Abstract
Aims
To determine whether altered beta-adrenergic responses contribute to early cardiac dysfunction in mdx (X-linked muscular dystrophy) mice, an animal model for human Duchenne muscular dystrophy.
Methods and results
Replacement fibrosis in mdx hearts gradually increased with age, suggesting a gradual loss of cardiomyocytes. Echocardiography and intra-left ventricular haemodynamic measurements detected baseline cardiac dysfunction in mdx mice at ≥8 months. However, a reduction of cardiac beta-adrenergic response to isoproterenol (ISO) was already present in mdx mice at 4 months. Ventricular myocytes (VMs) isolated from 4- and 8-month-old mdx mice had greater baseline contractile function {fractional shortening, [Ca2+]i, and sarcoplasmic reticulum (SR) Ca2+ content} and ICa-L than age-matched control VMs and than myocytes isolated from 2-month-old mdx mice. ISO increased myocyte function in the VMs of 4- and 8-month-old mdx mice to the same level as in age-matched control VMs. In the VMs of 12-month-old mdx mice, ISO failed to increase myocyte function to the level in VMs of 12-month-old control mice and could not further increaseICa-L. No differences were observed in the expression of Cav1.2α1c, Cav1.2β1, Cav1.2β2, sarco/endoplasmic reticulum Ca2+ ATPase (SERCA), and the Na+/Ca2+ exchanger. In contrast, total ryanodine receptor 2 (RyR2) and basal phosphorylation of RyR2, phospholamban, and Cav1.2α1c were found to be increased in hearts of 4-month-old mdx mice; baseline protein kinase A activity was also increased. After ISO treatment, phosphorylation levels were the same in mdx and control hearts. VMs of 4-month-old mdx mice had reduced beta1-adrenergic receptor (β1-AR) density and beta-adrenergic sensitivity.
Conclusion
In young mdx mice, the myocyte increases its contractile function to compensate for myocyte loss. However, these myocytes with enhanced baseline function have reduced potential for stimulation, decreased β1-AR density/sensitivity, leading to blunted cardiac beta-adrenergic response.
Keywords: Duchenne muscular dystrophy, Heart, Beta-adrenergic response, Myocyte contraction, Calcium
1. Introduction
Duchenne muscular dystrophy (DMD) is a highly lethal X-linked recessive (mdx) genetic disease, affecting 1 in 3500 male births worldwide. The disease is caused by mutations in the dystrophin gene which is located on the X-chromosome, resulting in non-functional dystrophin.1 Dystrophin normally links cytoskeletal proteins to sarcolemmal glycoproteins to form the dystrophin-associated glycoprotein complex. The loss of dystrophin leads to unstable membranes and increased sensitivity to mechanical stress in muscle cells,2 causing muscle degeneration due to Ca2+ overload. An early manifestation of DMD is skeletal muscle weakness,3 which impairs the diaphragm, ultimately leading to respiratory failure in DMD patients.1 At the late stage of DMD, cardiac dysfunction is an evident feature and a significant contributing factor for mortality.4 Now supported by mechanical ventilators, more DMD patients are surviving with cardiac dysfunction and eventually dilated cardiomyopathy.3
An important function of the heart is to adapt to increased needs for tissue perfusion under normal physiological conditions. This adaptation is achieved by the activation of the sympathoadrenergic system (SAS). The SAS can persistently be activated when the heart is under stress, leading to a reduced beta-adrenergic response. The reduction in cardiac beta-adrenergic reserve in response to increased demand of cardiac output is an early feature of diseased hearts.5,6 In the clinic, the treadmill test and dobutamine stress test have widely been used to evaluate cardiac reserve. However, to the best of our knowledge, there is no report on cardiac reserve in DMD patients. Furthermore, since cardiac dysfunction in DMD patients is usually diagnosed at rest and can only be detected at a late stage, the treatment for cardiac dysfunction is usually only initiated at that stage.7
The mdx mouse is a model of human DMD, carrying a stop codon mutation in exon 23 of the dystrophin gene. In mdx mice, as in human DMD patients, cardiac dysfunction has been monitored only at a late stage of the disease.8 Few studies have been conducted to determine cardiac reserve in mdx mice, which is the topic of this study. We determined in vivo and ex vivo cardiac beta-adrenergic responses in mdx and control mice at different ages and further explored the cellular and molecular mechanisms for the altered beta-adrenergic response in mdx mice. Our data suggest that loss of cardiomyocytes in the early stages is compensated by a significant enhancement of myocyte contractile function to maintain whole-heart function in mdx mice, which is brought about by increasing basal phosphorylation of Ca2+ handling proteins. This remodelling combined with reduced myocyte beta1-adrenergic receptor (β1-AR) density decreased the residual whole-heart beta-adrenergic response in young mdx mice.
2. Methods
2.1. Animals
All mice were handled in compliance with the ‘Principles of Laboratory Animal Care’ formulated by the National Society for Medical Research and the ‘Guide for the Care and Use of Laboratory Animals’ published by the National Institutes of Health (NIH publication 85-23, revised 1996). All animal protocols were approved by the Animal Care and Use Committee of Temple University. Mdx (C57BL/10ScSn-Dmdmdx/J mice) and control mice (C57BL/10ScSn) breeding pairs were purchased from the Jackson Laboratory. Animals were studied at the ages of 2, 3, 4, 5, 6, 7, 8, and 12 months for echocardiography (ECHO) analysis and at 2, 4, 8, and 12 months for all other investigations. Tissue harvesting and myocyte isolation were performed after animal euthanasia using 120 mg/kg sodium pentobarbital (ip). Tribromoethanol (250 mg/kg, ip) was used for non-survival surgery to measure intra-left ventricle (LV) haemodynamics. For ECHO, mice were anaesthetized with 2% isoflurane initially, then with 1%. Tail and toe pinch reflexes were checked to ensure adequate anaesthesia.
2.2. Cardiac fibrosis and function evaluation
Standard histology and Masson's trichrome staining were used to quantitate myocardial fibrosis.9 ECHO was performed with a VisualSonics Vevo 770 machine9 to evaluate cardiac function and cardiac geometry in mdx and control mice.
Intra-LV pressure was measured with a 1.4-F Millar catheter (SPR-839, Millar Instruments, Houston, TX, USA) before and after isoproterenol (ISO) as described previously.10 Eight doses of ISO [10−16–10−9 g ISO/g body weight (BW)] were applied through the jugular vein cannula. ISO was selected because it has equal affinity for both β1- and β2-ARs and it is not known which type of receptor is altered in mdx animals.
2.3. Mouse Langendorff heart perfusion
The Langendorff-perfused heart preparation was established as described previously.11 Eight doses of ISO (10−12–10−5 M) were applied through the perfusate to stimulate the heart.
2.4. Contraction, sarcomere length, Ca2+ transient, and sarcoplasmic reticulum load measurements
Adult mouse ventricular myocyte (VM) isolation was done as previously described.10 VMs were stimulated at 0.5 Hz, cytosolic Ca2+ ([Ca2+]i, Fluo-4 AM), and cell length changes were recorded simultaneously before and after the application of 10−7 M ISO or of a dose series (10−9, 5 × 10−9, 10−8, and 10−7 M).10 Sarcomere length was measured in paced myocytes (0.5 Hz) after a full relaxation. Sarcoplasmic reticulum (SR) Ca2+ content was determined using 20 mM caffeine rapidly applied with a Pico spritzer.10
2.5. Ca2+ current and L-type calcium channel charge movement (Qon and Qoff) measurements
Whole-cell Ca2+ current (ICa) was measured in Na+- and K+-free solutions at 35°C as described previously.1 Charge movement, Qon and Qoff, of the L-type calcium channel in mdx and control myocytes were measured as described previously.10
2.6. Western blotting
Standard western blot procedures were performed to detect alterations in the abundance of calcium handling proteins and their phosphorylation, and the β-ARs.
2.7. Protein kinase A activity measurement
Protein kinase A (PKA) activity of non-stimulated or ISO-stimulated hearts was measured with an enzyme-linked immunosorbent assay (ELISA)-based PKA activity kit (Assay Design, Ann Arbor, MI, USA) according to the manufacturer's instructions. Mouse ventricle samples were homogenized in lysis buffer supplemented with 0.4 mM IBMX (3-isobutyl-methylxanthine, a non-specific inhibitor of phosphodiesterases). PKA activity in the homogenate was then determined.
2.8. Statistics
Data are reported as mean ± standard error of the mean. Unpaired t-test, two-way analysis of variance (ANOVA), or two-way ANOVAs for repeated measures were used to detect statistical significance with GraphPad Prism.5 All cell measurements from the same heart were averaged as one averaged data point. At least three cells from each heart were studied.
For three-factor (genotype, age, and drug) statistical analysis, a mixed procedure was performed for covariance analysis and overall significance evaluation, followed by effect size testing to determine the effect of each factor on the analysed parameter. Post hoc test with Bonferroni adjustment was done to determine pairwise differences.
In all tests, P-values of ≤0.05 were considered significant.
Detailed ‘Materials and methods’ are described in Supplementary materials online.
3. Results
3.1. Myocardial fibrosis in mdx mice begins at the age of 2 months, but baseline cardiac dysfunction and morphological changes are only detected from 8 months onwards
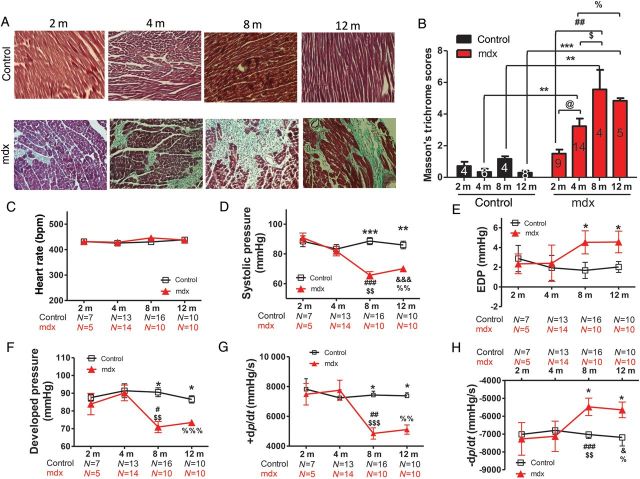
Mdx mice are characterized by the loss of cardiomyocytes and replacement fibrosis. While there was a little change in collagen content in control mice during the 1-year study, fibrosis in mdx mice began to appear at the age of 2 months and gradually reached its maximum at the age of 8 months (Figure 1A and B).
Figure 1.
Cardiac fibrosis starts at a young age (2 months) in mdx mice, but baseline cardiac dysfunction is only detected from 8 months onwards. (A) Images (×200) of Masson's trichrome staining of myocardial fibrosis (blue). (B) Fibrosis scores in ventricular tissue at different ages (2, 4, 8, and 12 months). (C–H) Systolic pressure (D), end-diastolic pressure (EDP; E), developed pressure (F), maximum +dp/dt (G), and minimum −dp/dt (H) determined by intra-LV haemodynamic measurements at the same heart rates (400–450 bpm, C) in control and mdx mice at different ages (2, 4, 8, and 12 months). *P < 0.05, **P < 0.01, ***P < 0.001 control vs. mdx mice at the same age, respectively; @P < 0.05; 4- vs. 2-month-old mdx mice; #P < 0.05, ##P < 0.01, ###P < 0.001 for 8- vs. 2-month-old mdx mice; $$P < 0.01, $$$P < 0.001 for 8- vs. 4-month-old mdx mice; &P < 0.05, &&&P < 0.001 for 12- vs. 2-month-old mdx mice; %P < 0.05, %%P < 0.01, %%%P < 0.001 for 12- vs. 4-month-old mdx mice. Two-way ANOVAs with the post hoc test (Bonferroni adjustment) were performed. Numbers of animals examined are indicated in the bars (B) or underneath the age group (C–H).
Chronic loss of cardiomyocytes and fibrosis may significantly impact cardiac function. Therefore, cardiac function of mdx mice was evaluated over time. At baseline, when the heart rate was controlled in the range of 400–450 bpm (Figure 1C and see Supplementary material online, Figure S1A), cardiac contractile function indicated by fractional shortening (FS; see Supplementary material online, Figure S1B), systolic pressure (Figure 1D), developed pressure (Figure 1F), and maximal +dp/dt (Figure 1G) was significantly depressed only in mdx mice aged ≥8 months compared with age-matched control mice. In contrast, the diastolic pressure (Figure 1E) was elevated, and relaxation (minimum −dp/dt, Figure 1H) was impaired in mdx mice at ≥8 months but not in control mice. It seems that significant basal cardiac contraction depression and relaxation impairment appeared at the age when there was maximum myocardial fibrosis, most probably because at this time point fibrosis impairs cardiac relaxation, with the surviving myocytes not able to compensate fully for the lost myocytes.
Morphologically, mdx mice had a thicker diastolic LV posterior wall (see Supplementary material online, Figure S1C) and interventricular septum (see Supplementary material online, Figure S1D) from 8 months onwards, causing slight decreases in diastolic LV internal diameter (see Supplementary material online, Figure S1E). Finally, the LV mass was significantly increased in 10- and 12-month-old mdx mice compared with age-matched control and 2-month-old mdx mice (see Supplementary material online, Figure S1F).
3.2. Young mdx hearts have reduced beta-adrenergic responses in vivo
It is well known that hearts at the compensatory stage during heart disease progression have a normal basal cardiac function but a reduced beta-adrenergic response.12 Therefore, the beta-adrenergic responses of 4-month-old mdx hearts with normal basal cardiac function and 8-month-old mdx hearts with depressed basal cardiac function were compared with those of age-matched control hearts. A high dose of ISO (2 mg/kg BW) was injected ip to test its effect on cardiac function as monitored by intra-LV pressure recording (Figure 2). Steady-state effects of ISO on cardiac performance were compared. Agreeing with the ECHO results, at baseline, 8-month-old mdx hearts had a decreased +dp/dt, increased end-diastolic pressure, and decreased relaxation rate (−dp/dt) compared with 8-month-old control and 4-month-old mdx hearts (Figure 2E, G, and H). ISO increased the heart rates in both groups to the same level (Figure 2A–C) at the ages of 4 and 8 months. However, ISO only increased systolic pressure, developed pressure, maximum +dp/dt, and minimum −dp/dt in control mice (Figure 2D and F–H), as previously reported.10 ISO instead decreased these parameters in 4- and 8-month-old mdx hearts. ISO also increased diastolic pressure in 8-month-old mdx hearts, but not in other groups (Figure 2E).
Figure 2.
Mdx hearts have reduced beta-adrenergic responses at 4 and 8 months. (A and B) Examples of intra-LV pressure recordings, derived dp/dt, and heart rates with a saturating dose of ISO (2 mg/kg BW) in 4-month-old control mice (A) and mdx mice (B). (C–H) Systolic pressure (D), diastolic pressure (E), developed pressure (F), maximum +dp/dt (G), and minimum −dp/dt (H) determined by intra-LV haemodynamic measurements at the same heart rates (400–450 bpm, C) in control and mdx mice at the ages of 4 and 8 months. Mixed procedure and post hoc test with Bonferroni adjustment were performed for statistical significance between groups with SAS 9.
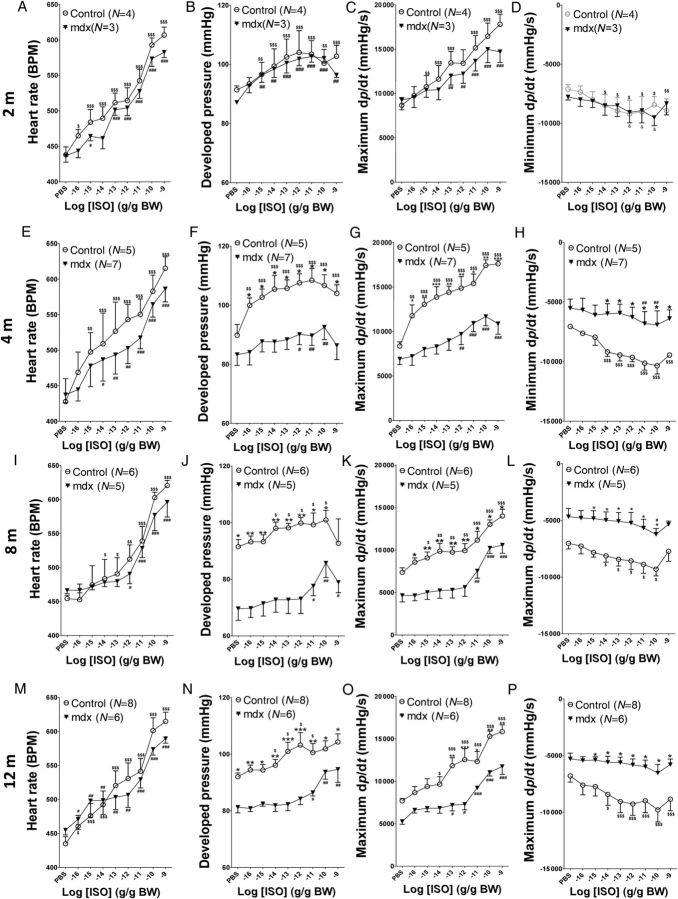
Since only a single high dose of ISO was used in Figure 2, subsaturating concentrations (10−16–10−9 g ISO/g BW, intrajugular vein injection) of ISO were used to better differentiate beta-adrenergic responses between mdx and control hearts at different ages (2, 4, 8, and 12 months) (Figure 3 and see Supplementary material online, Figure S2). In mdx mice of all ages, the heart rate had responses to different doses of ISO comparable with that in age-matched control mice. ISO at these concentrations had comparable effects on maximum +dp/dt, minimum −dp/dt, and developed pressure in 2-month-old mdx and control mice (Figure 3A–D). In 4-month-old animals, ISO did not enhance cardiac function in mdx hearts to the same extent as in control hearts (Figure 3E–H). At the ages of 8 and 12 months, there was decreased baseline maximum +dp/dt, minimum −dp/dt, and developed pressure in mdx mice, and these parameters could not be increased to the same extent in mdx hearts as in control hearts (Figure 3I–P). Basal and maximum response to ISO of the heart rate, developed pressure, maximum +dp/dt, and minimum −dp/dt in control and mdx mice are depicted in Supplementary material online, Figure S2. Taken together, these results suggest that ≥4-month-old mdx ventricles have blunted beta-adrenergic responses.
Figure 3.
In vivo mdx hearts have reduced beta-adrenergic responses starting at the age of 4 months. Intra-LV pressure was recorded under basal conditions and with different concentrations of ISO (10−16–10−9 g ISO/g BW) applied through the jugular vein serially at different ages. The heart rate (A, E, I, and M), developed pressure (B, F, J, and N), maximum +dp/dt (C, G, K, and O), and minimum −dp/dt (D, H, L, and P) were analysed at different ages (A–D; 2 months; E–H, 4 months; I–L, 8 months; M–P, 12 months). $P < 0.05, $$P < 0.01, $$$P < 0.001 vs. control baseline, respectively; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. mdx baseline, respectively; *P < 0.05, **P < 0.01, ***P < 0.001 control vs. mdx at the same age, respectively. Two-way ANOVAs with repeated measurements and post hoc test (Bonferroni corrections) were performed.
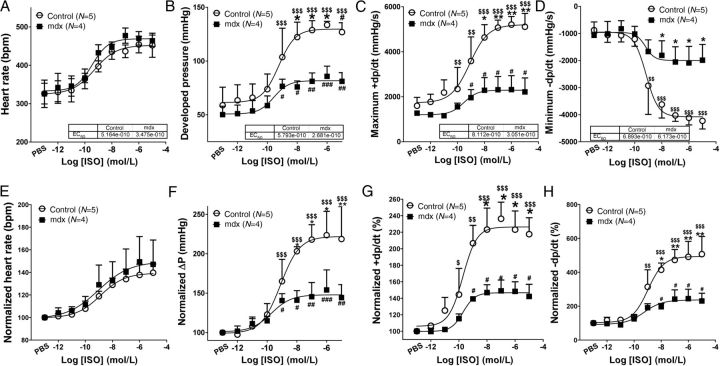
3.3. Reduced beta-adrenergic responses are the characteristic of young mdx hearts
Since in vivo studies may be complicated by feedback regulatory mechanisms such as concomitant activation of the parasympathetic system, we further tested if the reduced beta-adrenergic response of 4-month-old mdx hearts was an intrinsic property of these hearts. We examined the responses of ex vivo hearts perfused on a Langendorff apparatus to different concentrations of ISO (10−12–10−5 M). Hearts from both groups of mice started to respond to ISO at 10−11 M and reached a maximal response at a concentration of 10−7 M. The EC50 of ISO on the heart rate, developed pressure, maximum +dp/dt, and minimum −dp/dt (∼5 × 10−10 M) of perfused control and mdx hearts were not significantly altered, but tended to be greater in mdx hearts. ISO at doses >10−10 M increased developed pressure, maximum +dp/dt, and minimum −dp/dt more in control hearts than in mdx hearts (Figure 4). These data suggest that the reduced cardiac beta-adrenergic response in mdx hearts is an intrinsic property of the heart.
Figure 4.
Ex vivo mdx hearts have reduced beta-adrenergic responses at the age of 4 months. Hearts perfused on Langendorff apparatus with different concentrations of ISO (10−12–10−5 M). Heart rates (A), developed pressure (B), maximum +dp/dt (C), and minimum −dp/dt (D) were analysed. (E–H) Normalized data of (A–D). $P < 0.05, $$P < 0.01, $$$P < 0.001 vs. control baseline, respectively; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. mdx baseline, respectively; *P < 0.05, **P < 0.01, ***P < 0.001 control vs. mdx at the same age, respectively. Two-way ANOVAs with repeated measurements and post hoc test (Bonferroni corrections) were performed.
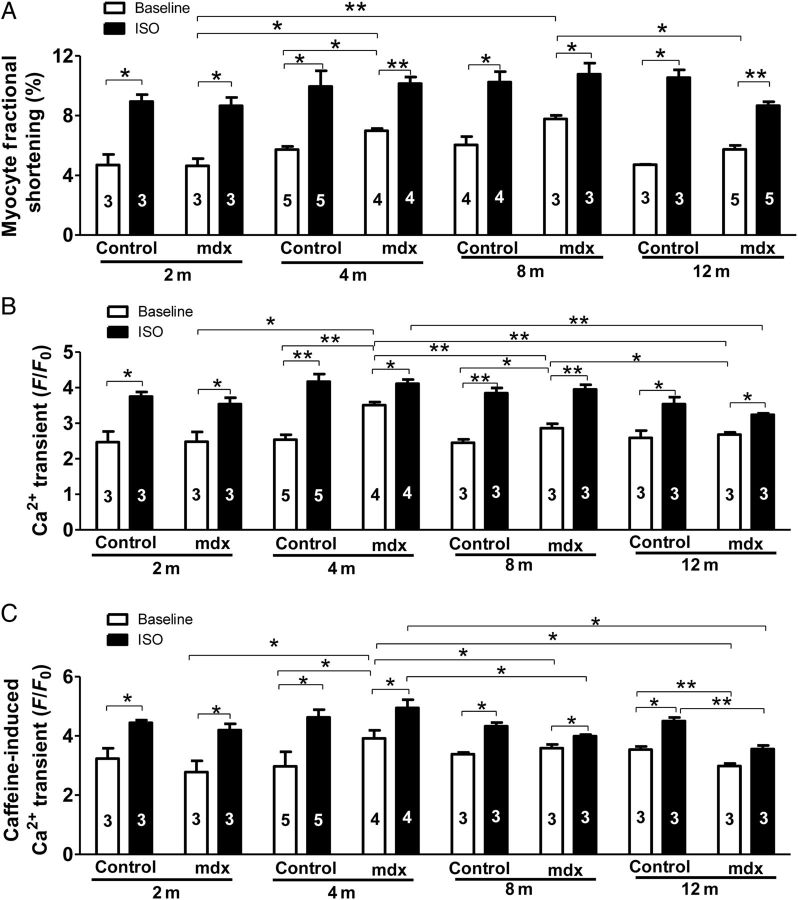
3.4. Myocytes from mdx hearts at ≥4 months are hypercontractile, but have reduced potential for stimulation by beta-adrenergic agonists
To explore the cellular mechanism for the reduced beta-adrenergic reserve, we measured myocyte contractions and Ca2+ transients and their responses to ISO in myocytes from 2-, 4-, 8-, and 12-month-old control and mdx hearts. Age did not change the amplitudes of myocyte FS and Ca2+ transients ([Ca2+]i) in control myocytes at baseline or after ISO, but affected both FS and [Ca2+]i at baseline and after ISO in mdx myocytes. Baseline FS and [Ca2+]i amplitudes were enhanced in mdx myocytes of 4- and 8-month-old mice compared with 2-month-old mdx and age-matched control myocytes and then decreased to the amplitudes comparable with 2-month-old mdx and 12-month-old control myocytes. At the ages of 2, 4, and 8 months, the amplitudes of myocyte FS and [Ca2+]i in mdx and control myocytes were increased to the same level by ISO. However, the amplitudes of FS and [Ca2+]i after ISO in 12-month-old mdx myocytes were less than in age-matched control myocytes and 2- and 4-month-old mdx myocytes (Figure 5A and B). However, diastolic sarcomere length was not different between 4-month-old mdx and control myocytes when stimulated at 0.5 Hz (see Supplementary material online, Figure S3). These data suggest that young myocytes in mdx hearts develop hypercontraction to compensate for the loss of myocytes, but this compensation leads to reduced potential for mdx myocytes to respond to maximal beta-adrenergic stimulation. In addition, in older mdx myocytes, there were reduced beta-adrenergic responses compared with age-matched control myocytes.
Figure 5.
Mdx myocytes from ≥4-month-old mice show enhanced contraction, intracellular Ca2+ transients, and SR Ca2+ content at baseline, but a blunted beta-adrenergic response compared with age-matched controls. The amplitudes of baseline myocyte contraction (A), Ca2+ transients (B), and SR Ca2+ content (C) in 2-, 4-, 8-, and 12-month-old control and mdx VMs before and after the application of 100 nM ISO. The numbers in the bars are animal numbers used for studies (3–15 cells were studied for each animal). A mixed-effects model was used to test differences at an overall P-value of 0.05 with Bonferroni adjustments. Post hoc tests were performed pairwise with Bonferroni adjustment. *P < 0.05, **P < 0.01, ***P < 0.001, respectively.
3.5. Increased SR Ca2+ content and ICa in mdx myocytes have reduced beta-adrenergic responses
Since myocyte contraction is determined by the amount of available Ca2+ in the SR (SR Ca2+ content) for release and the size of the trigger (ICa) causing the release of Ca2+ from the SR,13 baseline and ISO-stimulated SR Ca2+ content and ICa were determined in mdx and control myocytes. Age did not significantly change the SR Ca2+ content at baseline and after ISO in control myocytes, but had a complex effect on the SR Ca2+ content in mdx myocytes. At baseline, the SR Ca2+ content was not changed in 2-month-old mdx myocytes, but was increased in 4-month-old mdx myocytes compared with 2-month-old mdx and age-matched control myocytes. Then, it was decreased to normal in 8-month-old mdx myocytes and to less than in 2-month-old mdx myocytes and age-matched control myocytes in 12-month-old mdx myocytes (Figure 5C). After ISO stimulation, SR Ca2+ content was significantly increased to the same in 2- and 4-month-old control and mdx myocytes, but reduced in 8- and 12-month-old mdx myocytes.
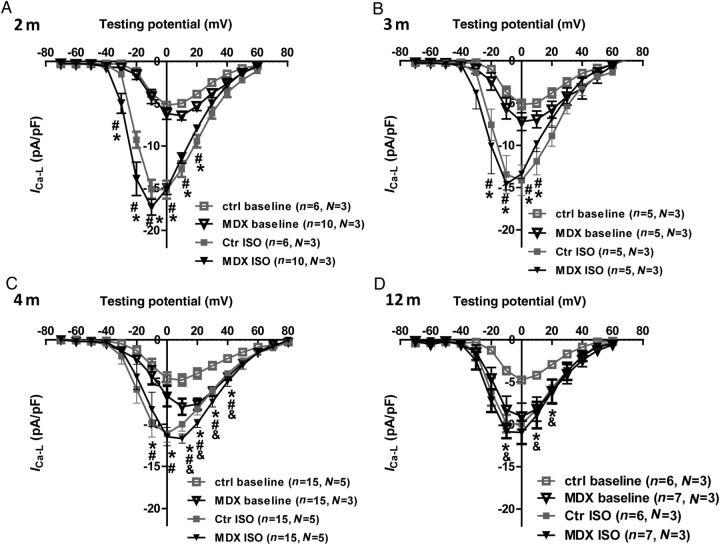
We further determined the ICa and its response to ISO in control and mdx myocytes at different ages. Age did not affect baseline ICa in control myocytes, but had a significant effect on ICa in mdx myocytes. Baseline ICa was not changed in 2- and 3-month-old mdx myocytes compared with age-matched control myocytes, but was significantly increased in 4- and 12-month-old mdx myocytes compared with 2- and 3-month-old mdx and age-matched control myocytes (Figure 6 and see Supplementary material online, Figure S4). When stimulated with ISO, maximum ICa was increased to the same amplitude in 2-, 3-, and 4-month-old mdx and control myocytes. However, baseline ICa in 12-month-old mdx myocytes was close to the ICa after ISO in 12-month-old control myocytes and was not significantly increased by ISO (Figure 6D and see Supplementary material online, Figure S4). These data suggest that, in mdx myocytes, ICa is gradually enhanced, contributing to hypercontractile function of mdx myocytes older than 4 months.
Figure 6.
Enhanced basal ICa but blunted beta-adrenergic response in ≥4-month-old mdx myocytes. ICa–voltage curves in 2- (A), 3- (B), 4- (C), and 12-month (D) control and mdx VMs before and after ISO. *P < 0.05, before vs. after ISO in control myocytes at the same test voltage at baseline; #P < 0.05, before vs. after ISO in mdx myocytes at the same test voltage; &P < 0.05, mdx vs. control at the same test voltage at baseline. Two-way ANOVAs with repeated measurements and post hoc test (Bonferroni corrections) were performed within the same age group.
3.6. Increased ryanodine receptor expression and baseline phosphorylation of Cav1.2α1c, phospholamban, and ryanodine receptor 2 in 4-month-old mdx myocytes
To explore the molecular mechanisms of the enhanced basal contractile function but reduced beta-adrenergic response in 4-month-old mdx myocytes, the expression of Ca2+ handling proteins and the phosphorylation state of phospholamban (PLB), ryanodine receptor 2 (RyR2), and Cav1.2α1c were determined. The abundance of PLB, sarco/endoplasmic reticulum Ca2+ ATPase 2a (SERCA2a), Na+/Ca2+ exchanger 1 (NCX1), Cav1.2α1c, Cav1.2β1, and Cav1.2β2 was not altered. In contrast, RyR2 was increased in 4-month-old mdx hearts compared with 4-month-old control hearts (Figure 7A and B). No change of Cav1.2α1c abundance was also supported by no difference in Cav1.2 charge movement between 4-month-old mdx and control myocytes (see Supplementary material online, Figure S5). The phosphorylation of Cav1.2α1c at Ser1928, RyR2 at the PKA site (Ser2809), PLB at the PKA site (Ser16), and the Ca2+/calmodulin-dependent kinase II (CaMKII) site (Thr17) was increased at baseline in 4-month-old mdx hearts compared with 4-month-old control hearts, but was not different between groups after ISO (10−7 M) (Figure 7). These results suggest that the activities of Cav1.2, SERCA2, and RyR2 could be enhanced at baseline in 4-month-old mdx hearts.13
Figure 7.
The expression and phosphorylation of calcium handling proteins in 4-month-old control and mdx hearts. (A and B) Western blot analysis of SERCA2a, PLB, NCX1, RyR2, Cav1.2α1c, Cav1.2β1, and Cav1.2β2 in 4-month-old mdx hearts was compared with that 4-month-old control hearts. (C–F) Western analysis of Cav1.2α1c phosphorylated at Ser1928 (C), RyR2 phosphorylated at Ser2815 and Ser2809 (D), PLB phosphorylated at Ser16 and Thr17 at baseline, and after ISO stimulation (E). The averaged phosphorylation level of these proteins is shown in (F). Student's t-test was done for each protein in (B) and two-way ANOVAs for phosphorylated proteins in (F).
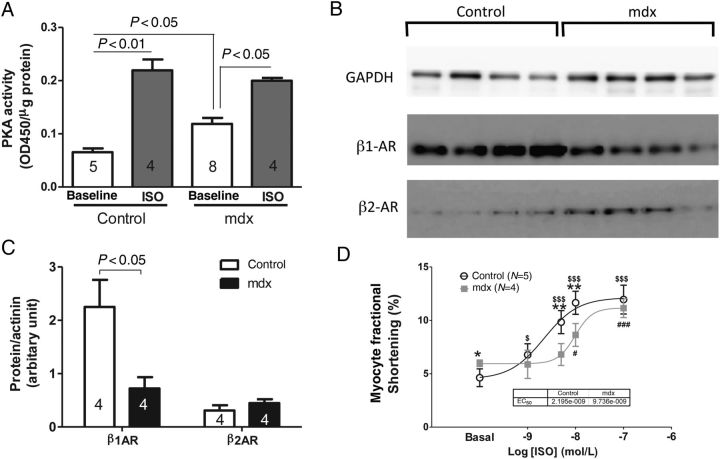
3.7. Increased basal PKA activity, but reduced β1-AR in mdx myocytes
The increases in the phosphorylation of Ca2+ handling proteins indicate that there could be an increase in basal PKA activity in 4-month-old mdx myocytes, which was confirmed by basal PKA activity measurement. After ISO, PKA activity was similar between these two groups of hearts (Figure 8A). We found that the abundance of beta1-AR but not beta2-AR was reduced in mdx, compared with control hearts (Figure 8B and C). We further determined the impact of reduced beta1-AR density on myocyte sensitivity to ISO stimulation by measuring myocyte FS at baseline and after the application of 1, 5, 10, and 100 nM ISO. To avoid desensitization after the application of ISO, all cells were only exposed to one dose of ISO. As shown in Figure 8D, the FS of 4-month-old mdx myocyte was significantly reduced in response to 5 and 10 nM ISO compared with 4-month-old control myocytes, indicating a reduced sensitivity of mdx myocytes to beta-adrenergic stimulation.
Figure 8.
PKA activity with or without ISO stimulation and beta-adrenergic receptors (β1-AR and β2-AR) abundance in 4-month-old mdx and control hearts. (A) PKA activity in 4-month-old control and mdx hearts without or with ISO. Western blots (B) and abundance (C) of β1-AR and β2-AR in 4-month-old control and mdx hearts. (D) Contractions of 4-month-old control and mdx myocytes in response to different doses of ISO. Student's t-test was used for (C); two-way ANOVAs with the post hoc test were performed for (A) and also with repeated measurements for (D). *P < 0.05, **P < 0.01 control vs. mdx at the same ISO dose; $P < 0.05, $$$P < 0.001 at the indicated dose of ISO vs. baseline in control myocytes; #P < 0.05, ###P < 0.001 at the indicated dose of ISO vs. baseline in mdx myocytes.
4. Discussion
It is still unknown if and how cardiac beta-adrenergic response, an indicator of early functional change of diseased hearts, is altered in young mdx hearts. In this study, we found that: (i) cardiac beta-adrenergic response of the heart in ≥4-month-old mdx mice was reduced in vivo and ex vivo compared with that in age-matched control and 2-month-old mdx hearts. (ii) The myocytes isolated from ≥4-month-old mdx hearts had enhanced basal contractile function, but a further increase by ISO (4-month-old mdx myocytes) was reduced though contraction still attained similar maximal levels to those in the controls. However, this maximum was reduced in 8- and 12-month-old mdx myocytes. (iii) SR Ca2+ content was not changed in 2-month-old mdx myocytes, was increased in 4-month-old mdx myocytes, and was decreased in 8- and 12-month-old mdx myocytes at baseline; ISO had reduced effects on the SR Ca2+ content in mdx myocytes at ≥4 months old. (iv) Though the baseline ICa did not change in control myocytes with age, it was increased in ≥4-month-old mdx myocytes. ISO enhanced ICa to the same amplitude as in age-matched control myocytes. (v) The abundance of Cav1.2α1c, Cav1.2β1, Cav1.2β2, PLB, NCX, and SERCA was not altered, while RyR abundance was increased in 4-month-old mdx hearts. (vi) Basal phosphorylation of Cav1.2α1c, RyR2, and PLB was increased in 4-month-old mdx hearts but, after ISO, the phosphorylation of these proteins was comparable in mdx and control hearts. (vii) Basal PKA activity was increased, but beta1-AR abundance was decreased in 4-month-old mdx mice at baseline; 4-month-old mdx myocytes had reduced sensitivity to ISO stimulation.
4.1. Potential mechanisms for the reduced beta-adrenergic response
We found that cardiac beta-adrenergic responses of mdx hearts at ≥4 months old were reduced compared with those of either age-matched control hearts or mdx hearts at 2 months, which is before the alterations in global baseline cardiac function. The reductions of beta-adrenergic responses of mdx hearts are probably due to the following reasons. (i) The loss of myocytes: in agreement with previous studies, our study also showed substantial loss of myocytes revealed by significant replacement fibrosis in ≥4-month-old mdx hearts. In 4- and 8-month-old mdx hearts, at baseline no cardiac dysfunction was observed, probably due to a full compensation of myocyte loss by enhancing myocyte contraction. However, since the maximum ISO effect increased myocyte contraction in 4- and 8-month-old mdx mice to the same extent as in control myocytes, mdx hearts with fewer myocytes had poorer function compared with control hearts after ISO. In 8- and 12-month-old mdx hearts, the myocytes had reduced response to beta-adrenergic stimulation, which further decreased mdx cardiac function after ISO. (ii) Hypercontractile function of mdx myocytes due to ‘hyper'phosphorylation of multiple Ca2+ handing proteins reduces the potential for ISO to increase myocyte function further. This could be a result of chronic stimulation by the SAS and other neurohormone systems. (iii) Desensitization of the beta-adrenergic signalling in the myocytes: the SAS could be activated in response to substantial loss of myocytes in ≥4-month-old mdx mice, suggested by heightened basal heart rates in DMD patients and mdx mice.14. Increased basal PKA activity and phosphorylation of Ca2+ handling proteins also suggest hyperactivity of the SAS in mdx mice (Figures 7 and 8). Persistent activation of the SAS could lead to desensitization of the beta-adrenergic signalling in cardiomyocytes.12 Our study shows that the expression of β1-AR is significantly reduced in 4-month-old mdx hearts, and mdx myocytes had reduced sensitivity to ISO. It has been reported that there is a reduction of beta1-AR density and sensitivity in 12-month-old mdx, but not in 3-month-old mdx, atria.15 The slight difference in the timing of the β1-AR decrease could be due to differences in ages (4 vs. 3 months) and tissues (ventricles vs. atria). The fact that the heart rates of 2-, 4-, 8-, and 12-month-old mdx hearts respond almost normally to different doses of ISO implies that the sinoatrial nodal cells might maintain a normal density of β-ARs, arguing for differential regulation of β-AR density in different parts of the mdx heart. The impairment of cardiac relaxation in ≥4-month-old mdx hearts after ISO and in mdx hearts older than 8 months at baseline could be related to both the alterations of myocyte function and the increases in myocardial fibrosis. Therefore, measurement of myocardial fibrosis with an advanced non-invasive technique could be a reliable and valuable prognostic marker for cardiac dysfunction.
4.2. Remodelling of calcium handling in young mdx myocytes
When a stressed heart is at its compensated stage, the myocytes may develop a hypercontractile function to overcome the stress and maintain cardiac output.13,16 In this study, young mdx myocytes were found to be hypercontractile. Enhanced myocyte contraction in young mdx myocytes may be due to increases in ICa, PLB phosphorylation, and resultant SR Ca2+ load. The increase in ICa may only partially account for the increase of SR Ca2+ load because there is increased Ca2+ influx via other routes.17 In addition, at baseline, enhanced phosphorylation of PLB could increase SERCA activity to allow more Ca2+ uptake into the SR. Previously, Williams and Allen16 found that the phosphorylation of PLB at the Ser16 site is decreased in both 2- to 3-month-old and 9- to 12-month-old mice. Another study by Sarma et al.18 found no change in PLB phosphorylation at Ser16 in 3- and 15-month-old hearts. These differences could be related to the differences in ages of animals used and the experimental conditions. In our study, we also showed increased RyR phosphorylation at both PKA and CaMKII sites that may cause Ca2+ to leak out of the SR. However, we observed a higher basal SR Ca2+ content in 4-month-old mdx myocytes, suggesting that the increased ICa and SERCA activity overcome the leaking of Ca2+ out of the SR at this age. Basal SR Ca2+ load in older (8 and 12 months) mdx myocytes tended to be decreased compared with that in aged-matched controls. Our data agree with a previous report showing increased basal SR Ca2+ load in 16-week-old mdx mice,16 but did not agree with a recent report by Kyrychenko et al.,19 who observed an increased SR Ca2+ load in 1-month-old mdx mice but no changes in the SR Ca2+ load in 3- to 4-month-old mdx myocytes. This might be related to the animals studied by Kyrychenko et al., which develop cardiomyopathy at a faster pace than our animals. Therefore, the SR Ca2+ load in mdx myocytes might be dependent on the balance between the increased Ca2+ influx and Ca2+ uptake into the SR and the leakage out of the SR. This could explain why the SR Ca2+ content is reduced and the [Ca2+]i transient was about normal while there is increased ICa in 12-month-old mdx myocytes. Also, the increase in SR Ca2+ leakage through RyR2 could be an additive effect of PKA- and CaMKII-dependent phosphorylation,18 nitrosylation,20 and oxidation.21
The phosphorylation levels of Cav1.2α1c, RyR, and PLB are increased in 4-month-old mdx hearts. Previously, it was found that there is increased phosphorylation of RyR at both PKA and CaMKII sites in 12-month-old mdx, but not in 3-month-old mdx, hearts.18 Currently, it is not known how these proteins become highly phosphorylated in mdx hearts. Both a high basal PKA and CaMKII activity and a low phosphatase activity could result in ‘hyper'phosphorylation of some molecules, as observed in isolated failing human myocytes.22,23 Our study shows that the baseline PKA activity is greater in isolated mdx hearts and myocytes. We have previously reported similar observations in mouse myocytes deficient in G-protein receptor kinase 224 and failing human VMs.25 The molecular mechanisms responsible for this increase in basal PKA activity remain to be determined and could be related to an enhanced basal activity of the β-AR/Gs/adenylyl cyclase signalling and/or a decrease in phosphodiesterase activity. These molecular alterations could account for the enhanced myocyte contraction.
4.3. Increased ICa in young mdx cardiomyocytes
We have shown that ICa density is increased in VMs of ≥4-month-old mdx mice. Significant increases in Cav1.2 density (measured by ligand binding), and Cav1.2α1c protein and mRNA abundance in 3-month-old mdx hearts, were reported previously.26 Previous studies of ICa in neonatal,27,28 1-month-old,21 and 2-month-old29 mdx mice showed no change of ICa density, as our study indicates that ICa change could only appear after the age of 4 months; Ullrich et al.30 showed a non-significant change of ICa (wild type, −5.36 ± 0.09, n = 20 vs. mdx −5.81 ± 0.37, n = 16; P = 0.20, two-tailed Student's t-test) in 6- to 12-month-old mdx mice. These data, together with our data (Figure 6), suggest a dynamic alteration and gradual increase of ICa during the progression of the disease. In our study, we did not detect changes in Cav1.2α1c expression by either western blot or charge movement measurement, but rather a significant increase in ICa density was found in VMs from 4-month-old mdx mice, indicating a significant increase in Cav1.2 activity in young mdx hearts. The increased phosphorylation of Cav1.2α1c could account for the increased Cav1.2 activity. In addition, the lack of an effect of dystrophin in decreasing the rate of ICa inactivation28 might also contribute to enhanced Cav1.2 activity in 4-month-old mdx myocytes.
4.4. Significance of our study
During the past decade, better support for respiratory function makes cardiomyopathy and arrhythmias important contributing factors to the mortality of DMD patients.31 Our study showed that, in young mdx mice, reduced cardiac beta-adrenergic responses are an indication of early cardiac dysfunction. It remains to be determined whether this also occurs in young DMD patients because of differences in the progression of cardiomyopathy in human DMD patients and mdx mice.8 Though it is possible to perform exercise or dobutamine tests in very young DMD patients who are still capable of exercise, such tests in DMD patients become difficult at later stages.8 Our study suggests that chronic activation of the SAS in mdx/DMD may promote the progression of cardiomyopathy, and treatments with beta-blockers or beta-blockers in combination with other drugs such as angiotensin-converting enzyme inhibitors for cardiac complications in DMD patients might be considered at a younger age. Future approaches could include blocking hyperactive PKA18 and CaMKII.32
Our study also suggests that the interplay between substantial cardiomyocyte loss and the activation of SAS to maintain cardiac function promotes cardiomyopathy. It seems that hyperactivity of the SAS and the hypercontractile function of cardiac myocytes compensate for substantial loss of cardiomyocytes in mdx hearts. However, these alterations promote myocyte death, forming a vicious cycle promoting cardiac failure in mdx mice and DMD patients.
5. Conclusion
Mdx hearts partially lose beta-adrenergic responses from a young age (4 months) because of myocyte loss, reduced potential for increasing myocyte contraction in mdx myocytes with enhanced myocyte contractile function via molecular remodelling, and cellular desensitization in response to the hyperactive SAS. Although cellular remodelling exerts some beneficial effects in mdx hearts, in the long run it may contribute to the progression of cardiomyopathy in mdx hearts.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This study was supported by NIH HL088243, HL088243-03S1, and AHA 0730347N to X.C., and by the Ministry of Health of China (grant no. 201202002), a grant from the CPLA Scientific Research Fund (no. BWS11J039), and the Key Project on Advanced Clinical Technology for Military Hospital (no. 2010gxjs068) to Y.P.
Supplementary Material
References
- 1.Pichavant C, Aartsma-Rus A, Clemens PR, Davies KE, Dickson G, Takeda S, Wilton SD, Wolff JA, Wooddell CI, Xiao X, Tremblay JP. Current status of pharmaceutical and genetic therapeutic approaches to treat DMD. Mol Ther. 2011;19:830–840. doi: 10.1038/mt.2011.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell KP, Kahl SD. Association of dystrophin and an integral membrane glycoprotein. Nature. 1989;338:259–262. doi: 10.1038/338259a0. [DOI] [PubMed] [Google Scholar]
- 3.Kaspar RW, Allen HD, Montanaro F. Current understanding and management of dilated cardiomyopathy in Duchenne and Becker muscular dystrophy. J Am Acad Nurse Pract. 2009;21:241–249. doi: 10.1111/j.1745-7599.2009.00404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mertens L, Ganame J, Claus P, Goemans N, Thijs D, Eyskens B, Van Laere D, Bijnens B, D'Hooge J, Sutherland GR, Buyse G. Early regional myocardial dysfunction in young patients with Duchenne muscular dystrophy. J Am Soc Echocardiogr. 2008;21:1049–1054. doi: 10.1016/j.echo.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, Lurie K, Billingham ME, Harrison DC, Stinson EB. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med. 1982;307:205–211. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- 6.Lymperopoulos A, Rengo G, Koch WJ. Adrenergic nervous system in heart failure: pathophysiology and therapy. Circ Res. 2013;113:739–753. doi: 10.1161/CIRCRESAHA.113.300308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bushby K, Connor E. Clinical outcome measures for trials in Duchenne muscular dystrophy: report from international working group meetings. Clin Investig (Lond) 2011;1:1217–1235. doi: 10.4155/cli.11.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shirokova N, Niggli E. Cardiac phenotype of Duchenne muscular dystrophy: insights from cellular studies. J Mol Cell Cardiol. 2013;58:217–224. doi: 10.1016/j.yjmcc.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X, Nakayama H, Zhang X, Ai X, Harris DM, Tang M, Zhang H, Szeto C, Stockbower K, Berretta RM, Eckhart AD, Koch WJ, Molkentin JD, Houser SR. Calcium influx through Cav1.2 is a proximal signal for pathological cardiomyocyte hypertrophy. J Mol Cell Cardiol. 2011;50:460–470. doi: 10.1016/j.yjmcc.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poller U, Fuchs B, Gorf A, Jakubetz J, Radke J, Ponicke K, Brodde OE. Terbutaline-induced desensitization of human cardiac beta 2-adrenoceptor-mediated positive inotropic effects: attenuation by ketotifen. Cardiovasc Res. 1998;40:211–222. doi: 10.1016/s0008-6363(98)00101-1. [DOI] [PubMed] [Google Scholar]
- 11.Choi DJ, Koch WJ, Hunter JJ, Rockman HA. Mechanism of beta-adrenergic receptor desensitization in cardiac hypertrophy is increased beta-adrenergic receptor kinase. J Biol Chem. 1997;272:17223–17229. doi: 10.1074/jbc.272.27.17223. [DOI] [PubMed] [Google Scholar]
- 12.Banday AA, Fazili FR, Lokhandwala MF. Insulin causes renal dopamine D1 receptor desensitization via GRK2-mediated receptor phosphorylation involving phosphatidylinositol 3-kinase and protein kinase C. Am J Physiol Renal Physiol. 2007;293:F877–F884. doi: 10.1152/ajprenal.00184.2007. [DOI] [PubMed] [Google Scholar]
- 13.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 14.Thomas TO, Morgan TM, Burnette WB, Markham LW. Correlation of heart rate and cardiac dysfunction in Duchenne muscular dystrophy. Pediatr Cardiol. 2012;33:1175–1179. doi: 10.1007/s00246-012-0281-0. [DOI] [PubMed] [Google Scholar]
- 15.Lu S, Hoey A. Age- and sex-associated changes in cardiac beta(1)-adrenoceptors from the muscular dystrophy (mdx) mouse. J Mol Cell Cardiol. 2000;32:1661–1668. doi: 10.1006/jmcc.2000.1200. [DOI] [PubMed] [Google Scholar]
- 16.Williams IA, Allen DG. Intracellular calcium handling in ventricular myocytes from mdx mice. Am J Physiol Heart Circ Physiol. 2007;292:H846–H855. doi: 10.1152/ajpheart.00688.2006. [DOI] [PubMed] [Google Scholar]
- 17.Petrof BJ. Molecular pathophysiology of myofiber injury in deficiencies of the dystrophin-glycoprotein complex. Am J Phys Med Rehabil. 2002;81:S162–S174. doi: 10.1097/00002060-200211001-00017. [DOI] [PubMed] [Google Scholar]
- 18.Sarma S, Li N, van Oort RJ, Reynolds C, Skapura DG, Wehrens XH. Genetic inhibition of PKA phosphorylation of RyR2 prevents dystrophic cardiomyopathy. Proc Natl Acad Sci USA. 2010;107:13165–13170. doi: 10.1073/pnas.1004509107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kyrychenko S, Polakova E, Kang C, Pocsai K, Ullrich ND, Niggli E, Shirokova N. Hierarchical accumulation of RyR post-translational modifications drives disease progression in dystrophic cardiomyopathy. Cardiovasc Res. 2013;97:666–675. doi: 10.1093/cvr/cvs425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fauconnier J, Thireau J, Reiken S, Cassan C, Richard S, Matecki S, Marks AR, Lacampagne A. Leaky RyR2 trigger ventricular arrhythmias in Duchenne muscular dystrophy. Proc Natl Acad Sci USA. 2010;107:1559–1564. doi: 10.1073/pnas.0908540107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamanushi TT, Shui Z, Leach RN, Dobrzynski H, Claydon TW, Boyett MR. Role of internalization of M2 muscarinic receptor via clathrin-coated vesicles in desensitization of the muscarinic K+ current in heart. Am J Physiol Heart Circ Physiol. 2007;292:H1737–H1746. doi: 10.1152/ajpheart.01287.2005. [DOI] [PubMed] [Google Scholar]
- 22.El-Armouche A, Zolk O, Rau T, Eschenhagen T. Inhibitory G-proteins and their role in desensitization of the adenylyl cyclase pathway in heart failure. Cardiovasc Res. 2003;60:478–487. doi: 10.1016/j.cardiores.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 23.Mardon K, Montagne O, Elbaz N, Malek Z, Syrota A, Dubois-Rande JL, Meignan M, Merlet P. Uptake-1 carrier downregulates in parallel with the beta-adrenergic receptor desensitization in rat hearts chronically exposed to high levels of circulating norepinephrine: implications for cardiac neuroimaging in human cardiomyopathies. J Nucl Med. 2003;44:1459–1466. [PubMed] [Google Scholar]
- 24.Raake PW, Zhang X, Vinge LE, Brinks H, Gao E, Jaleel N, Li Y, Tang M, Most P, Dorn GW, II, Houser SR, Katus HA, Chen X, Koch WJ. Cardiac G-protein-coupled receptor kinase 2 ablation induces a novel Ca2+ handling phenotype resistant to adverse alterations and remodeling after myocardial infarction. Circulation. 2012;125:2108–2118. doi: 10.1161/CIRCULATIONAHA.111.044255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen X, Piacentino V, III, Furukawa S, Goldman B, Margulies KB, Houser SR. L-type Ca2+ channel density and regulation are altered in failing human ventricular myocytes and recover after support with mechanical assist devices. Circ Res. 2002;91:517–524. doi: 10.1161/01.res.0000033988.13062.7c. [DOI] [PubMed] [Google Scholar]
- 26.Woolf PJ, Lu S, Cornford-Nairn R, Watson M, Xiao XH, Holroyd SM, Brown L, Hoey AJ. Alterations in dihydropyridine receptors in dystrophin-deficient cardiac muscle. Am J Physiol Heart Circ Physiol. 2006;290:H2439–H2445. doi: 10.1152/ajpheart.00844.2005. [DOI] [PubMed] [Google Scholar]
- 27.Koenig X, Dysek S, Kimbacher S, Mike AK, Cervenka R, Lukacs P, Nagl K, Dang XB, Todt H, Bittner RE, Hilber K. Voltage-gated ion channel dysfunction precedes cardiomyopathy development in the dystrophic heart. PLoS ONE. 2011;6:e20300. doi: 10.1371/journal.pone.0020300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sadeghi A, Doyle AD, Johnson BD. Regulation of the cardiac L-type Ca2+ channel by the actin-binding proteins alpha-actinin and dystrophin. Am J Physiol Cell Physiol. 2002;282:C1502–C1511. doi: 10.1152/ajpcell.00435.2001. [DOI] [PubMed] [Google Scholar]
- 29.Viola HM, Davies SM, Filipovska A, Hool LC. The L-type Ca2+ channel contributes to alterations in mitochondrial calcium handling in the mdx ventricular myocyte. Am J Physiol Heart Circ Physiol. 2013;304:H767–H775. doi: 10.1152/ajpheart.00700.2012. [DOI] [PubMed] [Google Scholar]
- 30.Ullrich ND, Fanchaouy M, Gusev K, Shirokova N, Niggli E. Hypersensitivity of excitation–contraction coupling in dystrophic cardiomyocytes. Am J Physiol Heart Circ Physiol. 2009;297:H1992–H2003. doi: 10.1152/ajpheart.00602.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yilmaz A, Sechtem U. Cardiac involvement in muscular dystrophy: advances in diagnosis and therapy. Heart. 2012;98:420–429. doi: 10.1136/heartjnl-2011-300254. [DOI] [PubMed] [Google Scholar]
- 32.Ather S, Wang W, Wang Q, Li N, Anderson ME, Wehrens XH. Inhibition of CaMKII phosphorylation of RyR2 prevents inducible ventricular arrhythmias in mice with Duchenne muscular dystrophy. Heart Rhythm. 2013;10:592–599. doi: 10.1016/j.hrthm.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.