Abstract
Background: Individuals with autoimmune diseases and severe infections have persistent or acutely elevated inflammatory biomarkers and increased risk of schizophrenia. We tested the hypothesis that baseline elevated plasma levels of the inflammatory biomarker, C-reactive protein (CRP), associate with increased risk of late- and very-late-onset schizophrenia in the general population, and if such an association possibly is causal. Method: We analyzed data from 78 810 men and women, aged 20–100 years, from 2 large population studies. Endpoints were hospitalization with schizophrenia and schizophrenia and schizophrenia-like psychosis combined. We performed prospective and cross-sectional analyses adjusted for potential confounders with up to 20 years of follow-up. Furthermore, we used genetic variants influencing plasma CRP levels to perform a Mendelian randomization study. Results: Age- and gender-adjusted hazard ratios vs individuals in the first quartile of CRP were 1.7 (95% CI: 0.3–8.9) for second quartile, 2.1 (0.4–10) for third quartile, and 11 (2.8–40) for fourth quartile individuals. The corresponding hazard ratio for fourth quartile individuals after multifactorial adjustment was 5.9 (1.4–24). Furthermore, individuals with vs without schizophrenia had 63% increased plasma levels of CRP (P = 1 × 10−4). Finally, when CRP was on a continuous scale, a doubling in CRP yielded an age- and gender-adjusted observational OR of 1.5 (1.3–1.8) and a corresponding causal OR of 1.4 (0.5–4.3) (observed vs causal: P = .89). Conclusion: Baseline elevated plasma CRP is associated with a 6- to 11-fold increased risk of late- and very-late-onset schizophrenia in the general population. We cannot exclude that this is a causal association. These are novel findings.
Key words: inflammation, epidemiology, schizophrenia, genetics
Introduction
Individuals with autoimmune diseases and severe infections have persistent or acutely elevated levels of inflammatory biomarkers in plasma,1 and such individuals were found to have increased risk of schizophrenia in a recent prospective study of 3.6 million individuals.2 Furthermore, from mainly case-control studies, there is increasing evidence that patients with schizophrenia have elevated levels of inflammatory markers, including cytokines in plasma.3,4 These findings suggest that inflammation could be involved in the pathogenesis of schizophrenia.
C-reactive protein (CRP) is one of the most commonly used markers of inflammation. During acute inflammation like bacterial infection, levels of CRP may increase up to 300-fold.1 In apparently healthy individuals, plasma levels of CRP are usually below 3mg/l but can be up to 10mg/l5; however, slightly elevated CRP levels below 10mg/l in normal subjects may indicate a state of low-grade inflammation,1 which has been associated with increased risk of several diseases, including major depression and bipolar disorder.6–8 Only few studies have examined the association between elevated CRP and schizophrenia, largely in case-control studies.9–15 Of these, 4 studies have reported an association between elevated CRP and schizophrenia,9–11,15 whereas the remaining studies reported a null association.12–14 Importantly, to our knowledge, there are no prospective studies of the general population examining the association between baseline elevated CRP and future risk of schizophrenia.
Schizophrenia is generally regarded as an illness with onset in late adolescence or early adult life, but ~24% of patients are >40 years of age at onset.16 Based on the International Consensus Criteria, early-onset schizophrenia is schizophrenia with onset before age 40, late-onset schizophrenia after age 40, and very-late-onset schizophrenia (also termed very-late-onset schizophrenia-like psychosis) is onset after age 60.17
First, we tested the hypothesis that baseline elevated plasma levels of CRP associate with increased risk of late- and very-late-onset schizophrenia in the general population; we were only able to examine late- and very-late onset schizophrenia because of the age range of our cohorts. We measured CRP in 78 810 individuals from 2 large independent general population studies, the Copenhagen General Population Study and the Copenhagen City Heart Study, and the individuals were followed up for up to 20 years. To ascertain schizophrenia, we used hospitalization with schizophrenia and schizophrenia-like psychosis from the national Danish Patient Registry,2,8 which covers all psychiatric and somatic hospitals in Denmark from 1977 onwards. Second, we compared plasma CRP levels between individuals with and without schizophrenia and schizophrenia and schizophrenia-like psychosis combined. Finally, the hypothesis that an association between CRP and schizophrenia is causal was tested using the genotypes of 78 810 individuals for 4 single nucleotide polymorphisms (SNPs) in the CRP gene to perform a Mendelian randomization study.18,19 We chose 4 SNPs that capture most of the variation in the CRP gene, including the most important 3-allelic promoter SNP20,21; the latter SNP causes lifelong elevated but fully functional CRP levels. These SNPs together explain approximately 2% of the variation in plasma CRP levels.21 We chose not to include SNPs outside the CRP gene that influence CRP levels,21,22 as such SNPs typically will have pleiotropic effects, making interpretation of data more difficult than when using SNPs solely influencing CRP levels, as done in the present study.
Methods
This study was approved by Herlev Hospital and Danish ethical committees (KF-100.2039/91 and H-KF-01-144/01).
Individuals
We included 78 810 men and women from 2 independent prospective cohort studies of the Danish general population: the Copenhagen General Population Study (n = 68780) and the Copenhagen City Heart Study (n = 10030)8,23–25; no individual appeared in both studies and the 2 studies were combined to achieve maximal statistical power. Individuals in both studies were aged 20–100 years at the time of inclusion. All individuals were white and of Danish descent (ie, the national Danish Civil Registration System showed that each participant and both parents were born in Denmark and were Danish citizens). Because all individuals in Denmark have a unique identification number, we used the national Danish Civil Registration System to recruit individuals at random and to register emigration or death on all individuals.26 Written informed consent was obtained from all individuals. Details on the studies can be seen in online supplementary material.
Biochemical Analyses
Plasma levels of CRP were measured at the Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital.27 CRP was measured with a high-sensitivity assay using latex-enhanced turbidimetry (Dako) or nephelometry (Dade Behring) blinded to schizophrenia status; results were similar for the 2 assays and, therefore, all statistical analyses were combined, but with adjustment for assay type and study. Four SNPs in the CRP gene were genotyped using TaqMan, ABI Prism 7900 HT Sequence Detection System (Applied Biosystems Inc). Details on other biochemical analyses and details on genotyping can be seen in online supplementary material.
Schizophrenia and Schizophrenia-Like Psychosis
Diagnoses of schizophrenia and schizophrenia-like psychoses and dates of diagnoses were obtained from the national Danish Patient Registry and the national Danish Causes of Death Registry.28 The national Danish Patient Registry has information on all hospital discharge diagnoses of psychiatric diseases from psychiatric and somatic hospitals since January 1977, including outpatients and emergency wards since 1995.29 Details on diagnoses can be seen in online supplementary material and supplementary tables 1 and 2.
Covariates
Individuals reported on smoking status (no. of cigarettes/day), alcohol consumption (g/week), level of education after primary and secondary lower school (no further education, education less than 3 years, higher education ≥3 years, and university education), level of income (lowest, middle, and highest), and use of statins (yes/no). Body mass index (BMI) was measured as weight in kilograms divided by height in meters squared. Chronic disease was ascertained by collecting information from the national Danish Patient Registry (see online supplementary material).
Statistical Analyses
Stata version 12.1 was used for all statistical analyses.
Two endpoints were used: (1) schizophrenia and (2) a combined endpoint of schizophrenia and schizophrenia-like psychosis. For the prospective analysis, individuals were a priori divided into groups based on CRP quartiles. This was done as no prior cutpoint for elevated CRP in relation to schizophrenia exist, and to achieve sufficient statistical power (see online supplementary material). We had 98% complete data on alcohol consumption, smoking status, income, and level of education. All missing values were imputed based on age and gender before multifactorial adjustment.30
First, we used a Cox proportional hazards regression model with age as the underlying time scale, which means that age is automatically adjusted for, and left truncation (=delayed entry) in 1991–1994, 2001–2003, or 2003–2010 as appropriate to calculate hazard ratios with 95% CIs. Individuals with previous or current schizophrenia at baseline (n = 34) were excluded from the analyses of schizophrenia, and individuals with previous or current schizophrenia and/or schizophrenia-like psychosis at baseline (n = 73) were excluded from the analyses of the combined endpoint. Follow-up began at blood sampling, and individuals were censored at hospitalization with schizophrenia (n = 36) or schizophrenia-like psychosis (n = 95), death (n = 6484), emigration (n = 379), or end of follow-up (June 2011), whichever came first. Models were adjusted either for age and gender alone or multifactorially for covariates associated with schizophrenia and/or plasma CRP levels, ie, for age, gender, alcohol consumption, smoking status, level of education, level of income, BMI, chronic disease, plasma cholesterol, plasma triglycerides, plasma high-density lipoprotein (HDL) cholesterol, and use of statins. We tested the proportional hazards assumption graphically by plotting −log[−log(survival)] vs log(age) and by using Schoenfeld residuals; no violations were detected. Because 4317 individuals had CRP measured in both the 1991–1994 and in 2001–2003 examinations of the Copenhagen City Heart Study, we were able to calculate a regression dilution ratio of 0.91 for CRP quartiles using the nonparametric method and to correct hazard ratios and CIs for regression dilution bias in order to avoid underestimation of the association; importantly however, significant levels are not influenced by this correction. It is sufficient to calculate a regression dilution ratio in a subsample and, then, apply this ratio to the entire study population.31
Second, we used Kaplan-Meier curves to plot cumulative incidence of schizophrenia and of the combined endpoint of schizophrenia and schizophrenia-like psychosis as a function of age. Based on results from the Cox regression analysis, we divided individuals into 2 groups based on CRP levels: 1 group including individuals with a CRP in the first to third quartile and 1 group including individuals with a CRP in the fourth quartile. We used log-rank tests to examine differences between groups.
Third, we examined differences in plasma levels of CRP between all individuals with and without a diagnosis of schizophrenia, and with and without the combined endpoint of schizophrenia and schizophrenia-like psychosis, ie, diagnoses before or after study entry combined. To reduce the influence of covariates on plasma CRP levels, we examined logarithmically transformed CRP levels in 3 ways: (1) unadjusted, (2) adjusted for age and gender, and (3) adjusted multifactorially for age, gender, alcohol consumption, smoking status, level of education, level of income, BMI, chronic disease, plasma cholesterol, plasma triglycerides, plasma HDL cholesterol, and use of statins. Two-tailed P values for differences between CRP levels were determined using multiple regression.
Fourth, in sensitivity analyses, we repeated analyses after excluding individuals with early-onset schizophrenia, individuals with imputed covariates, individuals with a diagnosis of dementia within 2 years after the diagnosis of schizophrenia, and individuals with a CRP >30mg/l.
Finally, we used a Mendelian randomization design18 to test a potential causal relationship between elevated CRP and schizophrenia.21,32 Using 4 SNPs in the CRP gene, we identified the genotype combinations with the largest effect on lifelong plasma CRP levels (see online supplementary figure 1). These genotype combinations are randomly assorted among individuals and unconfounded by other factors (see online supplementary tables 4 and 5). We then tested whether each of the CRP SNPs and the genotype combinations were associated with schizophrenia in 2 ways (ie, if the SNPs and the genotype combinations are not associated with the endpoint in a Mendelian randomization study, it is unlikely that CRP is a causal factor for schizophrenia and vice versa18). First, we assumed that genetically elevated plasma CRP levels confer the same increase in risk of schizophrenia as that observed for elevated plasma CRP levels in the general population. Thus, the increase in OR for schizophrenia for a 1% increase in plasma CRP was used to calculate the theoretically predicted risk based on the changes in CRP levels for each SNPs and the genotype combinations. Theoretically predicted risk was then compared with observed risk of schizophrenia. The observed risk for each of the SNPs and the genotype combinations was calculated using logistic regression. Second, we performed an instrumental variable analysis using 2-stage least squares regression.18 In this model, we used the genotype combinations as an instrument (ie, a proxy) to estimate the causal effect of CRP on risk of schizophrenia. F-statistics evaluated the strength of the instrument (F > 10 indicates that the instrument is sufficiently correlated with the variable [CRP] of interest). Causal ORs were calculated using a multiplicative generalized method of moments estimation, which is implemented in the Stata command ivpois.33 The unadjusted observed and causal models were compared using a Wald test.
Results
Baseline characteristics of the 78 810 individuals based on CRP quartiles and endpoints are listed in table 1 and online supplementary table 3.
Table 1.
Baseline Characteristics of 78 810 Individuals from the General Population by C-Reactive Protein Quartiles
| C-Reactive Protein | |||||
|---|---|---|---|---|---|
| First Quartile | Second Quartile | Third Quartile | Fourth Quartile | P Trend | |
| No. | 19 707 | 19 705 | 19 698 | 19 700 | |
| Plasma level, mg/l, median (interquartile range) | 0.8 (0.4–1.0) |
1.3 (1.2–1.4) |
1.9 (1.7–2.1) |
4.3 (3.2–7.0) |
|
| Age, years, median (interquartile range) | 54 (45–64) |
55 (46–65) |
59 (48–68) |
61 (51–70) |
<1 × 10− 300 |
| Women, no. (%) | 10 693 (54) |
10 891 (55) |
10 816 (55) |
11 364 (58) |
4 × 10− 10 |
| Alcohol consumption, g/week, median (interquartile range) | 108 (49–180) |
108 (48–180) |
108 (48–184) |
106 (36–180) |
8 × 10− 11 |
| Smoking, no. of cigarettes/day, mean (interquartile range)a | 2.6 (0–0) |
2.7 (0–0) |
3.6 (0–0) |
5.1 (0–8) |
6 × 10− 228 |
| Less than 3 years of educationb, no. (%) | 10 373 (53) |
10 321 (52) |
12 122 (61) |
13 940 (71) |
<1 × 10− 300 |
| Low income, no. (%) | 2651 (13) |
2514 (13) |
3599 (18) |
5371 (27) |
<1 × 10− 300 |
| Body mass index, kg/m2, median (interquartile range) | 24 (22–26) |
25 (23–27) |
26 (24–29) |
27 (25–31) |
<1 × 10− 300 |
| Chronic disease, no. (%) | 5962 (30) |
6541 (33) |
7919 (40) |
10 056 (51) |
<1 × 10− 300 |
| Plasma cholesterol, mmol/l, median (interquartile range) | 5.5 (4.8–6.2) |
5.6 (4.9–6.3) |
5.7 (5.0–6.5) |
5.7 (5.0–6.5) |
4 × 10− 121 |
| Plasma triglyceride, mmol/l, median (interquartile range) | 1.2 (0.9–1.8) |
1.3 (0.9–1.9) |
1.5 (1.1–2.2) |
1.7 (1.3–2.0) |
<1 × 10− 300 |
| Plasma HDL cholesterol, mol/l, median (interquartile range) | 1.7 (1.3–2.0) |
1.6 (1.3–2.0) |
1.5 (1.2–1.9) |
1.4 (1.1–1.8) |
<1 × 10− 300 |
| Statin use, no. (%) | 1496 (8) |
1818 (9) |
1331 (10) |
1998 (10) |
3 × 10− 20 |
Note: HDL, high-density lipoprotein. Baseline characteristics for participants in the Copenhagen General Population Study and the Copenhagen City Heart Study combined.
aNumber of cigarettes/day is shown as mean because medians in all quartiles are 0.
bEducation after primary and secondary lower school. Further details on education and income can be seen in online supplementary table 6.
In total, 70 individuals had a diagnosis of schizophrenia and 168 had a diagnosis of schizophrenia or schizophrenia-like psychosis combined. The mean follow-up to a diagnosis of schizophrenia or schizophrenia and schizophrenia-like psychosis combined was 7.3 years (range: 268 days to 19 years) and 8.2 years (range: 23 days to 31 years), respectively.
Relative Risk
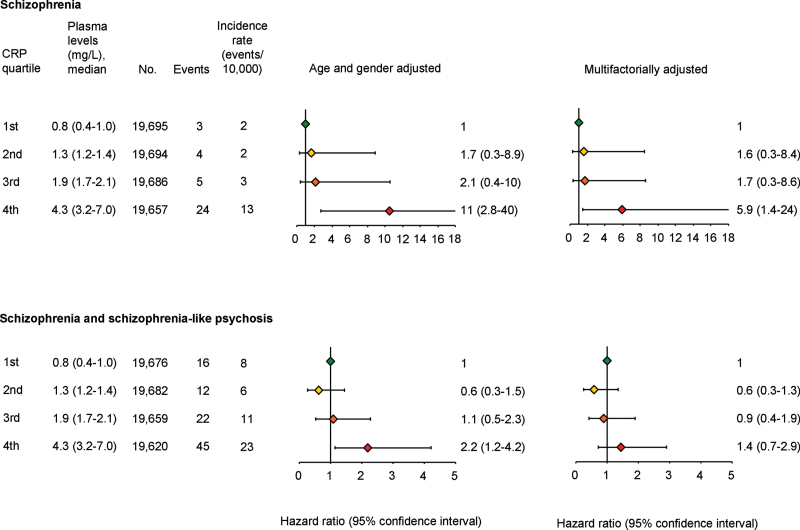
For schizophrenia, age- and gender-adjusted hazard ratios vs individuals in the first quartile were 1.7 (95% CI: 0.3–8.9) for second quartile, 2.1 (0.4–10) for third quartile, and 11 (2.8–40) for fourth quartile individuals (figure 1). The corresponding hazard ratio for fourth quartile individuals was 5.9 (1.4–24) after multifactorial adjustment.
Fig. 1.
Prospective analyses of the associations between schizophrenia and C-reactive protein (CRP) quartiles (top) and between schizophrenia and schizophrenia-like psychosis combined and CRP quartiles (bottom) in the general population. Based on 78 810 individuals from the Copenhagen General Population Study and the Copenhagen City Heart Study combined, followed for up to 20 years; individuals with a hospitalization with the relevant endpoint before CRP measurements were excluded (schizophrenia, n = 34; schizophrenia + schizophrenia-like psychosis, n = 73). Multifactorially adjusted was for age, gender, alcohol consumption, smoking, education, income, body mass index, chronic disease, plasma cholesterol, plasma triglycerides, plasma high-density lipoprotein cholesterol, and use of statins. For a color version, see this figure online.
For the combined endpoint of schizophrenia and schizophrenia-like psychosis, age- and gender-adjusted hazard ratios vs individuals in the first quartile were 0.6 (95% CI: 0.3–1.5) for second quartile, 1.1 (0.5–2.3) for third quartile, and 2.2 (1.2–4.2) for fourth quartile individuals (figure 1). The corresponding hazard ratio for fourth quartile individuals was 1.4 (0.7–2.9) after multifactorial adjustment.
The hazard ratios for each of the covariates for each of the above-mentioned multifactorially adjusted models are shown in online supplementary table 8. Middle and high vs low level of income were associated with decreased risk of both schizophrenia and the combined endpoint of schizophrenia and schizophrenia-like psychosis. Male gender, increased smoking, and chronic disease were associated with increased risk of the combined endpoint of schizophrenia and schizophrenia-like psychosis, but this was not statistically significant for schizophrenia alone.
Cumulative Incidence
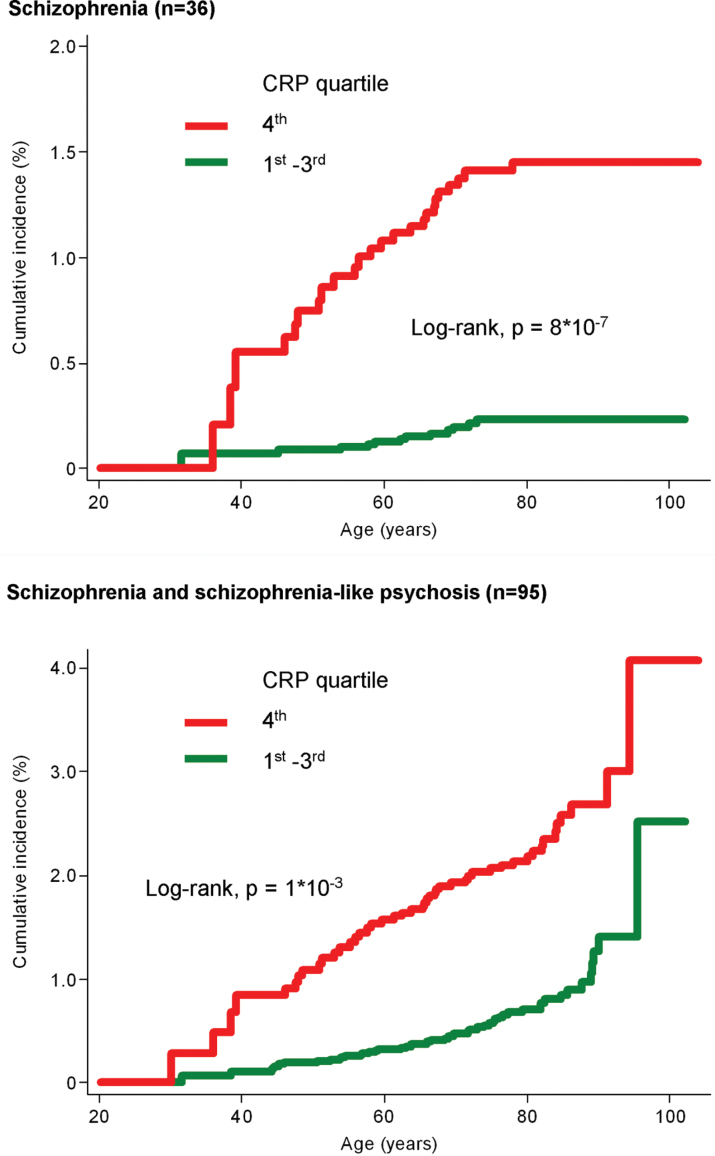
The cumulative incidence of schizophrenia and the combined endpoint of schizophrenia and schizophrenia-like psychosis was increased in individuals with a CRP in the fourth quartile compared to individuals with a CRP in the first to third quartiles (log-rank, P = 8 × 10− 7 and 1 × 10− 3) (figure 2).
Fig. 2.
Cumulative incidence of schizophrenia (top) and the combined endpoint of schizophrenia and schizophrenia-like psychosis (bottom) as a function of age by quartiles of C-reactive protein (CRP). Based on 78 810 individuals from the Copenhagen General Population Study and the Copenhagen City Heart Study combined, followed for up to 20 years; individuals with a hospitalization with the relevant endpoint before CRP measurements were excluded (schizophrenia, n = 34; schizophrenia + schizophrenia-like psychosis, n = 73). For a color version, see this figure online.
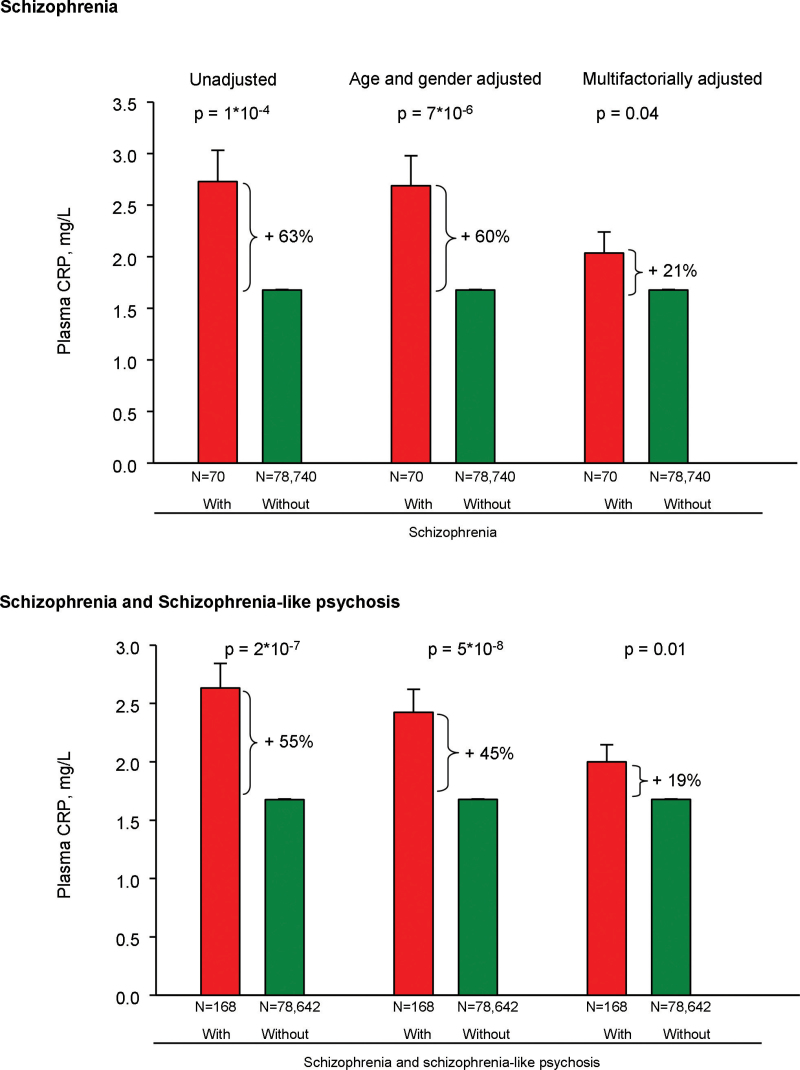
Plasma CRP
Mean CRP levels were 63% higher for individuals with schizophrenia compared to those without (P = 1 × 10− 4) (figure 3). This difference remained significant when we adjusted either for age and gender (P = 7 × 10− 6) and when we adjusted multifactorially (P = .04). Similarly, mean CRP levels were 55% higher for individuals with schizophrenia or schizophrenia-like psychosis combined compared to those without (P = 2 × 10− 7) (figure 3). This difference remained significant when we adjusted either for age and gender (P = 5 × 10− 8) or multifactorially (P = .01). The regression coefficients for association with CRP levels from the multifactorially adjusted models in all 78 810 participants are shown in online supplementary table 9.
Fig. 3.
Plasma C-reactive protein (CRP) levels in individuals with and without schizophrenia (top) or the combined endpoint of schizophrenia and schizophrenia-like psychosis (bottom) before or after CRP measurement. Based on 78 810 individuals from the Copenhagen General Population Study and Copenhagen City Heart Study combined. Multifactorially adjusted was for age, gender, alcohol consumption, smoking, education, income, body mass index, chronic disease, plasma cholesterol, plasma triglycerides, plasma high-density lipoprotein cholesterol, and use of statins. Plasma CRP is shown as mean ± SE. For a color version, see this figure online.
Sensitivity Analysis
When we excluded those with early-onset schizophrenia (11% of cases), individuals with imputed covariates (2% of the study population), individuals with a diagnosis of dementia within 2 years after the diagnosis of schizophrenia (3% of cases), and individuals with a CRP >30mg/l (0.6% of the study population), the results were found to be similar to those in the main analyses (data not shown).
Mendelian Randomization
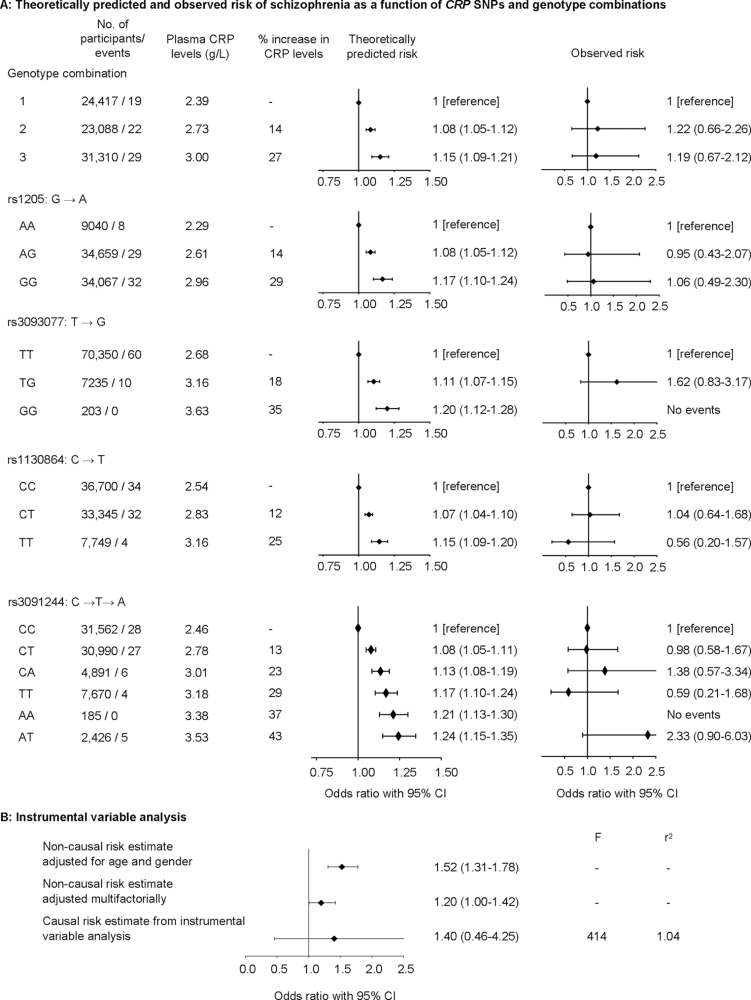
Theoretically Predicted vs Observed Risk.
For the genotype combinations resulting in increased CRP (P trend = 5×10−210), there was an increased theoretically predicted risk of schizophrenia with an OR of 1.08 (1.05–1.11) for genotype combination 2 and 1.15 (1.09–1.21) for genotype combination 3 vs genotype combination 1 (figure 4A). For individuals with these genotype combinations, calculation of observed risk yielded corresponding ORs of 1.22 (0.66–2.26) and 1.19 (0.67–2.12). Similar results for the 4 individual SNPs are also shown in this figure.
Fig. 4.
Mendelian randomization. (A) Theoretically predicted and observed risk of schizophrenia as a function of C-reactive protein (CRP) gene SNPs and genotype combination. (B) Instrumental variable analysis. Based on 78 810 individuals from the Copenhagen General Population Study and the Copenhagen City Heart Study combined. Theoretically predicted risk was adjusted for age and gender (A). Multifactorially adjusted was for age, gender, alcohol consumption, smoking, education, income, body mass index, chronic disease, plasma cholesterol, plasma triglycerides, plasma high-density lipoprotein cholesterol, and use of statins (B). Causal risk estimates were not adjusted.
Instrumental Variable Analysis: Observational vs Causal Risk Estimates.
The observational risk estimate for a doubling in CRP levels was an age- and gender-adjusted OR of 1.52 (1.31–1.78) and a multifactorially adjusted OR of 1.20 (1.00–1.42) (figure 4B). The instrumental variable analysis resulted in a causal risk estimate with an OR of 1.40 (0.46–4.25) (observational vs causal model: P = .89).
Discussion
In this prospective study of 78 810 individuals from the general population, we found that elevated plasma levels of CRP were associated with a 6- to 11-fold increased risk of late- or very-late-onset schizophrenia. These novel findings remained significant after multifactorial adjustment. Furthermore, we showed that individuals with vs without schizophrenia had increased CRP levels. Finally, we cannot exclude that elevated CRP levels per se may be causally associated with schizophrenia.
Supporting our results is a study of 3.6 million individuals reporting that prior hospitalization for autoimmune disease or severe infection, ie, diseases resulting in persistent or acutely elevated levels of CRP,34 was associated with increased future risk of schizophrenia.2 Although no previous studies have examined the association between elevated CRP and schizophrenia prospectively, our results of elevated CRP levels in individuals with vs without schizophrenia are consistent with results in previous case-control studies of 90–400 individuals.9–11,15 Other studies have reported no difference in CRP levels between individuals with vs without schizophrenia12–14; however, compared to CRP levels typically reported for the general population,35 these studies reported much lower mean CRP levels of <0.3mg/l in both individuals with and without schizophrenia. In contrast, in the former studies showing higher CRP levels in individuals with vs without schizophrenia,10,11,15 CRP levels in those without schizophrenia were similar to the mean CRP level in our study of 1.7mg/l, based on 78 810 individuals from the general population.
The biological mechanism linking inflammation and schizophrenia is not fully understood. A possible causal role of CRP per se could be CRP’s disruptive effect on the blood-brain barrier, which has been shown in animals.36 Such a disruption might lead to increased permeability for proinflammatory cytokines and/or autoantibodies, both of which have been associated with schizophrenia and psychotic symptoms.37 Indeed, as the causal and observational estimates for risk of schizophrenia did not differ statistically and both suggested increased risk in our study, we cannot exclude a causal relationship between elevated CRP levels and increased risk of schizophrenia.
When we included participants with schizophrenia-like psychosis, risk estimates were attenuated. This might suggest that elevated CRP is less or not associated with schizophrenia-like psychoses. One reason could be that schizophrenia is characterized by more chronic symptoms and longer duration than that of psychosis and, thus, more strongly associated with baseline CRP which, in this study, was measured on average of 7–8 years before hospitalization with schizophrenia or schizophrenia-like psychosis.
Elevated CRP can be caused by chronic diseases27,38 but also lifestyle factors.38,39 We were also able to show these associations in our study, where CRP levels were significantly associated with smoking, alcohol consumption, education, income, plasma cholesterol, plasma triglycerides, plasma HDL cholesterol, BMI, and chronic disease. Many of these covariates also varied between participants with and without late- and very-late-onset schizophrenia and/or schizophrenia-like psychosis (online supplementary table 3), and they were, therefore, included as covariates in the multifactorially adjusted analyses in order to reduce the risk of confounding.
It is well known that patients with schizophrenia have increased risk of low socioeconomic status, possibly due to the impairments of social functioning caused by the disease,40 in accordance with the present data. That higher income is associated with decreased future risk of hospitalization with schizophrenia in our study is likely caused by generally reduced income for participants prior to hospitalization with schizophrenia rather than higher income being protective against schizophrenia. No other covariates were associated with future risk of schizophrenia, but this might be caused by limited statistical power due to the low number of participants with schizophrenia.
Strengths of our study include the large number of individuals from the general population. Furthermore, we had up to 20 years of follow-up, and the present study is the first to examine the association between elevated CRP levels and schizophrenia prospectively. Also, due to the completeness of the national Danish Patient Registry and the national Danish Civil Registration System, we had 100% complete data on hospitalization with schizophrenia and schizophrenia-like psychoses, on emigrations, and on deaths. Finally, we had CRP measurements from both 1991–1994 and 2001–2003 examinations on 4317 individuals, which meant we were able to correct our results for regression dilution bias. Though CRP is known to be relatively constant over time in each individual,5 having only 1 measurement of CRP may introduce bias caused by measurement bias and intraindividual variation. Because we had a second CRP measurement on 4317 individuals approximately 10 years after inclusion in the study, we were able to calculate a regression dilution ratio of 0.91 that we used to correct our risk estimates. This reduces the effect of regression towards the mean, which might otherwise have lead to an underestimation of risk estimates.31
A potential limitation of this study is that no individual was younger than 20 years at the time of inclusion. Therefore, most of the individuals who received their first diagnosis of schizophrenia after inclusion were individuals with late-onset or very-late-onset schizophrenia, which is a selected group of patients. However, we are not aware of studies suggesting that the association between inflammation and schizophrenia should vary between patients with early-onset or late- or very-late-onset schizophrenia. Patients with late- and very-late-onset schizophrenia are more often women (in our sample, we had 50% women), and their symptoms are often more dominated by positive than negative symptoms.41 It is important to note that some psychoses in elderly people can be caused by dementia. However, when we excluded the individuals with a diagnosis of dementia within 2 year after a psychosis (only 2 participants), the results were similar. Furthermore, individuals with schizophrenia may not participate as often as healthy individuals, which can also be seen in the prevalence of schizophrenia of 0.09% in our study, whereas the prevalence in Denmark is approximately 0.5%–1%.42 The prevalence of schizophrenia and the combined endpoint of schizophrenia and schizophrenia-like-psychosis in the Copenhagen City Heart Study was 138% and 98% higher among nonresponders compared to responders, and the cumulative incidence was significantly higher among nonresponders compared to responders (online supplementary figure 2). However, despite these limitations, we were able to show an association between baseline elevated CRP and future risk of schizophrenia. Furthermore, despite adjustment for multiple covariates, we can not exclude residual confounding or reverse causation. Another potential limitation is that we did not have any validated diagnostic scoring scale of schizophrenia such as the Positive and Negative Syndrome Scale,43 and, therefore, we could not differentiate between negative, cognitive and positive symptoms, and we could not estimate the severity of symptoms. Finally, yet another potential limitation of our study is that we studied whites only, and, therefore, our results may not necessarily apply to other races.
In conclusion, we found that elevated plasma levels of CRP were associated with a 6- to 11-fold increased risk of late- or very-late-onset schizophrenia in a prospective study of the general population. These findings are novel.
Supplementary Material
Supplementary material is available at http://schizophre niabulletin.oxfordjournals.org.
Funding
Herlev Hospital, Copenhagen University Hospital ; The Danish Council for Independent Research, Medical Sciences.
Supplementary Material
Acknowledgments
The funding source had no role in the design and conduct of the study, the collection and management of data, analysis, and interpretation of the data, or the preparation of the manuscript. We thank individuals and staff of the Copenhagen General Population Study and the Copenhagen City Heart Study for their important contributions. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454 [DOI] [PubMed] [Google Scholar]
- 2. Benros ME, Nielsen PR, Nordentoft M, Eaton WW, Dalton SO, Mortensen PB. Autoimmune diseases and severe infections as risk factors for schizophrenia: a 30-year population-based register study. Am J Psychiatry. 2011;168:1303–1310 [DOI] [PubMed] [Google Scholar]
- 3. Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70:663–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry. 2008;63:801–808 [DOI] [PubMed] [Google Scholar]
- 5. Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Berardis D, Conti CM, Campanella D, et al. Evaluation of C-reactive protein and total serum cholesterol in adult patients with bipolar disorder. Int J Immunopathol Pharmacol. 2008;21:319–324 [DOI] [PubMed] [Google Scholar]
- 7. Maes M, Delange J, Ranjan R, et al. Acute phase proteins in schizophrenia, mania and major depression: modulation by psychotropic drugs. Psychiatry Res. 1997;66:1–11 [DOI] [PubMed] [Google Scholar]
- 8. Wium-Andersen MK, Ørsted DD, Nielsen SF, Nordestgaard BG. Elevated C-reactive protein levels, psychological distress, and depression in 73, 131 individuals. JAMA Psychiatry. 2013;70:176–184 [DOI] [PubMed] [Google Scholar]
- 9. Akanji AO, Ohaeri JU, Al-Shammri S, Fatania HR. Association of blood levels of C-reactive protein with clinical phenotypes in Arab schizophrenic patients. Psychiatry Res. 2009;169:56–61 [DOI] [PubMed] [Google Scholar]
- 10. Carrizo E, Fernández V, Quintero J, et al. Coagulation and inflammation markers during atypical or typical antipsychotic treatment in schizophrenia patients and drug-free first-degree relatives. Schizophr Res. 2008;103:83–93 [DOI] [PubMed] [Google Scholar]
- 11. Fawzi MH, Fawzi MM, Fawzi MM, Said NS. C-reactive protein serum level in drug-free male Egyptian patients with schizophrenia. Psychiatry Res. 2011;190:91–97 [DOI] [PubMed] [Google Scholar]
- 12. Fernandez-Egea E, Bernardo M, Donner T, et al. Metabolic profile of antipsychotic-naive individuals with non-affective psychosis. Br J Psychiatry. 2009;194:434–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hope S, Dieset I, Agartz I, et al. Affective symptoms are associated with markers of inflammation and immune activation in bipolar disorders but not in schizophrenia. J Psychiatr Res. 2011;45:1608–1616 [DOI] [PubMed] [Google Scholar]
- 14. Sarandol A, Kirli S, Akkaya C, Ocak N, Eroz E, Sarandol E. Coronary artery disease risk factors in patients with schizophrenia: effects of short term antipsychotic treatment. J Psychopharmacol. 2007;21:857–863 [DOI] [PubMed] [Google Scholar]
- 15. Suvisaari J, Loo BM, Saarni SE, et al. Inflammation in psychotic disorders: a population-based study. Psychiatry Res. 2011;189:305–311 [DOI] [PubMed] [Google Scholar]
- 16. Harris MJ, Jeste DV. Late-onset schizophrenia: an overview. Schizophr Bull. 1988;14:39–55 [DOI] [PubMed] [Google Scholar]
- 17. Howard R, Rabins PV, Seeman MV, Jeste DV. Late-onset schizophrenia and very-late-onset schizophrenia-like psychosis: an international consensus. The International Late-Onset Schizophrenia Group. Am J Psychiatry. 2000;157:172–178 [DOI] [PubMed] [Google Scholar]
- 18. Lawlor DA, Harbord RM, Sterne JAC, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27:1133–1163 [DOI] [PubMed] [Google Scholar]
- 19. Smith GD, Ebrahim S. Mendelian randomization: prospects, potentials, and limitations. Int J Epidemiol. 2004;33:30–42 [DOI] [PubMed] [Google Scholar]
- 20. CRP CHD Genetics Collaboration. Collaborative pooled analysis of data on C-reactive protein gene variants and coronary disease: judging causality by Mendelian randomisation. Eur J Epidemiol. 2008;23:531–540 [DOI] [PubMed] [Google Scholar]
- 21. Zacho J, Tybjærg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med. 2008;359:1897–1908 [DOI] [PubMed] [Google Scholar]
- 22. Dehghan A, Dupuis J, Barbalic M, et al. Meta-analysis of genome-wide association studies in >80 000 subjects identifies multiple loci for C-reactive protein levels. Circulation. 2011;123:731–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kamstrup PR, Benn M, Tybjærg-Hansen A, Nordestgaard BG. Extreme lipoprotein(a) levels and risk of myocardial infarction in the general population: the Copenhagen City Heart Study. Circulation. 2008;117:176–184 [DOI] [PubMed] [Google Scholar]
- 24. Nordestgaard BG, Benn M, Schnohr P, Tybjærg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299–308 [DOI] [PubMed] [Google Scholar]
- 25. Nordestgaard BG, Palmer TM, Benn M, et al. The effect of elevated body mass index on ischemic heart disease risk: causal estimates from a Mendelian randomisation approach. PLoS Med. 2012;9:e1001212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39:22–25 [DOI] [PubMed] [Google Scholar]
- 27. Allin KH, Bojesen SE, Nordestgaard BG. Baseline C-reactive protein is associated with incident cancer and survival in patients with cancer. J Clin Oncol. 2009;27:2217–2224 [DOI] [PubMed] [Google Scholar]
- 28. Helweg-Larsen K. The Danish Register of Causes of Death. Scand J Public Health. 2011;39:26–29 [DOI] [PubMed] [Google Scholar]
- 29. Andersen TF, Madsen M, Jørgensen J, Mellemkjoer L, Olsen JH. The Danish National Hospital Register. A valuable source of data for modern health sciences. Dan Med Bull. 1999;46:263–268 [PubMed] [Google Scholar]
- 30. Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med. 1991;10:585–598 [DOI] [PubMed] [Google Scholar]
- 31. Clarke R, Shipley M, Lewington S, et al. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol. 1999;150:341–353 [DOI] [PubMed] [Google Scholar]
- 32. Benn M, Tybjærg-Hansen A, McCarthy MI, Jensen GB, Grande P, Nordestgaard BG. Nonfasting glucose, ischemic heart disease, and myocardial infarction: a Mendelian randomization study. J Am Coll Cardiol. 2012;59:2356–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Palmer TM, Sterne JAC, Harbord RM, et al. Instrumental variable estimation of causal risk ratios and causal odds ratios in Mendelian randomization analyses. Am J Epidemiol. 2011;173:1392–1403 [DOI] [PubMed] [Google Scholar]
- 34. Casas JP, Shah T, Hingorani AD, Danesh J, Pepys MB. C-reactive protein and coronary heart disease: a critical review. J Intern Med. 2008;264:295–314 [DOI] [PubMed] [Google Scholar]
- 35. Nordestgaard BG. Does elevated C-reactive protein cause human atherothrombosis? Novel insights from genetics, intervention trials, and elsewhere. Curr Opin Lipidol. 2009;20:393–401 [DOI] [PubMed] [Google Scholar]
- 36. Kuhlmann CRW, Librizzi L, Closhen D, et al. Mechanisms of C-reactive protein-induced blood-brain barrier disruption. Stroke. 2009;40:1458–1466 [DOI] [PubMed] [Google Scholar]
- 37. Benros ME, Mortensen PB, Eaton WW. Autoimmune diseases and infections as risk factors for schizophrenia. Ann N Y Acad Sci. 2012;1262:56–66 [DOI] [PubMed] [Google Scholar]
- 38. Black S, Kushner I, Samols D. C-reactive protein. J Biol Chem. 2004;279:48487–48490 [DOI] [PubMed] [Google Scholar]
- 39. Musunuru K, Kral BG, Blumenthal RS, et al. The use of high-sensitivity assays for C-reactive protein in clinical practice. Nat Clin Pract Cardiovasc Med. 2008;5:621–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Salkever DS, Karakus MC, Slade EP, et al. Measures and predictors of community-based employment and earnings of persons with schizophrenia in a multisite study. Psychiatr Serv. 2007;58:315–324 [DOI] [PubMed] [Google Scholar]
- 41. Wynn Owen PA, Castle DJ. Late-onset schizophrenia: epidemiology, diagnosis, management and outcomes. Drugs Aging. 1999;15:81–89 [DOI] [PubMed] [Google Scholar]
- 42. Danish National Board of Health. Sundhedsstyrelsens referenceprogram for skizofreni 2004. Copenhagen, Denmark: Sundhedsstyrelsen; 2004 [Google Scholar]
- 43. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.