Abstract
Background: Sensory gating deficits are among the core features of schizophrenia. Recently, we reported significantly increased sensorimotor gating following additional administration of single dosages of clonidine to the treatment of stably medicated patients with schizophrenia who, in spite of their medication, showed gating deficits. In the current study, we investigated whether this result is generalizable to filtering of sensory information as a whole, by examining clonidine’s effect on P50 suppression in the same group of patients. Methods: In a double-blind, placebo-controlled, randomized yet balanced cross-over design, 20 male schizophrenia patients on stable medication were assessed in a psychophysiological test battery, including a sensory gating paradigm on 5 occasions: once after oral administration of placebo and after single doses of 25, 50, 75, and 150 µg of clonidine. Their results were compared with 20 age-matched healthy male volunteers, who received no treatment. Results: Patients showed significantly reduced levels of P50 suppression in the placebo session compared with controls. All dosages of clonidine significantly diminished these deficits to such levels that they no longer differed significantly from the healthy controls (except the highest dose). Conclusions: This is the first study to show that even a single low dose of clonidine administered to stably medicated patients with schizophrenia not only significantly increases their levels of P50 suppression but also normalizes them. The results indicate that α2-noradrenergic agonists are capable of normalizing levels of P50 gating, which has a potentially high clinical relevance for the medical treatment of schizophrenia.
Key words: chronic schizophrenia, P50 suppression, clonidine, noradrenaline
Introduction
Schizophrenia is a severe, chronic brain disorder that is not only characterized by positive and negative symptoms but also by impaired cognition and deficits in basic information processing, such as sensory gating. Sensory gating can be defined as the brain’s ability to successfully filter irrelevant sensory stimuli out of the stream of sensory information that an individual is subjected to at any given moment in time, securing further processing of only those stimuli that are considered relevant for the individual.1 Defects or flaws in these early sensory filter mechanisms have been proposed leading to cognitive fragmentation and ultimately even to hallucinations and delusions (eg, Perry et al2 and Potter et al3) although the actual relationship between sensory gating and cognition has not been studied very well yet.3
Standard medical treatment in schizophrenia is usually directed towards a reduction of dopamine D2 and/or combined dopamine D2 and serotonin 5-HT2A (5-hydroxytryptamine) activity by means of antipsychotics. Although the currently available antipsychotics are quite effective in reducing positive symptoms in schizophrenia, they are far less effective in reducing negative symptoms and even less in normalizing cognition. In fact, a large number of the reports on cognitive enhancement by second-generation antipsychotics in patients with schizophrenia may have been flawed because they did not take practice effects into account.4 Similarly, there is little indication that the currently available first- or second-generation antipsychotics are able to attenuate deficient sensory gating3 although there are exceptions to this rule,5 see also below.
Besides involvement of dopamine D2 and serotonin-2A receptors, there is evidence for involvement of a large number of other neurotransmitter systems in schizophrenia, the noradrenergic system is one among them.6–8 Noradrenaline is one of the key neurotransmitters involved in cognition (eg, Clark et al9), especially it is involved in the cognitive functions that involve the prefrontal cortex (PFC).10 Evidence is also accumulating for an involvement of the PFC in both sensory5,11 and sensorimotor12,13 gating, including studies from our own laboratory. Unsurprisingly, therefore, there is substantial evidence pointing towards a noradrenergic involvement in a large number of the cognitive deficits that are usually found in patients with schizophrenia.8,14,15 Recent research suggests that the stress-induced high levels of noradrenaline in the PFC impair its functions through stimulation of noradrenergic-α1 receptors, whereas increased noradrenergic-α2 activity appears to improve PFC functions and its cognitive performance.10 Importantly, noradrenergic-α1 receptors appear less sensitive to noradrenaline than noradrenergic-α2 receptors.10,16 It is, therefore, likely that the contrasting sensitivity of these 2 noradrenergic receptor subtypes results in improved PFC function in the presence of moderate levels of noradrenaline (by stimulating the sensitive α2-receptors) and decreased PFC function in the presence of higher levels of noradrenaline (by stimulating the less sensitive α1-receptors).10
Up until the mid 90s, research on noradrenergic involvement in schizophrenia was particularly focused on the effects of clonidine (specific noradrenergic α2-agonist) either administered in monotherapy or in combination with antipsychotics. These studies indicated that clonidine not only improved cognition but also reduced positive and even negative symptoms (eg, Fields et al17 and Freedman et al18). For reasons unknown to us, these studies were not followed up, and from a more general perspective, investigators appeared to had lost interest in the involvement of the entire noradrenergic system in schizophrenia until recently. Unfortunately, this loss of interest is also reflected in the currently available pharmacological treatment for schizophrenia: antipsychotics were not specifically manufactured to target the noradrenergic system; if antipsychotics happen to show affinity for the noradrenergic receptors, then it is usually in one direction only: they block noradrenergic receptors (eg, Litman and Pickar14 and Svensson19). Given the above lines of reasoning, blocking noradrenergic α2-receptors would impair PFC functions, which (at best) would improve neither sensory gating nor cognition in patients with schizophrenia. This blocking of α2-receptors may very well contribute to the fact that the currently available antipsychotics are only modestly able to improve sensory gating and cognition in schizophrenia.
Levels of P50 suppression and prepulse inhibition (PPI) of the startle reflex are widely believed to reflect an individual’s sensory and sensorimotor gating abilities, respectively.1,20 Recent studies from our laboratory showed significantly improved sensory and sensorimotor gating in initially antipsychotic-naive, first-episode patients with schizophrenia following a 6-month treatment period with the second-generation antipsychotic quetiapine.5,21 The first metabolite of quetiapine, norquetiapine, is a rather potent blocker of the noradrenergic transporter (NET).22 As described above, norquetiapine may have increased PPI and P50 suppression in our patients by counterbalancing some of quetiapine’s antagonistic actions of α2-noradrenergic activity in the PFC by inhibiting NET, and as such restoring some of its functions. Indeed, recently, we reported the PPI results of the psychophysiological test battery, where we found significantly increased PPI following administration of single dosages of clonidine to stably medicated patients with schizophrenia.23 Currently, we investigated whether this last result is generalizable to sensory gating as a whole, by examining clonidine’s effect on the P50 suppression paradigm of this test battery. To our knowledge, there are no previous reports on the effects of clonidine on P50 suppression in literature. However, other studies have indicated that yohimbine (a noradrenergic α2-antagonist) disrupts sensory gating.24,25 This, together with our previous results on the effects of clonidine and quetiapine on sensory and/or sensorimotor gating,5,21,23 made us expect significantly increased P50 suppression following administration of single dosages of clonidine to our group of schizophrenia patients who were stable on their current medical treatment.
Methods
Subjects
The study was approved by the Ethics Committee of the Capital Region (H-KF-2006–6813), Copenhagen, with regards to the ethical principles for medical research involving human subjects as stated in the declaration of Helsinki (amendment of Washington, 2002). Written and oral information was given, after which written informed consent was obtained from all subjects. Chronic, yet clinically stable, medicated male patients, aged between 25 and 50, with schizophrenia were recruited from psychiatric hospitals and related outpatient clinics in the Copenhagen municipality and county. All included patients fulfilled the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for schizophrenia, confirmed with the Schedule for Clinical Assessment in Neuropsychiatry (SCAN; version 2.1).26 The patients were age matched to healthy male controls. The healthy controls were recruited from the community and had no history of psychiatric illness (ascertained with the SCAN interview). This cohort consisting of both patients and healthy controls was identical to that included in our previously published data on the effects of clonidine on PPI of the startle reflex23 and, thus, has been described before. In short: both patients and healthy controls passed a physical examination before inclusion to ascertain that they were all physically healthy. Exclusion criteria were coercive measures, a history of mental retardation, organic brain damage or organic psychosis. Substance dependence (as defined by DSM-IV criteria) was an exclusion criterion. Healthy controls with a history of mental illness in first-degree relatives were also not included in the study. Subjects were screened for absence of overt hearing deficits at 500, 1000, and 6000 Hz (40 dB). Due to the naturalistic design, concomitant treatment with benzodiazepines and antidepressants was allowed. In total, 20 male patients (mean age: 37.5 y, SD = 6.4) and 20 age-matched healthy male control subjects (mean age: 36.5 y, SD = 6.5) completed the project. Two patients were treated with typical antipsychotics, 15 with atypical, and 3 with a combination of typical and atypical medication. Besides antipsychotics, the medical treatment of 5 patients included benzodiazepines, and also 5 patients were simultaneously treated with antidepressants (only treatment with selective serotonin reuptake inhibitors with very low noradrenergic affinity [Ki < 1000 nM] was allowed) during their participation in the project (the medication of 3 patients included both antidepressants and benzodiazepines). Importantly, none of the patients had any change in their medical treatment during the participation in the project, and 14 of the patients were smokers, whereas 4 of the controls were smokers. Three patients dropped out before the project was completed: 1 patient did not participate in the 25-µg clonidine session, 1 not in the 150-µg clonidine session, while 1 patient only participated in the 25-µg clonidine session. Table 1 shows the characteristics of all subjects.
Table 1.
Demographics, Clinical Characteristics and P50 Amplitudes
| Controls | Patients | Patients | Patients | Patients | Patients | |
|---|---|---|---|---|---|---|
| Placebo | 25-µg clonidine | 50-µg clonidine | 75-µg clonidine | 150-µg clonidine | ||
| Mean age (SEM) | 36.5 y (1.5) | 37.5 y (1.4) | ||||
| Average PANSS scores (SEM) | ||||||
| Positive | 13.0 (1.2) | 14.0 (1.6) | 12.7 (1.1) | 13.8 (1.6) | 12.5 (0.9) | |
| Negative | 14.3 (1.2) | 15.6 (1.0) | 15.4 (1.4) | 15.4 (1.2) | 14.3 (1.1) | |
| General | 25.4 (1.6) | 27.1 (1.9) | 25.1 (1.7) | 25.7 (1.6) | 24.4 (1.2) | |
| Total | 52.6 (3.4) | 56.1 (3.4) | 53.1 (3.4) | 54.8 (3.9) | 50.8 (2.4) | |
| Average P50 amplitudes (SEM) | ||||||
| C stimuli | 2.07 (0.24) | 1.54a (0.32) | 1.25 (0.23) | 1.17 (0.19) | 1.47 (0.21) | 1.34 (0.26) |
| T stimuli | 0.39 (0.13) | 0.65 (0.17) | 0.43b (0.12) | 0.42 (0.20) | 0.32b (0.11) | 0.38 (0.11) |
| C-T | 1.67 (0.19 | 0.89a (0.38) | 0.83 (0.18) | 0.75 (0.19) | 1.15 (0.18) | 0.95 (0.26) |
Note: SEM, standard error of the mean; PANSS, Positive and Negative Syndrome Scale. Characteristics and raw P50 amplitude scores of patients and matched healthy controls show significant differences neither in age between patients and controls nor in PANSS scores in the patients over the 5 different test sessions. Please see text for further details on the P50 amplitude scores.
aPlacebo value significantly different compared with that for controls.
bSignificantly decreased value compared with (patients) placebo session.
Experimental Design
In a double-blind, placebo-controlled, pseudorandomized (balanced) cross-over experiment, all patients were tested in the Copenhagen Psychophysiological Test Battery (CPTB) on 5 occasions, separated by a minimum of 1 week: once after oral administration of placebo and once after a single dose of 25, 50, 75, and 150 µg of clonidine (Catapresan). To avoid order effects as much as possible, we used a pseudorandomized design: randomly chosen, an equal number of patients per session started with each dose (ie, 4 patients started with 0 [placebo] and 4 with 25, 50, 75, or 150 µg of clonidine), while the order of the subsequent 4 doses was randomized. Both placebo and clonidine tablets were administered 4 h before the psychophysiological assessments, in opaque white capsules. On all 5 test days, before administration of the capsule, the patients’ symptomatology was rated with the Positive and Negative Syndrome Scale (PANSS).27 It should be realized that the data of the controls were only used to assess whether patients showed significant deficits in our dependent variables (P50 suppression and its components) in spite of them being clinically stable on their medication and to evaluate the level of change in these variables as a result of clonidine administration. Therefore, the controls were assessed only once without being administered any compounds. The CPTB includes a PPI, P50 suppression, mismatch negativity, and selective attention and paradigm, and is always assessed in this order. To keep the study focused, only the results on P50 gating will be reported in the present article, the results on PPI have been published elsewhere,23 and so will those of the other paradigms. At inclusion, neither the patients nor the healthy controls had ever participated in psychophysiological research before. To avoid acute and/or withdrawal effects of nicotine,1 smoking was not allowed from 1 h prior to testing. To avoid influence of sedatives, patients were requested not to take any benzodiazepines from the evening before a test day (from 23.00 o’ clock) until the completion of the tests. Furthermore, urine samples of the subjects were screened for drug abuse (cannabis, cocaine, and amphetamines).
P50 Suppression Paradigm.
The method has been des cribed before.5 In short, subjects were seated in a room with a sound level below 40 dB. Subjects were instructed to sit still, to keep their eyes fixed on a spot on the wall directly in front of them and were asked to stay awake. During P50 gating testing, 3 experimental blocks were presented, each consisting of 40 pairs of bursts (1.5ms and 80 dB) of white noise, with an instantaneous rise time, an interstimulus interval of 500 ms, and a fixed intertrial interval of 10 s.
Signal Recording.
Electroencephalography (EEG) recor dings were performed with BioSemi hardware (BioSemi), using a cap with 64 active electrodes. All signals were digitized online by a computer at a rate of 4096 Hz.
P50 Suppression Assessment.
Brain Electrical Source Analysis (BESA) software (version 5.2.4; MEGIS Software GmbH) was used for further processing of the data. First, the EEG data were down-sampled to a rate of 250 Hz after which it was corrected for eye movement by applying the surrogate model of BESA.28 Thereafter, correction of movement- and other nonparadigm-related artifacts was performed by excluding those epochs in which amplitude differences between maximum and minimum in the epoch exceeded 100 µV. Data were epoched and averaged between 100ms prestimulus and 400ms poststimulus. Averaged epochs were then filtered (high-pass filter: 1.6 Hz, 24 dB/octave; low-pass filter: 70 Hz, 24 dB/octave). P50 amplitudes were scored from Cz (midline central) only, with average reference. P50 amplitude was defined as the largest trough to peak amplitude within an interval of 40–90ms following the first (conditioning or “C”) stimulus in each paired click. The P50 amplitude following the second (testing or “T”) stimulus was identified as the largest trough to peak amplitude within an interval of ±10ms of the latency of the maximum P50 amplitude to the C stimulus. P50 suppression was expressed as the ratio “T/C.”
Statistical Analysis
All statistical analyses were performed with SPSS (version 11.0; SPSS Inc.). Initially, the P50 amplitude and P50 suppression data were analyzed with univariate ANCOVA in which gender, current smoking (yes/no), history of substance abuse, and current use of benzodiazepines and/or antidepressants (yes/no) were used as covariates: except from smoking, none of these factors covariated significantly. Therefore, only smoking was used as a covariate in all further analyses. Because the P50 suppression data (ratio T/C) was nonnormally distributed (Kolmogorov-Smirnov), we normalized the T/C scores by a logarithmic transformation (10Log). However, using these normalized data instead of the unaltered data did not alter any of the outcomes, indicating once more the relative insensitivity of ANOVAs for nonnormally distributed data. Therefore, we will only report the analyses of the unaltered P50 suppression data. Whenever sphericity of the data was violated, Greenhouse-Geisser values are reported.
Psychopathology (positive, negative, general, and total PANSS score) was analyzed with ANOVA to evaluate the clinical stability of the patients over the period that they participated in this project.
Results
Psychopathology
No significant differences were found in the patients PANSS scores in the 5 different sessions (assessed before administration of clonidine on each test day), indicating that the patients’ psychopathology was stable over time (for more details, see Oranje and Glenthoj23).
P50 Amplitude and P50 Suppression Data
Although we balanced the order of the 5 treatment sessions of the patients, we screened our data for order effects with univariate ANOVA; P levels ranged from .26 to .76 for P50 suppression data (T/C), from .58 to .87 for amplitudes to C stimuli, and from .34 to .90 for amplitudes to T stimuli, indicating no significant order effects.
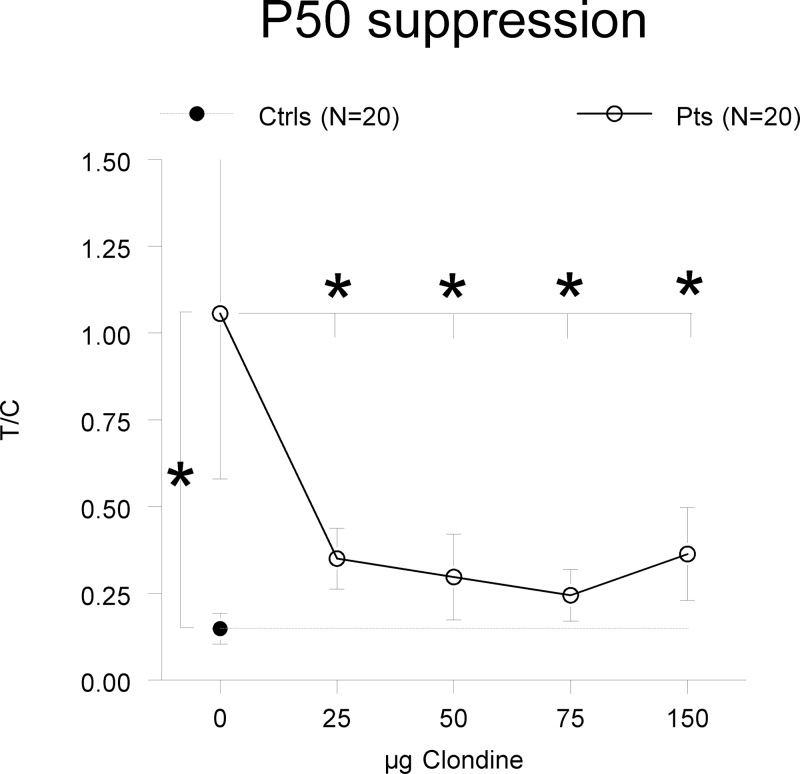
The analysis of the P50 suppression data (see figure 1) revealed a highly significant increased T/C ratio in the placebo session of the patients compared with controls [F(1,36) = 8.49, P = .008], indicating P50 gating deficits in the patients in spite of their medical treatment. This increased T/C ratio appeared to be based on a significantly lower P50 amplitude to C stimuli in the patients compared with the controls [F(1,36) = 2.94, P = .036]. The patients’ P50 amplitudes to T stimuli were on average increased, but this did not reach statistical significance [F(1,36) = 1.07, P = .31]. To test whether clonidine was able to ameliorate the deficient levels of P50 gating of the patients, we used a repeated measures ANCOVA with within factor “dose” (0-, 25-, 50-, 75-, and 150-µg clonidine with smoking as a covariate), which revealed a significant effect of dose [F(4,29) = 5.12, P = .012]. This effect appeared solely based on the difference in T/C ratio in the placebo session compared with those of the clonidine sessions because no significant ratio differences were found between the 4 clonidine sessions [F(4,29) = 2.27, P = .13]. Further testing of this treatment effect revealed that all dosages of clonidine significantly decreased the patients’ T/C ratios: 25 µg: F(1,17) = 8.68, P = .009; 50 µg: F(1,16) = 6.15, P = .025; 75 µg: F(1,17) = 9.24, P = .007]; and 150 µg: [F(1,15) = 6.28, P = .024]. Comparison of these decreased levels of T/C ratios with those of the healthy volunteers showed no significant differences anymore (P > .16), except for the highest dose, where patients still showed significantly higher T/C ratios than controls [F(1,15) = 6.28, P = .045] (see also figure 1). This decrease in T/C ratio appeared predominantly caused by clonidine, reducing the P50 amplitude to T stimuli (within factor “dose,” smoking as covariate: [F(4,56) = 2.94, P = .028]). Clonidine did not significantly affect the P50 amplitudes to C stimuli ([F(4,56) = 0.86, P = .49]). In the placebo session, patients showed lower P50 difference scores (C-T) than controls [F(1,36) = 6.63, P = .014], and none of the dosages of clonidine affected these scores (P > .28) (see table 1). For readers interested in the effects of clonidine on the N100 amplitude, please refer to online supplementary table S1.
Fig. 1.
P50 suppression. P50 suppression (T/C ± SEM) for patients (pts) and controls (ctrls) is specified for all treatments. The patients showed significantly increased T/C ratios in the placebo treatment compared with the controls. All dosages of clonidine significantly decreased the T/C ratios of the patients compared with placebo, the 3 lower ones to levels that were no longer significantly different from that of the controls. Please note that the controls were assessed only once. *P < .05.
Discussion
To our knowledge, this is the first study reporting the effects of clonidine on deficient sensory gating in patients with schizophrenia. In fact, there appears to be no literature on the effects of α2-noradrenergic agonists on human P50 suppression in general. The main results indicate that much similar to our previous report on the effects of clonidine on sensorimotor gating,23 even low doses of clonidine added to the patients’ medical treatment significantly increased their P50 suppression to such levels that it was not significantly different from the controls anymore. However, although the highest dose of clonidine (150 µg) significantly increased the patients’ level of P50 suppression, it was still significantly lower than that of the healthy controls.
The locus coeruleus (LC) is responsible for approximately 90% of the noradrenergic innervation of the forebrain, including the PFC.29 The PFC is not only known for its involvement in a large number of cognitive functions, eg, executive functions, attention, and working memory (eg, Ramos and Arnsten10 and Reichenberg and Harvey30), but also for its involvement in P50 suppression.5,11,31 The finding that all of our 4 different single dosages of clonidine increased P50 suppression in our schizophrenia patients is in agreement with our hypothesis that aberrant filtering of sensory information, as generally assumed to be reflected in deficient P50 suppression and/or reduced PPI, is at least in part caused by aberrant noradrenergic activity. Because α2-noradrenergic receptors are located both in the LC (presynaptically) as well as in the PFC (postsynaptically),16 it would be logical to assume that clonidine acted either through reduction of noradrenergic activity—by stimulating presynaptic α2-receptors in the LC—or by stimulating postsynaptic α2-receptors in the PFC. However, our patients were on stable medication before administration of clonidine. In spite of that they still showed deficient levels of P50 suppression in the placebo treatment compared with the healthy controls. A large number of the currently most prescribed antipsychotics—particularly, the atypical ones that were also used by the majority of our patients—reduce noradrenergic-α2 activity,32 which means that reducing noradrenergic activity alone is not effective, or at least not sufficient, in ameliorating P50 suppression deficits. Clonidine’s ability to increase P50 suppression in our stably medicated patients might, therefore, indicate that it acted by stimulation of postsynaptic α2-receptors in the PFC. This would not only agree with a steadily growing literature pointing towards an involvement of the PFC in both P50 suppression and PPI5,11,31 but also with the above-mentioned studies, suggesting that stimulation of α1-receptors impairs normal PFC functions, while stimulation of α2-receptors strengthens its functions (eg, Ramos and Arnsten10 and Arnsten16). However, the fact that many other studies suggest involvement of, for instance, dopaminergic and serotonergic activity in sensory gating,5,33,34 as well either in the PFC or in unspecified areas of the brain, makes it clear that more research is necessary to reach a better insight in the neurochemistry and neuroanatomy behind sensory gating.
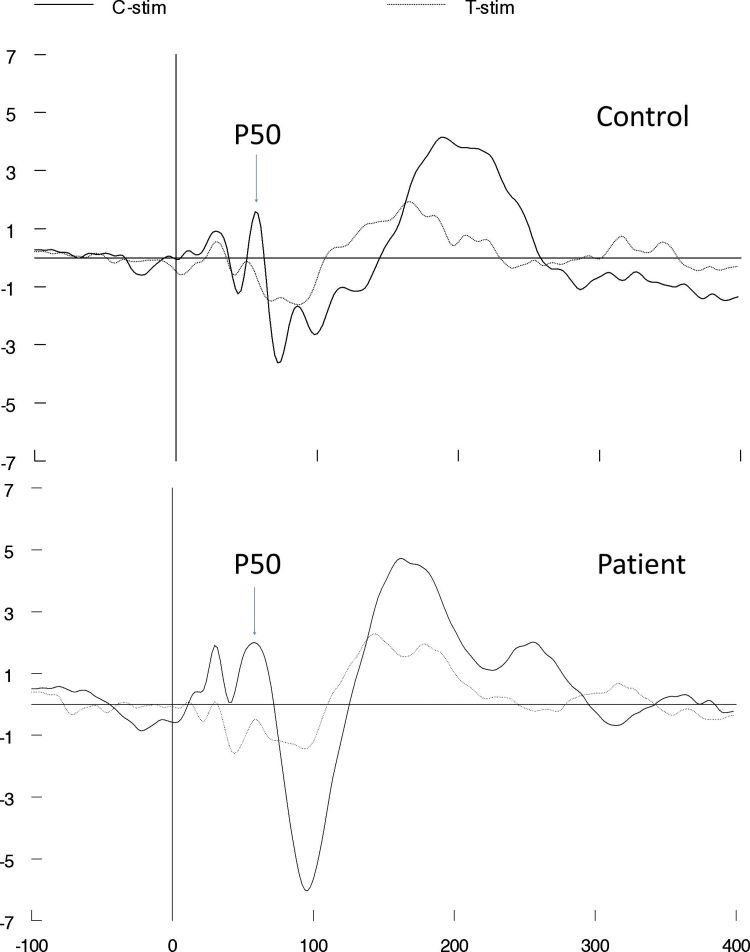
Clonidine appeared to increase P50 suppression (more specifically, the T/C ratio) in our study by significantly and selectively reducing the response of the patients to the second (T) stimulus of the click pairs, while not significantly affecting the patients’ response to the first (C) stimulus. In line with the hypothesis suggested by Adler et al,35 deficient gating reflects the brain’s incapacity to gate the processing of stimuli (eg, the T stimulus) while it is still busy processing earlier stimuli (eg, the C stimulus). According to this hypothesis, clonidine truly enhanced sensory gating in the current study, which is in line with the results of our previous report on the effects of clonidine on sensorimotor gating (PPI).23 It is, however, important to realize that the significantly increased T/C ratio in the placebo treatment of the patients was due to a significantly reduced C amplitude (figure 2) compared with the controls, and not due to a significantly increased T amplitude (although the response of the patients to T stimuli on average was higher than that of the controls, this did not reach statistical significance). This finding is more indicative of sensory registration issues, then of sensory filtering issues. Clonidine, however, normalized the patients’ T/C ratio by significantly reducing the T amplitudes, while not significantly affecting the C amplitudes. This last finding indicates that clonidine increased sensory gating in the patients, ie, the same level of processing of the C stimulus results in less processing of the T stimulus with clonidine than in the placebo treatment. In other words, our 2 studies combined provide the first evidence that α2-noradrenergic agonists are able to increase sensory filtering in patients, as assessed with P50 suppression (as a measure of sensory gating) and PPI (as a measure of sensorimotor gating). However, interpretation of the true nature of our findings remains difficult, since it seems as if clonidine increased sensory filtering in our patients, while they appeared to suffer from sensory registration issues. Clonidine did not restore another measure of sensory filtering that was significantly reduced in patients in the placebo session compared with controls, the P50 difference score (C-T). This is probably due to the fact that clonidine did not significantly affect the amplitudes to the C stimuli but only significantly reduced amplitudes to T stimuli, which are usually rather small: the resulting effects on the difference waves were, therefore, probably too small to be detected.
Fig. 2.
Response of a typical patient and control. Average response of a typical patient and typical control in the placebo treatment indicates reduced P50 amplitude to C stimuli of the patient compared with the control but indicates comparable P50 amplitudes to T stimuli.
Deficits in filtering of sensory information are thought to lead to cognitive fragmentation and ultimately even to hallucinations and delusions (eg, Braff et al1 and Perry et al2). The relationship between P50 suppression and cognition is not well studied, but there is some evidence suggesting that P50 suppression deficits are related to impairments in sustained attention and vigilance.3 Deficient PPI appears to correlate with measures of perceptual and reasoning disturbances,2 and also with aspects of hallucinations.36 Increasing sensory and sensorimotor gating in schizophrenia may, therefore, set the stage for improving cognitive abilities and/or symptomatology, for which early studies on the effects of α2-agonists in schizophrenia already have provided some evidence (eg, Fields et al17 and Freedman et al18). Obviously however, more research is necessary to evaluate the clinical significance of our findings.
There are strengths, but also limitations to the current study. An obvious strength is that the essential part of this study had a within-subject design, where patients randomly received not only 1 but 4 different single dosages of clonidine, showing that 3 of these dosages not only significantly increased but also largely normalized the patients’ levels of P50 suppression. A limitation of the current study is that only the effects of single dosages of clonidine on P50 suppression were studied. Therefore, investigation of long-term treatment effects of clonidine on sensory and sensorimotor gating, symptomatology, and cognition in schizophrenia is warranted. Another limitation might be the fact that we tested our healthy controls only once, whereas the patients were tested 5 times. However, the order of the 5 test sessions of the patients was randomized, and we found no significant order effects in our data, which makes it highly unlikely that the multiple test exposures, instead of the effects of clonidine, accounted for our current results. Finally, given the explorative nature of this study, and to the fact that the T/C ratio across the 4 different clonidine sessions did not differ significantly from each other, we decided not to correct for multiple testing; it should be noted, however, that the improved P50 ratio following administration of the 50 and 150 µg dosages of clonidine do not survive Bonferroni correction.
Summarized, the current study is the first to show that levels of P50 suppression in patients with schizophrenia on stable medication can be effectively normalized by adding a single low dosage of clonidine to their current treatment, this in spite of the fact that clonidine did not ameliorate the patients’ sensory registration issues. Together with previous results from our laboratory, this indicates that α2-receptor agonists are capable of normalizing both levels of P50 suppression and PPI in schizophrenia, presumably by restoring some of the PFC functions. Future research should focus on long-term treatment effects of clonidine on basic information processing deficits, cognition, and symptomatology in schizophrenia.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
The study was sponsored by The Danish Council for Independent Research-Medical Sciences (271-06-0308) and the Lundbeck foundation (R25-A2701).
Supplementary Material
Acknowledgments
The authors would like to thank the contributions of Gitte Saltoft Andersen and Katherina Alfsen. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl). 2001;156:234–258 [DOI] [PubMed] [Google Scholar]
- 2. Perry W, Geyer MA, Braff DL. Sensorimotor gating and thought disturbance measured in close temporal proximity in schizophrenic patients. Arch Gen Psychiatry. 1999;56:277–281 [DOI] [PubMed] [Google Scholar]
- 3. Potter D, Summerfelt A, Gold J, Buchanan RW. Review of clinical correlates of P50 sensory gating abnormalities in patients with schizophrenia. Schizophr Bull. 2006;32:692–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goldberg TE, Goldman RS, Burdick KE, et al. Cognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia: is it a practice effect? Arch Gen Psychiatry. 2007;64:1115–1122 [DOI] [PubMed] [Google Scholar]
- 5. Oranje B, Aggernaes B, Rasmussen H, Ebdrup BH, Glenthoj BY. Sensory gating and its neural generators in antipsychotic-naïve, first-episode schizophrenia before and after 6 months of quetiapine treatment. Schizophr Bull. 2012;39:472–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Powchik P, Davidson M, Haroutunian V, et al. Postmortem studies in schizophrenia. Schizophr Bull. 1998;24:325–341 [DOI] [PubMed] [Google Scholar]
- 7. Farley IJ, Price KS, McCullough E, Deck JH, Hordynski W, Hornykiewicz O. Norepinephrine in chronic paranoid schizophrenia: above-normal levels in limbic forebrain. Science. 1978;200:456–458 [DOI] [PubMed] [Google Scholar]
- 8. Yamamoto K, Hornykiewicz O. Proposal for a noradrenaline hypothesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:913–922 [DOI] [PubMed] [Google Scholar]
- 9. Clark CR, Geffen GM, Geffen LB. Catecholamines and attention. II: pharmacological studies in normal humans. Neurosci Biobehav Rev. 1987;11:353–364 [DOI] [PubMed] [Google Scholar]
- 10. Ramos BP, Arnsten AF. Adrenergic pharmacology and cognition: focus on the prefrontal cortex. Pharmacol Ther. 2007;113:523–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bak N, Glenthoj BY, Rostrup E, Larsson HB, Oranje B. Source localization of sensory gating: a combined EEG and fMRI study in healthy volunteers. Neuroimage. 2011;54:2711–2718 [DOI] [PubMed] [Google Scholar]
- 12. Hammer TB, Oranje B, Bro H, Glenthoj BY. Stability of prepulse inhibition and habituation of the startle reflex in schizophrenia: a 6-year follow-up study of initially antipsychotic-naïve, first-episode schizophrenia patients. Int J Neuropsychopharmacol. 2011;4:1–13 [DOI] [PubMed] [Google Scholar]
- 13. Kumari V, Fannon D, Geyer MA, et al. Cortical grey matter volume and sensorimotor gating in schizophrenia. Cortex. 2008;44:1206–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Litman RE, Pickar D. Noradrenergic systems: a target for augmenting pharmacotherapy. In: Breier A, ed. The New Pharmacotherapy of Schizophrenia (Clinical Practice Series). Washington, DC: American Psychiatric Press; 1996:133–152 [Google Scholar]
- 15. Friedman JI, Adler DN, Davis KL. The role of norepinephrine in the pathophysiology of cognitive disorders: potential applications to the treatment of cognitive dysfunction in schizophrenia and Alzheimer’s disease. Biol Psychiatry. 1999;46:1243–1252 [DOI] [PubMed] [Google Scholar]
- 16. Arnsten AFT. Norepinephrine and cognitive disorders. In: Ordway GA, Schwartz MA, Frazer A, eds. Brain Norepinephrine, Neurobiology and Therapeutics. Cambridge, UK: Cambridge University Press; 2007:408–435 [Google Scholar]
- 17. Fields RB, Van Kammen DP, Peters JL, et al. Clonidine improves memory function in schizophrenia independently from change in psychosis. Preliminary findings. Schizophr Res. 1988;1:417–423 [DOI] [PubMed] [Google Scholar]
- 18. Freedman R, Kirch D, Bell J, et al. Clonidine treatment of schizophrenia. Double-blind comparison to placebo and neuroleptic drugs. Acta Psychiatr Scand. 1982;65:35–45 [DOI] [PubMed] [Google Scholar]
- 19. Svensson TH. Alpha-adrenoceptor modulation hypothesis of antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1145–1158 [DOI] [PubMed] [Google Scholar]
- 20. Bramon E, Rabe-Hesketh S, Sham P, Murray RM, Frangou S. Meta-analysis of the P300 and P50 waveforms in schizophrenia. Schizophr Res. 2004;70:315–329 [DOI] [PubMed] [Google Scholar]
- 21. Aggernaes B, Glenthoj BY, Ebdrup BH, Rasmussen H, Lublin H, Oranje B. Sensorimotor gating and habituation in antipsychotic-naive, first-episode schizophrenia patients before and after 6 months’ treatment with quetiapine. Int J Neuropsychopharmacol. 2010;13:1383–1395 [DOI] [PubMed] [Google Scholar]
- 22. McIntyre RS, Muzina DJ, Adams A, et al. Quetiapine XR efficacy and tolerability as monotherapy and as adjunctive treatment to conventional antidepressants in the acute and maintenance treatment of major depressive disorder: a review of registration trials. Expert Opin Pharmacother. 2009;10:3061–3075 [DOI] [PubMed] [Google Scholar]
- 23. Oranje B, Glenthøj BY. Clonidine normalizes sensorimotor gating deficits in patients with schizophrenia on stable medication. Schizophr Bull. 2013;39:684–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adler LE, Hoffer L, Nagamoto HT, Waldo MC, Kisley MA, Giffith JM. Yohimbine impairs P50 auditory sensory gating in normal subjects. Neuropsychopharmacology. 1994;10: 249–257 [DOI] [PubMed] [Google Scholar]
- 25. Stevens KE, Meltzer J, Rose GM. Disruption of sensory gating by the alpha 2 selective noradrenergic antagonist yohimbine. Biol Psychiatry. 1993;33:130–132 [DOI] [PubMed] [Google Scholar]
- 26. Wing JK, Babor T, Brugha T, et al. SCAN. Schedules for Clinical Assessment in Neuropsychiatry. Arch Gen Psychiatry. 1990;47:589–593 [DOI] [PubMed] [Google Scholar]
- 27. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276 [DOI] [PubMed] [Google Scholar]
- 28. Berg P, Scherg M. A multiple source approach to the correction of eye artifacts. Electroencephalogr Clin Neurophysiol. 1994;90:229–241 [DOI] [PubMed] [Google Scholar]
- 29. Simpson KL, Lin RCS. Neuroanatomical and chemical organization of the locus coeruleus. In: Ordway GA, Schwartz MA, Frazer A, eds. Brain Norepinephrine, Neurobiology and Therapeutics. Cambridge, UK: Cambridge University Press; 2007:9–52 [Google Scholar]
- 30. Reichenberg A, Harvey PD. Neuropsychological impairments in schizophrenia: integration of performance-based and brain imaging findings. Psychol Bull. 2007;133:833–858 [DOI] [PubMed] [Google Scholar]
- 31. Weiland BJ, Boutros NN, Moran JM, Tepley N, Bowyer SM. Evidence for a frontal cortex role in both auditory and somatosensory habituation: a MEG study. Neuroimage. 2008;42:827–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Blake TJ, Tillery CE, Reynolds GP. Antipsychotic drug affinities at alpha 2-adrenoceptor subtypes in post-mortem human brain. J Psychopharmacol. 1998;12:151–154 [DOI] [PubMed] [Google Scholar]
- 33. Csomor PA, Stadler RR, Feldon J, Yee BK, Geyer MA, Vollenweider FX. Haloperidol differentially modulates prepulse inhibition and p50 suppression in healthy humans stratified for low and high gating levels. Neuropsychopharmacology. 2008;33:497–512 [DOI] [PubMed] [Google Scholar]
- 34. Quednow BB, Schmechtig A, Ettinger U, et al. Sensorimotor gating depends on polymorphisms of the serotonin-2A receptor and catechol-O-methyltransferase, but not on neuregulin-1 Arg38Gln genotype: a replication study. Biol Psychiatry. 2009;66:614–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Adler LE, Pachtman E, Franks RD, Pecevich M, Waldo MC, Freedman R. Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biol Psychiatry. 1982;17:639–654 [PubMed] [Google Scholar]
- 36. Kumari V, Peters ER, Fannon D, et al. Uncontrollable voices and their relationship to gating deficits in schizophrenia. Schizophr Res. 2008;101:185–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.