Abstract
Self-experience anomalies are elementary features of schizophrenic pathology. Such deficits can have a profound impact on self-other relationship, but how they are related through aberrant brain function remains poorly understood. In this functional magnetic resonance imaging (fMRI) study, we provide new evidence for a cortical link between aberrant self-experience and social cognition in first-episode schizophrenia (FES). As identified in previous studies, ventral premotor cortex (vPMC) and posterior insula (pIC) are candidate brain regions underlying disturbances in both self-experience and self-other relationship due to their processing of predominantly externally guided (vPMC; goal-oriented behavior) and internally guided (pIC; interoception) stimuli. Results from functional interaction analysis in a sample of 24 FES patients and 22 healthy controls show aberrant functional interactions (background/intrinsic connectivity) of right vPMC and bilateral pIC with posterior cingulate cortex (PCC), a midline region that has been shown central in mediating self-experience. More specifically, our results show increased functional coupling between vPMC and PCC, which positively correlated with basic symptoms (subjective self-experience disturbances). pIC showed reduced functional coupling with PCC and postcentral gyrus and increased functional interactions with anterior insula. Taken together, our results suggest an imbalance in the processing between internally and externally guided information and its abnormal integration with self-referential processing as mediated by PCC. Due to our correlation findings, we suggest this imbalance to be closely related to basic symptoms in FES and thus anomalous self-experience. The findings further disentangle the cortical basis of how self-experience anomalies may pervade the social domain.
Key words: psychosis, posterior insula, ventral premotor cortex, posterior cingulate cortex, functional magnetic resonance imaging, connectivity
Introduction
Schizophrenia is a pervasive and complex neuropsychiatric disorder with basic deficits in self-experience that can have a profound impact on self-other relationship. Since the early 19th century, a crucial role has been attributed to the self and its prereflective attunement with the external world in schizophrenic psychopathology.1,2 More recently, schizophrenia has been primarily characterized as a disorder of self-experience and awareness that entails anomalies in self-other relationship (see among others).3–8 Self-experience deficits also have been approximated by basic symptoms, reflecting a variety of anomalies of subjective experience in the domains of cognition, perception, bodily experience, action, and emotion.9,10 Disentangling the neural mechanism underlying an anomalous link between aberrant self-experience and self-other relationship will provide a better understanding not only of the nature of elementary features of schizophrenia but also of how the self-other relationship is grounded in self-experience.
Several empirical studies suggested that self-experience disturbances could be closely associated with anomalies in the self-other relationship. Schizophrenic patients with high self-monitoring skills were reported to have better social skills.11 Others revealed confusion in the attribution of events to their origin, including the attribution of self-produced sensory experiences and actions to external sources.12–17 An increased tendency to incorporate the experiences of external bodies has been reported too. For instance, the rubber hand illusion (a condition in which an observed rubber hand is subjectively experienced as if it actually were one’s own hand)18 has been found enhanced in schizophrenic pathology.19,20 Thus, along with anomalous self-experiences, social interactions may confuse with a blurred distinction between self and other.
In a previous functional magnetic resonance imaging (fMRI) study investigating social perception (observation of others’ sensory-affective experiences),21 we showed that deficits in self-other relationship in first-episode schizophrenia (FES) could have strong roots in disturbances of self-experience, including impaired multisensory integration and self-other distinction associated with ventral premotor cortex (vPMC) and posterior insula (pIC) dysfunction, respectively. Hence, abnormal functioning of these regions may be involved in the link between self-experience disturbances and some aspects of social dysfunction in schizophrenia.
From a general neurobiological point of view on schizophrenia, aberrant neural connectivity has been proposed as an elementary aspect in its pathophysiology.22–25 Neuroimaging research seems to suggest a widespread and possibly context-independent alteration of functional connectivity in schizophrenia26–30 and aberrant structural functional connectivity patterns.31,32 Topologically, functional brain networks in schizophrenia could be characterized by a reduced clustering and small-worldness, associated with a less hub-dominated organization.33 However, the specific links between neural connectivity and social dysfunction are poorly understood, partially due to a lack of systematic research on this topic.
This study aimed at providing more insight in how impairments in self-other relationship in schizophrenia could be related to altered functional interaction patterns. Specifically, we examined (1) whether vPMC and pIC could be characterized by altered neurofunctional interactions, (2) whether these could provide information complementary to task-evoked activation patterns, and (3) whether they could be related to symptomatology, especially disturbances of self-experience.
A peculiar role could be expected for posterior cingulate cortex (PCC) as one of the most central hubs in the human brain in terms of connectivity patterns.34,35 PCC is a crucial node of the Default Mode Network,36,37 allows the communication between different cortical modules or cross-network interactions, 38,39 and is commonly implicated in schizophrenia.32,40 Of particular interest in relation to the disability to distinguish between self and other in schizophrenia,41 PCC often is associated with self-referential processing,42–45 integrating self-experience,46 and regulating the balance between internally and externally directed cognition.47,48 The self also has been conceived of as a basic precognitive structure related to such a balance as predisposed by the brain’s intrinsic activity in cortical midline structures including PCC,42,49 whereas abnormalities in intrinsic functioning of these structures have been linked with aberrant self-referential processing in schizophrenia.8
In order to address these issues, fMRI functional interaction analysis was performed on the same data set as used for our previous study21 by measuring the statistical dependence between low-frequency blood oxygen level dependent (BOLD) signals across brain voxels (“background” or “intrinsic” connectivity).50,51 Functional interaction analysis during specific task contexts also has been shown to be complementary to the analysis of task-evoked neural activation patterns.52,53 Individual functional interaction indices were related to symptomatology, including positive and negative symptoms54 and basic symptoms, ie, subjective disturbances of self-experience.9,55
Methods
Participants
Twenty-four outpatients with FES and 22 matched healthy control (HC) participants were included in this study and were the same as in Ebisch et al.21 All participating FES patients had a history of a single psychotic episode, and all received a diagnosis of schizophrenia according to DSM-IV criteria 6 months after the episode. Detailed participant characteristics and inclusion criteria are described in the supplementary material and table 1. FES patients were rated for symptom severity with the Positive and Negative Symptom Scale (PANSS)56 and evaluated for the presence of basic symptoms by means of the schizophrenia proneness instrument—adult version (SPI-A)57 by trained psychiatrists. The study was approved by the local Ethics Committee. Written informed consent was obtained from all participants after full explanation of the procedure of the study, in line with the Declaration of Helsinki.
Table 1.
Demographic Information About the First-Episode Schizophrenia (FES) Group and Healthy Control (HC) Group (Adapted from Ebisch et al, Soc Cogn Affect Neurosci 2013)
| FES Group (N = 24) | HC Group (N = 22) | |
|---|---|---|
| Agea (mean ± SD) | 27.3±4.8 | 27.5±3.3 |
| Mean time from psychotic episode (months, mean ± SD) | 8±5 | n.a. |
| Handedness score,a (mean ± SD) | 65.3±18.1 | 69.3±15.8 |
| Male/femalea | 16/8 | 12/10 |
| Diagnosis | (First-episode) schizophrenia | n.a. |
| Intelligence quotient (mean ± SD) | 100±8.5 | n.a. |
| Empathy quotient,b mean ± SD (cognitive empathyc/emotional reactivityc/social skillsd) | 38±11.4 (12.4±5.4/12.2±5.2/6.3±2.8) | 45.4±9.7 (14.5±4.6/14.6±4.4/8.1±2.3) |
| SCID-II Cluster A | n.a. | Negative |
| SCID-II Cluster B | n.a. | Negative |
| SCID-II Cluster C | n.a. | Negative |
| PANSS Positive scale individual scores (mean ± SD) | 16 10 9 14 17 16 21 14 12 16 18 12 10 12 13 11 19 13 8 13 9 10 11 15 (13.3±3.4) | n.a. |
| PANSS Negative scale individual scores (mean ± SD) | 8 10 10 9 10 11 12 16 12 24 12 8 10 9 11 9 11 22 9 14 9 8 12 22 (12±4.5) | n.a. |
| PANSS General Psychopathology scale individual scores (mean ± SD) | 22 20 20 30 18 24 32 25 22 37 22 22 20 20 23 21 25 25 19 25 20 19 22 35 (23.6±5) | n.a. |
| SPI-A total individual scores (mean ± SD) | 138 28 74 91 115 71 67 5 0 114 40 36 97 17 12 27 42 22 83 82 45 40 49 85 (61.1±38.4) | n.a. |
| Medicatione | 6 Quetiapine | n.a. |
| 7 Risperidone | ||
| 3 Paliperidone | ||
| 4 Aripiprazole | ||
| 3 Olanzapine | ||
| 1 Drug free |
Notes: SCID-II, Structured Clinical Interview for DSM-IV Axis II personality disorders; PANSS, Positive and Negative Symptom Scale; SPI-A, schizophrenia proneness instrument; n.a., not applicable.
aNo significant differences between the HC and FES group.
bSignificant difference between the HC and FES group (P = .02).
cNo significant differences between the HC and FES group.
dSignificant difference between the HC and FES group (P = .02).
eClorpromazine equivalent mean dose = 422mg/day, SD = 395.5 (calculated on 21 patients because no equivalents are available for paliperidone).
fMRI Preprocessing and Analysis
The same data set as in Ebisch et al21 was used for the present fMRI study. Data acquisition parameters and the first fMRI preprocessing procedures were the same. Additionally, for fMRI functional interaction analysis, a second step of data preprocessing was performed by using self-devised MATLAB scripts (The Mathworks Inc) including (1) bandpass filtering between 0.009 and 0.08 Hz51,58,59; (2) regression of global, white matter, ventricle signals, and their first derivatives50,60; (3) regression of 3-dimensional motion parameters and their first derivatives; (4) regression of task-related BOLD fluctuations50,52,61,62; and (5) scrubbing of motion-affected functional volumes.63
Seed-based analysis of long-range functional interactions was performed identifying temporally correlated patterns of brain activity across brain regions.50,64 Functional interaction maps were calculated by means of voxel-wise, whole brain analyses for the 3 seed regions of interest (ROIs) defined as spheres with a 6-mm radius. ROI coordinates were based on Ebisch et al21: right vPMC (Talairach coordinates: 50, 7, 32) and bilateral pIC (Talairach coordinates: right pIC 38, −12, 7 and left pIC −38, −10, 7).
For all participants, we calculated correlations of BOLD fluctuations over continuous fMRI time series between the seed ROI time courses and the time courses of all individual brain voxels. After applying Fisher’s r-to-z transformation65 to each correlation map, random-effect analysis was performed independently for each of the two groups in order to reveal functional interaction patterns that were consistent across participants. Statistical significance was determined by means of one-sample t tests. Group statistical maps were thresholded at P < .001 corrected for multiple comparisons by the false discovery rate (FDR).66
To test for significant differences between the FES and HC groups, independent-samples t tests between the functional interaction maps for the 3 seed ROIs in right vPMC and bilateral pIC were performed. Statistical maps of these between-group contrasts were thresholded at P < .01 FDR corrected with a cluster size of k > 8.
Additional ROI-based analyses were performed to investigate the relationship between functional interaction indices and symptomatology (SPI-A: basic symptoms; PANSS: positive, negative, and general symptoms) and to control for possibly confounding effects due to medication dosage (chlorpromazine [CP] equivalence values), illness duration, general cognitive ability (intelligence quotient [IQ]), task-evoked neural activation patterns. A priori ROIs were defined as spheres with a 6-mm radius and included the original seed ROIs (right vPMC, bilateral pIC) and the brain regions showing significant differences in coupling with the seed ROIs between the HC and FES groups (see table 2).
Table 2.
Location and Statistical Information Regarding the Voxel Clusters Showing Altered Functional Coupling With the Seed Regions of Interest (ROIs) in the FES Group, Compared With the HC Group
| Brain Region | Talairach Coordinates | Cluster Size | t-value HC | t-value FES | t-value HC vs FES |
|---|---|---|---|---|---|
| Seed ROI: RH vPMC | |||||
| PCC | 2, −32, 45 | 270 | −5.26 | 1.30 | −4.52 |
| Seed ROI: LH pIC | |||||
| LH aIC | −40, 22, 12 | 1512 | 0.82 | 8.66 | −4.81 |
| RH aIC | 44, 1, 15 | 540 | 3.12 | 11.07 | −4.44 |
| PCC | −1, −26, 45 | 270 | 8.06 | −0.81 | 4.36 |
| RH PPC | 38, −56, 33 | 135 | −7.18 | −2.36 | −4.32 |
| LH PPC | −34, −56, 24 | 324 | −4.57 | 1.87 | −4.58 |
| RH aFC | 32, 46, 24 | 2970 | −8.07 | −1.22 | −6.23 |
| LH aFC | −34, 46, 15 | 351 | −5.22 | 1.32 | −4.51 |
| Seed ROI: RH pIC | |||||
| RH aIC | 26, 19, 3 | 243 | −1.19 | 5.02 | −4.35 |
| LH PostCG | −49, −35, 48 | 432 | 5.47 | −0.50 | 4.12 |
| RH PPC | 29, −62, 24 | 702 | −4.57 | 1.99 | −4.65 |
| LH PPC | −34, −56, 26 | 324 | −7.50 | −0.23 | −4.46 |
Note: RH, right hemisphere; LH, left hemisphere; vPMC, ventral premotor cortex; pIC, posterior insula; PCC, posterior cingulate cortex; aIC, anterior insula; PPC, posterior parietal cortex; aFC, anterior frontal cortex; PostCG, postcentral gyrus.
Consensus maps,67 consisting of the sum of different binarized correlation maps (threshold corresponding to P < .05 FDR), allowed to test which voxel clusters showed altered functional connections in the FES group, compared with the HC group, in common between the seed ROIs (right hemisphere vPMC and bilateral pIC).
Detailed information about preprocessing and ROI-based analysis procedures is provided in the supplementary material.
Results
Functional Interaction Analysis: Between-Group Comparisons
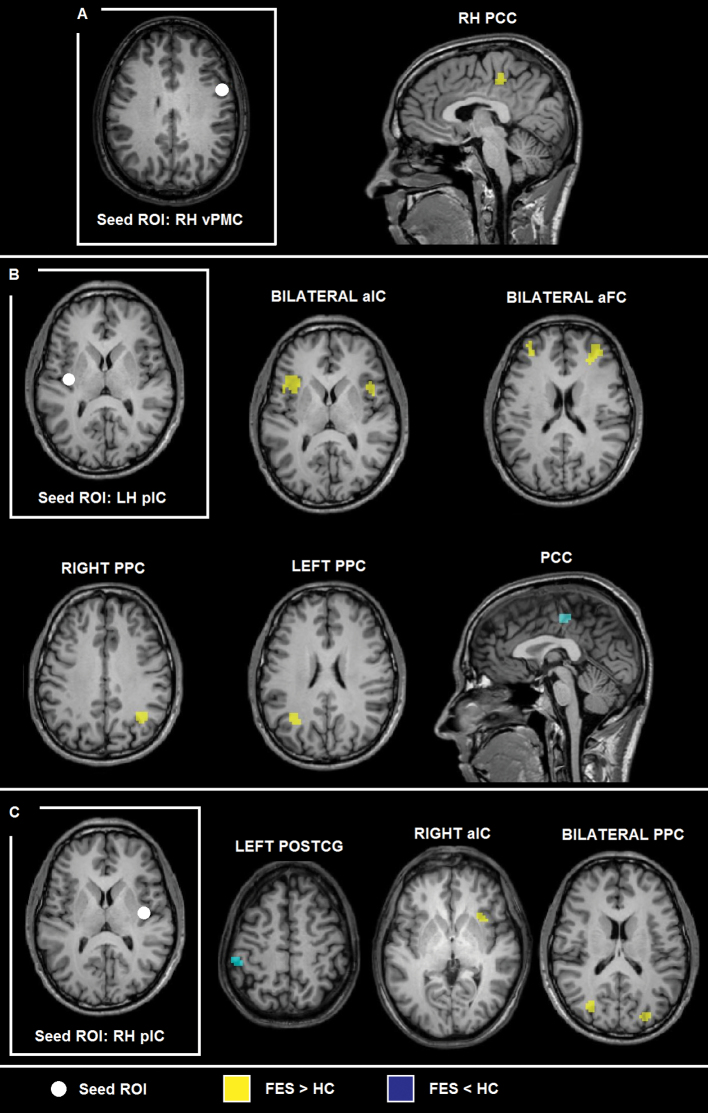
The functional interaction maps for the different seed ROIs calculated individually for the HC and FES groups are described in the supplementary material and visualized in supplementary figure 1. Regarding between-group contrasts, seed-based, voxel-wise analysis of functional interactions yielded an increased functional coupling between the seed ROI in right hemisphere vPMC and right PCC in the FES group, compared with the HC group (figure 1a, table 2).
Fig. 1.
Group statistical maps (P < .01 false discovery rate corrected, k > 8) showing altered functional connectivity patterns for (A) right ventral premotor cortex, (B) left anterior insula, and (C) right posterior insula in the first-episode schizophrenia group, compared with the healthy control group.
With respect to the seed ROI in left hemisphere pIC, the results showed an increased functional coupling in the FES group with bilateral anterior insular cortex (aIC), bilateral posterior parietal cortex (PPC), and right anterior frontal cortex, whereas a decreased functional coupling in the FES group was detected between left pIC and right PCC, compared with the HC group (figure 1a, table 2).
When using the right hemisphere pIC as seed ROI, we found increased functional interactions in the FES group with right aIC and bilateral PPC. We also found decreased functional coupling between right pIC and left postcentral gyrus (PostCG)/somatosensory cortex for the FES group, compared with the HC group (figure 1c, table 2).
A ROI-based analysis of covariance of functional interactions between the seed ROIs (right vPMC, left pIC, right pIC) and the voxel clusters showing altered functional interactions in the FES group as detected by means of the voxel-wise functional interaction analyses showed that these alterations in the FES group were independent of BOLD responses to the experimental stimuli (task-evoked activation patterns) and of IQ. Further correlation analyses based on a similar ROI-based approach in the FES group showed that there was no significant, linear relationship between alterations in functional interactions in the FES group and CP, illness duration, or IQ (all r < 0.37; P ≥ .1).
Relationship Between Functional Interactions and Symptomatology
Applying an ROI-based approach, partial correlation analysis showed a significant relationship of functional interaction between right vPMC and PCC with basic symptoms in the FES group (r = 0.89; P ≤ .05 FDR corrected), while excluding effects due to possible residuals of BOLD responses to the experimental stimuli, IQ, illness duration, and CP. No significant relationship was detected between vPMC functional interactions and PANSS scores (positive scale, negative scale, general psychopathology scale; P > .1). Moreover, no significant relationship between symptomatology and long-range functional interactions was detected for the seed ROIs in left or right pIC (P > .1).
Using a similar ROI-based approach, additional multiple regression with functional interaction indices as dependent variable and symptoms (basic symptoms as well as negative, positive and general symptoms) as independent variables showed that only basic symptoms significantly predicted vPMC-PCC functional interaction in the FES group (β = .539, P ≤ .01), but not PANSS scores (negative symptoms, positive symptoms, general symptoms, all P ≥ 25), confirming the specific relevance of basic symptoms.
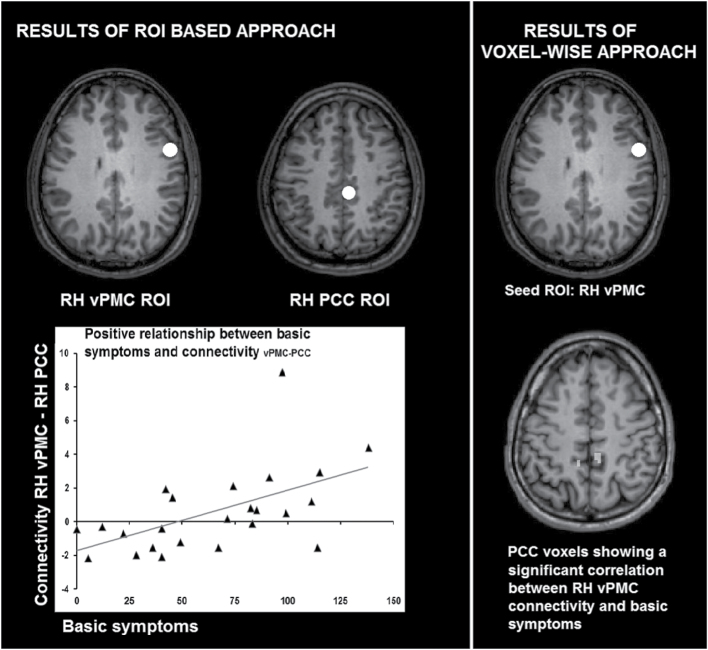
A seed-based, whole brain covariance analysis with right vPMC as seed ROI and basic symptoms as covariate confirmed a positive relationship (r > .62, P < .001, k > 5) between right vPMC-right PCC coupling and basic symptoms (Talairach coordinates of PCC voxel cluster obtained by means of seed-based analysis: 7, −37, 45).
The relationship between vPMC-PCC coupling and basic symptoms is visualized in figure 2 (left: ROI-based analysis; right: seed-based analysis).
Fig. 2.
The relationship between ventral premotor cortex-posterior cingulate cortex functional interactions and basic symptoms.
Functional Interaction Consensus Map
Consensus maps showed a voxel cluster in dorsal anterior PCC (ROI size = 243) characterized by altered functional interactions with 2 out of the 3 seed ROIs (2/3 consensus). Three voxels of the PCC cluster also showed altered functional coupling with all 3 seed ROIs: vPMC and bilateral pIC (3/3 consensus; Talairach coordinates: 2, −26, 45). Supplementary figure 2 shows the consensus maps of the FES-HC contrasts for the 3 seed ROIs.
ROI-based analysis with right vPMC, bilateral pIC, and PCC (3/3 consensus) as a priori ROIs confirmed an altered functional coupling for the two ROI pairs left pIC-PCC and right vPMC-PCC (P < .05 FDR corrected) in the FES group, whereas the difference between the FES group and HC group approached significance for the third ROI pair right pIC-right vPMC (P < .08 FDR corrected).
Discussion
This study aimed at investigating the functional interactions of right vPMC and bilateral pIC in FES. In accordance with the hypothesis, one of the main results regards an abnormal coupling of the seed ROIs with PCC in FES. First, functional interaction between vPMC and PCC significantly correlated with basic symptom severity. Second, consensus maps suggested that PCC could be a central brain region underlying altered functional interactions of both vPMC and pIC. Control analyses demonstrated that the reported effects were independent of medication dosage (CP), illness duration, task-evoked neural activity, and general cognitive ability (IQ) and likely reflected functional abnormalities in the intrinsic functional organization of the brain in FES. Furthermore, a subdivision in different frequency bands of BOLD fluctuations68,69 suggested that differences between FES and HC more specifically concerned the frequency range between 0.01 and 0.027 Hz (slow-5) but not the 0.027–0.07 Hz (slow-4) frequency range (see supplementary material and supplementary table 1).
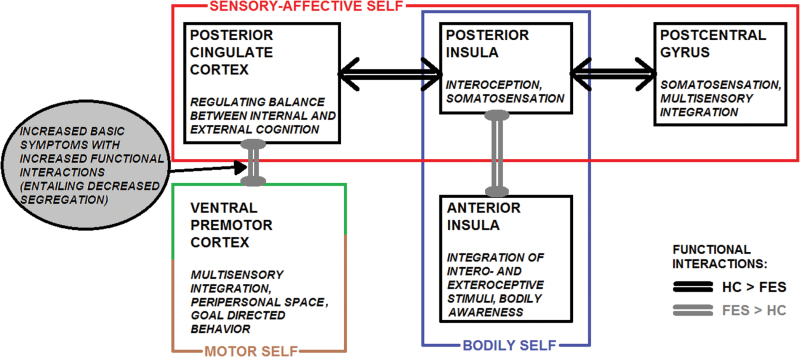
PCC connectivity has been indicated consistently in schizophrenic pathology.70–73 In this study, we found increased functional interactions between vPMC and PCC in the FES group. In line with previous studies on the intrinsic functional organization of the brain,60,74,75 PCC and vPMC appeared to belong to distinct, possibly antagonistic functional networks in the HC group. Based on the functions of vPMC in multisensory integration, peripersonal space representation, and goal-directed behavior76–81 and of PCC in mediating self-experience,43,45,46 we propose that such a decreased functional segregation could lead to a deranged relationship between the intrinsic self (self-referential cognition) and the extrinsic self (the self interacting with the environment) in FES. Interestingly, gamma amino butyric acid, an inhibitory neurotransmitter in the brain, in cortical midline structures including PCC likely has a relevant role in mediating between internally directed and externally directed thought,48 providing a possible neurochemical basis of such alterations in FES.82 Because cortical midline (PCC) and vPMC regions also have complementary functions in relating the self to its social environment,45,83–85 altered vPMC-PCC interactions likely disrupt some aspects of social cognition too.
The detected positive correlation between vPMC-PCC coupling and basic symptoms highlights the clinical relevance of a reduced functional segregation between the two nodes in schizophrenia. Basic symptoms approximate subjective disturbances of self-experience.9,10 This suggests that an abnormally strong vPMC-PCC coupling is related to a disrupted sense of a coherent self in everyday life. Interestingly, basic symptoms remain stable during the entire disease progression, including the prodromal phase of schizophrenia. Moreover, basic symptoms also represent a link between a more phenomenological European approach to psychopathology and a more categorical approach based on positive and negative symptoms typical of the Anglo-Saxon tradition. Specifically, basic symptoms, appearing more or less continuously even many years before psychosis onset, possibly are related to a predisposition and reflect the first changes in experience that set off the development of schizophrenic psychotic symptoms.86–88 Indeed, the occurrence of a basic symptom could gradually increase in number and severity and, in most cases, ultimately develop into psychotic symptoms, such as negative and positive symptoms.87,88 Hence, the study of their relationship with cortical processes has a peculiar relevance also from a clinical point of view, especially in the light of preventive approaches and an early diagnosis of schizophrenia.55,89 In this light, it is recommended to apply the current approach to at risk of psychosis states and their transition to FES.
With respect to pIC, functional interactions with PCC and PostCG were decreased in the FES group. pIC is generally involved in processing somatosensory stimuli with affective or motivational significance and is considered a central brain region for interoception, self-awareness, and bodily awareness.90–95 Regarding the social functions of pIC, in a previous study, we showed that pIC activity distinguished between self and other in social situations, especially where affective experiences are implicated.96 Of particular relevance, this function appears disrupted in schizophrenic pathology.21 The present findings of a diminished functional coupling in FES of pIC with PCC and PostCG, together representing a functional, sensory network in HC,47,97–100 essentially extend these previous results in terms of pIC functional interactions underlying a deficit in the self-other distinction. Considering the sensory-affective functions of pIC and PostCG, and the mediation of self-experience in PCC,43,45,46 reduced integrity within this network arguably can be associated with a deficit in the processing of bodily sensory-affective information as belonging to the self.
Another remarkable finding was that the FES group exhibited a disproportionally strong coupling between pIC and aIC. Different from pIC, along with the integration of cognitive and emotional responses, aIC underpins the conscious evaluation of affective experience, ie, the subjective awareness of bodily feeling states.95,101,102 Accordingly, insula abnormalities and deficits associated with insula damage, including disruption in self-awareness and self-other discrimination, are commonly reported in schizophrenic pathology.103,104 Although pIC and aIC are anatomically connected,105,106 in contrast to the present results in the FES group, functional connectivity studies in HC consistently suggest that they belong to distinct functional networks with minimal correlation of neural activity between them.97–100
Taken together, the altered pIC functional interactions reported in this study provide further evidence for an abnormal functional organization of neural networks involved in the awareness of the sensory-affective self. Such alterations could be at the basis of anomalous self-experiences commonly reported in schizophrenia, like ownership of experience and agency of action.104,107,108 Regarding the social domain, insula dysfunction also could lead to difficulties in experiencing the self as distinct from the other.21,103 For example, patients with schizophrenia may experience confusion in the attribution of internal sensory information to an external source or vice versa reflecting a blurred distinction between self and other.6–8
Finally, although in our previous study we provided evidence for impaired functioning of vPMC and pIC during social perception,21 still little is known about how they might relate to each other. Aberrant functional connectivity of these regions has been implicated in schizophrenic pathology by previous studies.33 Here we show that altered functional interactions of pIC and vPMC converged on PCC. Relevantly, PCC represents a cortical hub in the human brain mediating between activity in distinct networks.35,38,39 As described above, on the one hand, dorsal anterior PCC has strong connections within the sensory network including pIC and PostCG.47 On the other hand, it shows an antagonistic relationship with vPMC60 involved in peripersonal space representation and goal-directed behavior.81,109 Both relationships appear anomalous in FES. Therefore, the present results could reflect a neural mechanism underlying a deregulated interaction between an afflicted sensory-affective self and its environment with a central role of PCC.
Some additional issues need to be mentioned. First, the present fMRI data did not include a task-free condition. Possibly, conditions with systematic task demands modulate functional coupling.50,62 It is recommended for further studies to include task-free periods for elucidating whether aberrant functional interaction patterns in FES reflect an intrinsic, neural predisposition of the brain to abnormally react to social stimuli.110–112 Nevertheless, given the regression of task-related BOLD signal and rigorous control analyses, we argue that it is very unlikely that the observed effects can be explained by task-evoked BOLD responses. Analysis of background functional interactions rather could provide information complementary to task-evoked BOLD responses in terms of brain long-range communication.
Second, no relationship was detected between brain long-range communication and positive and negative symptoms, and correlation between altered functional interaction and basic symptoms was independent of positive and negative symptoms. This may underscore the relevance and specificity of basic symptoms, tapping into distinct neural mechanisms. A possible alternative explanation is that the included patients had a very recent illness onset and relatively low PANSS scores. Further studies will be needed to investigate patients with more pronounced positive and negative symptoms and chronic samples for a better understanding of the relationship between psychotic symptoms and its progress over time.
Third, some previous studies showed that antipsychotic medication influenced functional connectivity of cortical networks,113,114 suggesting that medication might have contributed to the results in this study. However, covariance and correlation analyses showed that altered functional interaction patterns in the FES group and their relation to symptomatology were independent of antipsychotic medication dosage. Moreover, the FES patients were treated by a single atypical antipsychotic drug for a relative short time. Therefore, we argue that it is unlikely that the presented results can be attributed to medication effects.
Finally, although correlations with particular symptoms suggest that some alterations in functional interaction patterns are related to specific aspects of schizophrenic pathology, optimally an additional psychiatric, nonschizophrenic control group would have been needed to obtain greater specificity in associating the present results with FES.
In conclusion, this study demonstrates altered neurofunctional interactions of vPMC and pIC in FES, compared with HC, as reflected by a reduced segregation between and a reduced integration within functional networks associated with sensory-affective processing, self-awareness, and interaction with the external world (figure 3). The same networks also have important functions in social cognition and interaction. The results suggest imbalance in the processing between internally and externally guided information and its abnormal integration with self-referential processing as mediated by the PCC. This imbalance could be closely related to anomalous self-experience in FES.
Fig. 3.
Schematic representation of the general study results.
Supplementary Material
Supplementary material is available at http://schizophre niabulletin.oxfordjournals.org.
Funding
EU grant Towards an Embodied Science of Inter Subjec- tivity to V.G.; Swiss National Science Foundation project 320030_146531 to D.M.; FP7 Marie Curie Career Integration (PCIG12-334039 to D.M.).
Supplementary Material
Acknowledgments
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Bleuler E. Dementia praecox oder Gruppe der Schizophrenien. In: Aschaffenburg G, ed. Handbuch der Psychiatrie. Leipzig, Germany: Deuticke; 1911 [Google Scholar]
- 2. Minkowski E. La schizophrénie. Psychopathologie des schizoïdes et des schizophrènes. Paris, France: Payot; 1927 [Google Scholar]
- 3. Parnas J, Bovet P, Zahavi D. Schizophrenic autism: clinical phenomenology and pathogenetic implications. World Psychiatry. 2002;1:131–136 [PMC free article] [PubMed] [Google Scholar]
- 4. Parnas J, Møller P, Kircher T, et al. EASE: Examination of Anomalous Self-Experience. Psychopathology. 2005;38: 236–258 [DOI] [PubMed] [Google Scholar]
- 5. Gallese V. The roots of empathy: the shared manifold hypothesis and the neural basis of intersubjectivity. Psychopathology. 2003;36:171–180 [DOI] [PubMed] [Google Scholar]
- 6. Sass LA, Parnas J. Schizophrenia, consciousness, and the self. Schizophr Bull. 2003;29:427–444 [DOI] [PubMed] [Google Scholar]
- 7. Sass LA. Self-disturbance and schizophrenia: structure, specificity, pathogenesis (Current issues, New directions). [published online ahead of print June 14, 2013]. Schizophr Res. 2013. pii S0920–9964(13)00272–7. 10.1016/j.schres.2013.05.017 [DOI] [PubMed] [Google Scholar]
- 8. Nelson B, Fornito A, Harrison BJ, et al. A disturbed sense of self in the psychosis prodrome: linking phenomenology and neurobiology. Neurosci Biobehav Rev. 2009;33:807–817 [DOI] [PubMed] [Google Scholar]
- 9. Huber G, Gross G. The concept of basic symptoms in schizophrenic and schizoaffective psychoses. Recenti Prog Med. 1989;80:646–652 [PubMed] [Google Scholar]
- 10. Klosterkötter J. The meaning of basic symptoms for the genesis of the schizophrenic nuclear syndrome. Jpn J Psychiatry Neurol. 1992;46:609–630 [DOI] [PubMed] [Google Scholar]
- 11. Ihnen GH, Penn DL, Corrigan PW, Martin J. Social perception and social skill in schizophrenia. Psychiatry Res. 1998;80:275–286 [DOI] [PubMed] [Google Scholar]
- 12. Bentall RP, Baker GA, Havers S. Reality monitoring and psychotic hallucinations. Br J Clin Psychol. 1991;30(Pt 3):213–222 [DOI] [PubMed] [Google Scholar]
- 13. Georgieff N, Jeannerod M. Beyond consciousness of external reality: a “who” system for consciousness of action and self-consciousness. Conscious Cogn. 1998;7:465–477 [DOI] [PubMed] [Google Scholar]
- 14. Blakemore SJ, Smith J, Steel R, Johnstone CE, Frith CD. The perception of self-produced sensory stimuli in patients with auditory hallucinations and passivity experiences: evidence for a breakdown in self-monitoring. Psychol Med. 2000;30:1131–1139 [DOI] [PubMed] [Google Scholar]
- 15. Franck N, Farrer C, Georgieff N, et al. Defective recognition of one’s own actions in patients with schizophrenia. Am J Psychiatry. 2001;158:454–459 [DOI] [PubMed] [Google Scholar]
- 16. Vinogradov S, Luks TL, Schulman BJ, Simpson GV. Deficit in a neural correlate of reality monitoring in schizophrenia patients. Cereb Cortex. 2008;18:2532–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Voss M, Moore J, Hauser M, Gallinat J, Heinz A, Haggard P. Altered awareness of action in schizophrenia: a specific deficit in predicting action consequences. Brain. 2010;133:3104–3112 [DOI] [PubMed] [Google Scholar]
- 18. Botvinick M, Cohen J. Rubber hands ‘feel’ touch that eyes see. Nature. 1998;391:756. [DOI] [PubMed] [Google Scholar]
- 19. Peled A, Ritsner M, Hirschmann S, Geva AB, Modai I. Touch feel illusion in schizophrenic patients. Biol Psychiatry. 2000;48:1105–1108 [DOI] [PubMed] [Google Scholar]
- 20. Morgan HL, Turner DC, Corlett PR, et al. Exploring the impact of ketamine on the experience of illusory body ownership. Biol Psychiatry. 2011;69:35–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ebisch SJ, Salone A, Ferri F, et al. Out of touch with reality? Social perception in first-episode schizophrenia. Soc Cogn Affect Neurosci. 2013;8:394–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Volkow ND, Wolf AP, Brodie JD, et al. Brain interactions in chronic schizophrenics under resting and activation conditions. Schizophr Res. 1988;1:47–53 [DOI] [PubMed] [Google Scholar]
- 23. Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin Neurosci. 1995;3:89–97 [PubMed] [Google Scholar]
- 24. Bullmore ET, Frangou S, Murray RM. The dysplastic net hypothesis: an integration of developmental and dysconnectivity theories of schizophrenia. Schizophr Res. 1997;28:143–156 [DOI] [PubMed] [Google Scholar]
- 25. Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci. 2006;7:818–827 [DOI] [PubMed] [Google Scholar]
- 26. Fornito A, Zalesky A, Pantelis C, Bullmore ET. Schizophrenia, neuroimaging and connectomics. Neuroimage. 2012;62:2296–2314 [DOI] [PubMed] [Google Scholar]
- 27. Pettersson-Yeo W, Allen P, Benetti S, McGuire P, Mechelli A. Dysconnectivity in schizophrenia: where are we now? Neurosci Biobehav Rev. 2011;35:1110–1124 [DOI] [PubMed] [Google Scholar]
- 28. Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113 [DOI] [PubMed] [Google Scholar]
- 29. Calhoun VD, Eichele T, Pearlson G. Functional brain networks in schizophrenia: a review. Front Hum Neurosci. 2009;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Woodward ND, Rogers B, Heckers S. Functional resting-state networks are differentially affected in schizophrenia. Schizophr Res. 2011;130:86–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Walterfang M, Wood SJ, Velakoulis D, Pantelis C. Neuropathological, neurogenetic and neuroimaging evidence for white matter pathology in schizophrenia. Neurosci Biobehav Rev. 2006;30:918–948 [DOI] [PubMed] [Google Scholar]
- 32. Skudlarski P, Jagannathan K, Anderson K, et al. Brain connectivity is not only lower but different in schizophrenia: a combined anatomical and functional approach. Biol Psychiatry. 2010;68:61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lynall ME, Bassett DS, Kerwin R, et al. Functional connectivity and brain networks in schizophrenia. J Neurosci. 2010;30:9477–9487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. de Pasquale F, Sabatini U, Della Penna S, et al. The connectivity of functional cores reveals different degrees of segregation and integration in the brain at rest. Neuroimage. 2013;69:51–61 [DOI] [PubMed] [Google Scholar]
- 35. Hagmann P, Cammoun L, Gigandet X, et al. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6:e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38 [DOI] [PubMed] [Google Scholar]
- 37. Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. de Pasquale F, Della Penna S, Snyder AZ, et al. A cortical core for dynamic integration of functional networks in the resting human brain. Neuron. 2012;74:753–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Uddin LQ, Kelly AM, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum Brain Mapp. 2009;30:625–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Northoff G, Richter A, Bermpohl F, et al. NMDA hypofunction in the posterior cingulate as a model for schizophrenia: an exploratory ketamine administration study in fMRI. Schizophr Res. 2005;72:235–248 [DOI] [PubMed] [Google Scholar]
- 41. Nelson B, Fornito A, Harrison BJ, et al. A disturbed sense of self in the psychosis prodrome: linking phenomenology and neurobiology. Neurosci Biobehav Rev. 2009;33:807–817 [DOI] [PubMed] [Google Scholar]
- 42. Qin P, Northoff G. How is our self related to midline regions and the default-mode network? Neuroimage. 2011;57:1221–1233 [DOI] [PubMed] [Google Scholar]
- 43. Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain–a meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–457 [DOI] [PubMed] [Google Scholar]
- 44. Northoff G, Qin P, Nakao T. Rest-stimulus interaction in the brain: a review. Trends Neurosci. 2010;33:277–284 [DOI] [PubMed] [Google Scholar]
- 45. Johnson MK, Raye CL, Mitchell KJ, Touryan SR, Greene EJ, Nolen-Hoeksema S. Dissociating medial frontal and posterior cingulate activity during self-reflection. Soc Cogn Affect Neurosci. 2006;1:56–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Northoff G, Bermpohl F. Cortical midline structures and the self. Trends Cogn Sci. 2004;8:102–107 [DOI] [PubMed] [Google Scholar]
- 47. Leech R, Braga R, Sharp DJ. Echoes of the brain within the posterior cingulate cortex. J Neurosci. 2012;32:215–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wiebking C, Duncan NW, Qin P, et al. External awareness and GABA-A multimodal imaging study combining fMRI and [(18) F]flumazenil-PET. [published online ahead of print September 21, 2012]. Hum Brain Mapp. 2012. doi: 10.1002/hbm.22166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Northoff G, Panksepp J. The trans-species concept of self and the subcortical-cortical midline system. Trends Cogn Sci. 2008;12:259–264 [DOI] [PubMed] [Google Scholar]
- 50. Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 2010;103:297–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711 [DOI] [PubMed] [Google Scholar]
- 52. Ebisch SJ, Mantini D, Romanelli R, et al. Long-range functional interactions of anterior insula and medial frontal cortex are differently modulated by visuospatial and inductive reasoning tasks. Neuroimage. 2013;78:426–438 [DOI] [PubMed] [Google Scholar]
- 53. Norman-Haignere SV, McCarthy G, Chun MM, Turk-Browne NB. Category-selective background connectivity in ventral visual cortex. Cereb Cortex. 2012;22:391–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276 [DOI] [PubMed] [Google Scholar]
- 55. Klosterkötter J, Hellmich M, Steinmeyer EM, Schultze-Lutter F. Diagnosing schizophrenia in the initial prodromal phase. Arch Gen Psychiatry. 2001;58:158–164 [DOI] [PubMed] [Google Scholar]
- 56. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276 [DOI] [PubMed] [Google Scholar]
- 57. Schultze-Lutter F, Addington J, Ruhrmann S, Klosterkötter J. Strumento di valutazione per la propensione alla schizofrenia. Versione per adulti. Trad. Giuliano Aiello. Rome, Italy: Giovanni Fioriti Editore srl; 2011 [Google Scholar]
- 58. Auer DP. Spontaneous low-frequency blood oxygenation level-dependent fluctuations and functional connectivity analysis of the ‘resting’ brain. Magn Reson Imaging. 2008;26:1055–1064 [DOI] [PubMed] [Google Scholar]
- 59. Cordes D, Haughton VM, Arfanakis K, et al. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol. 2001;22:1326–1333 [PMC free article] [PubMed] [Google Scholar]
- 60. Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101:3270–3283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron. 2007;53:905–918 [DOI] [PubMed] [Google Scholar]
- 62. Fair DA, Schlaggar BL, Cohen AL, et al. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage. 2007;35:396–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ebisch SJ, Gallese V, Willems RM, et al. Altered intrinsic functional connectivity of anterior and posterior insula regions in high-functioning participants with autism spectrum disorder. Hum Brain Mapp. 2011;32:1013–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zar JH. Biostatistical Analysis. Upper Saddle River, NJ: Prentice-Hall; 1996 [Google Scholar]
- 66. Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878 [DOI] [PubMed] [Google Scholar]
- 67. Mantini D, Gerits A, Nelissen K, et al. Default mode of brain function in monkeys. J Neurosci. 2011;31:12954–12962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Huang Z, Dai R, Wu X, et al. The self and its resting state in consciousness: An investigation of the vegetative state. [published online ahead of print July 01, 2013]. Hum Brain Mapp. 2013. doi: 10.1002/hbm.22308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zuo XN, Di Martino A, Kelly C, et al. The oscillating brain: complex and reliable. Neuroimage. 2010;49:1432–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bluhm RL, Miller J, Lanius RA, et al. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull. 2007;33:1004–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mannell MV, Franco AR, Calhoun VD, Cañive JM, Thoma RJ, Mayer AR. Resting state and task-induced deactivation: A methodological comparison in patients with schizophrenia and healthy controls. Hum Brain Mapp. 2010;31:424–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Skudlarski P, Jagannathan K, Anderson K, et al. Brain connectivity is not only lower but different in schizophrenia: a combined anatomical and functional approach. Biol Psychiatry. 2010;68:61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mazza M, Catalucci A, Pino MC, et al. Dysfunctional neural networks associated with impaired social interactions in early psychosis: an ICA analysis. [published online ahead of print March 12, 2013]. Brain Imaging Behav. 2013. doi: 10.1007/s11682-013-9223-6. [DOI] [PubMed] [Google Scholar]
- 74. Hampson M, Peterson BS, Skudlarski P, Gatenby JC, Gore JC. Detection of functional connectivity using temporal correlations in MR images. Hum Brain Mapp. 2002;15:247–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fogassi L, Gallese V, Fadiga L, Luppino G, Matelli M, Rizzolatti G. Coding of peripersonal space in inferior premotor cortex (area F4). J Neurophysiol. 1996;76:141–157 [DOI] [PubMed] [Google Scholar]
- 77. Graziano MS. Neuroscience. Awareness of space. Nature. 2001;411:903–904 [DOI] [PubMed] [Google Scholar]
- 78. Rizzolatti G, Fogassi L, Gallese V. Motor and cognitive functions of the ventral premotor cortex. Curr Opin Neurobiol. 2002;12:149–154 [DOI] [PubMed] [Google Scholar]
- 79. Bremmer F, Schlack A, Shah NJ, et al. Polymodal motion processing in posterior parietal and premotor cortex: a human fMRI study strongly implies equivalencies between humans and monkeys. Neuron. 2001;29:287–296 [DOI] [PubMed] [Google Scholar]
- 80. Galati G, Committeri G, Sanes JN, Pizzamiglio L. Spatial coding of visual and somatic sensory information in body-centred coordinates. Eur J Neurosci. 2001;14:737–746 [DOI] [PubMed] [Google Scholar]
- 81. Serino A, Canzoneri E, Avenanti A. Fronto-parietal areas necessary for a multisensory representation of peripersonal space in humans: an rTMS study. J Cogn Neurosci. 2011;23:2956–2967 [DOI] [PubMed] [Google Scholar]
- 82. Newell KA, Zavitsanou K, Jew SK, Huang XF. Alterations of muscarinic and GABA receptor binding in the posterior cingulate cortex in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:225–233 [DOI] [PubMed] [Google Scholar]
- 83. Caggiano V, Fogassi L, Rizzolatti G, Thier P, Casile A. Mirror neurons differentially encode the peripersonal and extrapersonal space of monkeys. Science. 2009;324:403–406 [DOI] [PubMed] [Google Scholar]
- 84. Uddin LQ, Iacoboni M, Lange C, Keenan JP. The self and social cognition: the role of cortical midline structures and mirror neurons. Trends Cogn Sci. 2007;11:153–157 [DOI] [PubMed] [Google Scholar]
- 85. Gallese V. The manifold nature of interpersonal relations: the quest for a common mechanism. Philos Trans R Soc Lond B Biol Sci. 2003;358:517–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Parnas J. From predisposition to psychosis: progression of symptoms in schizophrenia. Acta Psychiatr Scand. 1999;395:20–29 [DOI] [PubMed] [Google Scholar]
- 87. Klosterkötter J, Hellmich M, Steinmeyer EM, Schultze-Lutter F. Diagnosing schizophrenia in the initial prodromal phase. Arch Gen Psychiatry. 2001;58:158–164 [DOI] [PubMed] [Google Scholar]
- 88. Schultze-Lutter F. Subjective symptoms of schizophrenia in research and the clinic: the basic symptom concept. Schizophr Bull. 2009;35:5–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Schultze-Lutter F. Subjective symptoms of schizophrenia in research and the clinic: the basic symptom concept. Schizophr Bull. 2009;35:5–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666 [DOI] [PubMed] [Google Scholar]
- 91. Tsakiris M, Hesse MD, Boy C, Haggard P, Fink GR. Neural signatures of body ownership: a sensory network for bodily self-consciousness. Cereb Cortex. 2007;17:2235–2244 [DOI] [PubMed] [Google Scholar]
- 92. Karnath HO, Baier B, Nägele T. Awareness of the functioning of one’s own limbs mediated by the insular cortex? J Neurosci. 2005;25:7134–7138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Farrer C, Franck N, Georgieff N, Frith CD, Decety J, Jeannerod M. Modulating the experience of agency: a positron emission tomography study. Neuroimage. 2003;18:324–333 [DOI] [PubMed] [Google Scholar]
- 94. Heydrich L, Blanke O. Distinct illusory own-body perceptions caused by damage to posterior insula and extrastriate cortex. Brain. 2013;136:790–803 [DOI] [PubMed] [Google Scholar]
- 95. Craig AD. How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70 [DOI] [PubMed] [Google Scholar]
- 96. Ebisch SJ, Ferri F, Salone A, et al. Differential involvement of somatosensory and interoceptive cortices during the observation of affective touch. J Cogn Neurosci. 2011;23:1808–1822 [DOI] [PubMed] [Google Scholar]
- 97. Taylor KS, Seminowicz DA, Davis KD. Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp. 2009;30:2731–2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb Cortex. 2013;23:739–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Deen B, Pitskel NB, Pelphrey KA. Three systems of insular functional connectivity identified with cluster analysis. Cereb Cortex. 2011;21:1498–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010;214:519–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195 [DOI] [PubMed] [Google Scholar]
- 102. Damasio AR, Grabowski TJ, Bechara A, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000;3:1049–1056 [DOI] [PubMed] [Google Scholar]
- 103. Wylie KP, Tregellas JR. The role of the insula in schizophrenia. Schizophr Res. 2010;123:93–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Nelson B, Whitford TJ, Lavoie S, Sass LA. What are the neurocognitive correlates of basic self-disturbance in schizophrenia? Integrating phenomenology and neurocognition. Source monitoring deficits. Schizophr Res. 2013. (Pt 1). pii: S0920–9964(13)00333–2. doi: 10.1016/j.schres.2013.06.033 [DOI] [PubMed] [Google Scholar]
- 105. Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev. 1996;22:229–244 [DOI] [PubMed] [Google Scholar]
- 106. Mesulam MM, Mufson EJ. Insula of the old world monkey. I. Architectonics in the insulo-orbito-temporal component of the paralimbic brain. J Comp Neurol. 1982;212:1–22 [DOI] [PubMed] [Google Scholar]
- 107. Sass LA, Parnas J. Schizophrenia, consciousness, and the self. Schizophr Bull. 2003;29:427–444 [DOI] [PubMed] [Google Scholar]
- 108. Farrer C, Franck N, Frith CD, et al. Neural correlates of action attribution in schizophrenia. Psychiatry Res. 2004;131:31–44 [DOI] [PubMed] [Google Scholar]
- 109. Rizzolatti G, Fogassi L, Gallese V. Motor and cognitive functions of the ventral premotor cortex. Curr Opin Neurobiol. 2002;12:149–154 [DOI] [PubMed] [Google Scholar]
- 110. Northoff G. What the brain’s intrinsic activity can tell us about consciousness? A tri-dimensional view. Neurosci Biobehav Rev. 2013;37:726–738 [DOI] [PubMed] [Google Scholar]
- 111. Northoff G. Unlocking the Brain. Volume 1: Coding. Oxford, UK: Oxford University Press; 2013 [Google Scholar]
- 112. Northoff G. Unlocking the brain. Volume 2: Consciousness. Oxford, UK: Oxford University Press; 2013 [Google Scholar]
- 113. Lui S, Li T, Deng W, et al. Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Arch Gen Psychiatry. 2010;67:783–792 [DOI] [PubMed] [Google Scholar]
- 114. Sambataro F, Blasi G, Fazio L, et al. Treatment with olanzapine is associated with modulation of the default mode network in patients with Schizophrenia. Neuropsychopharmacology. 2010;35:904–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.