Abstract
Patients with schizophrenia perform poorly on cognitive skill learning tasks. This study is the first to investigate the neural basis of impairment in cognitive skill learning in first-degree adolescent relatives of patients with schizophrenia. We used functional magnetic resonance imaging to compare activation in 16 adolescent siblings of patients with childhood-onset schizophrenia (COS) and 45 adolescent controls to determine whether impaired cognitive skill learning in individuals with genetic risk for schizophrenia was associated with specific patterns of neural activation. The siblings of patients with COS were severely impaired on the Weather Prediction Task (WPT) and showed a relative deactivation in frontal regions and in the striatum after extensive training on the WPT compared with controls. These differences were not accounted for by performance differences in the 2 groups. The results suggest that corticostriatal dysfunction may be part of the liability for schizophrenia.
Key words: cognitive skill learning, striatal dysfunction, genetic risk, fMRI
Patients with schizophrenia perform poorly on cognitive skill learning tasks,1–5 consistent with the hypothesis that the pathophysiology of schizophrenia involves dysfunction of corticostriatal circuits.6–8 The corticostriatal system plays an important role in skill learning, as revealed by neuropsychological9–12 and neuroimaging studies.13–18 There is some evidence that patients with schizophrenia demonstrate relatively specific impairment of corticostriatal function; they show an impairment in a task that taps the cognitive corticostriatal loop (the caudate nucleus, dorsolateral prefrontal cortex [PFC], and ventral striatum/orbitofrontal cortex) and normal performance on a task that taps the motor corticostriatal loop (including putamen and motor cortical regions).4
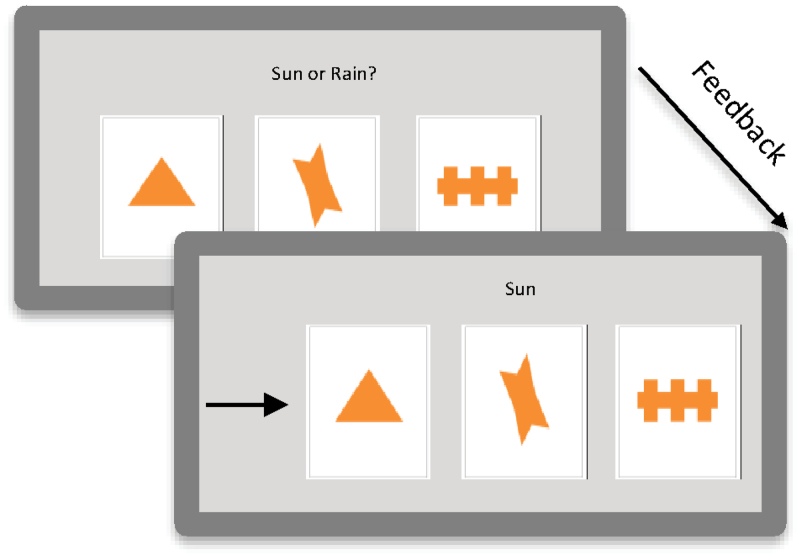
One cognitive skill learning task that has been used extensively in the neuropsychological literature is the Weather Prediction Task (WPT).19 The WPT is a probabilistic classification task that requires participants to learn the probabilistic associations between cues and binary outcomes by attending to visual stimuli presented on a computer screen after which participants are provided feedback about the correctness of their response (figure 1; online supplementary table 1). Performance on the WPT is impaired in patients with schizophrenia.4,21–23 Patients with schizophrenia also show deficits on other cognitive skill learning tasks such as the Tower of Toronto and the Tower of Hanoi.1,3 However, these tasks demand considerable executive control resources, and thus impairments may also reflect deficits in executive function. Because of its relatively simple task demands, the WPT may be a more specific measure of cognitive skill learning that taps corticostriatal dysfunction.
Fig. 1.
The Weather Prediction Task. Participants were told to predict the weather (sun or rain) based on cues. On every trial, between 1 and 3 cues (out of 4 possibilities) could appear, yielding 14 possible combinations. The cues were probabilistically related to the outcomes. The association of the different cues with different probabilities was randomized across participants. The cue strength of each of the 14 resulting stimuli were such that the overall probability associating each cue with sun or rain was .727, .556, .409, and .280 across the task. Because feedback was probabilistic, a response was considered correct if it matched the outcome most strongly associated with a stimulus, regardless of feedback. Thus, a response could be “correct” even if feedback reported an incorrect answer. Therefore, the percentage correct score reflected how well the subjects learned the cue-outcome associations20. The cues are shown on the screen for a maximum of 3 seconds, the feedback is shown on the screen for 1 second, and the time between trials is 0.5 seconds..
While deficits in cognitive skill learning appear to be present in patients with schizophrenia, there is some question about the meaning of those deficits. The patients in studies in which cognitive skill learning deficits were detected were receiving antipsychotic medications to control their psychotic symptoms. Antipsychotic medications have effects on striatal structure (enlargement of the volume of the basal ganglia) and alter striatal D2 receptors.24,25 It is possible that these medications impair striatal function and thereby adversely impact cognitive skill learning in patients receiving antipsychotic treatment. One way to address this issue is to study the nonpsychotic first-degree relatives of patients with schizophrenia. These individuals share some of the familial liability to schizophrenia with patients with schizophrenia but because they do not exhibit symptoms of the disease, they are not receiving antipsychotic medications. Thus, if they show cognitive skill learning deficits, those deficits would appear to reflect familial liability to schizophrenia, not the effects of antipsychotic medication.
There are 2 prior studies of cognitive skill learning in first-degree relatives of patients with schizophrenia. Weickert et al5 compared adult patients with schizophrenia, their adult siblings, and controls on the WPT. The patients demonstrated a severe learning deficit, while there was no significant difference between the adult siblings and the controls. However, when subjects were separated into good and poor learners, the siblings of first-degree relatives of patients with schizophrenia were disproportionately represented in the poor-learner group. In a recent study by Wagshal et al,26 nonpsychotic adolescent siblings of patients with childhood-onset schizophrenia (COS) exhibited deficits in the WPT. In this study, controls showed significant learning in the first 50 trials while the sibling did not, and even after extensive training, the COS siblings reached a lower level of asymptotic performance than controls. The adolescent COS siblings demonstrated impaired early and late learning on the WPT compared with controls. The siblings of COS patients may have exhibited a greater deficit than the siblings of adult-onset patients in the Weickert et al5 study because COS appears to be a more severe form of the disorder and may have a more pronounced genetic component than adult-onset schizophrenia.27 Second, in Wagshal et al,26 we tested adolescent subjects, and thus it is also possible that the impairment in the COS siblings represents a developmental delay.
In the present study, we investigate the neural basis of impaired cognitive skill learning in adolescent siblings of patients with COS. We used functional magnetic resonance imaging (fMRI) to compare the pattern of activation before and after training on the WPT in a subset of the participants in the Wagshal et al26 study to determine whether impaired cognitive skill learning in individuals with genetic risk for schizophrenia was associated with specific patterns of neural activation. Identifying alterations in the neural networks, supporting cognitive skill learning in the siblings of patients with schizophrenia, is an important step in demonstrating that those networks are associated with liability to schizophrenia. In Wagshal et al,26 the siblings of COS probands exhibited impaired performance during the first 50 trials of training, during which much of the learning took place in controls. The siblings of COS probands also exhibited a reduced level of asymptotic performance compared with controls after 800 trials of training. In the present study, we included a scanning session at the onset of training and after extended period of training in order to compare neural activation in the 2 groups during both time periods.
While studies of the genetic liability for schizophrenia generally examine individuals who are relatives of patients with adult-onset schizophrenia, here we examined siblings of COS patients. There is an increased aggregation of schizophrenia and schizophrenia spectrum disorders in first-degree relatives of patients with COS compared with first-degree relatives of patients with adult onset of schizophrenia.28 First-degree relatives of COS probands show neurocognitive impairments similar to those shown by patients with schizophrenia.29–32 The genetic liability for schizophrenia may be greater in first-degree relatives of COS patients than relatives of adult-onset schizophrenia patients. Thus, this group may be particularly informative in determining if the patterns of brain activation associated with impaired cognitive skill learning are associated with liability to schizophrenia without the confound of medication effects.
Methods
Participants
Sixteen adolescent siblings (age range: 8–16) of COS patients and 45 adolescent controls (age range: 8–16), who were right-handed and were matched in age, education, and gender to the COS siblings, participated in the experiment (online supplementary table 2). These subjects were a subset of the participants in our previous behavioral study of WPT learning in siblings of COS patients.26 Twenty controls and 6 siblings of COS patients were excluded from analysis based on computer malfunction, not responding on more than 10% of the trials, excessive movement of 3 mm or greater, or not completing both days of training. The siblings of COS probands were recruited through previous participation in family studies of COS at the University of California, Los Angeles (UCLA). Families of potential control subjects who lived within a 25-mile radius of UCLA were identified by a survey research firm and were contacted by phone. All participants provided informed consent according to the procedures of the UCLA Institutional Review Board. Potential participants in both groups were screened and excluded for reports of prior treatments for psychiatric disorders including psychosis, attention-deficit hyperactivity disorder, learning disabilities, Tourette’s syndrome, traumatic brain injury, drug and alcohol abuse, and other neurological disorders that affect cognitive functioning or the presence of any psychotic symptoms. Control subjects were also excluded if a first-degree relative had been reported to have been diagnosed with psychosis.
Task Design
Participants were administered the WPT.19 The MATLAB (The MathWorks, Inc., Natick, MA) Psychophysics Toolbox33 version 7.4 was used to present the stimuli and to record responses on an Apple G4 PowerBook using the OSX operating system.
Weather Prediction Task.
The WPT19 is a probabilistic classification task in which participants are told that they have to predict the weather (sun or rain) based on cues by pressing one of 2 buttons that correspond to either sun or rain (figure 1; online supplementary table 1). The cues are probabilistically related to the outcomes. On every trial, between 1 and 3 cues (out of 4 possibilities) can appear, yielding 14 stimuli consisting of combinations of cues. The association of the different cues with different probabilities was randomized across participants. The cue strength of each of the 14 resulting combinations was such that the overall probability associating each individual cue with sun or rain was .727, .556, .409, and .280 across the trials. A response was counted as correct on a trial if it matched the outcome most strongly associated with a stimulus; thus, a response would be counted as correct even if, on that trial, the participant received feedback that their response was incorrect, which occurred because the cue-outcome associations were probabilistic, not deterministic. For example, a particular cue is associated 72.7% of the time with sun. This means that 27.3% of the time the cue will be associated with rain. The percentage correct score reflects how well the subjects learned the cue-outcome associations.20
When the subjects were completing the task in the fMRI scanner, a baseline condition was interleaved between trial blocks, which consisted of the subjects always pressing the same button to the same 3 cues on the screen. Each trial block consisted of 2 sets of 5 task trials, with each set followed by 3 baseline trials. The baseline was the same task for both days of training in the scanner.
Procedure
Testing occurred in 2 sessions. All behavioral testing was completed in the Semel Institute for Neuroscience and Behavior at UCLA. On the first day, participants were screened for neurological or psychiatric disorders by a clinical psychologist and completed the Wechsler Abbreviated Scale of Intelligence (WASI) Vocabulary and Block Design subtests (online supplementary table 2). Afterwards, the subjects completed the 50 trials of the WPT in the fMRI scanner. This was considered “early training.” Within 1 week, participants returned and first attended a behavioral testing session that lasted approximately 2 hours where the subjects were trained for an additional 800 trials of the WPT occurring in 2 blocks of 400 trials with a rest break of 30 minutes between training blocks. During a subset of the trials (81–160 in the first block and trials 641–720 in the second block), subjects performed a tone counting task concurrently with the WPT. After these 800 training trials, subjects then completed 50 additional trials of the WPT while in the scanner (late training). Therefore, the WPT was performed in the scanner 2 times: once before the extended practice and once after the extended practice. Two different sequences of trials were generated for the scanner and behavioral version of the WPT, and order was counterbalanced across participants.
Imaging Procedure
Scanning was performed on a 3-Tesla Siemens Allegra head-only MRI scanner in the Ahmanson-Lovelace Brain Mapping Center and Ronald Reagan Hospital at UCLA. The scanning sessions occurred in a building near the Semel Institute where behavioral training occurred. For the functional runs, T2*-weighted echoplanar images (EPIs) were collected (34 slices, slice thickness = 4 mm, repetition time [TR] = 2000 ms, echo time [TE] = 30 ms, voxel size = 3.1 × 3.1 × 4.00 mm, no gap, flip angle = 90°, matrix size = 64 × 64, field of view = 200). Structural images were collected as well that included: a T2-weighted matched-bandwidth high-resolution scan (34 slices, slice thickness = 4 mm, TR = 5000 ms, TE = 33 ms, voxel size = 1.6 × 1.6 × 4.0 mm, no gap, flip angle = 90°, matrix size = 128 × 128, field of view = 200) and a magnetization-prepared rapid acquisition gradient echo image (MPRage; 160 sagittal slices, slice thickness = 1 mm, TR = 2300 ms, TE = 2.1 ms, voxel size = 1.3 × 1.3 × 1.0 mm, 0.5mm gap, flip angle = 8°, matrix = 192 × 192, field of view = 256).
Stimulus presentation was controlled by an Apple G4 Powerbook, and participants viewed the stimuli through MRI compatible goggles and responded with an MRI compatible response box connected directly to the computer. Head movement was minimized with foam padding in a standard radiofrequency single channel head coil. Two volumes were acquired at the beginning of each functional scan to allow equilibration to steady state and were subsequently excluded from the analysis.
Behavioral Data Analysis
All behavioral data analysis was conducted using the Statistical Package for the Social Sciences (SPSS) 16 (SPSS, Chicago, IL). The variable of interest was accuracy. The data were analyzed using a MANOVA with the Huynh-Feldt correction for nonsphericity. The control vs sibling group was the between-group subject factor. We tested a directional hypothesis: siblings of COS patients would perform worse than healthy, age-matched controls.
Imaging Data Preprocessing and Analysis
Data preprocessing was conducted using functional magnetic resonance imaging of the brain (FMRIB) Software Library (FSL) version 3.3 (www.fmrib.ox.ac.uk/fsl). Images were motion-corrected using FMRIB’s Motion Correction Linear Image Registration Tool (McFLIRT) using a normalized correlation ratio cost function and linear interpolation.34 Skulls were stripped using the Brain Extraction Tool (BET).35
Data analysis was conducted in FSL version 4.1 using the fMRI Expert Analysis Tool (FEAT version 5.98), first at an individual subject-level (using fixed-effects model) and then using a mixed-effects model at the group analysis level contrasts. For the individual subject-level analysis, data were spatially smoothed using a 5-mm full-width-half-maximum Gaussian kernel, intensity normalized, and filtered with a nonlinear high-pass filter (Gaussian-weighted least-squares straight line fitting, with sigma 100.0 s). A 3-step registration process aligned individual participant data into standard Montreal Neurological Institute (MNI) space. EPIs were registered to the matched-bandwidth image (7 degrees of freedom [df]), then to the MPRage image (7 df), and finally to MNI space using FMRIB’s Linear Image Registration Tool (FLIRT)34 using an affine transformation with 12 df.
We then conducted an individual subject-level analysis in FEAT with a contrast of interest of task vs baseline for early training on day 1 and a separate analysis for late training on day 2. For all time-series statistical analyses, FMRIB’s Improved Linear Model (FILM) with local autocorrelation correction36 was used. Regressors of interest were created using a delta function with trial onset times convolved with a canonical (double gamma) hemodynamic response function, along with the temporal derivative. Higher-level analyses using a mixed-effects model compared differences between contrast of interest between days within and between groups as well as the interaction between day and group. For all contrasts of interest, Z-statistic images were created using an uncorrected cluster-forming threshold of z >2.3. We then applied the cluster-based theory of Gaussian random fields,37 which determines a null distribution for cluster sizes based on the smoothness and size of the search volume. The whole-brain corrected P value for each observed cluster was determined using the cluster-based correction, and those with corrected P <.05 are reported; the smallest observed cluster size surviving the corrected extent threshold in the analysis was 700 voxels (FSL does not report the actual extent threshold).
Results
Behavioral Results of the fMRI Subjects
As in previous studies of first-degree relatives of patients with schizophrenia, the siblings of COS probands had significantly lower scores on 2 WASI subscales (Vocabulary: t(32) = 4.165, P < .001 and Block Design: t(30) = 3.279, P = .003) than controls. One of the siblings of COS probands was not tested on the WASI subtests, and 2 control participants were not tested on the Block Design subtest due to time constraints and were not included in these analyses.
Consistent with the results of our larger behavioral study26 from which the participants in the current study were drawn, the siblings of COS probands exhibited performance deficits on the WPT. It is important to note that these results are not a replication of our prior results because all of the participants were a subset of the subjects presented in Wagshal et al26 from which the behavioral data were collected. Below we present the behavioral data from the scanning sessions for subjects who contributed to the fMRI results reported here.
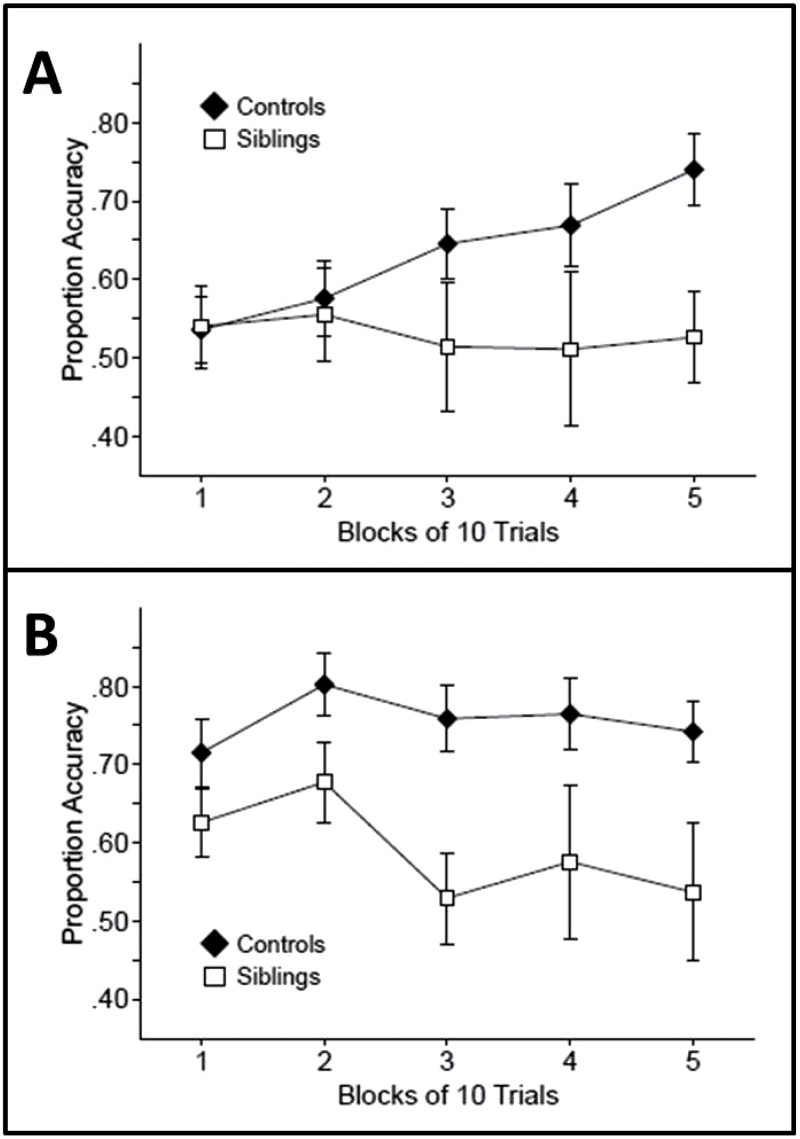
We first examined accuracy during early learning, which we defined as learning on day 1 (the first 5 blocks of 10 trials, 50 trials). Controls demonstrated learning in the first 50 trials, in that performance was not significantly above chance (50%) on the first block of 10 trials (t[24] = 0.85, P > .2) but they did perform significantly above chance in the third block of 10 trials and in subsequent blocks (ts[24] > 3.25, Ps < .02). In contrast, the siblings of COS probands did not perform significantly above chance on any block during this period (ts[9] < 0.77, Ps > .2) (figure 2A). During the 50 trials of training on day 1, there was a main effect of group (F(1, 33) = 4.134, P = .025) with significantly better performance by the controls than siblings of COS probands. There was no main effect of block or an interaction between group and block (F < 1).
Fig. 2.
Weather Prediction Task accuracy of the controls and childhood-onset schizophrenia siblings (A) in early training (first 50 trials) and (B) after extensive training (last 50 trials, trials 851–900). Error bars represent the SE of the mean.
We next analyzed accuracy during the second day of training (the last 5 blocks of 10 trials: trials 851–800). Figure 2B presents accuracy for the 2 groups during this late practice. In the first block of training on day 2, control participants performed numerically better than the siblings of COS probands, but there were no significant differences between the groups initially. However, as practice continued, a difference emerged between the groups. During this period, the control group performed above chance on each 10 trial block (ts[24] > 5.03, Ps < .001). In contrast, the siblings of COS probands only performed above chance on the first 2 blocks (ts[9] > 2.87, Ps < .01; but not in the last 3 blocks, ts[9] < 0.77, Ps > .2). The siblings of COS probands reached a lower level of asymptotic performance than controls, even after extensive training. During this later learning, there was a significant main effect of group (F(1, 33) = 11.328, P = .001) but no main effect of block or an interaction between group and block (F < 1). Thus, as in the Wagshal et al26 study, the controls demonstrated significant learning in the first 50 trials, while the siblings did not. Even after extended training when performance was asymptotic, the COS sibling group never achieved the same level as controls.
fMRI Results
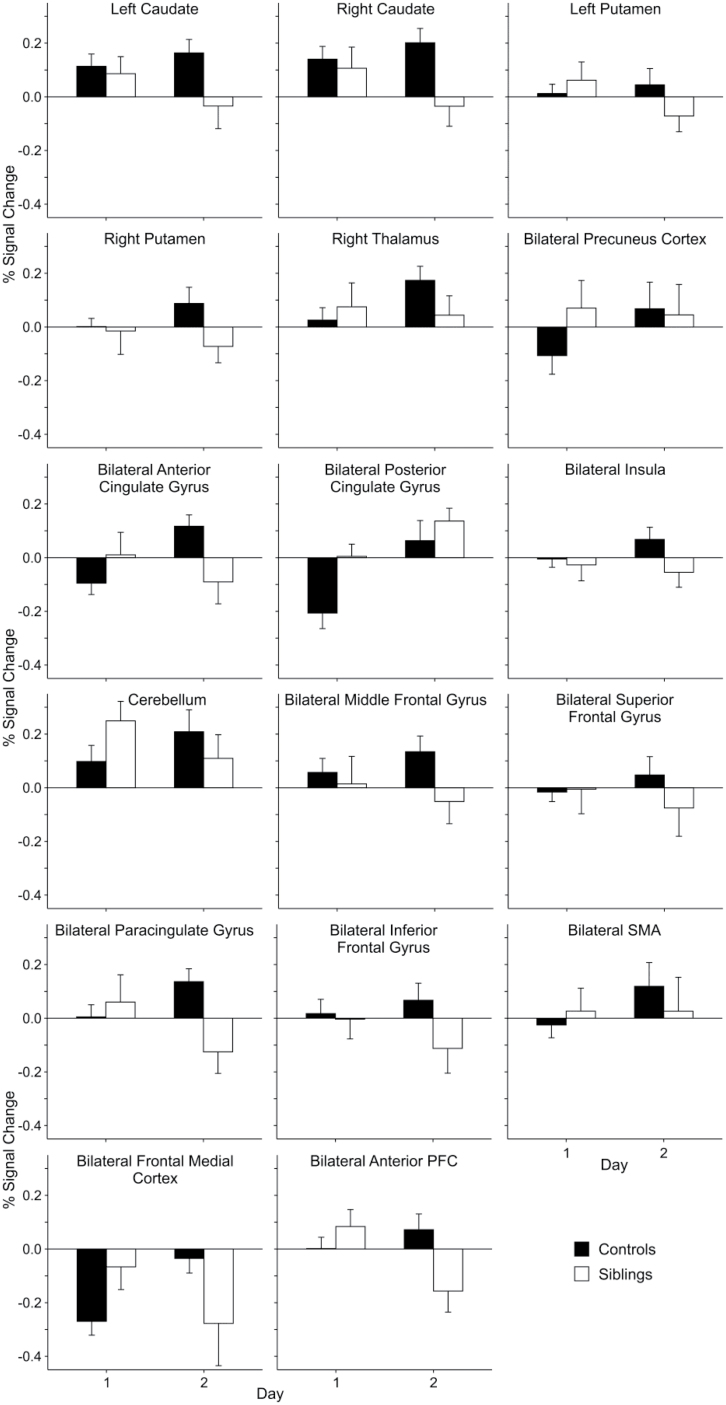
We examined neural activation during performance of the WPT using a task-baseline contrast in a whole-brain group-level voxel-wise analysis during early and late learning. We first identified regions in which there was a significant Day × Group interaction in blood-oxygen-level-dependent (BOLD) activation and found multiple regions in which the change in activation across training differed between the groups. These regions include: bilateral caudate, bilateral putamen, right thalamus, bilateral precuneus cortex, bilateral anterior and posterior cingulate gyrus, bilateral middle and superior frontal gyrus, bilateral paracingulate gyrus, bilateral inferior frontal gyrus, bilateral supplementary motor area (SMA), bilateral frontal medial cortex, bilateral anterior PFC, bilateral insula, and cerebellum (figure 3; online supplementary table 3). Activity in these regions increased or was maintained across training in controls but did not increase or actually decreased in the siblings of COS probands. There were no regions in which there was a greater increase in activation in the COS siblings across training than in the controls.
Fig. 3.
Percent signal change during the task compared with baseline within the regions that demonstrated areas of significant difference between childhood-onset schizophrenia siblings and controls during a Day × Group interaction. Error bars represent the SE of the mean.
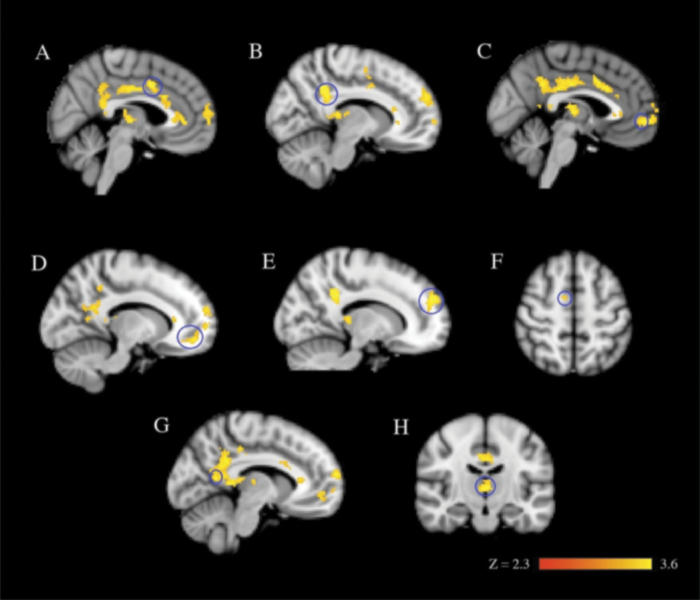
We next examined group differences separately at the 2 timepoints during training. During early learning (the first 50 trials), there were several regions that were significantly more active in the siblings of COS probands than in controls: bilateral anterior and posterior cingulate gyrus, bilateral precuneus cortex, bilateral thalamus, bilateral SMA, bilateral paracingulate cortex, bilateral anterior PFC, and the bilateral frontal medial cortex (figure 4; online supplementary table 4). Of these regions that significantly passed threshold, online supplementary figure 1 demonstrates that all the regions were more active in the siblings of COS probands than in controls, except for the bilateral frontal medial cortex, which was less deactivated relative to the baseline condition in the siblings of COS probands than in the controls. There were no regions that were more active in controls compared with the siblings of COS probands.
Fig. 4.
Early learning (first 50 trials) in the Weather Prediction Task. Images are from the group-level analysis (z > 2.3, cluster-corrected thresholded at P = .05). (A) bilateral anterior cingulate gyrus, (B) bilateral posterior cingulate gyrus, (C) bilateral paracingulate cortex, (D) bilateral anterior prefrontal cortex, (E) bilateral frontal medial cortex, (F) bilateral supplementary motor area, (G) bilateral precuneus cortex, and (H) right and left thalamus for childhood-onset schizophrenia siblings >controls for task vs baseline. Regions circled in blue correspond to their labeled region.
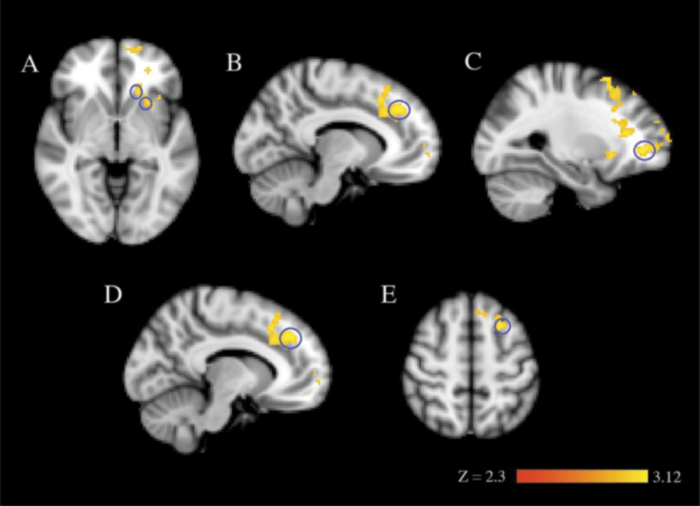
During late learning (the last 50 trials on day 2 after the additional 800 behavioral training trials, trials 851–900), there were several regions that were significantly more active in controls than in the siblings of COS probands. Two regions (the bilateral paracingulate cortex and the bilateral anterior PFC) had been more active in the siblings of COS probands early in training but decreased in activation, falling below that of controls at the end of training. There were other regions in which activation was not significantly different between the groups in early training but was significantly lower in the siblings of COS probands during later training. These areas included the left caudate and putamen, and the bilateral superior and middle frontal gyri (figure 5; online supplementary figure 2 and supplementary table 4). There were no regions that were more active in the siblings of the COS proband group compared with controls at the late timepoint.
Fig. 5.
Extended training (after 850 additional trials) in the Weather Prediction Task. Images are from the group-level analysis (z > 2.3, cluster-corrected thresholded at P = .05). (A) left caudate and left putamen, (B) bilateral paracingulate cortex, (C) bilateral anterior prefrontal cortex, (D) bilateral superior frontal gyrus, and (E) bilateral middle frontal gyrus for controls >childhood-onset schizophrenia siblings for task vs baseline. Regions circled in blue correspond to their labeled region.
Because the performance of the COS siblings was significantly worse than that of controls, it is possible that the pattern of activation seen in the COS siblings was characteristic of poorly performing subjects in general and not specific to genetic liability for schizophrenia. To examine this possibility, we conducted a secondary analysis comparing the COS group to the subset of controls whose performance was below the overall control median (online supplementary table 2 and supplementary figure 3). This subgroup of controls performed at a similar level to the COS siblings (ie, there was no difference in performance between the groups, F(1, 21) = 0.381, P = .544). While this comparison had reduced statistical power compared to the analysis including the larger group of control subjects, the results were remarkably similar. During early learning, the regions that were significantly more active in the siblings of COS probands than in the performance-matched controls included bilateral anterior and posterior cingulate gyrus, bilateral precuneus cortex, left thalamus, bilateral paracingulate cortex, and bilateral anterior PFC (online supplementary table 5). These were the same regions that showed increased activation in the COS sibling group compared with the larger control group as well. Only the increases in SMA and medial frontal regions that were present in the main analysis did not emerge in the performance-matched analysis. As in the larger analysis, there were no regions that were more active in performance-matched controls compared with the siblings of COS probands.
Comparing the performance-matched groups late in training also revealed a pattern similar to the larger analysis. Similar regions that were significantly more active in the controls than in the siblings of COS probands were also more active in the subset of performance-matched controls: bilateral anterior PFC, bilateral paracingulate cortex, and bilateral superior and middle frontal gyri (online supplementary table 4). There was also a trend for the left putamen (P uncorrected = .004) and bilateral caudate (P uncorrected = .0091) to display more activation in the performance-matched controls compared with the COS siblings. In the analysis with the larger group of controls, the difference in the striatum achieved statistical significance. One additional difference emerged in the comparison of performance-matched subjects that was not present in the comparison including all controls. In the insula, the performance-matched controls showed greater activation than the COS siblings late in training.
The results of the analysis of performance-matched groups indicate that the pattern shown by the siblings of COS patients of increased activation during early learning followed by reduced activation after extended training is not characteristic of poor performance in general on the WPT. Rather, the siblings of COS probands exhibit a different pattern of activation compared with control subjects performing at the same level.
Discussion
Adolescent siblings of COS patients performed significantly more poorly on the WPT and exhibited different patterns of neural activation during learning of this task compared with controls. Early in practice, there was clear learning by the controls and no evidence of learning in the siblings of COS probands. There was also a lower level of asymptotic performance in the siblings of COS probands after extended practice compared with controls. The fMRI data revealed that in a number of frontal regions, the siblings of COS probands demonstrated increased activation compared with controls early in training and decreased activation compared with controls after extensive training. In the control group, the level of activation in the striatum was consistent across training. In contrast, the activation in the left striatum in the siblings of COS probands decreased from early to late in practice. A secondary analysis comparing the COS siblings to a subset of controls matched for performance revealed a similar pattern as seen in the more inclusive analysis. Although the performance-matched analysis had lower statistical power, the same differences in frontal and cingulate/paracingulate regions emerged across both days of training, and trend-level group differences in the striatum were present after extended training.
Previous neuropsychological and neuroimaging studies have shown that performance on the WPT is supported by multiple neural systems. Very early learning can be supported by a declarative system with a habit system supporting learning as training progresses.38 The present results suggest disruption of both components of performance in the siblings of COS probands. Corticostriatal circuits play an important role in learning in the WPT, particularly when declarative memory is compromised or after extensive training.10,39–41 The fact that the control subjects maintained striatal activity across learning is consistent with previous work. While the siblings of COS probands showed task-related striatal activity early in training, this activation decreased significantly later in training. Even when performance was matched, there was a trend for COS siblings to show lower activation than controls that emerged at the late timepoint. This pattern may reflect a lack of effective utilization of striatal circuits in the siblings of COS patients, that may have contributed to their poorer level of asymptotic performance on the WPT.
Because we collected fMRI data during both initial learning and asymptotic performance, we were able to compare the change in neural activation in the 2 groups across training. In control subjects, 3 regions (anterior and posterior cingulate and frontal medial cortex) were deactivated relative to baseline early in training, and then activation increased to baseline levels at asymptotic performance. This pattern is consistent with these areas being part of a default mode network, which is deactivated during attention-demanding tasks, reflecting a redistribution of processing resources.42–47 Early in learning, the WPT was likely to be attention demanding but with extensive training performance became relatively automatic,26 and the default mode network was reinstituted in controls. In contrast, in the siblings of COS probands, the activity in these regions showed the opposite pattern, with activity decreasing in these regions across training. In the siblings of COS probands, deactivation did not occur in these regions early in training, but there was deactivation after extensive training. In adult patients with schizophrenia and their relatives, altered default mode network activity has been demonstrated.48–51 Altered default mode network function in schizophrenia has been conjectured to be involved in the complex pattern of symptoms observed in schizophrenia involving attending to internal and external stimuli and self-referential processing.52 The present results suggest that this alteration may be part of the genetic liability for schizophrenia and not merely related to the presence of psychotic symptoms.
There were group differences in the activation of bilateral anterior PFC and paracingulate cortex. In control subjects, there was no task-specific activation in these regions early in training, with marginal increases at the end of training. In the siblings of COS probands, however, these regions showed above baseline activation early in training, with sharp reductions later in training to below baseline levels. The anterior PFC has been implicated in the integration of cognitive processes and memory retrieval.53–55 The paracingulate cortex is a cingulo-frontal transition area that has been termed the “cognitive” division of the anterior cingulate cortex and has reciprocal connections with PFC regions, including anterior PFC.56–59 While these regions do not appear to play a role in learning in the control group, it may be that the siblings of COS probands activate these regions to a greater extent early in learning as a compensatory strategy. The sharp decrease in activity in these regions as performance becomes asymptotic in the siblings of COS probands suggests that these regions are engaged in learning in this group.
The results provide evidence that corticostriatal dysfunction may be part of the genetic liability for schizophrenia independent of the effects of medication and the illness. Also, the sharp decrease in activation in the anterior cingulate gyrus and a number of frontal regions in the siblings of COS probands compared with controls late in training may reflect an ineffective utilization of cortico-cingulate circuits, which may have contributed in the poorer level of asymptotic performance on the WPT.
In the present study, adolescents were tested. Thus, the impaired behavioral performance and alterations in functional brain activation in the siblings of COS probands could reflect a developmental delay rather than an enduring deficit. Given findings of a normalization of gray matter abnormalities in siblings of COS patients when they reach adulthood,60 it is particularly important to conduct longitudinal studies in this group to see if functional brain abnormalities persist into adulthood. It would also be important to know whether the abnormalities in neural activation seen here are associated with conversion to schizophrenia. It is possible that some of the siblings of COS patients will go on to develop psychosis during adulthood, and the abnormalities in neural activation described here may reflect precursors of disease rather than purely genetic liability.
In our study, there was little or no relationship within each group between measures of intellectual function and learning across both days of training on the WPT. For the COS sibling group, there was no relationship between scores on the Block Design or Vocabulary Design subtests of the WASI and either early or late performance (Ps > .05). For the control participants, there were modest correlations between scores on the WASI subtests and WPT performance. There were significant correlations only for early learning and Vocabulary Design (r(25) = .401, P < .047) and for asymptotic performance and Block Design (r(23) = .436, P = .038). Finally, a regression analysis showed that the IQ measures did not significantly predict performance during either early learning or asymptotic performance (P > .05). Based on the lack of a strong relationship between either measures of verbal or performance IQ and WPT performance, it is unlikely that differences in general intellectual function between the groups could substantially account for the findings of our study. However, we cannot rule out the possibility that these differences may have made some contribution to the pattern of results. Future work with larger samples are needed that can better assess the relationship between individual cognitive or behavioral factors and WPT performance.
Future work with larger samples could also potentially reveal differences in strategy use in the WPT (eg, whether choices are based on single cues or cue combinations) in relatives of COS probands. While Weickert et al5 did not find evidence of different strategy use in the WPT in siblings of adult-onset patients, it is possible that relatives of COS probands would show such a difference. In addition, studies with larger sample sizes of relatives of COS probands are needed to examine whether the abnormal patterns of BOLD signal activity are correlated with behavioral deficits in cognitive skill learning in this group.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
Della Martin Foundation and a National Institute of Mental Health grant (MH 72697).
Supplementary Material
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Giménez M, Junqué C, Pérez M, et al. Basal ganglia N-acetylaspartate correlates with the performance in the procedural task ‘Tower of Hanoi’ of neuroleptic-naive schizophrenic patients. Neurosci Lett. 2003;347:97–100 [DOI] [PubMed] [Google Scholar]
- 2. Schröder J, Tittel A, Stockert A, Karr M. Memory deficits in subsyndromes of chronic schizophrenia. Schizophr Res. 1996;21:19–26 [DOI] [PubMed] [Google Scholar]
- 3. Purdon SE, Woodward N, Lindborg SR, Stip E. Procedural learning in schizophrenia after 6 months of double-blind treatment with olanzapine, risperidone, and haloperidol. Psychopharmacology (Berl). 2003;169:390–397 [DOI] [PubMed] [Google Scholar]
- 4. Foerde K, Poldrack RA, Khan BJ, et al. Selective corticostriatal dysfunction in schizophrenia: examination of motor and cognitive skill learning. Neuropsychology. 2008;22:100–109 [DOI] [PubMed] [Google Scholar]
- 5. Weickert TW, Goldberg TE, Egan MF, et al. Relative risk of probabilistic category learning deficits in patients with schizophrenia and their siblings. Biol Psychiatry. 2010;67:948–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buchanan RW, Breier A, Kirkpatrick B, et al. Structural abnormalities in deficit and nondeficit schizophrenia. Am J Psychiatry. 1993;150:59–65 [DOI] [PubMed] [Google Scholar]
- 7. Buchsbaum MS. Frontal lobes, basal ganglia, temporal lobes–three sites for schizophrenia? Schizophr Bull. 1990;16:377–378 [DOI] [PubMed] [Google Scholar]
- 8. Kleist K. Schizophrenic symptoms and cerebral pathology. J Ment Sci. 1960;106:246–255 [DOI] [PubMed] [Google Scholar]
- 9. Heindel WC, Salmon DP, Shults CW, Walicke PA, Butters N. Neuropsychological evidence for multiple implicit memory systems: a comparison of Alzheimer’s, Huntington’s, and Parkinson’s disease patients. J Neurosci. 1989;9:582–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273:1399–1402 [DOI] [PubMed] [Google Scholar]
- 11. Doyon J, Bellec P, Amsel R, et al. Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav Brain Res. 2009;199:61–75 [DOI] [PubMed] [Google Scholar]
- 12. Peigneux P, Maquet P, Meulemans T, et al. Striatum forever, despite sequence learning variability: a random effect analysis of PET data. Hum Brain Mapp. 2000;10:179–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grafton ST, Hazeltine E, Ivry R. Functional mapping of sequence learning in normal humans. J Cogn Neurosci. 1995;7:497–510 [DOI] [PubMed] [Google Scholar]
- 14. Rauch SL, Whalen PJ, Savage CR, et al. Striatal recruitment during an implicit sequence learning task as measured by functional magnetic resonance imaging. Hum Brain Mapp. 1997;5:124–132 [PubMed] [Google Scholar]
- 15. Poldrack RA, Gabrieli JD. Characterizing the neural mechanisms of skill learning and repetition priming: evidence from mirror reading. Brain. 2001;124:67–82 [DOI] [PubMed] [Google Scholar]
- 16. Schiltz C, Bodart JM, Michel C, Crommelinck M. A pet study of human skill learning: changes in brain activity related to learning an orientation discrimination task. Cortex. 2001;37:243–265 [DOI] [PubMed] [Google Scholar]
- 17. Aron AR, Shohamy D, Clark J, Myers C, Gluck MA, Poldrack RA. Human midbrain sensitivity to cognitive feedback and uncertainty during classification learning. J Neurophysiol. 2004;92:1144–1152 [DOI] [PubMed] [Google Scholar]
- 18. Moody TD, Bookheimer SY, Vanek Z, Knowlton BJ. An implicit learning task activates medial temporal lobe in patients with Parkinson’s disease. Behav Neurosci. 2004;118:438–442 [DOI] [PubMed] [Google Scholar]
- 19. Knowlton BJ, Squire LR, Gluck MA. Probabilistic classification learning in amnesia. Learn Mem. 1994;1:106–120 [PubMed] [Google Scholar]
- 20. Marsh R, Alexander GM, Packard MG, et al. Habit learning in Tourette syndrome: a translational neuroscience approach to a developmental psychopathology. Arch Gen Psychiatry. 2004;61:1259–1268 [DOI] [PubMed] [Google Scholar]
- 21. Kéri S, Juhász A, Rimanóczy A, et al. Habit learning and the genetics of the dopamine D3 receptor: evidence from patients with schizophrenia and healthy controls. Behav Neurosci. 2005;119:687–693 [DOI] [PubMed] [Google Scholar]
- 22. Horan WP, Green MF, Knowlton BJ, Wynn JK, Mintz J, Nuechterlein KH. Impaired implicit learning in schizophrenia. Neuropsychology. 2008;22:606–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weickert TW, Terrazas A, Bigelow LB, et al. Habit and skill learning in schizophrenia: evidence of normal striatal processing with abnormal cortical input. Learn Mem. 2002;9:430–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Paquet F, Soucy JP, Stip E, Lévesque M, Elie A, Bédard MA. Comparison between olanzapine and haloperidol on procedural learning and the relationship with striatal D2 receptor occupancy in schizophrenia. J Neuropsychiatry Clin Neurosci. 2004;16:47–56 [DOI] [PubMed] [Google Scholar]
- 25. Kumari V, Gray JA, Honey GD, et al. Procedural learning in schizophrenia: a functional magnetic resonance imaging investigation. Schizophr Res. 2002;57:97–107 [DOI] [PubMed] [Google Scholar]
- 26. Wagshal D, Knowlton BJ, Cohen JR, et al. Deficits in probabilistic classification learning and liability for schizophrenia. Psychiatry Res. 2012;200:167–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Asarnow RF. Childhood schizophrenia. In: Beauchaine TP, Hinshaw SP, eds. Child and Adolescent Psychopathology. 2nd ed. Hoboken, NJ: John Wiley and Sons; 2013:685–714 [Google Scholar]
- 28. Asarnow RF, Nuechterlein KH, Fogelson D, et al. Schizophrenia and schizophrenia-spectrum personality disorders in the first-degree relatives of children with schizophrenia: the UCLA family study. Arch Gen Psychiatry. 2001;58:581–588 [DOI] [PubMed] [Google Scholar]
- 29. Asarnow RF. Neurocognitive impairments in schizophrenia: a piece of the epigenetic puzzle. Eur Child Adolesc Psychiatry. 1999;8(suppl 1):I5–I8 [DOI] [PubMed] [Google Scholar]
- 30. Gold JM, Harvey PD. Cognitive deficits in schizophrenia. Psychiatr Clin North Am. 1993;16:295–312 [PubMed] [Google Scholar]
- 31. Kuperberg G, Heckers S. Schizophrenia and cognitive function. Curr Opin Neurobiol. 2000;10:205–210 [DOI] [PubMed] [Google Scholar]
- 32. Riley EM, McGovern D, Mockler D, et al. Neuropsychological functioning in first-episode psychosis–evidence of specific deficits. Schizophr Res. 2000;43:47–55 [DOI] [PubMed] [Google Scholar]
- 33. Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10:433–436 [PubMed] [Google Scholar]
- 34. Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841 [DOI] [PubMed] [Google Scholar]
- 35. Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–1386 [DOI] [PubMed] [Google Scholar]
- 37. Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp. 1994;1:214–220 [DOI] [PubMed] [Google Scholar]
- 38. Shohamy D, Myers CE, Kalanithi J, Gluck MA. Basal ganglia and dopamine contributions to probabilistic category learning. Neurosci Biobehav Rev. 2008;32:219–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Foerde K, Knowlton BJ, Poldrack RA. Modulation of competing memory systems by distraction. Proc Natl Acad Sci U S A. 2006;103:11778–11783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Poldrack RA, Clark J, Paré-Blagoev EJ, et al. Interactive memory systems in the human brain. Nature. 2001;414:546–550 [DOI] [PubMed] [Google Scholar]
- 41. Poldrack RA, Prabhakaran V, Seger CA, Gabrieli JD. Striatal activation during acquisition of a cognitive skill. Neuropsychology. 1999;13:564–574 [DOI] [PubMed] [Google Scholar]
- 42. McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15:394–408 [DOI] [PubMed] [Google Scholar]
- 43. Tomasi D, Volkow ND, Wang R, et al. Dopamine transporters in striatum correlate with deactivation in the default mode network during visuospatial attention. PLoS One. 2009;4:e6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ogg RJ, Zou P, Allen DN, Hutchins SB, Dutkiewicz RM, Mulhern RK. Neural correlates of a clinical continuous performance test. Magn Reson Imaging. 2008;26:504–512 [DOI] [PubMed] [Google Scholar]
- 45. Ungar L, Nestor PG, Niznikiewicz MA, Wible CG, Kubicki M. Color Stroop and negative priming in schizophrenia: an fMRI study. Psychiatry Res. 2010;181:24–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sepede G, Ferretti A, Perrucci MG, et al. Altered brain response without behavioral attention deficits in healthy siblings of schizophrenic patients: an event-related fMRI study. Neuroimage. 2010;49:1080–1090 [DOI] [PubMed] [Google Scholar]
- 47. Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978 [DOI] [PubMed] [Google Scholar]
- 48. Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164:450–457 [DOI] [PubMed] [Google Scholar]
- 49. Zhou Y, Liang M, Tian L, et al. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophr Res. 2007;97:194–205 [DOI] [PubMed] [Google Scholar]
- 50. Pomarol-Clotet E, Salvador R, Sarró S, et al. Failure to deactivate in the prefrontal cortex in schizophrenia: dysfunction of the default mode network? Psychol Med. 2008;38:1185–1193 [DOI] [PubMed] [Google Scholar]
- 51. Whitfield-Gabrieli S, Thermenos HW, Milanovic S, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106:1279–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rotarska-Jagiela A, van de Ven V, Oertel-Knöchel V, Uhlhaas PJ, Vogeley K, Linden DE. Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr Res. 2010;117:21–30 [DOI] [PubMed] [Google Scholar]
- 53. Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat Rev Neurosci. 2004;5:184–194 [DOI] [PubMed] [Google Scholar]
- 54. Düzel E, Cabeza R, Picton TW, et al. Task-related and item-related brain processes of memory retrieval. Proc Natl Acad Sci U S A. 1999;96:1794–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kapur S, Craik FI, Jones C, Brown GM, Houle S, Tulving E. Functional role of the prefrontal cortex in retrieval of memories: a PET study. Neuroreport. 1995;6:1880–1884 [DOI] [PubMed] [Google Scholar]
- 56. Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118 (Pt 1):279–306 [DOI] [PubMed] [Google Scholar]
- 57. Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222 [DOI] [PubMed] [Google Scholar]
- 58. Vogt BA, Nimchinsky EA, Vogt LJ, Hof PR. Human cingulate cortex: surface features, flat maps, and cytoarchitecture. J Comp Neurol. 1995;359:490–506 [DOI] [PubMed] [Google Scholar]
- 59. Mega MS Cummings JL. The cingulate and cingulate syndromes. In: Trimble MR, Cummings JL, eds. Contemporary Behavioural Neurology. Boston, MA: Butterworth-Heinemann; 1997:189–213 [Google Scholar]
- 60. Rapoport JL, Gogtay N. Childhood onset schizophrenia: support for a progressive neurodevelopmental disorder. Int J Dev Neurosci. 2011;29:251–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.