Abstract
Algae are significant members of Earth’s biodiversity. Having been studied for a long time, the discovery of new algal phyla is extremely unusual. Recently, the enigmatic ‘Picobiliphyta’, a group of uncultured eukaryotes unveiled using molecular tools, were claimed to represent an unrecognized early branching algal lineage with a nucleomorph (remnant nucleus of a secondary algal endosymbiont) in their plastids. However, subsequent studies rejected the presence of a nucleomorph, and single-cell genomic studies failed to detect any plastid-related genes, ruling out the possibility of plastid occurrence. The isolation of the first ‘picobiliphyte’, Picomonas judraskeda, a tiny organism that feeds on very small (<150 nm) organic particles, came as final proof of their non-photosynthetic lifestyle. Consequently, the group has been renamed Picozoa. The passage from ‘picobiliphytes’ to ‘picozoa’ illustrates the crucial role that classical protistology should play to provide sound biological context for the wealth of data produced by modern molecular techniques.
Introduction
Cyanobacteria, plants, and a variety of eukaryotic algae are responsible for the release of oxygen to the Earth’s atmosphere and also for the most significant part of CO2 fixation into organic matter [1-4]. Among these organisms, cyanobacteria and many unicellular algae are microscopic, but the presence of photosynthetic and other accessory pigments in their cells makes them easily visible under the microscope. This is why, to the detriment of the many colorless heterotrophic species that coexist with them, microalgae became a favored target of study since the early days of optical microscopy and why they figure among the best known microorganisms. A vast amount of microalgal morphology and cell ultrastructural data has been collected during the last five decades and has served for phenotype-based taxonomy. In more recent years, this body of information has been completed by molecular phylogeny analyses of conserved gene markers, which has been instrumental in attempting to establish a natural, phylogeny-based taxonomy. These studies led to classify eukaryotic algae into a relatively small number of lineages [5]. Three algal groups (Glaucophyta, Rhodophyta and Chlorophyta) harbor plastids that derive from the original endosymbiosis of a cyanobacterium within an initial heterotrophic eukaryotic cell (primary endosymbiosis) [6,7]. The rest of the eukaryotic algae have plastids acquired through the endosymbiosis of red or green algae within different eukaryotic hosts (secondary endosymbioses), although in some occasions tertiary endosymbioses or secondary plastid replacements may also have occurred [8]. These secondary and tertiary events gave rise to a variety of algal groups that are distributed across the eukaryotic tree. These include the Euglenida, Chlorarachniophyta, Cryptophyta, Haptophyta, various groups within the Heterokonta or Stramenopiles (Bacillariophyta, Phaeophyta, Xantophyta, etc.) and, within the Alveolata, the Dinophyceae and a group that secondarily lost the photosynthetic capacity, the parasitic Apicomplexa. As mentioned above, these groups have been known for a long time, so the discovery of new algal lineages can be regarded as highly improbable, something only comparable to the discovery of new mammalian species or new animal groups. In fact, a recent analysis using accumulation curves for high level algal taxa (families, orders, and classes) has showed that the curves are already close to saturation, which suggests that most of those taxa have already been discovered [9].
Unexpectedly, the existence of three new groups of putative algae has been reported recently. One of them, the Chromerida, represented by its first described species Chromera velia, is related to the once-photosynthetic Apicomplexa and contains plastids derived from red algae [10-12]. This algal group is now firmly established based on abundant sequence information as well as morphological, ultrastructural and biochemical data, and its diversity has been expanded with the isolation of the new species Vitrella brassicaformis [13-15]. The second group, informally called “rappemonads”, has been characterized by a combination of the analysis of plastid 16S rRNA gene sequences retrieved from environmental surveys of marine and freshwater environments and fluorescent in situ hybridization (FISH) experiments with specific plastid-targeted probes [16]. Rappemonads appear to contain several plastids in their cells and branch as sister-group of the haptophytes in plastid rRNA gene phylogenies. Thus, it is unclear whether these organisms actually represent a new algal group or just an uncharacterized deeply-diverging haptophyte lineage. The third group of putative new algae was probably the most enigmatic. Initially detected in 18S rDNA environmental libraries as a lineage with unclear affinity to any other eukaryotic group, their members were subsequently visualized using FISH with specific 18S rDNA-targeted probes. FISH experiments and fluorescent microscopy revealed very small cells (<5 μm) showing orange autofluorescence, which might indicate the presence of phycobilin pigments (known to occur in cyanobacteria and in plastids of glaucophytes, red algae, and some other algae containing plastids derived from red algae [17]). Surprisingly, DNA staining with fluorescent intercalating agents appeared to show the presence of a DNA-containing organelle resembling a plastid with a nucleomorph. Nucleomorphs are remnants of red or green algal nuclei in plastids derived from secondary endosymbionts [18]. Their occurrence in these tiny organisms would indicate that they are algae containing plastids acquired by secondary endosymbiosis. Accordingly, the authors of these observations concluded that these organisms were possibly photosynthetic and coined for them the term Picobiliphyta, in reference to their small size, pigmentation, and presumed algal nature [19].
However, these conclusions were based on indirect observations in natural environments, since no picobiliphyte representative was available in culture. Nonetheless, the discovery of a new algal group attracted much attention and efforts were made to gain more knowledge from this potentially pivotal algal group. As data began to accumulate, doubts were raised as to the photosynthetic nature of these organisms and, finally, a new conclusion on these organisms’ lifestyle was reached. We will discuss here these recent advances as an example that illustrates the necessity of a renaissance of classical protistology to complement modern molecular methods of microbial diversity analysis in order to fully understand the biology of new microbial eukaryotic lineages.
‘Picobiliphytes’ are widespread in oceans but lack a nucleomorph
As mentioned above, ‘picobiliphytes’ (also known as ‘biliphytes’), were first detected as a separate group of sequences in 18S rDNA libraries constructed from marine surface plankton DNA samples [19]. A subsequent study by Cuvelier et al. retrieved additional ‘picobiliphyte’ sequences in warm and cold surface samples from very distant marine locations, indicating that this group was globally distributed, since they were present in very different oceanic regions [20]. Phylogenetic analysis of the complete set of 18S rDNA sequences led these authors to divide the ‘picobiliphytes’ into three groups (BP1, BP2, and BP3) containing sequences from different locations, without any apparent pattern of distribution related to geography or temperature adaptation. In contrast with the strong statistical support found for group BP2, the groups BP1 and BP3 were weakly supported [20]. The subsequent accumulation of new ‘picobiliphyte’ 18S rDNA sequences allowed more accurate phylogenetic analyses that confirmed the monophyly of group BP2 and that BP3 and, especially, BP1 were not monophyletic and could be subdivided into several smaller clades [21]. Nevertheless, the most interesting result of Cuvelier et al. was the absence of clear evidence for the presence of a nucleomorph. This was tested by direct cell observation after FISH and DNA staining, which did not reveal any DNA in the cell area showing phycobilin-like fluorescence (the putative plastid). In addition, primers designed to amplify nucleomorph and/or rhodophyte 18S rDNA failed to yield any sequence from the ‘picobiliphyte’ cells. Thus, the authors concluded that “understanding the origin of biliphyte plastids will likely require ultrastructural, molecular phylogenetic and genomic investigations” [20]. Such investigations came a few years later thanks to the work of several teams.
Single-cell genomics and deep-sea sequences - Where is the plastid?
Recent technical advances in single-cell manipulation and in working with small amounts of starting material have facilitated the development of single-cell genomics, a discipline that is likely to revolutionize many scientific areas, in particular microbiology [22]. Using this approach, Yoon et al. isolated single ‘picobiliphyte’ cells by fluorescence activated cell sorting (FACS) and used multiple displacement amplification to amplify their genomic DNA. They used 454 pyrosequencing to obtain ~5 Mbp of assembled contigs per cell followed by Illumina sequencing on one of the amplified genomes, which led to ~28 Mbp of assembled contigs [23]. They then looked for plastid and nuclear-encoded plastid-targeted gene sequences in all these datasets but were unable to identify any convincing hit. As is often the case for this type of approach, the single-cell genome data obtained were partial. However, given that sequenced genomes of eukaryotic plants and algae contain a significant proportion of genes related to photosynthesis and other plastid-related activities scattered in the genomes [24,25], they interpreted the complete absence of photosynthesis-related genes from single-cell genome sequence data as evidence against a photosynthetic lifestyle for the ‘picobiliphytes’. Moreover, the three ‘picobiliphyte’ cells examined had been retrieved from the colorless, chlorophyll-lacking planktonic fraction of a seawater sample, reinforcing the idea that these organisms were not photosynthetic. This result was consistent with the finding of ‘picobiliphyte’ cells recognized by specific FISH probes but lacking the orange phycobilin-like fluorescence [16].
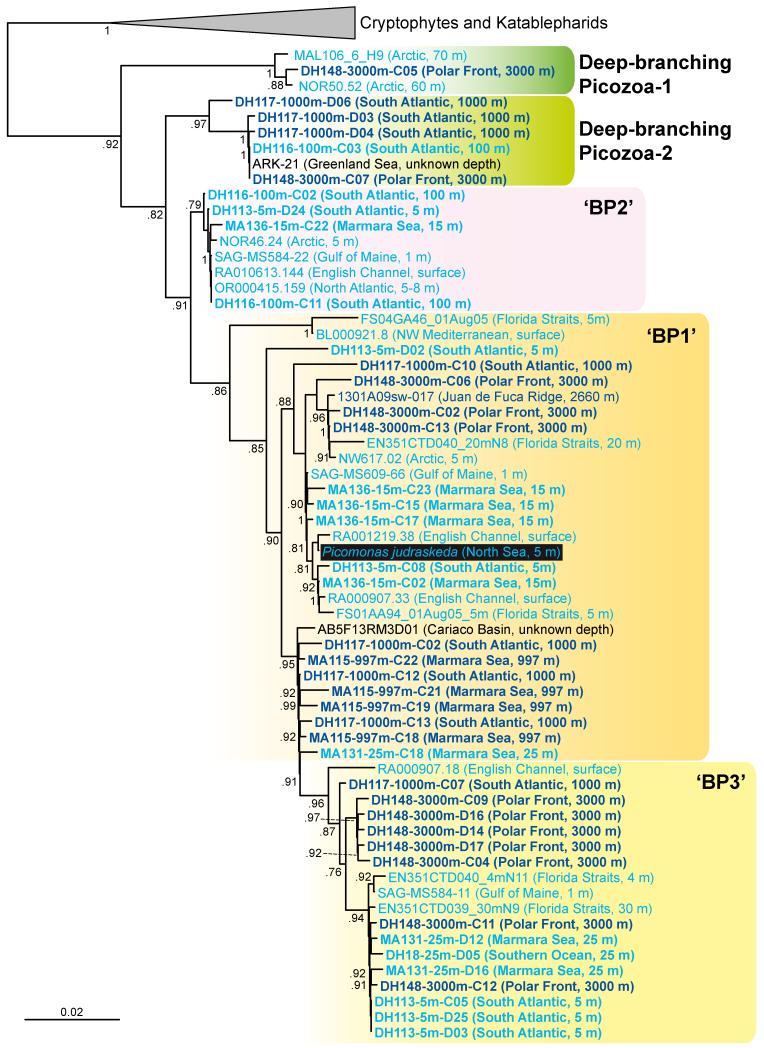
In addition to those studies, the use of ‘picobiliphyte’-specific primers allowed us to detect a large diversity of ‘picobiliphyte’ 18S rDNA sequences in different marine samples, including not only surface but also deep-sea ones. Thus, surprisingly, we found very diverse sequences belonging to the groups BP1 and BP3 defined by Cuvelier et al. [20] not only in surface waters, as expected, but also in very deep samples, down to 3,000 m (Fig. 1). So far, only one ‘picobiliphyte’ sequence, SSRPB47, a partial sequence retrieved at 500 m depth in the Sargasso Sea and belonging to group BP1, had been found in deep sea samples [19]. Thus, whereas group BP2 appears to occur in surface waters, groups BP1 and BP3 do not show any clear distribution pattern related to depth, being found all along the water column. In addition to BP1 and BP3 sequences, we also retrieved sequences that define two new early-diverging groups sister to the already known picobiliphyte clades (Deep-branching groups 1 and 2, see Fig. 1). One of them, group 2, appears to be dominated by deep ocean sequences, although this has to be confirmed by more extensive sampling Altogether, these results suggest that ‘picobiliphytes’ are at least as diverse in the deep dark oceanic regions as in surface waters, which is difficult to imagine for supposedly photosynthetic organisms.
Figure 1.
Phylogenetic analysis of ‘pycobiliphyte’ 18S rDNA sequences. Environmental sequences retrieved from the epipelagic region are shown in light blue; deep-sea sequences are highlighted in dark blue. Sequences from our study are in bold (accession numbers KJ173835-KJ173876). 18S rDNA was amplified using the ‘picobiliphyte’-specific primer picolF (5′-CGTTTATTTGATGATCTCTTG) and general eukaryotic reverse primers from a collection of marine plankton DNA from the South Atlantic/Southern Ocean and the Marmara Sea used in previous studies following the same technical procedures [33,34], The name of the only isolated species, Picomonas jadrciskeda, is indicated by a black background. Groups BP1, BP2 and BP3 are named according to Cuvelier et al. [20], The tree was reconstructed by Bayesian analysis using 1,057 positions. The scale bar indicates the estimated number of nucleotide substitutions per site.
An intriguing phylogenetic position
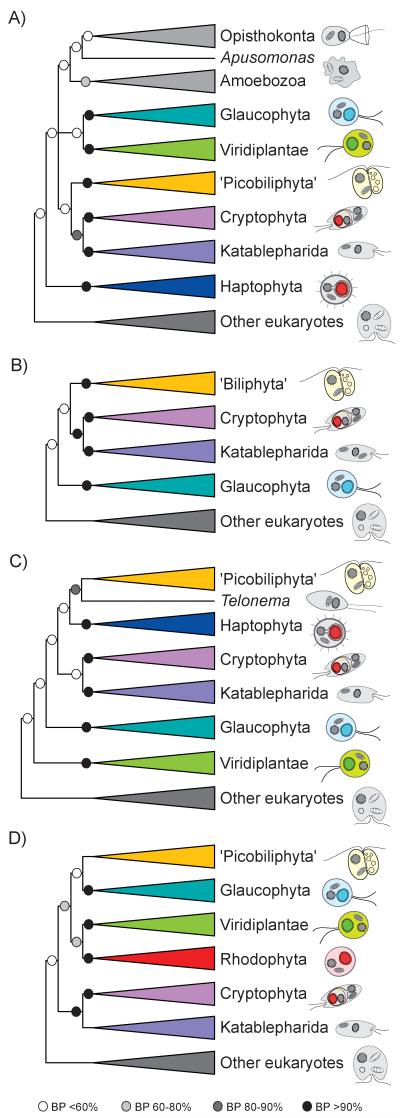
The first insights into the phylogenetic position of ‘picobiliphytes’ were based on trees reconstructed using 18S rDNA sequences [19]. In these trees, ‘picobiliphytes’ branched close to the cryptophytes and a group of essentially heterotrophic flagellates that had been considered incertae sedis for a long time, the katablepharids, although with moderate statistical support (Fig. 2A). In a subsequent analysis, Cuvelier et al. confirmed this result and also the proximity of a clade containing the ‘picobiliphytes’, cryptophytes and katablepharids to the glaucophytes (Fig. 2B) although, once again, with weak statistical support [20]. This relationship was unexpected since glaucophytes resulted from a primary endosymbiotic event with cyanobacteria and are assumed to form a monophyletic group with red algae and green algae and plants rather than with cryptophytes [6,7]. Indeed, 18S rDNA alone does not contain enough phylogenetic signal as to allow reconstruction of all evolutionary relationships among the different eukaryotic groups: access to additional genomic information from ‘picobiliphytes’ was necessary to try to resolve their phylogenetic position.
Figure 2.
Schematic phylogenetic trees showing the positions proposed for the ‘picobiliphytes’ by A: Not et al. [19], based on 18S rDNA sequence analyses; B: Cuvelier et al. [20], based on 18S rDNA sequence analyses; C: Yoon et al. [23], based on the analysis of 7 conserved proteins; and D: Burki et al. [26], based on the analysis of 258 conserved proteins. Circles at branches indicate the statistical support (bootstrap proportions, BP) found by maximum parsimony [19], maximum likelihood [20,23], and Bayesian inference [26],
Thus, in addition to providing material to test the presence of a putative plastid, the single-cell genome study also offered, in principle, abundant sequence data to address this question. Among approximately 2,000 picobiliphyte predicted proteins bearing similarity with sequences in other eukaryotic species, Yoon et al. selected 7 conserved markers to carry out a phylogenetic analysis. It retrieved the ‘picobiliphytes’ as a deep branch related to the telonemids (another group of heterotrophic protists of unresolved position), within a clade also containing the cryptophytes, haptophytes and katablepharids (Fig. 2C), although with moderate statistical support [23]. This study still used a relatively small amount of data, which could explain such limited resolution. A more comprehensive phylogenetic analysis was carried out by Burki et al. using a much richer dataset containing 258 protein markers. In their tree, ‘picobiliphytes’ emerged close to the primary-plastid-bearing glaucophytes (Fig. 2D), far from the telonemids [26]. Surprisingly, despite the large amount of sequence data used, the statistical support for the position of the ‘picobiliphytes’ remained low. This could be due, at least in part, to the large amount of missing data for this taxon (66% of the genes employed by Burki et al. were not found in the single-cell genome data available for the ‘picobiliphytes’), as missing data can reduce the resolving power of phylogenetic analysis [27]. In fact, one problem frequently encountered in single-cell genomic experiments is the incomplete and biased amplification of the genomic DNA by multiple displacement amplification or related amplification techniques, which leads to incomplete genome coverage [28]. Thus, genome sequence data from other picobiliphyte species, ideally from cultured strains to avoid the DNA limitation inherent to single-cell approaches, or from single-cell amplified genomes using improved amplification methods, remain necessary to solve the phylogenetic position of this group. In this sense, the recovery of genome sequence data from the newly identified deep-branching groups 1 and 2 (Fig. 1) would be of particular interest.
Classical protistology discloses a lifestyle mystery
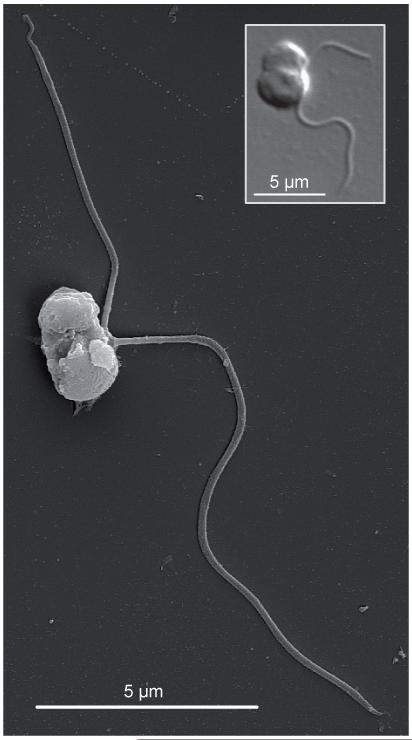
Since their discovery, ‘picobiliphytes’ were comprehensively studied using 18S rDNA libraries, FISH and even single-cell genomics, but the major question of whether they had a photosynthetic or heterotrophic lifestyle remained open since only indirect and partial evidence was available. A formal proof remained necessary. Such a proof has been provided very recently thanks to the isolation of the first ‘picobiliphyte’ species, Picomonas judraskeda [21]. It was cultured from a surface seawater sample previously filtered through 2 μm pore size filters. Accordingly, Picomonas cells turned out to be of tiny size, 2.5-3.8 × 2-2.5 μm in average. These cells had a very unusual structure, as they were divided into two very distinct parts separated by a deep groove (Fig. 3). One part contained all the major cell organelles (the nucleus, a single mitochondrion, a Golgi body and the flagellar apparatus) and the second part was specialized in feeding, containing the “mouth” (cytostome) and a number of vacuoles and vesicles involved in digestion. These two parts were separated by a large vacuolar cisterna of unknown function, though it has been speculated that it may serve as a physical barrier to protect the cell against infection by viruses ingested by the cell region specialized in feeding. In addition to this unusual structure, Picomonas displayed an atypical movement that was described as a “jump, drag, and skedaddle” cycle [21]. The combination of all these morphological and behavioral traits is unique to Picomonas judraskeda.
Figure 3.
Scanning electron microscopy image of a cell of Picomonas judraskeda. An optical microscopy image is shown in the inlet. The difference in cell size between the two images is due to the chemical fixation prior to optical microscopy, which slightly increases the actual cell size. Photographs courtesy of R. Seenivasan and M. Melkonian
An important observation in the nicely detailed ultrastructural characterization carried out by Seenivasan et al. was to show that plastids were absent from Picomonas. In addition to previous studies that failed to find any typical photosynthetic trait in the ‘picobiliphytes’, ultrastructure provided the missing proof required to ascertain the heterotrophic nature of these organisms. Moreover, the food vacuoles found in Picomonas appeared to contain only particles of less than 150 nm in size, much less than the typical size of prokaryotic cells, which suggested that Picomonas probably feed on small colloidal food particles (such as bacterial membrane debris) and, perhaps, also on small viruses. This is an important observation because it rules out the possibility that ‘picobiliphytes’ may ingest eukaryotic algae to recover and enslave their plastids (a process called kleptoplastidy) or even cyanobacteria, as both types of cells are larger than 150 nm in diameter. This also excludes the possibility that the pattern of orange autofluorescence observed in a number of uncultured picobiliphyte cells is due to the presence of ingested photosynthetic cells, as had been advanced [19], and rather suggests some kind of fixation or staining artifact [21].
The morphological characteristics of Picomonas are unique among the known eukaryotic species and do not show any specific affinity to those found in groups such as cryptophytes, katablepharids or glaucophytes, which appear to be related to ‘picobiliphytes’ in phylogenetic analyses, albeit always with weak support (Fig. 2). The uniqueness of the cell structure and the lack of close phylogenetic relatives justify the erection of a new phylum, which Seenivasan et al. proposed to call “Picozoa” to highlight the heterotrophic nutrition mode of these organisms, in clear contrast with the semantics of the previously proposed name “Picobiliphyta”. The dismissal of the photosynthetic nature of these organisms may be perceived as a disappointing result. However, Picozoa constitute a bona fide new eukaryotic lineage, widespread across very different oceanic locations and depths. This makes them good candidates to play a relevant ecological role in marine ecosystems, maybe as specialists in the degradation of very small particles, including marine colloids which, incidentally, are the most abundant particles in the ocean and represent up to 50% of the dissolved organic carbon [29]. Although quantitative data are scarce for this group, it has already been shown that picozoan cells can be abundant in certain locations. For example, FISH experiments have revealed that they can reach densities between 80 and 300 cells ml-1 and account for >1% of all planktonic picoeukaryotic cells in several surface marine locations [19]. Thus, they are most likely significant actors of organic matter recycling in oceans. In addition, they seem to encompass a large diversity, which remains to be fully explored, and branch in a very difficult-to-resolve region of the eukaryotic tree, where photosynthetic and heterotrophic lineages appear intermixed [26]. Thus, isolating new species and sequencing their genomes will be crucial to understand the evolution of these eukaryotic lineages and resolve their phylogenetic relationships as well as to understand their ecological role.
Conclusions and outlook
Picozoa represent a novel diverse group of small marine heterotrophic protists branching deeply in the eukaryotic tree. Unfortunately, the Picomonas strain isolated by Seenivasan et al. was lost after a relatively short time of successful cultivation in the laboratory [21]. Nevertheless, their work demonstrated two important things. First, that new eukaryotic lineages can be put into culture not only by chance but also targeting them using FISH, flow cytometry sorting or other techniques to enrich the desired group from natural samples. Second, that, as could be expected, having the organisms in culture allows their accurate characterization, including morphological, ultrastructural, behavioral, genomic and other aspects. This illustrates that, despite the amazing power of new molecular techniques, traditional protistology remains fundamental to understand the biology of eukaryotic microorganisms. Regrettably, at the same time as molecular approaches became more and more prevalent, classical specialists about many microbial eukaryotic groups retired, so that the vast body of classical knowledge on these organisms became highly endangered and can get lost forever. Of course, this problem is not limited to protistology but to all branches of Biology, as traditional whole-organism approaches are being increasingly deserted [30-32]. The story of the raise and fall of the Picobiliphyta, a potentially novel unique algal group, that suddenly became the Picozoa, a phylum of heterotrophic protists feeding on tiny particles, is a paradigmatic example of the importance of combining molecular and classical tools to fully characterize new lineages.
Acknowledgements
We thank Ramkumar Seenivasan and Michael Melkonian (University of Cologne) for the generous gift of Picomonas judraskeda pictures, and Giselle Walker and two anonymous reviewers for useful comments. The research leading to these results has received funding from the European Research Council under the European Union’s Seventh Framework Programme ERC Grant Agreement 322669 ‘ProtistWorld’.
References
- 1.Field CB, Behrenfeld MJ, Randerson JT, Falkowski P. Primary production of the biosphere: integrating terrestrial and oceanic components. Science. 1998;281:237–40. doi: 10.1126/science.281.5374.237. [DOI] [PubMed] [Google Scholar]
- 2.Arrigo KR. Marine microorganisms and global nutrient cycles. Nature. 2005;437:349–55. doi: 10.1038/nature04159. [DOI] [PubMed] [Google Scholar]
- 3.Falkowski PG, Fenchel T, Delong EF. The microbial engines that drive Earth’s biogeochemical cycles. Science. 2008;320:1034–9. doi: 10.1126/science.1153213. [DOI] [PubMed] [Google Scholar]
- 4.Jiao N, Zheng Q. The microbial carbon pump: from genes to ecosystems. Appl Environ Microbiol. 2011;77:7439–44. doi: 10.1128/AEM.05640-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adl SM, Simpson AG, Lane CE, Lukes J, Bass D, et al. The revised classification of eukaryotes. J Eukaryot Microbiol. 2012;59:429–93. doi: 10.1111/j.1550-7408.2012.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moreira D, Le Guyader H, Philippe H. The origin of red algae and the evolution of chloroplasts. Nature. 2000;405:69–72. doi: 10.1038/35011054. [DOI] [PubMed] [Google Scholar]
- 7.Rodríguez-Ezpeleta N, Brinkmann H, Burey SC, Roure B, Burger G, et al. Monophyly of primary photosynthetic eukaryotes: green plants, red algae. Curr Biol. 2005;15:1325–30. doi: 10.1016/j.cub.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 8.Keeling PJ. The number, speed, and impact of plastid endosymbioses in eukaryotic evolution. Annu Rev Plant Biol. 2013;64:583–607. doi: 10.1146/annurev-arplant-050312-120144. [DOI] [PubMed] [Google Scholar]
- 9.De Clerck O, Guiry MD, Leliaert F, Samyn Y, Verbruggen H. Algal taxonomy: a road to nowhere? J Phycol. 2013;49:215–25. doi: 10.1111/jpy.12020. [DOI] [PubMed] [Google Scholar]
- 10.Moore RB, Obornik M, Janouskovec J, Chrudimsky T, Vancova M, et al. A photosynthetic alveolate closely related to apicomplexan parasites. Nature. 2008;451:959–63. doi: 10.1038/nature06635. [DOI] [PubMed] [Google Scholar]
- 11.Janouskovec J, Horak A, Obornik M, Lukes J, Keeling PJ. A common red algal origin of the apicomplexan, dinoflagellate, and heterokont plastids. Proc Natl Acad Sci U S A. 2010;107:10949–54. doi: 10.1073/pnas.1003335107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Obornik M, Vancova M, Lai DH, Janouskovec J, Keeling PJ, et al. Morphology and ultrastructure of multiple life cycle stages of the photosynthetic relative of apicomplexa, Chromera velia. Protist. 2011;162:115–30. doi: 10.1016/j.protis.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Obornik M, Modry D, Lukes M, Cernotikova-Stribrna E, Cihlar J, et al. Morphology, ultrastructure and life cycle of Vitrella brassicaformis n. sp., n. gen., a novel chromerid from the Great Barrier Reef. Protist. 2012;163:306–23. doi: 10.1016/j.protis.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Woehle C, Dagan T, Martin WF, Gould SB. Red and problematic green phylogenetic signals among thousands of nuclear genes from the photosynthetic and apicomplexa-related Chromera velia. Genome Biol Evol. 2011;3:1220–30. doi: 10.1093/gbe/evr100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burki F, Flegontov P, Obornik M, Cihlar J, Pain A, et al. Re-evaluating the green versus red signal in eukaryotes with secondary plastid of red algal origin. Genome Biol Evol. 2012;4:626–35. doi: 10.1093/gbe/evs049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim E, Harrison JW, Sudek S, Jones MD, Wilcox HM, et al. Newly identified and diverse plastid-bearing branch on the eukaryotic tree of life. Proc Natl Acad Sci U S A. 2011;108:1496–500. doi: 10.1073/pnas.1013337108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oheocha C. Biliproteins of algae. Annu R Plant Physiol. 1965;16:415–34. [Google Scholar]
- 18.Cavalier-Smith T. Nucleomorphs: enslaved algal nuclei. Curr Opin Microbiol. 2002;5:612–9. doi: 10.1016/s1369-5274(02)00373-9. [DOI] [PubMed] [Google Scholar]
- 19.Not F, Valentin K, Romari K, Lovejoy C, Massana R, et al. Picobiliphytes: a marine picoplanktonic algal group with unknown affinities to other eukaryotes. Science. 2007;315:253–5. doi: 10.1126/science.1136264. [DOI] [PubMed] [Google Scholar]
- 20.Cuvelier ML, Ortiz A, Kim E, Moehlig H, Richardson DE, et al. Widespread distribution of a unique marine protistan lineage. Environ Microbiol. 2008;10:1621–34. doi: 10.1111/j.1462-2920.2008.01580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seenivasan R, Sausen N, Medlin LK, Melkonian M. Picomonas judraskeda gen. et sp. nov.: the first identified member of the Picozoa phylum nov., a widespread group of picoeukaryotes, formerly known as ‘picobiliphytes’. PLoS One. 2013;8:26. doi: 10.1371/journal.pone.0059565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stepanauskas R, Sieracki ME. Matching phylogeny and metabolism in the uncultured marine bacteria, one cell at a time. Proc Natl Acad Sci U S A. 2007;104:9052–7. doi: 10.1073/pnas.0700496104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoon HS, Price DC, Stepanauskas R, Rajah VD, Sieracki ME, et al. Single-cell genomics reveals organismal interactions in uncultivated marine protists. Science. 2011;332:714–7. doi: 10.1126/science.1203163. [DOI] [PubMed] [Google Scholar]
- 24.Timmis JN, Ayliffe MA, Huang CY, Martin W. Endosymbiotic gene transfer: organelle genomes forge eukaryotic chromosomes. Nat Rev Genet. 2004;5:123–35. doi: 10.1038/nrg1271. [DOI] [PubMed] [Google Scholar]
- 25.Reyes-Prieto A, Hackett JD, Soares MB, Bonaldo MF, Bhattacharya D. Cyanobacterial contribution to algal nuclear genomes is primarily limited to plastid functions. Curr Biol. 2006;16:2320–5. doi: 10.1016/j.cub.2006.09.063. [DOI] [PubMed] [Google Scholar]
- 26.Burki F, Okamoto N, Pombert JF, Keeling PJ. The evolutionary history of haptophytes and cryptophytes: phylogenomic evidence for separate origins. Proc Biol Sci. 2012;279:2246–54. doi: 10.1098/rspb.2011.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roure B, Baurain D, Philippe H. Impact of missing data on phylogenies inferred from empirical phylogenomic data sets. Mol Biol Evol. 2013;30:197–214. doi: 10.1093/molbev/mss208. [DOI] [PubMed] [Google Scholar]
- 28.Worden AZ, Dupont C, Allen AE. Genomes of uncultured eukaryotes: sorting FACS from fiction. Genome Biol. 2011;12:117. doi: 10.1186/gb-2011-12-6-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wells M. Marine colloids: A neglected dimension. Nature. 1998;391:530–1. [Google Scholar]
- 30.Samyn Y, Massin C. Taxonomists’ requiem? Science. 2002;295:276–7. doi: 10.1126/science.295.5553.276. [DOI] [PubMed] [Google Scholar]
- 31.Wagele H, Klussmann-Kolb A, Kuhlmann M, Haszprunar G, Lindberg D, et al. The taxonomist - an endangered race. A practical proposal for its survival. Front Zool. 2011;8:25. doi: 10.1186/1742-9994-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heger TJ, Edgcomb VP, Kim E, Lukes J, Leander BS, et al. A resurgence in field research is essential to better understand the diversity, ecology, and evolution of microbial eukaryotes. J Eukaryot Microbiol. 2014;61:214–223. doi: 10.1111/jeu.12095. [DOI] [PubMed] [Google Scholar]
- 33.López-García P, Rodríguez-Valera F, Pedrós-Alió C, Moreira D. Unexpected diversity of small eukaryotes in deep-sea Antarctic plankton. Nature. 2001;409:603–7. doi: 10.1038/35054537. [DOI] [PubMed] [Google Scholar]
- 34.Lara E, Moreira D, Vereshchaka A, Lopez-Garcia P. Pan-oceanic distribution of new highly diverse clades of deep-sea diplonemids. Environ Microbiol. 2009;11:47–55. doi: 10.1111/j.1462-2920.2008.01737.x. [DOI] [PubMed] [Google Scholar]