Abstract
Previous studies suggest that elevated blood homocysteine levels and the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism are risk factors for schizophrenia. However, the effects of gender and MTHFR C677T genotypes on blood homocysteine levels in schizophrenia have not been consistent. We first investigated whether plasma total homocysteine levels were higher in patients with schizophrenia than in controls with stratification by gender and by the MTHFR C677T genotypes in a large cohort (N = 1379). Second, we conducted a meta-analysis of association studies between blood homocysteine levels and schizophrenia separately by gender (N = 4714). Third, we performed a case-control association study between the MTHFR C677T polymorphism and schizophrenia (N = 4998) and conducted a meta-analysis of genetic association studies based on Japanese subjects (N = 10 378). Finally, we assessed the effect of plasma total homocysteine levels on schizophrenia by a mendelian randomization approach. The ANCOVA after adjustment for age demonstrated a significant effect of diagnosis on the plasma total homocysteine levels in all strata, and the subsequent meta-analysis for gender demonstrated elevated blood homocysteine levels in both male and female patients with schizophrenia although antipsychotic medication might influence the outcome. The meta-analysis of the Japanese genetic association studies demonstrated a significant association between the MTHFR C677T polymorphism and schizophrenia. The mendelian randomization analysis in the Japanese populations yielded an OR of 1.15 for schizophrenia per 1-SD increase in plasma total homocysteine. Our study suggests that increased plasma total homocysteine levels may be associated with an increased risk of schizophrenia.
Key words: mendelian randomization, SNP, Japanese, plasma homocysteine
Introduction
Schizophrenia is a devastating psychiatric disorder with a median lifetime prevalence rate of 0.7%–0.8%.1 Accumulating evidence has shown that alterations in 1-carbon metabolism might play an important role in the pathogenesis of schizophrenia.2,3 A number of studies have been conducted to evaluate the association between blood homocysteine levels and schizophrenia. The majority of these studies have demonstrated elevated blood homocysteine levels in patients with schizophrenia compared with controls.4–24 However, several studies have reported no significant diagnostic differences in the blood homocysteine levels between the 2 groups.25–31
To date, 1 study has examined an association between blood homocysteine levels and schizophrenia by conducting a meta-analysis of 8 case-control studies (a total number of 812 cases with schizophrenia and 2113 control subjects) and demonstrated that a 5 μmol/l increase in homocysteine concentration was associated with a higher risk of schizophrenia (OR = 1.7; 95% CI = 1.27–2.29).32 However, this meta-analysis was performed without consideration of the effect of gender. Higher blood homocysteine levels in men than in women have been reported,33 and the results of the previous association studies between blood homocysteine levels and schizophrenia with stratification by gender are inconclusive. In some studies, elevated blood homocysteine levels were observed in only male patients with schizophrenia and not in female patients,5,6,10,19 whereas other studies have demonstrated that both male and female patients with schizophrenia had increased blood homocysteine levels.11,17
Blood homocysteine levels are also influenced by genetic variations.34,35 Among these variants, 1 common functional single nucleotide polymorphism (SNP) of the methylenetetrahydrofolate reductase (MTHFR) gene, C677T (rs1801133), has been investigated well. The MTHFR C677T polymorphism results in amino acid substitution (Ala222Val) and causes a reduction of enzyme activity and higher homocysteine levels.36 The results of the previous association studies between blood homocysteine levels and schizophrenia with stratification by C677T genotypes are inconclusive. A significant diagnostic difference in blood homocysteine levels has been found only in the subjects carrying the CT genotype or only in the subjects carrying the TT genotype.7,18 However, Feng et al16 showed a significant diagnostic difference for both CT and TT genotype carriers.
Many genetic case-control association studies between the MTHFR C677T polymorphism and schizophrenia have been performed in various populations, and the results of these association studies are not consistent. Only 1 study of the Japanese population reported a significant association between the MTHFR C677T polymorphism and schizophrenia, while the other 3 studies of the Japanese population have not replicated this positive finding.37–40 However, several meta-analyses of association studies have revealed a significant association between this SNP and schizophrenia.39,41–47
In this study, we first investigated whether plasma total homocysteine levels were higher in patients with schizophrenia than in nonpsychiatric controls with stratification by gender and by the MTHFR C677T genotypes in a large cohort (N = 1379). Second, we conducted a meta-analysis of association studies between the blood homocysteine levels and schizophrenia separately by gender to evaluate a precise estimation of the association (N = 4714). Third, we performed a case-control association study between the MTHFR C677T polymorphism and schizophrenia (N = 4998) and carried out a meta-analysis of genetic association studies of this SNP with schizophrenia based on Japanese subjects to determine whether the MTHFR C677T polymorphism was genetically implicated in schizophrenia in the Japanese population (N = 10 378). Finally, we assessed the effect of plasma total homocysteine levels on schizophrenia by a mendelian randomization (MR) approach, a useful tool for assessing causal associations in observational data.48,49
Methods
Subjects of the Association Study Between the Plasma Total Homocysteine Levels and Schizophrenia
Three hundred and eighty-one patients with schizophrenia (225 men, mean age: 58.2±9.3 y; 156 women, mean age: 59.4±9.7 y) were recruited from Tokushima University Hospital in Japan. The diagnosis of schizophrenia was made according to Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV criteria by at least 2 expert psychiatrists on the basis of extensive clinical interviews and a review of medical records. None of the patients had any psychiatric comorbidity or cardiovascular diseases. All patients were treated with various antipsychotic drugs. The mean chlorpromazine equivalent dose was 689.6±581.3mg/d. Nine hundred and ninety-eight control subjects (331 men, mean age: 38.3±12 y; 667 women, mean age: 43.0±11.9 y) were selected from volunteers who were recruited from hospital staff, students, and company employees documented to be free from psychiatric problems and past histories of mental illness. All subjects who participated in this study were of Japanese origin. All subjects signed written informed consent approved by the institutional ethics committees of the University of Tokushima Graduate School. Of 1379 subjects used in this association study, 1357 with genomic DNA (377 patients and 980 controls) were used in the next genetic association study.
Subjects of the Association Study Between the MTHFR C677T Polymorphism and Schizophrenia
Two case-control sets were used: the Tokushima sample set (A southern island of Japan) and the Osaka sample set (Midwestern Japan). Both sets have been described in previous studies.50,51 For the Tokushima sample set, 1149 patients with schizophrenia (676 males, 473 females, mean age: 54.6±14.9 y) were recruited from the Tokushima and Kochi University Hospitals in Japan. The diagnosis of schizophrenia was made according to DSM-IV criteria. A total of 2742 control subjects (1230 males, 1512 females, mean age: 38.8±12.6 y) were selected from volunteers. For the Osaka sample set, 621 patients with schizophrenia (302 males, 319 females, mean age: 46.5±15.8 y) were recruited from Osaka University Hospital in Japan. The diagnosis of schizophrenia was made according to DSM-IV criteria. A total of 486 control subjects (231 males, 255 females, mean age: 35.0±12.7 y) were selected from volunteers. All subjects signed written informed consent approved by the institutional ethics committees of the University of Tokushima Graduate School, Kochi Medical School, and University of Osaka Graduate School.
Plasma Total Homocysteine Analysis
Plasma total homocysteine levels were measured by high-performance liquid chromatography. Homocysteine was labeled with 4-fluoro-7-sulfamoylbenzofurazan and detected by a fluorescent detector according to the method of a previous study.52
MTHFR Genotyping
We genotyped the MTHFR C677T polymorphism by using a commercially available TaqMan probe with the Applied Biosystems 7500 Fast Real Time PCR System, according to the protocol recommended by the manufacturer (Applied Biosystems, California, USA). Twelve percent of the genotypes were genotyped again, and there were no mismatches between the 2 genotyping steps.
Study Selection for the Meta-analysis of Association Studies Between Blood Homocysteine Levels and Schizophrenia
Eligible studies were identified using the PubMed search engine with the terms “homocysteine,” “hyperhomocysteinemia,” and “schizophrenia.” We also conducted an additional manual search of reference lists and review articles. Studies meeting the following criteria were included for further meta-analysis: (1) included laboratory assessment of serum or plasma homocysteine levels, (2) performed a case-control study (schizophrenia vs control), (3) provided raw data of homocysteine levels separately by gender, and (4) published in an English language. The 2 reviewers (N.S. and K.K.) selected the articles independently according to the inclusion criteria, and then discussed the articles until they reached a consensus on every study used for the meta-analysis.
Statistical Methods
A linear regression analysis was used to examine the effects of diagnosis, age, gender, and the MTHFR C677T genotypes on the plasma total homocysteine. An ANCOVA was performed to examine the presence of the differences in the plasma total homocysteine between the 2 groups (schizophrenia vs control) separately by gender and by the 3 MTHFR C677T genotypes (total of 6 strata). Allelic and genotypic frequencies of the MTHFR C677T polymorphism in patients and control subjects were compared using the χ2 test. In order to quantify the strength of association between plasma total homocysteine and schizophrenia, an MR approach was used, as in a previous study.53 The risk estimate in gene-schizophrenia association for the TT genotypes of the MTHFR C677T polymorphism (vs the CC genotypes; ORSCZ/TT) was from the current meta-analysis of the Japanese genetic association studies. For gene-homocysteine association, the effect of the TT genotypes (vs the CC genotypes) on plasma total homocysteine levels (betahcy/TT) was estimated using the Japanese control subjects from the Tokushima homocysteine study under a multivariate linear regression model including age and gender as covariates. The effect for the TT genotypes in the gene-homocysteine association was expressed as 1-SD increase in plasma total homocysteine. From these 2 estimates, an MR estimate of the effect of plasma total homocysteine on the risk of schizophrenia (ORSCZ/hcy) was calculated as follows: log ORSCZ/hcy = (log ORSCZ/TT)/betahcy/TT. The MR estimate represented the OR for schizophrenia risk per 1-SD increase in plasma total homocysteine. The standard error (SE) of the MR estimate was calculated by the Delta method.54,55
Meta-analysis
The meta-analysis of association studies between the blood homocysteine levels and schizophrenia was performed on the standardized mean differences (SMD). The meta-analysis of association studies between the MTHFR C677T polymorphism and schizophrenia was performed for 5 genetic models, recessive (CC/CT vs TT genotypes), dominant (CC vs CT/TT genotypes), codominant (CC vs TT genotypes), codominant (CT vs TT genotypes), and allele frequency (C-allele vs T-allele), as had been done in a previous study.45 Heterogeneity was assessed using the I 2 statistic. If heterogeneity across studies was found, then a random-effects model was applied; otherwise, a fixed-effects model was applied. Publication bias was assessed using funnel plots and a regression test.56 OR and 95% CI were calculated by “metafor,” an R package.
Results
Differences in the Plasma Total Homocysteine Levels Between Patients With Schizophrenia and Controls
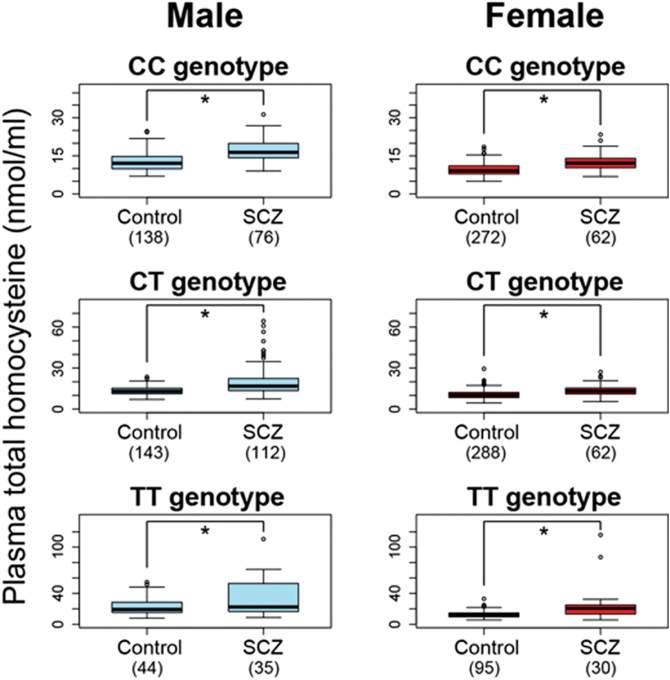
A linear regression analysis showed significant effects of diagnosis (higher in schizophrenic patients than in controls), MTHFR C677T genotypes (higher in CT carriers than in CC carriers, and higher in TT carriers than in CC carriers), age (decreases with age), and gender (higher in males than in females) on the plasma total homocysteine levels (diagnosis P = 3.4 × 10–29, genotype P [CT vs CC] = 4.7 × 10–3, genotype P [TT vs CC] = 5.8 × 10–43, age P = 1.0 × 10–2, and gender P = 1.3 × 10–26). Next, an ANCOVA was performed to examine the presence of the differences between the 2 groups in the plasma total homocysteine separately by gender and by the 3 MTHFR C677T genotypes (total of 6 strata), and a significant effect of diagnosis (higher in patients with schizophrenia than in the control) was still observed in all strata (diagnosis P of male-genotype CC = 2.4 × 10–8, diagnosis P of male-genotype CT = 3.2 × 10−10, diagnosis P of male-genotype TT = 2.3 × 10–4, diagnosis P of female-genotype CC = 1.1 × 10–8, diagnosis P of female-genotype CT = 3.2 × 10–8, and diagnosis P of female-genotype TT = 1.2 × 10–5, respectively) after adjustment for age (figure 1).
Fig. 1.
Differences in the plasma total homocysteine levels between patients with schizophrenia and controls separately by gender and by the methylenetetrahydrofolate reductase (MTHFR)C677T genotypes. The ANCOVA demonstrated that the plasma total homocysteine levels were significantly higher in patients with schizophrenia than in controls in each of the 6 strata (*age-adjusted P < .001).
A Meta-analysis of the Blood Homocysteine Levels in Schizophrenia
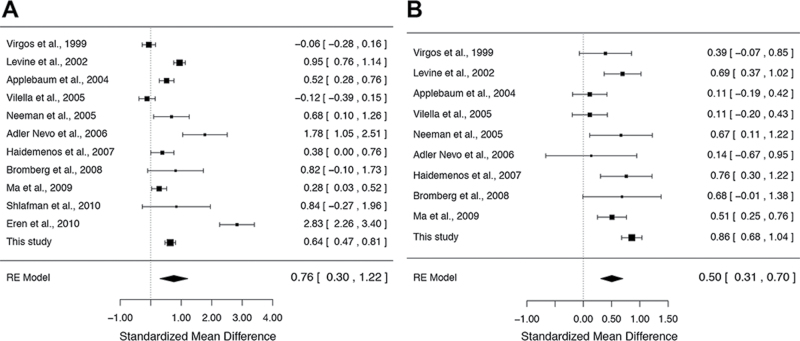
We performed a meta-analysis of previous association studies between the blood homocysteine levels and schizophrenia separately by gender. The studies included in this meta-analysis are shown in the supplementary table 1. For the meta-analysis of males, data were obtained from 12 association studies,5,6,8,10,11,13,17,20,21,25,28 including our data, for a total of 1079 patients with schizophrenia and 1559 control subjects. As shown in figure 2A, the random-effects model showed that the blood homocysteine levels were significantly higher in male patients with schizophrenia than in the male controls (SMD = 0.76; 95% CI = 0.30–1.22; P = 1.2 × 10–3) with significant heterogeneity among studies (I 2 = 96.3%; P < .05). The funnel plot analysis indicated no evidence of publication bias in the male association studies (P = .13; supplementary figure 1). For the meta-analysis of females, data were obtained from 10 association studies,5,6,8,10,11,13,17,25,28 including our data, for a total of 615 patients with schizophrenia and 1461 control subjects. As shown in figure 2B, the random-effects model showed that the blood homocysteine levels were significantly higher in female patients with schizophrenia than in the female controls (SMD = 0.50; 95% CI = 0.31–0.70; P = 5.9 × 10–7) with significant heterogeneity among the studies (I 2 = 65.7%; P < .05). The funnel plot analysis indicated no evidence of publication bias in the female association studies (P = .73; supplementary figure 2).
Fig. 2.
Meta-analyses of association studies between the blood homocysteine levels and schizophrenia. (A) The result of the meta-analysis of 12 male association studies (N = 2638). The blood homocysteine levels were significantly higher in male patients with schizophrenia than in the male controls (standardized mean difference [SMD] = 0.76; 95% CI = 0.30–1.22; P = 1.2×10–3 in the random-effects model). (B) The result of the meta-analysis of 10 female association studies (N = 2076). The blood homocysteine levels were significantly higher in female patients with schizophrenia than in the female controls (SMD = 0.50; 95% CI = 0.31–0.70; P = 5.9×10–7 in the random-effects model).
A Case-Control Association Study Between the MTHFR C677T Polymorphism and Schizophrenia
Two case-control data sets were evaluated: one is the Tokushima sample set (case = 1149, control = 2742), and the other is the Osaka sample set (case = 621, control = 486). The genotypic distributions of rs1801133 did not deviate significantly from the Hardy-Weinberg equilibrium (HWE) in the control groups of these 2 sample sets (P > .05). Significant difference was observed between the controls and patients with schizophrenia in the allelic frequencies of the Tokushima sample set (P = .025). On the other hand, no significant differences were observed in the genotypic and allelic frequencies of the Osaka sample set (genotype P = .98, and allele P = .97, respectively).
A Meta-analysis of Genetic Association Studies Between the MTHFR C677T Polymorphism and Schizophrenia
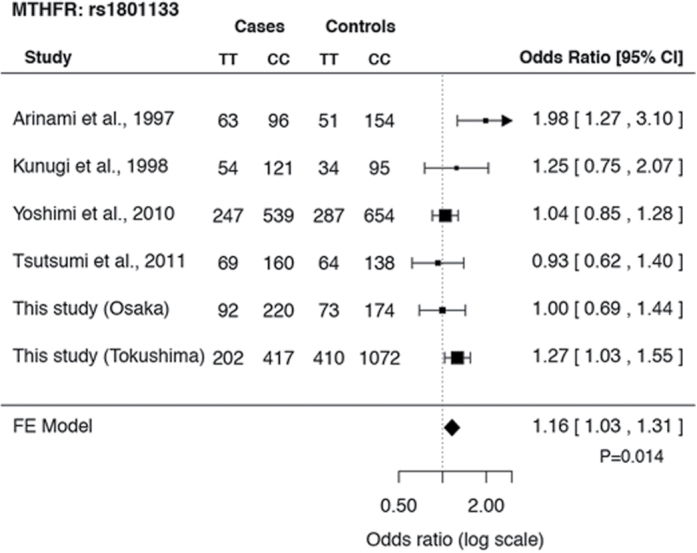
Six association studies on Japanese subjects, including the 2 data sets from this study, were used for the meta-analysis.37–40 The genotypic distributions and allelic frequencies of rs1801133 in each study are shown in the supplementary table 2. The diagnosis of schizophrenia was made according to DSM-IV criteria in 5 studies, and it was made according to DSM-III criteria in the remaining study. A total of 4316 cases and 6062 controls were included in this analysis. Genotypic distribution of this SNP did not deviate significantly from the HWE in any control group across the 6 studies (P > .05). Significant heterogeneity was not detected at this SNP among the studies for the 5 genetic models (P > .05). The funnel plot analysis indicated no evidence of publication bias for all genetic models (P > .05). The results of ORs and CI analyzed by the fixed-effects model for all genetic models and the risk of schizophrenia are shown in table 1. Of these 5 genetic models, significant associations were found in 4 models. The highest OR was observed in the codominant model (CC vs TT genotypes; figure 3; OR = 1.16, 95% CI = 1.03–1.31, P = 1.4 × 10–2, in the fixed-effects model).
Table 1.
The Results of OR and 95% CI Analyzed by the Fixed-Effects Model for All Genetic Models and Each P Value in the Meta-analysis for Methylenetetrahydrofolate reductase C677T
| Model | OR | 95% CI | P Value |
|---|---|---|---|
| Recessive (CC/CT vs TT) | 1.14 | 1.03–1.27 | .016 |
| Dominant (CC vs CT/TT) | 1.06 | 0.98–1.16 | .147 |
| Codominant (CC vs TT) | 1.16 | 1.03–1.31 | .014 |
| Codominant (CT vs TT) | 1.13 | 1.00–1.26 | .042 |
| Allelic (C vs T) | 1.07 | 1.01–1.13 | .022 |
Fig. 3.
A meta-analysis of genetic association studies on Japanese subjects between the MTHFR C677T polymorphism and schizophrenia. Six association studies on Japanese subjects, including the 2 data sets from this study, were used for the meta-analysis (N = 10 378). Significant associations between the MTHFR C677T polymorphism and schizophrenia were found in 4 models, and the result of the codominant model (CC vs TT genotypes) is shown (OR = 1.16, 95% CI = 1.03–1.31, P = 1.4×10–2 in the fixed-effects model).
Effect of Plasma Total Homocysteine Levels on Schizophrenia Risk in an MR Study
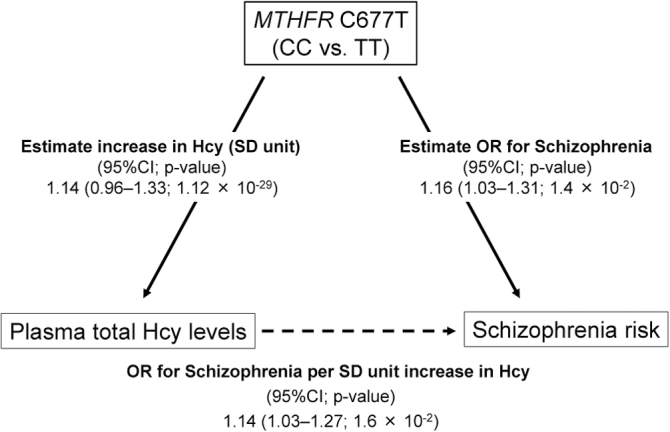
From the current meta-analysis of the Japanese genetic association studies, the pooled OR (the TT vs CC genotypes) for the effect of the MTHFR C677T polymorphism on schizophrenia risk was 1.16 (95% CI = 1.03–1.31). In multivariate gene-homocysteine association analysis in the Japanese control subjects genotyped (n = 980 with a SD for plasma total homocysteine levels of 4.84 nmol/ml), the effect on plasma total homocysteine levels, expressed as 1-SD increase in homocysteine, was estimated to be 1.14 (95% CI = 0.96–1.33; P = 1.1 × 10–29) for the TT genotypes of the MTHFR C677T polymorphism (vs the CC genotypes), after adjustment for age and gender. When combining these 2 estimates by an MR approach, the effect of plasma total homocysteine on schizophrenia risk was statistically significant, representing the OR of 1.14 (95% CI = 1.03–1.27; P = 1.6 × 10–2) for schizophrenia per 1-SD increase in plasma total homocysteine (figure 4).
Fig. 4.
Graphical representation of the mendelian randomization approach. The pooled OR (the TT vs CC genotypes) for the effect of the MTHFR C677T polymorphism on schizophrenia risk was 1.16 (95% CI = 1.03–1.31). The OR (the TT vs CC genotypes) for the effect of the MTHFR C677T polymorphism on plasma total homocysteine levels, expressed as 1-SD increase in homocysteine, was 1.14 (95% CI = 0.96–1.33; P = 1.1×10–29). The effect of plasma total homocysteine on schizophrenia risk by a mendelian randomization analysis was statistically significant, representing the OR of 1.14 (95% CI = 1.03–1.27; P = 1.6×10–2) for schizophrenia per 1-SD increase in plasma total homocysteine.
Discussion
In this study, we performed an association study between the plasma total homocysteine and schizophrenia with stratification by gender and by the MTHFR C677T genotypes and demonstrated significantly elevated plasma total homocysteine levels in patients with schizophrenia compared with controls, in both male and female subjects. The subsequent meta-analysis for gender supported this finding. To our knowledge, this is the first study to conduct a meta-analysis according to gender. The significant decrease in plasma total homocysteine levels with age in our multivariate linear regression analysis was not consistent with a previous finding.33 This discrepancy might be caused by disease status and gender of the subjects analyzed. When we examined the relationship between age and the plasma total homocysteine levels in a univariate linear regression model, a significant negative correlation was observed only in male subjects with schizophrenia (supplementary figure 3). Consistent with this finding, Levine et al5 reported that the difference in the plasma total homocysteine levels between patients and controls was attributable to young male patients with schizophrenia.
We also conducted a meta-analysis of genetic association studies between the MTHFR C677T polymorphism and schizophrenia based on Japanese subjects and demonstrated that the MTHFR C677T polymorphism was a risk factor for developing schizophrenia in the Japanese population, which is consistent with the results of previous meta-analyses.39,41–47,57 On the other hand, this polymorphism has not been identified as a risk locus for schizophrenia in the large genome-wide association studies.58,59 This discrepancy might be caused by ethnic differences (risk allele [T] frequencies from HapMap; Japanese 0.39, Caucasian 0.31, African American 0.12, and Mexican American 0.41) and a lack of adequate statistical power to detect the relatively small genetic effect of this polymorphism on schizophrenia at the genome-wide significant threshold.
Importantly, we demonstrated that increased homocysteine levels may be associated with an increased risk of developing schizophrenia by an MR approach. Hyperhomocysteinemia has been proposed as being part of the pathophysiology of schizophrenia due to its various biological effects, such as acting as a partial antagonist of the glutamate site of the N-methyl-d-aspartate receptor23 and causing subtle placental vascular damage that interferes with oxygen delivery to the fetus,60 DNA damage and cell cytotoxicity,61 neuronal apoptosis,62 and mitochondrial nitric oxide accumulation.63 Homocysteine acts as a methyl donor when it is converted to S-adenosyl-methionine, and we recently demonstrated a significant association between the plasma total homocysteine and DNA methylation in schizophrenia, which suggests that homocysteine might play a role in the pathogenesis of schizophrenia via alterations to DNA methylation.64 Homocysteine, S-adenosyl-methionine, DNA methylation, MTHFR, folate, and vitamin B12 are involved in 1-carbon metabolism, and abnormalities of these components in schizophrenia have been reported in previous studies.2,3,65 These lines of evidence suggest that disrupted 1-carbon metabolism may be an important role in the pathophysiology of schizophrenia.
The benefits of homocysteine-reducing strategies in schizophrenia have been shown in several studies. Levine et al66 reported an improvement in the clinical symptoms of schizophrenic patients with hyperhomocysteinemia who were treated with folate, vitamin B12, and pyridoxine. Hill et al67 reported an improvement in the negative symptoms of schizophrenic patients who were treated with folate when the MTHFR C677T genotype was taken into account. Roffman et al68 reported an improvement in the negative symptoms of schizophrenic patients who were treated with folate and vitamin B12 when 4 variants in the FOLH1, MTHFR, MTR, and COMT genes were taken into account. Further research will be necessary to identify the features of patients with schizophrenia who would benefit from homocysteine-reducing treatments. In cardiovascular diseases, which are also associated with hyperhomocysteinemia, clinical trials to identify a subgroup that appeared to benefit from homocysteine-lowering intervention have been performed69,70 although no benefits of homocysteine-lowering intervention on cardiovascular outcomes have been reported in randomized controlled trials.71
Many studies have indicated the potential contributions of the MTHFR C677T polymorphism to the pathophysiology of schizophrenia. This risk SNP has been associated with schizophrenic negative symptoms, aggressive behavior, and various phenotypes related to schizophrenia, such as cognitive function, episodic memory, gray matter density, and prefrontal function.72–80 Interestingly, pharmacogenetic studies have demonstrated that this risk SNP has also been involved in the antipsychotic drug response and metabolic syndrome treated with antipsychotics in schizophrenia.81–84
There are some limitations to this study. First, we did not obtain genomic DNA from all participants in the association study between the plasma total homocyateine levels and schizophrenia. Second, all patients were treated with various antipsychotic drugs, and these medications might influence the outcome. When we examined the relationship between equivalent dose and the plasma total homocysteine levels in subjects with schizophrenia by a univariate linear regression model, a positive correlation was observed in female subjects (P = .03). However, it did not reach statistical significance after correction for multiple comparisons. Third, there was heterogeneity among the studies in the meta-analysis for blood homocysteine, while significant heterogeneity was not observed in the meta-analysis for genetic association studies. This heterogeneity might be caused by other genetic variations, the clinical heterogeneity of the patients included, medications, and environmental factors, such as folic acid, vitamin B6, vitamin B12, obesity, smoking status, and caffeine consumption, although we did not take these confounding factors into consideration in our analysis. Fourth, the use of “well controls” in this case-control analysis might accentuate such confounding influences.85 Fifth, this is a cross-sectional study, and MR has limitations on the ability to establish causal relationships between risk factors and outcomes.86 So, the causality between schizophrenia and blood homocysteine levels must be still cautious. Notably, elevated maternal levels of homocysteine during the third trimester have been found to increase the risk of schizophrenia in the offspring.60 Finally, hyperhomocysteinemia has been identified as an independent risk factor for several neurological disorders in addition to schizophrenia, such as depression and dementia.87,88 Further studies to examine how hyperhomocysteinemia is involved in the pathophysiology of each disease will be necessary.
In conclusion, to the best of our knowledge, this is the first meta-analysis of association studies between blood homocysteine levels and schizophrenia according to gender, and we demonstrated elevated blood homocysteine levels in both male and female subjects with schizophrenia. The meta-analysis of genetic association studies using the Japanese subjects provided stringent evidence of association between the MTHFR C677T polymorphism and schizophrenia. Our MR analysis using the Japanese subjects suggests that increased plasma total homocysteine levels may be associated with an increased risk of developing schizophrenia.
Supplementary Material
Supplementary material is available at http://schizophre niabulletin.oxfordjournals.org.
Funding
Core Research for Evolutional Science and Technology, Japan Science and Technology Agency; Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology (24791216); SENSHIN Medical Research Foundation; Research Group For Schizophrenia.
Supplementary Material
Acknowledgments
The authors would like to thank all of the volunteers who understood the purpose of our study and participated in this study, as well as the physicians who helped us to collect clinical data and blood samples at the mental hospitals. The authors would also like to thank Mrs Akemi Okada for her technical assistance. All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1. Saha S, Chant D, Welham J, McGrath J. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005;2:e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Frankenburg FR. The role of one-carbon metabolism in schizophrenia and depression. Harv Rev Psychiatry. 2007;15:146–160 [DOI] [PubMed] [Google Scholar]
- 3. Krebs MO, Bellon A, Mainguy G, Jay TM, Frieling H. One-carbon metabolism and schizophrenia: current challenges and future directions. Trends Mol Med. 2009;15:562–570 [DOI] [PubMed] [Google Scholar]
- 4. Regland B, Johansson BV, Grenfeldt B, Hjelmgren LT, Medhus M. Homocysteinemia is a common feature of schizophrenia. J Neural Transm Gen Sect. 1995;100:165–169 [DOI] [PubMed] [Google Scholar]
- 5. Levine J, Stahl Z, Sela BA, Gavendo S, Ruderman V, Belmaker RH. Elevated homocysteine levels in young male patients with schizophrenia. Am J Psychiatry. 2002;159:1790–1792 [DOI] [PubMed] [Google Scholar]
- 6. Applebaum J, Shimon H, Sela BA, Belmaker RH, Levine J. Homocysteine levels in newly admitted schizophrenic patients. J Psychiatr Res. 2004;38:413–416 [DOI] [PubMed] [Google Scholar]
- 7. Muntjewerff JW, Hoogendoorn ML, Kahn RS, et al. Hyperhomocysteinemia, methylenetetrahydrofolate reductase 677TT genotype, and the risk for schizophrenia: a Dutch population based case-control study. Am J Med Genet B Neuropsychiatr Genet. 2005;135B:69–72 [DOI] [PubMed] [Google Scholar]
- 8. Neeman G, Blanaru M, Bloch B, et al. Relation of plasma glycine, serine, and homocysteine levels to schizophrenia symptoms and medication type. Am J Psychiatry. 2005;162:1738–1740 [DOI] [PubMed] [Google Scholar]
- 9. Lee YS, Han DH, Jeon CM, et al. Serum homocysteine, folate level and methylenetetrahydrofolate reductase 677, 1298 gene polymorphism in Korean schizophrenic patients. Neuroreport. 2006;17:743–746 [DOI] [PubMed] [Google Scholar]
- 10. Adler Nevo G, Meged S, Sela BA, Hanoch-Levi A, Hershko R, Weizman A. Homocysteine levels in adolescent schizophrenia patients. Eur Neuropsychopharmacol. 2006;16:588–591 [DOI] [PubMed] [Google Scholar]
- 11. Haidemenos A, Kontis D, Gazi A, Kallai E, Allin M, Lucia B. Plasma homocysteine, folate and B12 in chronic schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1289–1296 [DOI] [PubMed] [Google Scholar]
- 12. Akanji AO, Ohaeri JU, Al-Shammri SA, Fatania HR. Associations of blood homocysteine concentrations in Arab schizophrenic patients. Clin Biochem. 2007;40:1026–1031 [DOI] [PubMed] [Google Scholar]
- 13. Bromberg A, Levine J, Nemetz B, Belmaker RH, Agam G. No association between global leukocyte DNA methylation and homocysteine levels in schizophrenia patients. Schizophr Res. 2008;101:50–57 [DOI] [PubMed] [Google Scholar]
- 14. Petronijević ND, Radonjić NV, Ivković MD, et al. Plasma homocysteine levels in young male patients in the exacerbation and remission phase of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1921–1926 [DOI] [PubMed] [Google Scholar]
- 15. Dietrich-Muszalska A, Olas B, Głowacki R, Bald E. Oxidative/nitrative modifications of plasma proteins and thiols from patients with schizophrenia. Neuropsychobiology. 2009;59:1–7 [DOI] [PubMed] [Google Scholar]
- 16. Feng LG, Song ZW, Xin F, Hu J. Association of plasma homocysteine and methylenetetrahydrofolate reductase C677T gene variant with schizophrenia: A Chinese Han population-based case-control study. Psychiatry Res. 2009;168:205–208 [DOI] [PubMed] [Google Scholar]
- 17. Ma YY, Shek CC, Wong MC, et al. Homocysteine level in schizophrenia patients. Aust N Z J Psychiatry. 2009;43:760–765 [DOI] [PubMed] [Google Scholar]
- 18. García-Miss Mdel R, Pérez-Mutul J, López-Canul B, et al. Folate, homocysteine, interleukin-6, and tumor necrosis factor alfa levels, but not the methylenetetrahydrofolate reductase C677T polymorphism, are risk factors for schizophrenia. J Psychiatr Res. 2010;44:441–446 [DOI] [PubMed] [Google Scholar]
- 19. Kale A, Naphade N, Sapkale S, et al. Reduced folic acid, vitamin B12 and docosahexaenoic acid and increased homocysteine and cortisol in never-medicated schizophrenia patients: implications for altered one-carbon metabolism. Psychiatry Res. 2010;175:47–53 [DOI] [PubMed] [Google Scholar]
- 20. Shlafman N, Shaldubin S, Applebaum J, Belmaker RH, Levine J. No gross abnormality of plasma homocysteine after acute methionine loading in clinically stabilized patients with schizophrenia. Asian J Psychiatr. 2010;3:64–66 [DOI] [PubMed] [Google Scholar]
- 21. Eren E, Yeğin A, Yilmaz N, Herken H. Serum total homocystein, folate and vitamin B12 levels and their correlation with antipsychotic drug doses in adult male patients with chronic schizophrenia. Clin Lab. 2010;56:513–518 [PubMed] [Google Scholar]
- 22. Kim TH, Moon SW. Serum homocysteine and folate levels in korean schizophrenic patients. Psychiatry Investig. 2011;8:134–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dietrich-Muszalska A, Malinowska J, Olas B, et al. The oxidative stress may be induced by the elevated homocysteine in schizophrenic patients. Neurochem Res. 2012;37:1057–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kevere L, Purvina S, Bauze D, et al. Elevated serum levels of homocysteine as an early prognostic factor of psychiatric disorders in children and adolescents. Schizophr Res Treatment. 2012;2012:373261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Virgos C, Martorell L, Simó JM, et al. Plasma homocysteine and the methylenetetrahydrofolate reductase C677T gene variant: lack of association with schizophrenia. Neuroreport. 1999;10:2035–2038 [DOI] [PubMed] [Google Scholar]
- 26. Muntjewerff JW, van der Put N, Eskes T, et al. Homocysteine metabolism and B-vitamins in schizophrenic patients: low plasma folate as a possible independent risk factor for schizophrenia. Psychiatry Res. 2003;121:1–9 [DOI] [PubMed] [Google Scholar]
- 27. Goff DC, Bottiglieri T, Arning E, et al. Folate, homocysteine, and negative symptoms in schizophrenia. Am J Psychiatry. 2004;161:1705–1708 [DOI] [PubMed] [Google Scholar]
- 28. Vilella E, Virgos C, Murphy M, et al. Further evidence that hyperhomocysteinemia and methylenetetrahydrofolate reductase C677T and A1289C polymorphisms are not risk factors for schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1169–1174 [DOI] [PubMed] [Google Scholar]
- 29. Ozcan O, Ipçioğlu OM, Gültepe M, Başoğglu C. Altered red cell membrane compositions related to functional vitamin B(12) deficiency manifested by elevated urine methylmalonic acid concentrations in patients with schizophrenia. Ann Clin Biochem. 2008;45:44–49 [DOI] [PubMed] [Google Scholar]
- 30. Bouaziz N, Ayedi I, Sidhom O, et al. Plasma homocysteine in schizophrenia: determinants and clinical correlations in Tunisian patients free from antipsychotics. Psychiatry Res. 2010;179:24–29 [DOI] [PubMed] [Google Scholar]
- 31. Gysin R, Kraftsik R, Boulat O, et al. Genetic dysregulation of glutathione synthesis predicts alteration of plasma thiol redox status in schizophrenia. Antioxid Redox Signal. 2011;15:2003–2010 [DOI] [PubMed] [Google Scholar]
- 32. Muntjewerff JW, Kahn RS, Blom HJ, den Heijer M. Homocysteine, methylenetetrahydrofolate reductase and risk of schizophrenia: a meta-analysis. Mol Psychiatry. 2006;11:143–149 [DOI] [PubMed] [Google Scholar]
- 33. Nygård O, Vollset SE, Refsum H, et al. Total plasma homocysteine and cardiovascular risk profile. The Hordaland Homocysteine Study. JAMA. 1995;274:1526–1533 [DOI] [PubMed] [Google Scholar]
- 34. Paré G, Chasman DI, Parker AN, et al. Novel associations of CPS1, MUT, NOX4, and DPEP1 with plasma homocysteine in a healthy population: a genome-wide evaluation of 13 974 participants in the Women’s Genome Health Study. Circ Cardiovasc Genet. 2009;2:142–150. 10.1161/CIRCGENETICS.108.829804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hazra A, Kraft P, Lazarus R, et al. Genome-wide significant predictors of metabolites in the one-carbon metabolism pathway. Hum Mol Genet. 2009;18:4677–4687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Frosst P, Blom HJ, Milos R, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111–113 [DOI] [PubMed] [Google Scholar]
- 37. Arinami T, Yamada N, Yamakawa-Kobayashi K, Hamaguchi H, Toru M. Methylenetetrahydrofolate reductase variant and schizophrenia/depression. Am J Med Genet. 1997;74:526–528 [DOI] [PubMed] [Google Scholar]
- 38. Kunugi H, Fukuda R, Hattori M, et al. C677T polymorphism in methylenetetrahydrofolate reductase gene and psychoses. Mol Psychiatry. 1998;3:435–437 [DOI] [PubMed] [Google Scholar]
- 39. Yoshimi A, Aleksic B, Kawamura Y, et al. Gene-wide association study between the methylenetetrahydrofolate reductase gene (MTHFR) and schizophrenia in the Japanese population, with an updated meta-analysis on currently available data. Schizophr Res. 2010;124:216–222 [DOI] [PubMed] [Google Scholar]
- 40. Tsutsumi A, Glatt SJ, Kanazawa T, et al. The genetic validation of heterogeneity in schizophrenia. Behav Brain Funct. 2011;7:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Allen NC, Bagade S, McQueen MB, et al. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat Genet. 2008;40:827–834 [DOI] [PubMed] [Google Scholar]
- 42. Gilbody S, Lewis S, Lightfoot T. Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: a HuGE review. Am J Epidemiol. 2007;165:1–13 [DOI] [PubMed] [Google Scholar]
- 43. Jönsson EG, Larsson K, Vares M, et al. Two methylenetetrahydrofolate reductase gene (MTHFR) polymorphisms, schizophrenia and bipolar disorder: an association study. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:976–982 [DOI] [PubMed] [Google Scholar]
- 44. Lewis SJ, Zammit S, Gunnell D, Smith GD. A meta-analysis of the MTHFR C677T polymorphism and schizophrenia risk. Am J Med Genet B Neuropsychiatr Genet. 2005;135B:2–4 [DOI] [PubMed] [Google Scholar]
- 45. Peerbooms OL, van Os J, Drukker M, et al. ; MTHFR in Psychiatry Group. Meta-analysis of MTHFR gene variants in schizophrenia, bipolar disorder and unipolar depressive disorder: evidence for a common genetic vulnerability? Brain Behav Immun. 2011;25:1530–1543 [DOI] [PubMed] [Google Scholar]
- 46. Shi J, Gershon ES, Liu C. Genetic associations with schizophrenia: meta-analyses of 12 candidate genes. Schizophr Res. 2008;104:96–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zintzaras E. C677T and A1298C methylenetetrahydrofolate reductase gene polymorphisms in schizophrenia, bipolar disorder and depression: a meta-analysis of genetic association studies. Psychiatr Genet. 2006;16:105–115 [DOI] [PubMed] [Google Scholar]
- 48. Davey Smith G, Ebrahim S. What can mendelian randomisation tell us about modifiable behavioural and environmental exposures? BMJ. 2005;330:1076–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Burgess S, Butterworth A, Malarstig A, Thompson SG. Use of Mendelian randomisation to assess potential benefit of clinical intervention. BMJ. 2012;345:e7325. [DOI] [PubMed] [Google Scholar]
- 50. Kinoshita M, Numata S, Tajima A, et al. Meta-analysis of association studies between DISC1 missense variants and schizophrenia in the Japanese population. Schizophr Res. 2012;141:271–273 [DOI] [PubMed] [Google Scholar]
- 51. Hashimoto R, Ohi K, Yasuda Y, et al. No association between the PCM1 gene and schizophrenia: a multi-center case-control study and a meta-analysis. Schizophr Res. 2011;129:80–84 [DOI] [PubMed] [Google Scholar]
- 52. Araki A, Sako Y. Determination of free and total homocysteine in human plasma by high-performance liquid chromatography with fluorescence detection. J Chromatogr. 1987;422:43–52 [DOI] [PubMed] [Google Scholar]
- 53. Pichler I, Del Greco M F, Gögele M, et al. ; PD GWAS Consortium; International Parkinson’s Disease Genomics Consortium; Wellcome Trust Case Control Consortium 2; Genetics of Iron Status Consortium. Serum iron levels and the risk of Parkinson disease: a mendelian randomization study. PLoS Med. 2013;10:e1001462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bautista LE, Smeeth L, Hingorani AD, Casas JP. Estimation of bias in nongenetic observational studies using “mendelian triangulation”. Ann Epidemiol. 2006;16:675–680 [DOI] [PubMed] [Google Scholar]
- 55. Thomas DC, Lawlor DA, Thompson JR. Re: Estimation of bias in nongenetic observational studies using “Mendelian triangulation” by Bautista et al. Ann Epidemiol. 2007;17:511–513 [DOI] [PubMed] [Google Scholar]
- 56. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yue WH, Wang HF, Sun LD, et al. Genome-wide association study identifies a susceptibility locus for schizophrenia in Han Chinese at 11p11.2. Nat Genet. 2011;43:1228–1231 [DOI] [PubMed] [Google Scholar]
- 58. Stefansson H, Ophoff RA, Steinberg S, et al. ; Genetic Risk and Outcome in Psychosis (GROUP). Common variants conferring risk of schizophrenia. Nature. 2009;460:744–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schizophrenia Psychiatric Genome-Wide Association Study (GWAS) Consortium. Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011;43:969–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Brown AS, Bottiglieri T, Schaefer CA, et al. Elevated prenatal homocysteine levels as a risk factor for schizophrenia. Arch Gen Psychiatry. 2007;64:31–39 [DOI] [PubMed] [Google Scholar]
- 61. Liu CC, Ho WY, Leu KL, Tsai HM, Yang TH. Effects of S-adenosylhomocysteine and homocysteine on DNA damage and cell cytotoxicity in murine hepatic and microglia cell lines. J Biochem Mol Toxicol. 2009;23:349–356 [DOI] [PubMed] [Google Scholar]
- 62. Kruman II, Culmsee C, Chan SL, et al. Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. J Neurosci. 2000;20:6920–6926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tyagi N, Moshal KS, Ovechkin AV, et al. Mitochondrial mechanism of oxidative stress and systemic hypertension in hyperhomocysteinemia. J Cell Biochem. 2005;96:665–671 [DOI] [PubMed] [Google Scholar]
- 64. Kinoshita M, Numata S, Tajima A, Shimodera S, Imoto I, Ohmori T. Plasma total homocysteine is associated with DNA methylation in patients with schizophrenia. Epigenetics. 2013;8:584–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kirkbride JB, Susser E, Kundakovic M, Kresovich JK, Davey Smith G, Relton CL. Prenatal nutrition, epigenetics and schizophrenia risk: can we test causal effects? Epigenomics. 2012;4:303–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Levine J, Stahl Z, Sela BA, et al. Homocysteine-reducing strategies improve symptoms in chronic schizophrenic patients with hyperhomocysteinemia. Biol Psychiatry. 2006;60:265–269 [DOI] [PubMed] [Google Scholar]
- 67. Hill M, Shannahan K, Jasinski S, et al. Folate supplementation in schizophrenia: a possible role for MTHFR genotype. Schizophr Res. 2011;127:41–45 [DOI] [PubMed] [Google Scholar]
- 68. Roffman JL, Lamberti JS, Achtyes E, et al. Randomized multicenter investigation of folate plus vitamin B12 supplementation in schizophrenia. JAMA Psychiatry. 2013;70:481–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291:565–575 [DOI] [PubMed] [Google Scholar]
- 70. Spence JD, Bang H, Chambless LE, Stampfer MJ. Vitamin Intervention For Stroke Prevention trial: an efficacy analysis. Stroke. 2005;36:2404–2409 [DOI] [PubMed] [Google Scholar]
- 71. Martí-Carvajal AJ, Solà I, Lathyris D, Karakitsiou DE, Simancas-Racines D. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2013;1:CD006612. [DOI] [PubMed] [Google Scholar]
- 72. Dong ZQ, Tian YY, Guan X, et al. Genetic polymorphism of methylenetetrahydrofolate reductase and risk of aggressive behaviour in schizophrenia. Psychiatry Res. 2012;200:1082. [DOI] [PubMed] [Google Scholar]
- 73. Kontis D, Theochari E, Fryssira H, et al. COMT and MTHFR polymorphisms interaction on cognition in schizophrenia: an exploratory study. Neurosci Lett. 2013;537:17–22 [DOI] [PubMed] [Google Scholar]
- 74. Roffman JL, Weiss AP, Deckersbach T, et al. Effects of the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism on executive function in schizophrenia. Schizophr Res. 2007;92:181–188 [DOI] [PubMed] [Google Scholar]
- 75. Roffman JL, Gollub RL, Calhoun VD, et al. MTHFR 677C –> T genotype disrupts prefrontal function in schizophrenia through an interaction with COMT 158Val –> Met. Proc Natl Acad Sci U S A. 2008;105:17573–17578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Roffman JL, Weiss AP, Purcell S, et al. Contribution of methylenetetrahydrofolate reductase (MTHFR) polymorphisms to negative symptoms in schizophrenia. Biol Psychiatry. 2008;63:42–48 [DOI] [PubMed] [Google Scholar]
- 77. Roffman JL, Brohawn DG, Friedman JS, et al. MTHFR 677C>T effects on anterior cingulate structure and function during response monitoring in schizophrenia: a preliminary study. Brain Imaging Behav. 2011;5:65–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Roffman JL, Nitenson AZ, Agam Y, et al. A hypomethylating variant of MTHFR, 677C>T, blunts the neural response to errors in patients with schizophrenia and healthy individuals. PLoS One. 2011;6:e25253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Roffman JL, Brohawn DG, Nitenson AZ, Macklin EA, Smoller JW, Goff DC. Genetic variation throughout the folate metabolic pathway influences negative symptom severity in schizophrenia. Schizophr Bull. 2013;39:330–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhang Y, Yan H, Tian L, et al. Association of MTHFR C677T polymorphism with schizophrenia and its effect on episodic memory and gray matter density in patients. Behav Brain Res. 2013;243:146–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ellingrod VL, Miller DD, Taylor SF, Moline J, Holman T, Kerr J. Metabolic syndrome and insulin resistance in schizophrenia patients receiving antipsychotics genotyped for the methylenetetrahydrofolate reductase (MTHFR) 677C/T and 1298A/C variants. Schizophr Res. 2008;98:47–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ellingrod VL, Taylor SF, Dalack G, et al. Risk factors associated with metabolic syndrome in bipolar and schizophrenia subjects treated with antipsychotics: the role of folate pharmacogenetics. J Clin Psychopharmacol. 2012;32:261–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Vehof J, Burger H, Wilffert B, Al Hadithy A, Alizadeh BZ, Snieder H; GROUP investigators. Clinical response to antipsychotic drug treatment: association study of polymorphisms in six candidate genes. Eur Neuropsychopharmacol. 2012;22:625–631 [DOI] [PubMed] [Google Scholar]
- 84. Joober R, Benkelfat C, Lal S, et al. Association between the methylenetetrahydrofolate reductase 677C–>T missense mutation and schizophrenia. Mol Psychiatry. 2000;5:323–326 [DOI] [PubMed] [Google Scholar]
- 85. Schwartz S, Susser E. The use of well controls: an unhealthy practice in psychiatric research. Psychol Med. 2011;41:1127–1131 [DOI] [PubMed] [Google Scholar]
- 86. Bochud M. On the use of Mendelian randomization to infer causality in observational epidemiology. Eur Heart J. 2008;29:2456–2457 [DOI] [PubMed] [Google Scholar]
- 87. Almeida OP, McCaul K, Hankey GJ, Norman P, Jamrozik K, Flicker L. Homocysteine and depression in later life. Arch Gen Psychiatry. 2008;65:1286–1294 [DOI] [PubMed] [Google Scholar]
- 88. Wald DS, Kasturiratne A, Simmonds M. Serum homocysteine and dementia: meta-analysis of eight cohort studies including 8669 participants. Alzheimers Dement. 2011;7:412–417 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.