt treatment in GFP-UbG76V-expressing cells (Figure 3A), indicating enhanced 26 proteasome functionality. Such an enhancement attributed, at least in part, to elevated protease-like activity (Figure 3B), which was confirmed with proteasome inhibitors (Figure 3C).
Figure 3.
Cobalt treatment increases 26S proteasome functionality in endothelial cells. The 26S proteasome reporter (UbG76V-GFP)-expressing endothelia cells were incubated with cobalt for up to 6 h. The cell lysates were subjected to (A) western blot for the levels of proteasome reporter protein and (B) assay of proteasome chymotrypsin-like activity. (C) Proteasome chymotrypsin-like activity in the presence of MG132 (0.1 µM, pre-incubation for 1 h) in cobalt-treated cells. All blots shown are representative of three independent experiments. The results (n = 3 for western blot and n = 5 for proteasome activity) were analysed by ANOVA with Tukey's tests as post hoc tests. *P < 0.05. Cobalt, cobalt chloride; Epo, epoxomicin; GFP, green fluorescent protein; Lac, lactacystin; Ub, ubiquitin.
3.6. Reactive oxygen or nitrogen species mediate cobalt-enhanced 26S proteasome activity in endothelial cells
Hypoxia could increase reactive oxygen or nitrogen species (ROS/RNS) in endothelial cells.43 We wondered whether hypoxia enhanced 26S proteasome functionality through ROS/RNS, like normoxia.34,44 To this end, we incubated the cells with each of the following agents before cobalt challenge: l-NAME (a selective inhibitor of nitric oxide synthase), mTempol (a mitochondria-targeted antioxidant with O2− scavenging properties),45 and uric acid (UA; a potent scavenger of ONOO− which is generated by O2− and NO). We observed that pre-incubation with l-NAME blocked the reduction of reporter protein levels (Figure 4A); similar results were obtained either with mTempol (Figure 4A) or with UA (Figure 4A), suggesting a key role ROS/RNS in the elevation of 26S proteasome activity.
Figure 4.
ROS/RNS mediates cobalt-enhanced 26S proteasome functionality and OGT reduction. The HUVECs were treated with cobalt in the presence or absence of ROS inhibitor followed by (A) proteasome chymotrypsin-like activity assay and (B–E) western blotting in the presence of (B) mTempol (1 mM, pre-incubated for 1 h); (C) adenoviral overexpression of either GFP or SOD1; (D) UA (100 µM, pre-incubated for 1 h); and (E) l-NAME (1 mM, pre-incubated for 1 h). The results (n = 5/group) were analysed by ANOVA with Tukey's tests as post hoc tests. *P < 0.05. Ad-, adenoviral infection; Cobalt, cobalt chloride; GFP, green fluorescent protein; l-NAME, l-nitroarginine methyl ester; OGT, O-GlcNAc transferase; NS, not significant vs. control; ROS, reactive oxygen species; SOD, superoxide dismutase; UA, uric acid.
3.7. Blockade of ROS/RNS reverses cobalt-induced OGT degradation in endothelial cells
To identify the downstream outcome of suppressed proteasome activities by ROS/RNS inhibitors, we measured OGT protein levels. As depicted, administration of mTempol (Figure 4B), UA (Figure 4C), or l-NAME (Figure 4D) abrogated OGT reduction. The effect of mTempol was further confirmed through SOD1 overexpression (Figure 4E). These data support the notion that ROS mediates hypoxia-enhanced 26S proteasome functionality and the resultant OGT reduction.
3.8. Up-regulation of OGT mitigates cobalt-induced inflammatory response in endothelial cells
To establish the role of OGT in hypoxia-induced inflammation, we took the approach of OGT up-regulation. Cobalt treatment reproducibly promoted the enhanced gene and protein expression profile of inflammatory cytokine/chemokine/adhesion molecule (Figure 5A/D, B/E, and C/F); however, incubation of UDP-GlcNAc, an activator of OGT (Figure 5A/D) or adenoviral overexpression of OGT (Figure 5B/E), significantly blocked the profile. Likewise, siRNA knocking down β-TrCP1 (which stabilized OGT as shown in Figure 2D) inhibited the profile (Figure 5C/F). In summary, these data support a causal role of OGT down-regulation in hypoxia-enhanced inflammatory response in endothelial cells.
Figure 5.
OGT up-regulation prevents inflammatory response in cobalt-treated endothelial cells. Profiles of inflammatory cytokine/chemokine/adhesion molecule gene and protein expression (MCP-1, IL-8, E-selectin, and IL-6) in HUVECs, which were treated with cobalt in the presence of (A/D) O-GlcNAc (25 µM, pre-incubation for 1 h); (B/E) overexpression of either GFP (control) or OGT by adenoviral infection; (C/F) infection with siRNA of either control or β-TrCP1. The results (n = 5/group) were analysed with ANOVA to compare means of different experimental groups with Tukey's tests as post hoc tests. *P < 0.05 vs. the vehicle-treated. Ad-, adenoviral infection; Cobalt, cobalt chloride; GFP, green fluorescent protein; MCP-1, monocyte chemoattractant protein-1; OGT, O-GlcNAc transferase.
3.9. Hypoxia enhances 26S proteasome activity, OGT protein reduction, and vascular inflammatory response in vivo
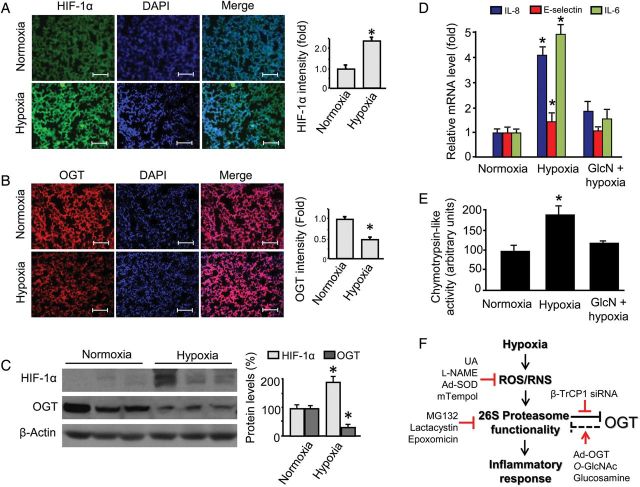
To demonstrate the findings in physiologically relevant settings, we adopted an OIR mouse model. OIR is an established model for ischaemic retinopathy study. However, recent studies reveal that hypoxic pathology extends beyond retina, e.g. to the lung in OIR.39 In this model, mouse pups (along with their nursing mothers) were exposed to 75% oxygen from postnatal days 7–12 in a Plexiglass chamber connected to an oxygen regulator (Pro-Ox, model 110; Reming Bioinstruments, Redfield, NY, USA). At postnatal day 12, the animals were returned to room air where the pups experienced relative hypoxia. The mice were sacrificed at postnatal day 17 for assays. Indeed, in the slides prepared from lung tissue of the OIR pups, the HIF-1α staining was elevated compared with the controls (Figure 6A). This elevation was accompanied by reduced OGT protein staining (Figure 6B). The reciprocal relationship between HIF-1α and OGT protein levels was further confirmed in western blot (Figure 6C), which also reproduced the findings in cultured cells (Figure 1B, C, E, and F). Importantly, the same tissues presented enhanced gene (Figure 6D) and protein (see Supplementary material online, Figure S2) expressions of pro-inflammatory cytokine/chemokine/adhesion molecule, like the cell model (Figure 1A). Furthermore, the hypoxic lung tissues increased proteasomal chymotrypsin-like activities (Figure 6E), nicely recapitulating the cell model (Figure 3B and C).
Figure 6.
Hypoxia enhances vascular inflammatory response, which is obliterated by administration of glucosamine in vivo. Male and age matched (7 days) wild-type (C57BL/6J) mice were used in an OIR model: mice were first subjected to high oxygen treatment (75% O2 chamber) for 5 days and transferred to room air environment for another 5 days, while the control mice were kept under room air all the time. Some hypoxia-exposed mice received treatment (glucosamine: 1 mg/g/days; vehicle: saline; ip, 5 days, n = 5/group) starting on the first day returned to room air environment. After treatment, lung tissues were collected for (A–B) immunohistochemistry staining of (A) HIF-1α and (B) OGT; (C) western blotting and quantification of OGT protein levels; and (D) gene expression profiles of MCP-1, IL-8, E-selectin, and IL-6. The results (n = 5/group) were analysed with ANOVA to compare means of different experimental groups with Tukey's tests as post hoc tests. *P < 0.05 vs. the vehicle- or control-siRNA-treated. (E) Proposed mechanism underlying hypoxia-enhanced vascular inflammatory response: role of ROS/RNS, 26S proteasome functionality, and OGT (see Discussion for details). Ad-, adenoviral overexpression; DAPI, 4′,6-diamidino-2-phenylindole; GlcN, glucosamine; l-NAME, l-nitroarginine methyl ester; OGT, O-GlcNAc transferase; ROS/RNS, reactive oxygen species/reactive nitrogen species; UA, uric acid. Denotations: ‘arrow’, activation or up-regulation; ‘inverted T’, inhibition or down-regulation; ‘line/arrow in red’, treatments or interventions employed in this study; ‘broken line’, based on published data. The scale bar indicates 50 μM.
3.10. Administration of glucosamine inhibits 26S proteasomes and obliterates hypoxia-induced inflammatory response in vivo
Next, we took a gain-of-function approach to establish the role of OGT. This was achieved by the administration of glucosamine (an OGT activator in vivo) in mice exposed to hypoxia in the OIR model, specifically, in the 5-day (postnatal day 12–17) period of relative hypoxia in this model mentioned earlier. Cytokine/chemokine/adhesion molecule profiles showed that administration of glucosamine significantly reduced the otherwise up-regulated gene (Figure 6D) and protein (see Supplementary material online, Figure S2) expression of IL-6, MCP-1, and E-selectin in lung tissues (n = 5/group, glucosamine vs. vehicle hypoxic mice, P < 0.05), in line with the impacts of O-GlcNAc administration in cobalt-treated endothelial cells (Figure 5A). Importantly, the suppressed cytokine/chemokine/adhesion molecule profiles were correlated with reduced chymotrypsin-like activity (glucosamine vs. vehicle in hypoxic mice, P < 0.05) (Figure 6E), reminiscent of the suppressive effect of O-GlcNAc on 26S proteasomes in endothelial cells under normoxia46 likely through OGT activation.28,46
4. Discussion
The connection between hypoxia and the resultant vascular inflammatory response is well recognized; however, regulation of this connection remains poorly defined. The present study provided the first evidence that stability of OGT played an important role in the vascular endothelial inflammatory response induced by hypoxia. Mechanistically, hypoxia elevated 26S proteasome functionality via generation of ROS/RNS. It was not the autophagy or caspase but the 26S proteasome that reduced OGT, involving an E3 ubiquitin ligase β-TrCP1. More importantly, up-regulation of OGT either through adenoviral overexpression or by administration of its activator UDP-GlcNAc (cell model) or glucosamine (mouse model) prevented hypoxia-mediated vascular inflammatory response. Given that results from the mouse model of hypoxia recapitulated the major findings in the cell models, we have uncovered an alternative mechanism by which hypoxia regulates inflammatory response involving up-regulation of 26S proteasome functionality and down-regulation of OGT in vascular endothelial cells (Figure 6F).
Hypoxia (diminished tissue oxygen supply) is a common physiological and pathophysiological occurrence; as a result, adaptation to hypoxia has become essential for survival.2 Cellular adaptation to hypoxia leads to the transcriptional induction of a series of genes that participate in angiogenesis, iron metabolism, glucose metabolism, and cell proliferation/survival. The transcriptional responses to hypoxia result in inflammatory cellular phenotypes; however, much less is known about the protein quality response to hypoxia in pro-inflammatory phenotype in vascular endothelial cells. To the best of our knowledge, this is the first demonstration in both cell and mouse models that hypoxia enhanced 26S proteasome functionality. Evidence provided in the present study support such a possibility that hypoxia-mimetic agent enhanced 26S proteasome functionality in a functional reporter system (Figure 3A), which was attributable to increased protease-like activity of the proteasome (Figure 3B and C), an effect recapitulated in hypoxia-exposed mice in vivo (Figure 6E). Of note, the involvement of ROS/RNS in the enhancement of 26S proteasome functionality under hypoxia is in line with those observed in normoxia.34,44,47 The enhanced 26S proteasome functionality seemingly resulted in elevated inflammatory response in both cell (Figures 1A and 5) and mouse (Figure 6D) models of hypoxia, provided the relationship between 26S proteasome functionality and inflammatory response demonstrated in normoxia.34 It is likely that hypoxia-boosted 26S proteasomes as an independent factor accelerate the degradation of proteins that have been identified as proteasome substrates in hypoxia, such as the alveolar epithelial Na+ channel,8 Aquaporin 5,9 nuclear factor Nrf1,10 myofibrillar protein (contributed to skeletal muscle atrophy),11 transcriptional factors SNAIL112 and STRA1,13 and oestrogen receptor alpha.14 If confirmed, it should attest to the notion that hypoxia-mediated enhancement of 26S proteasome functionality has broader implications in various cellular consequences.
Identification of OGT as a substrate of 26S proteasomes in hypoxia represented another novel aspect of the present study. The novelty is two-fold. First, OGT was demonstrated here as a functional substrate of 26S proteasomes: while proteasomal degradation of OGT was closely correlated with hypoxia-mediated vascular inflammatory response, up-regulation of OGT either genetically or pharmacologically attenuated the response (Figure 5A and B and Figure 6D and E). So is the direct correlation between down-regulation of OGT and up-regulation of proteasome activity (Figures 1C vs. 3B). Since OGT activation increased O-GlcNAc modification which could block 26S proteasome functionality,29 modulation of OGT may represent a new strategy in treating hypoxia-induced inflammatory deregulation (Figure 6F, broken line). Secondly, the study identified β-TrCP1 (not CHIP) as the E3 ubiquitin ligase responsible for OGT degradation in hypoxia, although CHIP-mediated OGT degradation induced by suppression of HSP90 in normoxia.40 Interestingly, β-TrCP1 is also the ligase responsible for I-κB degradation, which could activate the NF-κB pathway and promote inflammatory reactions induced either by lipopolysaccharide (LPS) or tumour necrosis factor (TNF)-α. Although it was not the focus to confirm this pathway in the present study, it could be expected that β-TrCP1 has a similar function in hypoxia as in normoxia at this point, provided the active role of β-TrCP1 in mediating OGT degradation (Figure 2D and E) and the elevated vascular inflammatory response (Figure 5C). Moreover, β-TrCP1 also targets other important proteins with diverse functions, such as p53, H-Ras, Smad4, and β-catenin for ubiquitin-mediated degradation. It would be interesting to determine whether these β-TrCP1 targets are also involved in hypoxia-mediated vascular inflammatory response.
The next important indication of the present study would be the connection of hypoxia-mediated vascular endothelial inflammatory response to OGT, a sensor of nutrition and metabolism. OGT functions as this sort of sensor largely because of its substrate and activator: UDP-GlcNAc, which is generated through the hexosamine biosynthetic pathway and is dependent on the state of metabolism.23 This connection is physiologically relevant and important, because metabolism not only responds to hypoxia but also regulates hypoxia-mediated response including vascular inflammation.48 The O-GlcNAc-increasing agents such as glucosamine prevented inflammation-induced vascular dysfunction.49 Similarly, increased O-GlcNAc levels have been shown to reverse NF-κB activation in macrophage50 and inhibit inflammatory responses to acute arterial injury.26 However, O-GlcNAc modification-associated NF-κB-activation has also been reported.51 The discrepancies among them may attribute to a major difference in modes of OGT activation, duration and site of GlcNAc modulation, type of inflammatory stimulator, cell and animal models adopted, and even the oxygen levels, since dynamics of OGT-mediated O-GlcNAc modification are complex and remain incompletely elucidated.21,52–54 Therefore, the functional outcome of OGT up-regulation (Figure 5A and B and Figure 6D and E) could be appropriately interpreted as a net result of the opposing effects, if any, of OGT. Chronic diabetes sustains low-grade inflammation by mechanisms not fully elucidated. Enhanced proteasome functionality has been reported in early diabetes.34 Given that proteasome-mediated OGT reduction connects inflammatory response, it would be interesting to investigate whether OGT becomes dysfunctional in chronic diabetes leading to sustained inflammation, although high glucose failed to change or reverse OGT reduction induced either by cobalt (see Supplementary material online, Figure S3A) or by hypoxia (see Supplementary material online, Figure S3B) in cultured endothelial cells.
Taken together, hypoxia through ROS/RNS enhances 26S proteasome functionality, which accelerates β-TrCP1-mediated proteasomal degradation of OGT and enhances vascular endothelial inflammatory response. We conclude that modulation of OGT regulates vascular endothelial inflammatory response in hypoxia, which bears the hope to develop pharmacological strategies to treat diseases characterized by hypoxic inflammation.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
Research reported in this study was supported in part by an Institutional Development Award (IDeA) the Center of Biomedical Research Excellence (COBRE) from the National Institute of General Medical Sciences of the National Institutes of Health (P20GM104934). This work was also supported in part by the National Scientist Development Grant (10SDG2600164) from the American Heart Association, a Junior Faculty Award (1-12-JF-58) from the American Diabetes Association, and a research award from the Oklahoma Center for Advancement of Science and Technology (HR11-200) (all to J.X.).
Supplementary Material
Acknowledgements
The authors thank Jeff Smith for his valuable technical assistance.
Conflict of interest: none declared.
References
- 1.Scholz CC, Taylor CT. Hydroxylase-dependent regulation of the NF-κβ pathway. Biol Chem. 2013;394:479–493. doi: 10.1515/hsz-2012-0338. [DOI] [PubMed] [Google Scholar]
- 2.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ye J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int J Obes (Lond) 2009;33:54–66. doi: 10.1038/ijo.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lavie L. Intermittent hypoxia: the culprit of oxidative stress, vascular inflammation and dyslipidemia in obstructive sleep apnea. Expert Rev Respir Med. 2008;2:75–84. doi: 10.1586/17476348.2.1.75. [DOI] [PubMed] [Google Scholar]
- 5.Ziesche R, Petkov V, Williams J, Zakeri SM, Mosgoller W, Knofler M, Block LH. Lipopolysaccharide and interleukin 1 augment the effects of hypoxia and inflammation in human pulmonary arterial tissue. Proc Natl Acad Sci USA. 1996;93:12478–12483. doi: 10.1073/pnas.93.22.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tripathy D, Sanchez A, Yin X, Luo J, Martinez J, Grammas P. Thrombin, a mediator of cerebrovascular inflammation in ad and hypoxia. Front Aging Neurosci. 2013;5:19. doi: 10.3389/fnagi.2013.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gille T, Randrianarison-Pellan N, Goolaerts A, Dard N, Uzunhan Y, Ferrary E, Hummler E, Clerici C, Planes C. Hypoxia-induced inhibition of ENAC in the lung: role of Nedd4-2 and the ubiquitin-proteasome pathway. Am J Respir Cell Mol Biol. 2013;50:526–537. doi: 10.1165/rcmb.2012-0518OC. [DOI] [PubMed] [Google Scholar]
- 9.Kawedia JD, Yang F, Sartor MA, Gozal D, Czyzyk-Krzeska M, Menon AG. Hypoxia and hypoxia mimetics decrease aquaporin 5 (AQP5) expression through both hypoxia inducible factor-1alpha and proteasome-mediated pathways. PLoS ONE. 2013;8:e57541. doi: 10.1371/journal.pone.0057541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chepelev NL, Bennitz JD, Huang T, McBride S, Willmore WG. The NRF1 CNC-BZIP protein is regulated by the proteasome and activated by hypoxia. PLoS ONE. 2011;6:e29167. doi: 10.1371/journal.pone.0029167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaudhary P, Suryakumar G, Prasad R, Singh SN, Ali S, Ilavazhagan G. Chronic hypobaric hypoxia mediated skeletal muscle atrophy: role of ubiquitin-proteasome pathway and calpains. Mol Cell Biochem. 2012;364:101–113. doi: 10.1007/s11010-011-1210-x. [DOI] [PubMed] [Google Scholar]
- 12.Vinas-Castells R, Beltran M, Valls G, Gomez I, Garcia JM, Montserrat-Sentis B, Baulida J, Bonilla F, de Herreros AG, Diaz VM. The hypoxia-controlled FBXl14 ubiquitin ligase targets SNAIL1 for proteasome degradation. J Biol Chem. 2010;285:3794–3805. doi: 10.1074/jbc.M109.065995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ivanova AV, Ivanov SV, Danilkovitch-Miagkova A, Lerman MI. Regulation of STRA13 by the von Hippel-Lindau Tumor suppressor protein, hypoxia, and the UBC9/ubiquitin proteasome degradation pathway. J Biol Chem. 2001;276:15306–15315. doi: 10.1074/jbc.M010516200. [DOI] [PubMed] [Google Scholar]
- 14.Stoner M, Saville B, Wormke M, Dean D, Burghardt R, Safe S. Hypoxia induces proteasome-dependent degradation of estrogen receptor alpha in ZR-75 breast cancer cells. Mol Endocrinol. 2002;16:2231–2242. doi: 10.1210/me.2001-0347. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz AL, Ciechanover A. The ubiquitin-proteasome pathway and pathogenesis of human diseases. Annu Rev Med. 1999;50:57–74. doi: 10.1146/annurev.med.50.1.57. [DOI] [PubMed] [Google Scholar]
- 16.Stangl K, Stangl V. The ubiquitin-proteasome pathway and endothelial (dys) function. Cardiovasc Res. 2010;85:281–290. doi: 10.1093/cvr/cvp315. [DOI] [PubMed] [Google Scholar]
- 17.Qureshi N, Vogel SN, Van Way C, III, Papasian CJ, Qureshi AA, Morrison DC. The proteasome: a central regulator of inflammation and macrophage function. Immunol Res. 2005;31:243–260. doi: 10.1385/IR:31:3:243. [DOI] [PubMed] [Google Scholar]
- 18.Lalu MM, Xu H, Sankaralingam S, Davidge ST. Proteasome inhibition decreases inflammation in human endothelial cells exposed to lipopolysaccharide. J Cardiovasc Pharmacol. 2012;60:381–389. doi: 10.1097/FJC.0b013e3182657eec. [DOI] [PubMed] [Google Scholar]
- 19.Storey KB, Wu CW. Stress response and adaptation: a new molecular toolkit for the 21st century. Comp Biochem Physiol A Mol Integr Physiol. 2013;165:417–428. doi: 10.1016/j.cbpa.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 20.Ngoh GA, Facundo HT, Hamid T, Dillmann W, Zachara NE, Jones SP. Unique hexosaminidase reduces metabolic survival signal and sensitizes cardiac myocytes to hypoxia/reoxygenation injury. Circ Res. 2009;104:41–49. doi: 10.1161/CIRCRESAHA.108.189431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–1022. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- 22.Darley-Usmar VM, Ball LE, Chatham JC. Protein O-linked beta-N-acetylglucosamine: a novel effector of cardiomyocyte metabolism and function. J Mol Cell Cardiol. 2012;52:538–549. doi: 10.1016/j.yjmcc.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanover JA, Krause MW, Love DC. The hexosamine signaling pathway: O-GlcNAc cycling in feast or famine. Biochim Biophys Acta. 2010;1800:80–95. doi: 10.1016/j.bbagen.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xing D, Gong K, Feng W, Nozell SE, Chen YF, Chatham JC, Oparil S. O-GlcNAc modification of NFκβ p65 inhibits TNF-α-induced inflammatory mediator expression in rat aortic smooth muscle cells. PLoS ONE. 2011;6:e24021. doi: 10.1371/journal.pone.0024021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajapakse AG, Ming XF, Carvas JM, Yang Z. O-linked beta-N-acetylglucosamine during hyperglycemia exerts both anti-inflammatory and pro-oxidative properties in the endothelial system. Oxid Med Cell Longev. 2009;2:172–175. doi: 10.4161/oxim.2.3.8482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xing D, Feng W, Not LG, Miller AP, Zhang Y, Chen YF, Majid-Hassan E, Chatham JC, Oparil S. Increased protein O-GlcNAc modification inhibits inflammatory and neointimal responses to acute endoluminal arterial injury. Am J Physiol Heart Circ Physiol. 2008;295:H335–H342. doi: 10.1152/ajpheart.01259.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones MB, Nasirikenari M, Lugade AA, Thanavala Y, Lau JT. Anti-inflammatory IgG production requires functional P1 promoter in β-galactoside α2,6-sialyltransferase 1 (ST6Gal-1) gene. J Biol Chem. 2012;287:15365–15370. doi: 10.1074/jbc.M112.345710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang F, Su K, Yang X, Bowe DB, Paterson AJ, Kudlow JE. O-GlcNAc modification is an endogenous inhibitor of the proteasome. Cell. 2003;115:715–725. doi: 10.1016/s0092-8674(03)00974-7. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt M, Finley D. Regulation of proteasome activity in health and disease. Biochim Biophys Acta. 2014;1843:13–25. doi: 10.1016/j.bbamcr.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slawson C, Zachara NE, Vosseller K, Cheung WD, Lane MD, Hart GW. Perturbations in O-linked beta-N-acetylglucosamine protein modification cause severe defects in mitotic progression and cytokinesis. J Biol Chem. 2005;280:32944–32956. doi: 10.1074/jbc.M503396200. [DOI] [PubMed] [Google Scholar]
- 31.Zeidan Q, Wang Z, De Maio A, Hart GW. O-GlcNAc cycling enzymes associate with the translational machinery and modify core ribosomal proteins. Mol Biol Cell. 2010;21:1922–1936. doi: 10.1091/mbc.E09-11-0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohta T, Xiong Y. Phosphorylation- and Skp1-independent in vitro ubiquitination of E2F1 by multiple ROC-cullin ligases. Cancer Res. 2001;61:1347–1353. [PubMed] [Google Scholar]
- 33.Heessen S, Dantuma NP, Tessarz P, Jellne M, Masucci MG. Inhibition of ubiquitin/proteasome-dependent proteolysis in Saccharomyces cerevisiae by a Gly-Ala repeat. FEBS Lett. 2003;555:397–404. doi: 10.1016/s0014-5793(03)01296-1. [DOI] [PubMed] [Google Scholar]
- 34.Liu H, Yu S, Xu W, Xu J. Enhancement of 26 s proteasome functionality connects oxidative stress and vascular endothelial inflammatory response in diabetes mellitus. Arterioscler Thromb Vasc Biol. 2012;32:2131–2140. doi: 10.1161/ATVBAHA.112.253385. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Liu H, Yu S, Zhang H, Xu J. Angiogenesis impairment in diabetes: role of methylglyoxal-induced receptor for advanced glycation endproducts, autophagy and vascular endothelial growth factor receptor 2. PLoS ONE. 2012;7:e46720. doi: 10.1371/journal.pone.0046720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y, Liu H, Xu QS, Du YG, Xu J. Chitosan oligosaccharides block LPS-induced O-GlcNAcylation of NF-κβ and endothelial inflammatory response. Carbohydr Polym. 2014;99:568–578. doi: 10.1016/j.carbpol.2013.08.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu J, Xie Z, Reece R, Pimental D, Zou MH. Uncoupling of endothelial nitric oxidase synthase by hypochlorous acid: role of NAD(P)H oxidase-derived superoxide and peroxynitrite. Arterioscler Thromb Vasc Biol. 2006;26:2688–2695. doi: 10.1161/01.ATV.0000249394.94588.82. [DOI] [PubMed] [Google Scholar]
- 38.Smith LE, Wesolowski E, McLellan A, Kostyk SK, D'Amato R, Sullivan R, D'Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- 39.Natoli R, Valter K, Barbosa M, Dahlstrom J, Rutar M, Kent A, Provis J. 670 nm photobiomodulation as a novel protection against retinopathy of prematurity: evidence from oxygen induced retinopathy models. PLoS ONE. 2013;8:e72135. doi: 10.1371/journal.pone.0072135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang F, Snead CM, Catravas JD. Hsp90 regulates O-linked beta-N-acetylglucosamine transferase: a novel mechanism of modulation of protein O-linked beta-N-acetylglucosamine modification in endothelial cells. Am J Physiol Cell Physiol. 2012;302:C1786–C1796. doi: 10.1152/ajpcell.00004.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dantuma NP, Lindsten K, Glas R, Jellne M, Masucci MG. Short-lived green fluorescent proteins for quantifying ubiquitin/proteasome-dependent proteolysis in living cells. Nat Biotechnol. 2000;18:538–543. doi: 10.1038/75406. [DOI] [PubMed] [Google Scholar]
- 42.Lindsten K, Menendez-Benito V, Masucci MG, Dantuma NP. A transgenic mouse model of the ubiquitin/proteasome system. Nat Biotechnol. 2003;21:897–902. doi: 10.1038/nbt851. [DOI] [PubMed] [Google Scholar]
- 43.Sohn HY, Krotz F, Gloe T, Keller M, Theisen K, Klauss V, Pohl U. Differential regulation of xanthine and NAD(P)H oxidase by hypoxia in human umbilical vein endothelial cells. Role of nitric oxide and adenosine. Cardiovasc Res. 2003;58:638–646. doi: 10.1016/s0008-6363(03)00262-1. [DOI] [PubMed] [Google Scholar]
- 44.Xu J, Wu Y, Song P, Zhang M, Wang S, Zou MH. Proteasome-dependent degradation of guanosine 5′-triphosphate cyclohydrolase I causes tetrahydrobiopterin deficiency in diabetes mellitus. Circulation. 2007;116:944–953. doi: 10.1161/CIRCULATIONAHA.106.684795. [DOI] [PubMed] [Google Scholar]
- 45.Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, Dikalov SI. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res. 2010;107:106–116. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu J, Wang S, Viollet B, Zou MH. Regulation of the proteasome by AMPK in endothelial cells: the role of O-GlcNAc transferase (OGT) PLoS ONE. 2012;7:e36717. doi: 10.1371/journal.pone.0036717. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Xu J, Wang S, Wu Y, Song P, Zou MH. Tyrosine nitration of pa700 activates the 26 s proteasome to induce endothelial dysfunction in mice with angiotensin II-induced hypertension. Hypertension. 2009;54:625–632. doi: 10.1161/HYPERTENSIONAHA.109.133736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walshe TE, D'Amore PA. The role of hypoxia in vascular injury and repair. Annu Rev Pathol. 2008;3:615–643. doi: 10.1146/annurev.pathmechdis.3.121806.151501. [DOI] [PubMed] [Google Scholar]
- 49.Hilgers RH, Xing D, Gong K, Chen YF, Chatham JC, Oparil S. Acute O-GlcNAcylation prevents inflammation-induced vascular dysfunction. Am J Physiol Heart Circ Physiol. 2012;303:H513–H522. doi: 10.1152/ajpheart.01175.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zou L, Yang S, Champattanachai V, Hu S, Chaudry IH, Marchase RB, Chatham JC. Glucosamine improves cardiac function following trauma-hemorrhage by increased protein O-GlcNAcylation and attenuation of NF-{kappa}B signaling. Am J Physiol Heart Circ Physiol. 2009;296:H515–H523. doi: 10.1152/ajpheart.01025.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang WH, Park SY, Nam HW, Kim do H, Kang JG, Kang ES, Kim YS, Lee HC, Kim KS, Cho JW. NF-κβ activation is associated with its O-GlcNAcylation state under hyperglycemic conditions. Proc Natl Acad Sci USA. 2008;105:17345–17350. doi: 10.1073/pnas.0806198105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hart GW, Copeland RJ. Glycomics hits the big time. Cell. 2010;143:672–676. doi: 10.1016/j.cell.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wells L, Vosseller K, Hart GW. Glycosylation of nucleocytoplasmic proteins: signal transduction and O-GlcNAc. Science. 2001;291:2376–2378. doi: 10.1126/science.1058714. [DOI] [PubMed] [Google Scholar]
- 54.Ozcan S, Andrali SS, Cantrell JE. Modulation of transcription factor function by O-GlcNAc modification. Biochim Biophys Acta. 2010;1799:353–364. doi: 10.1016/j.bbagrm.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.