Abstract
Background: The “mirror-neuron system” has been proposed to be a neurophysiological substrate for social cognition (SC) ability. We used transcranial magnetic stimulation (TMS) paradigms to compare putative mirror neuron activity (MNA) in 3 groups: antipsychotic-naive, medicated schizophrenia patients, and healthy comparison subjects. We also explored the association between MNA and SC ability in patients. Methods: Fifty-four consenting right-handed schizophrenia patients (33 antipsychotic naive) and 45 matched healthy comparison subjects completed a TMS experiment to assess putative premotor MNA. We used 4 TMS paradigms of eliciting motor-evoked potentials (MEP) in the right first dorsal interosseous (FDI) muscle. These were applied while the subjects observed a goal-directed action involving the FDI (actual action and its video) and a static image. The difference in the amplitude of the MEP while they observed the static image and the action provided a measure of MNA. Subjects also underwent SC assessments (theory of mind [ToM], emotion processing, and social perception). Results: Two-way repeated measures ANOVA revealed significant group × occasion interaction effect in 3 TMS paradigms, indicating deficient motor facilitation during action observation relative to rest state in antipsychotic-naive schizophrenia patients as compared with the other two groups. Among patients, there were significant direct correlations between measures of MNA and ToM performance. Conclusions: Antipsychotic-naive schizophrenia patients have poorer MNA than medicated patients and healthy controls. Measures of putative MNA had significant and consistent associations with ToM abilities. These findings suggest a possibility of deficient mirror neuron system underlying SC deficits in schizophrenia.
Key words: mirror neurons, social cognition, mental state attribution, psychosis, antipsychotic-naive, embodied simulation
Introduction
Deficits in social cognition (SC) have been amply demonstrated across different stages of schizophrenia.1,2 SC encompasses mental operations underlying social interactions, embracing subdomains of theory of mind (ToM), emotion processing, social perception and knowledge, and attributional styles.3 These deficits are more closely linked to functional outcome than general/nonsocial cognitive deficits.4 They are also associated with disorganization, reality distortion, and negative symptoms of schizophrenia.5
Mirror neurons are specialized nerve cells that are activated not only while performing an action oneself, but also while observing someone else perform that action.6 Mirror neuron-driven embodied simulation, captured by the “neural exploitation hypothesis,” has been proposed as a physiological substrate of social cognitive abilities in humans.7 This theory suggests that we reuse our own mental states represented with a bodily format in functionally attributing them to others. Here, neural exploitation refers to key aspects of human SC being produced by the exaptation of brain mechanisms originally evolved for sensory motor integration. Likewise, the social projection theory8 suggests that knowledge of oneself is used as a platform from which we understand others. Both these theories are grounded on the findings that mirror neurons become active “as if” we were executing the very same action that we are observing, thus mediating SC. In humans, mirror neuron activity (MNA) has been demonstrated using both direct (single cell recordings)9 and indirect methods, such as blood oxygenation level–dependent changes measured using magnetic resonance imaging (MRI),10 blood flow changes using positron emission tomography,11 mu rhythm suppression using electroencephalography (EEG),12 alpha band suppression and gamma band amplifications using magnetoencephalography,13,14 and motor-evoked potential (MEP) enhancement in transcranial magnetic stimulation (TMS) studies.15 A recent meta-analysis identifies various brain regions with mirror mechanism in humans, including inferior frontal gyrus, ventral premotor cortex, and inferior parietal lobule, being the most consistent.16
Studies exploring mirror mechanisms in schizophrenia have yielded contrasting findings. Greater mu wave suppression over the left hemisphere (representing greater MNA) was detected in actively psychotic schizophrenia subjects when compared with healthy participants and patients with residual symptoms.12 In contrast, a magnetoencephalographic study in antipsychotic-naive schizophrenia patients demonstrated that during action observation they exhibited fewer waveforms and equivalent current dipoles in the right parietal lobe than healthy comparison subjects, thus reflecting a possible deficit in MNA.14 Another magnetoencephalographic study also demonstrated deficient MNA in schizophrenia patients compared with their discordant healthy twins.13 A study using TMS showed significantly lower MEP during action observation, than healthy comparison subjects, indicating poorer MNA in the patient group.17 The contrasting results across these studies maybe due to relatively small sample sizes, effect of medications, and differing methods used to assess MNA. Such work warrants replication as sound knowledge on MNA and its influence on SC in schizophrenia could have translational potential to guide newer treatment strategies.18
Crucial phenotypic manifestations of abnormal MNA (eg, gesture imitation) have been shown to be impaired in schizophrenia.19 It has also been demonstrated that schizophrenia patients with flat affect do not activate their prefrontal cortices during emotion processing, reflecting a lack of emotional resonance.20 Moreover, there is emerging empirical evidence for the association between putative MNA measured using TMS paradigms and social impairments in adults with autism.21 These findings provide a mechanistic basis to understand the possible links between abnormal MNA and impaired SC and functioning in schizophrenia.
TMS is a noninvasive method to transiently excite the underlying cerebral cortex.22 When applied to the primary motor cortex, it produces peripheral MEPs, which are recorded using electromyography from hand muscles. Observation of actions using specific muscle enhances the amplitude of MEPs recorded from those muscles—this enhancement has been purported to be reflective of the mirror neuron system activity.23
In this study we aimed to (a) compare a putative measure of MNA (motor facilitation during action observation relative to rest states) using different cortical excitability TMS paradigms, in antipsychotic-naive and medicated patients with schizophrenia and healthy comparison subjects; and (b) study the relation between measures of MNA and SC in the patient group. We hypothesized that (a) schizophrenia patients would have reduced MNA and impaired SC compared with healthy comparison subjects; and (b) MNA would positively correlate with SC in the patient group.
Methods
Subjects
We recruited 54 right-handed schizophrenia patients (33 antipsychotic-naive [never exposed to any antipsychotic medication] and 21 medicated) from the inpatient and outpatient services of the National Institute of Mental Health & Neurosciences, Bangalore. Thirty-three patients had no prior engagement with psychiatric services and were antipsychotic naive. Twenty-one patients were prescribed antipsychotic medications and their adherence to medications was confirmed by self-report and report from family caregivers who lived with them. They were diagnosed independently by two qualified psychiatrists according to Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM IV)24 criteria, and confirmed using the Mini-International Neuropsychiatric Interview.25 Patients with substance dependence in the previous 6 months (except nicotine), presence of comorbid neurological or medical disorder, clinically diagnosable or self-reported visual or auditory impairment, current pregnant or postpartum state, and a score of ≤19 on the Hindi Mental Status Examination26 were excluded from the study. Patients were compared with 45 healthy comparison subjects, recruited from among acquaintances of the research staff (hospital staff and members from the community) and screened to rule out Axis-1 psychiatric disorders using Mini-International Neuropsychiatric Interview—Screening.25 None of the healthy comparison subjects had family history of psychotic disorder in first- and second-degree relatives as assessed by clinical interview. All subjects were assessed with (a) the Edinburgh inventory for handedness27 and (b) TMS Adult Safety Screen28 to screen for their potential to develop complications. The institute’s ethics committee approved the study. After complete description of the study to the subjects, written informed consent was obtained.
Assessments
Symptoms.
Patients’ symptoms were assessed using the Positive and Negative Syndrome scale (PANSS).29
Social Cognition.
ToM and social perception were assessed using the Social Cognition Rating Tools in Indian Setting (SOCRATIS).30 Emotion processing was assessed using the Tool for Recognition of Emotions in Neuropsychiatric Disorders (TRENDS).31
Theory of Mind
Tasks included 2 each of first-order (based on Sally-Anne [Wimmer and Perner32] and Smarties [Perner et al33] tasks), second-order false belief picture stories (based on ice cream van [Perner and Wimmer34] and missing cookies [Stone et al35] tasks), 2 metaphor-irony stories,36 and 10 faux pas recognition stories.35 These story-based tasks examined the ability, at different complexity levels, to “meta-represent” mental states of others (eg, Suresh thinks that Rani will go to the temple area to buy the ice cream because she has not seen the ice cream man go toward the school).30
Social Perception
A set of 18 true/false questions were asked on social (eg, Ali asked many questions about the movie because he was trying to impress Sunil) and nonsocial cues (eg, Harish and Lakshmi were looking over a book together) after showing the subjects 4 each of low and high emotion videos depicting a social interaction.30 This test was adapted from the social cue recognition test.37
Emotion Processing
Facial emotion recognition ability was assessed using 52 static images and 28 dynamic videos portraying 2 different intensities (low and high) of 6 basic human emotions (happy, sad, fear, anger, surprise, and disgust) depicted by 4 trained actors (1 young male, 1 young female, 1 older male, and 1 older female).31
Both SOCRATIS and TRENDS have been validated in the Indian cultural setting with satisfactory psychometrics. Each test provides an index of the respective test performance, which is equivalent to the score of an individual on the test divided by the maximum score possible.30,31 We considered metaphor and irony detection as first- and second-order ToM, respectively.30 Faux pas recognition is often described as a higher order ToM ability.38 However, in addition to mental state attribution (ie, purely ToM), it taps affective processing39 as well—for instance, one of the clarifying questions assesses how a person in the story felt during the faux pas. Thus, we had 4 final scores that were used in the analysis, namely, ToM (combined first and second order), faux pas, social perception (combined low and high emotion), and emotion recognition (combined static and dynamic portrayals) indices.
TMS Experiment to Assess Putative MNA
Subjects underwent an experiment to assess motor cortical excitability during goal-directed action observation, relative to rest states, to elicit a putative measure of MNA. Similar approaches have been previously reported in normal subjects23,40 and various patient populations.17,21
Subjects were seated comfortably in a chair, in a silent room, 50cm from the presentation monitor, with their elbows flexed at 90° and hands rested on the armrest of the chair in a prone position.15 Single-pulse TMS was applied using a 70-mm figure-of-eight coil (MagPro R30 with MagOption; MagVenture, Farum, Denmark) positioned tangentially over the hand area of the left motor cortex, with the handle pointing posterolaterally at a 45° angle to the sagittal plane.
For each subject, the optimal coil position was determined based on standard methods described in previous studies41,42 for localizing the scalp area from which TMS elicited motor potentials of maximal amplitude in the right first dorsal interosseous (FDI) muscle. This site was marked with a skin marker pen to ensure uniformity of coil positioning throughout the experiment. The coil was held with both hands bracing the coil against the head.42 Magnetic pulses activate cortical pyramidal neurons, leading to corticospinal output that can be measured peripherally as a MEP using electromyography. Initially, all participants underwent a calibration session during which their motor thresholds (MTs) were determined. Resting MT (RMT) was defined as the minimum stimulation intensity (measured in percentage of maximum machine output) required, to evoke a >50-μV MEP in the resting, right FDI muscle in at least 6 out of 10 consecutive trials, measured using electromyography.43 MT of 1 mV (MT1) was defined as the minimum stimulation intensity, evoking 1 mV peak-to-peak amplitude in the resting, right FDI muscle in at least 6 out of 10 successive recordings.43 Both RMT and MT1 were calculated using progressive reduction of stimulator intensity from suprathreshold levels in 1% decrements as described earlier.41
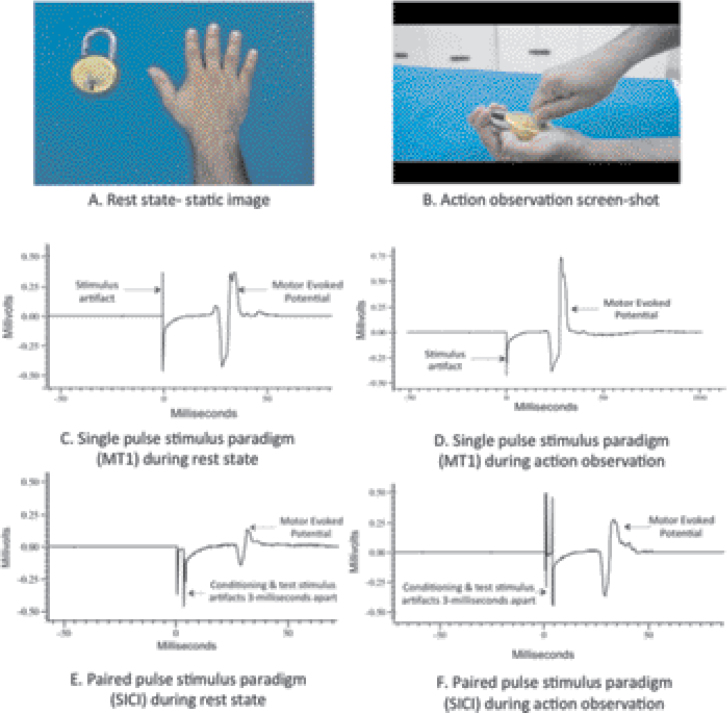
Next, participants underwent the experiment session (see figure 1 for illustration). Four stimulus paradigms (two single-pulse and two paired-pulse paradigms) were used to study cortical excitability while the participants watched three different action-related visual displays (see below).
Fig. 1.
Depiction of the rest/action observation states subjects were made to visualize during the transcranial magnetic stimulation experiment and the expected changes in motor-evoked potentials. (A and B) Represent the static image (A) of a hand/lock and key and the screen-shot (B) of video/actual action, depicting a goal-directed action that uses the right first dorsal interosseous muscle. Subjects watched these during rest state and action observation respectively. (C and D) Represent motor evoked potential (MEP) recordings with single pulse paradigm (motor threshold-1 stimulus-MT1) and expected changes in MEP during rest state (C) and action observation (D). Note the increase in MEP amplitude during action observation, with the use of same stimulus intensity. Recordings with the other single pulse paradigm (120% of resting motor threshold) would also appear similar. (E and F) Represent the MEP recordings using paired pulse stimulus paradigm (short interval intracortical inhibition-SICI) during rest state (E) and action observation (F). Note the increase in MEP amplitude during action observation, with the use of same stimulus intensity. Long interval intracortical inhibition (conditioning and test pulses separated 100 milliseconds apart) changes during rest state and action observation would also appear similar. Note: Increase in MEP amplitude during action observation is reflective of mirror neuron activity in both single and paired pulse paradigms. This MEP facilitation is hypothesized to be due to cortico-cortical connections between the premotor mirror neuron regions and the motor cortex 15. While single pulse stimuli reflect neuronal cell membrane excitability 44, paired pulse paradigms reflect inhibitory GABAergic neurotransmission 46, 48 mediating putative mirror neuron activity. Please see text in methods section for further details.
Single-Pulse Paradigms
120% of RMT
MEPs obtained with stimulus intensity equal to 120% of RMT were recorded. This stimulus intensity has been the most commonly implemented in studying putative mirror mechanisms using TMS.17,42
Motor Threshold-1
MEPs obtained with stimulus intensity equal to MT1 were recorded.
Both 120% RMT and MT1 are a measure of membrane excitability of pyramidal neurons, being influenced by voltage-gated sodium channels.44
Paired-Pulse Paradigms
Short Interval Intracortical Inhibition
A subthreshold conditioning stimulus (80% of RMT) was given 3 milliseconds before a suprathreshold test stimulus (MT1) with the right hand at rest. The subthreshold conditioning stimulus excites only the cortical interneurons and therefore inhibits the MEP response to the test stimulus (conditioned MEP).45 Gamma aminobutyric acid-type A (GABAA) receptor agonists potentiate short interval intracortical inhibition (SICI), thus suggesting that SICI may be mediated by GABAA receptor-mediated neurotransmission.46
Long Interval Intracortical Inhibition
A suprathreshold conditioning stimulus (MT1) is given 100 milliseconds before a suprathreshold test stimulus (MT1).47 The suprathreshold conditioning stimulus activates GABAB receptor-mediated inhibitory postsynaptic potentials, thus inhibiting the MEP response to the test stimulus (conditioned MEP). Thus, long interval intracortical inhibition (LICI) is thought to reflect cortical inhibition mediated through GABAB receptor-mediated neurotransmission, based, eg, on findings that baclofen, a specific GABAB receptor agonist enhances LICI.48
Both the paired-pulse paradigms (SICI and LICI) assess inhibitory functions of the motor cortex (ie, the extent to which the conditioning stimulus inhibits the response of the suprathreshold test stimulus). SICI and LICI were expressed as a percentage of the ratio between the conditioned MEPs and the nonconditioned MEPs with stimulus intensity of MT1; ie, (conditioned MEP/nonconditioned MEP) × 100.49
Ten MEP recordings, using each of these 4 stimulus paradigms (total of 40 recordings), were elicited in random sequence with 5-second intervals, while the subjects observed each of the following:
1. Actual observation of an action with active FDI: The subjects observed the experimenter’s hand, holding a key in lateral pinch grip (grasping objects between the side of the index finger and the thumb) to perform locking/unlocking actions. This action requires contraction of the FDI to abduct the index finger.50
2. Virtual observation: The subjects observed a video showing the above action (see figure 1).
3. Rest state: The subjects observed a still image of a hand and a lock displayed on the monitor (see figure 1).
The sequence of displaying these experimental states to each subject was randomized. In order to guarantee optimal attention allocation during the TMS experiments, subjects were instructed to pay attention to all the stimuli throughout the experiment.42 In addition, to further ensure attention, a second experimenter monitored the subjects’ behavior as has been done in earlier studies.51 All subjects who completed the TMS experiment were found to be attentive during the experiment as assessed during behavioral observation. Data acquisition and analysis were done using Signal-4 Software (Cambridge Electronic Devices, Cambridge, UK).
Calculation of MNA.
For single-pulse paradigms, the difference in MEP between rest and action-observation states (average across virtual and actual action observation) formed the measure of putative MNA. For paired-pulse paradigms, the difference in cortical inhibition (expressed as SICI and LICI) between rest and action-observation states formed the measure of putative MNA.
Statistical Analyses
Univariate statistics (ANOVA and chi-square tests) were used to compare clinical, demographic, SC variables, and baseline cortical excitability parameters across the 3 groups (antipsychotic-naive patients, medicated patients, and healthy comparison subjects). To examine putative MNA, we compared the MEP amplitudes (with the 4 stimulus paradigms described above) during “rest” state and “action-observation” state using 1-way repeated measures ANOVA (RMANOVA), separately for the 3 groups. To compare putative MNA across the 3 groups, we used 2-way RMANOVA for each of the 4 stimulus paradigms. Finally, to examine the association of magnitude of MNA with SC test scores and PANSS symptom scores, we conducted a correlational analysis. All statistical tests were two tailed and significance was set at an error probability of .05.
Results
Demographic and Clinical Variables
As summarized in table 1, the 3 groups were comparable for age, gender, and education. Patients in the medicated group were on atypical antipsychotics (12 taking risperidone, 4 olanzapine, 3 risperidone + olanzapine, 1 olanzapine + amisulpride, and 1 aripiprazole) with median duration of treatment with antipsychotics being 60 days and mean chlorpromazine equivalents of 413.43±226.95mg/d.52 The medicated patients had significantly greater proportion of inpatients than the antipsychotic-naive group. The diagnosis of paranoid subtype of schizophrenia was also more common in this group, albeit at trend level.
Table 1.
Demographic and Clinical Characteristics of the Antipsychotic-Naive Schizophrenia Patients, Medicated Schizophrenia Patients, and Healthy Comparison Subjects
| Antipsychotic-Naive Schizophrenia Patients (n = 33) | Medicated Schizophrenia Patients (n = 21) | Healthy Comparison Group (n = 45) | F/t/χ 2 | P | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||||
| Age (years) | 33.60 | 9.74 | 29.19 | 6.60 | 30.68 | 9.57 | 1.72 | .184 | |
| Education (years) | 11.75 | 2.25 | 13.09 | 2.80 | 13.13 | 3.50 | 2.28 | .107 | |
| Illness duration (months) | 41.12 | 44.20 | 50.42 | 42.65 | — | −0.764 | .448 | ||
| PANSS total score | 88.06 | 21.72 | 84.04 | 23.36 | — | 0.643 | .523 | ||
| PANSS-positive symptoms | 24.39 | 5.47 | 23.42 | 5.77 | — | 0.619 | .539 | ||
| PANSS-negative symptoms | 23.48 | 9.54 | 21.76 | 8.99 | — | 0.661 | .511 | ||
| N | % | N | % | N | % | ||||
| Female | 15 | 45.5 | 12 | 57.1 | 22 | 50 | 0.701 | .704 | |
| Inpatient status | 6 | 18.2 | 11 | 52.4 | — | 6.958 | .008 | ||
| Paranoid | 19 | 57.6 | 17 | 81 | 3.156 | .076 | |||
| Type of schizophrenia | Others | 14 | 42.4 | 4 | 19 | — | |||
Note: PANSS, Positive and Negative Syndrome scale.
Baseline Cortical Excitability
MTs (RMT and MT1) were comparable across the 3 groups. MEP amplitudes recorded during the static image observation state for single-pulse paradigms were also comparable. Baseline SICI, but not LICI, was less in the antipsychotic-naive patient group compared with the other 2 groups (supplementary table 1).
Performance on SC Tests
Patients demonstrated significant deficits across all SC domains when compared with healthy comparison subjects as demonstrated in table 2. Post hoc analysis (Tukey’s test) revealed better ToM and emotion recognition scores in medicated patients than antipsychotic-naive patients.
Table 2.
Social Cognition Performance of the Antipsychotic-Naive Schizophrenia Patients, Medicated Schizophrenia Patients, and Healthy Comparison Subjects
| Antipsychotic-Naive Schizophrenia Patients (n = 33) | Medicated Schizophrenia Patients (n = 21) | Healthy Comparison Group (n = 45)a | F b | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Theory of mind indexc | 0.48 | 0.20 | 0.60 | 0.21 | 0.84 | 0.13 | 41.48 |
| Faux pas composite index | 0.41 | 0.26 | 0.44 | 0.21 | 0.84 | 0.18 | 43.99 |
| Emotion recognition indexc | 0.53 | 0.15 | 0.66 | 0.07 | 0.78 | 0.08 | 45.59 |
| Social perception index | 0.49 | 0.16 | 0.56 | 0.16 | 0.83 | 0.06 | 77.24 |
aPost hoc analysis using Tukey’s HSD (honestly significant difference) test revealed significantly better social cognition test performance across all indices in the healthy comparison subjects compared with both the patient groups.
bSignificance at P < .0001, degrees of freedom = 2,96.
cTukey’s HSD also revealed significantly better theory of mind (P = .049) and emotion recognition indices (P = .0003) in the medicated patient group than the antipsychotic naive patient group.
MNA Across the 3 Groups
In healthy comparison subjects, the test MEP was significantly higher during action observation than rest state for 120% RMT (1-way RMANOVA: F (df) = 5.72 (1,44), P = .021), MT1 (F (df) = 6.54 (1,44), P = .014), and SICI (F (df) = 4.16 (1,44), P = .04) stimulus paradigms, suggesting mediation of MNA. In contrast, antipsychotic-naive schizophrenia patients showed no significant difference between rest and action-observation states for 120% RMT (F (df) = 2.74 (1,32), P = .11), MT1 (F (df) = 2.34 (1,32), P = .136), SICI (F (df) = 0.96 (1,32), P = .335) stimulus paradigms. In the medicated patient group, there were mixed results: MNA mediation was observed with MT1 (F (df) = 4.88 (1,20), P = .039) and SICI (F (df) = 7.66 (1,20), P = .012) stimulus paradigms, but not with 120% RMT (F (df) = 0.38 (1,20), P = .545) (see supplementary figure 1). The LICI stimulus paradigm did not reveal modulation by action observation (no influence of MNA) in any of these groups (data not shown).
Two-way RMANOVA revealed significant group × occasion interaction effect for all stimulus paradigms except LICI, indicating deficient MNA in antipsychotic-naive schizophrenia patients as compared with the other 2 groups (see table 3 and supplementary figure 1). Separate 2-way RMANOVAs to examine the difference between medicated and antipsychotic-naive patients revealed a significant group × occasion interaction effect for MT1 (F (df) = 8.31 (1,53), P = .006) and SICI (F (df) = 7.29 (1,53), P = .009) stimulus paradigms, and a trend level significance for 120% RMT (F (df) = 3.78 (1,53), P = .058) stimulus paradigm, indicating better MNA in medicated than in antipsychotic-naive patients.
Table 3.
Motor-Evoked Potentials (in Millivolts) With Single- and Paired-Pulse Stimulation Paradigms During Rest and Action Observation Experimental States in Antipsychotic-Naive/Medicated Schizophrenia Patients and Healthy Subjects
| MEP Stimulus Paradigms | Experimental State | Antipsychotic-Naive Schizophrenia Patients (n = 33) | Medicated Schizophrenia Patients (n = 21) | Healthy Comparison Group (n = 45) | F Statisticsa | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | F 1 | F 2 | F 3 | ||
| 120% RMT | Rest | 0.69 | 0.35 | 0.67 | 0.39 | 0.76 | 0.31 | 0.92 | 1.94 | 3.91* |
| Action observation | 0.65 | 0.37 | 0.69 | 0.38 | 0.85 | 0.27 | ||||
| Motor threshold-1 | Rest | 0.89 | 0.28 | 0.79 | 0.32 | 0.89 | 0.24 | 5.42* | 1.08 | 4.74* |
| Action observation | 0.85 | 0.27 | 0.89 | 0.24 | 0.96 | 0.2 | ||||
| SICI | Rest | 83.84 | 31.07 | 70.51 | 41.41 | 64.11 | 27.90 | 6.05* | 1.26 | 3.21* |
| Action observation | 80.29 | 34.01 | 86.90 | 52.73 | 76.01 | 41.61 | ||||
| LICI | Rest | 35.82 | 26.33 | 27.89 | 19.09 | 42.18 | 45.38 | 0.88 | 1.45 | 1.29 |
| Action observation | 29.83 | 23.53 | 27.95 | 21.84 | 42.63 | 47.98 | ||||
Notes: SICI and LICI were expressed as a percentage of the ratio between the conditioned MEPs and the nonconditioned MEPs with stimulus intensity of MT1; ie, (conditioned MEP/nonconditioned MEP) × 100. LICI, long interval intracortical inhibition; MEP, motor-evoked potentials; MT1, motor threshold of 1 mV; RMT, resting motor threshold; SICI, short interval intracortical inhibition.
aTwo-way repeated measures ANOVA: F 1 = occasion effect, F 2 = group effect, F 3 = group × occasion interaction effect, with significance at *P < .05.
Relationship of MNA With SC and Symptom Scores
We conducted separate Pearson’s correlation analyses (table 4) for the 2 patient groups. MNA measured using the 120% RMT stimulus paradigm had a significant correlation with ToM index in the antipsychotic-naive patient group. MNA measured using MT1 and SICI paradigms had trend level correlations with the ToM index. The correlation coefficients in the medicated patient group were largely comparable to those in the antipsychotic-naive group, but were not statistically significant.
Table 4.
Correlations (Pearson’s r) Between Putative Mirror Neuron Activity and Measures of Social Cognition and Symptoms in Patients
| MNA Using MT1 Stimulus | MNA Using 120% RMT Stimulus | MNA Using SICI | MNA Using LICI | |
|---|---|---|---|---|
| Antipsychotic-naive schizophrenia patients (n = 33) | ||||
| Theory of mind index | 0.324*** | 0.443** | 0.324*** | 0.18 |
| Faux pas composite index | 0.121 | 0.247 | 0.038 | −0.14 |
| Emotion recognition index | 0.062 | 0.17 | 0.208 | 0.123 |
| Social perception index | 0.06 | 0.039 | 0.273 | −0.112 |
| PANSS-positive symptoms | 0.018 | 0.093 | 0.143 | 0.314*** |
| PANSS-negative symptoms | −0.034 | −0.086 | −0.051 | 0.1 |
| Schizophrenia patients prescribed medication (n = 21) | ||||
| Theory of mind index | 0.396*** | −0.083 | 0.275 | -0.18 |
| Faux pas composite index | 0.293 | 0.225 | 0.189 | 0.05 |
| Emotion recognition index | 0.242 | −0.249 | 0.311 | 0.066 |
| Social perception index | 0.118 | 0.098 | −0.03 | 0.357 |
| PANSS-positive symptoms | −0.359 | −0.253 | −0.304 | −0.178 |
| PANSS-negative symptoms | 0.025 | 0.068 | 0.217 | −0.397*** |
| Combined patient group (n = 54) | ||||
| Theory of mind index | 0.413** | 0.275* | 0.361** | 0.101 |
| Faux pas composite index | 0.196 | 0.247*** | 0.106 | −0.092 |
| Emotion recognition index | 0.228 | 0.144 | 0.32* | 0.149 |
| Social perception index | 0.145 | 0.096 | 0.203 | 0.036 |
| PANSS-positive symptoms | −0.17 | −0.06 | −0.061 | 0.159 |
| PANSS-negative symptoms | −0.04 | −0.05 | −0.02 | −0.038 |
Notes: Abbreviations are explained in the first footnote to table 3. MNA, mirror neuron activity—measured as the difference between motor-evoked potentials at rest and action observation states using motor threshold-1 stimulus, 120% resting motor threshold stimulus, and short and long interval intracortical inhibition.
Significance at **P < .01, *P < .05, trend level correlation at ***P < .08.
Subsequently, we conducted Pearson’s correlational analyses between measures of MNA and SC in the combined patient group (antipsychotic-naive and medicated patients; n = 54). Significant positive correlation between putative MNA (measured using 120% RMT, MT1, and SICI stimulus paradigms) and ToM index was observed (table 4 and supplementary figure 2). In addition, MNA measured using the SICI stimulus paradigm also revealed a significant correlation with emotion recognition index. No other SC ability had significant correlations with measures of MNA. Further, there was no correlation between MNA and symptom scores (table 4). In healthy comparison subjects, none of the MNA measures had a significant correlation with any of the SC scores (data not shown). Given that the medicated group had a greater proportion of inpatients (P = .008) and paranoid subtype of schizophrenia (P = .076), we examined if illness subtype and inpatient status altered the relationship between ToM index and individual measures of MNA, using multiple-linear regression analyses. We found that the relationship between MNA and SC indices (ToM and emotion recognition) remained significant even after controlling for inpatient status and illness subtype (see supplementary table 2).
Discussion
In this study, we (a) compared putative MNA measured using TMS in patients with schizophrenia and matched healthy comparison subjects, and (b) explored the association between putative MNA and SC abilities in schizophrenia patients. We found greater enhancement of MEP during action observation relative to rest states in healthy comparison subjects and medicated patients when compared with antipsychotic-naive schizophrenia patients. This finding suggests that antipsychotic-naive schizophrenia patients have significant deficits in putative MNA when compared with the other 2 groups. We also observed that in the combined patient group, there was a significant association between measures of MNA and performance on ToM tasks. Further, MNA measured using SICI stimulus paradigm had a significant association with emotion-processing abilities in addition to ToM. These associations were observed even after controlling for the effects of inpatient status and illness subtype.
The association between putative MNA measures and ToM index was more consistent in the antipsychotic-naive group, than in the medicated patient group. All measures of MNA except that measured using LICI paradigm had at least trend level significance of association with ToM index; 1 measure (120% RMT stimulus) reached significance of P < .05. The correlation coefficients (r) for this association in the medicated patient group were comparable to those observed in the antipsychotic-naive group. However, this did not reach statistical significance. As the correlation coefficients were comparable, the lack of statistical significance appears to be due to a type-2 error—it may be noted that the sample size in the medicated patient group was small (n = 21).
Our fairly consistent demonstration of poor MNA in antipsychotic-naive schizophrenia patients compared with medicated patients and healthy comparison subjects, replicates earlier findings of MNA deficits in patients with schizophrenia.13,14,17 Medicated schizophrenia patients showed better MNA than antipsychotic-naive schizophrenia patients. Both the antipsychotic-naive and medicated patient groups had comparable symptom severity ratings, possibly because many of the medicated patients were symptomatic inpatients, admitted for symptom exacerbations after stopping previously prescribed antipsychotics or having poor response to antipsychotics. Evidently, the difference in MNA across these 2 groups was not due to differences in their symptom severity. Moreover, we did not find any correlation between MNA and symptom severity scores.
It has been demonstrated that unmedicated schizophrenia patients have reduced GABA neurotransmission-driven cortical inhibition41,53 and MT (reflective of membrane excitability),41 compared with patients on antipsychotics and healthy controls. These findings have kindled hypotheses that antipsychotics may improve cortical inhibition and membrane excitability in schizophrenia by their property of modulating the baseline dopaminergic tone. In the context of this study, such an influence of antipsychotics on motor cortex excitability is likely to occur during the rest state as well as during action observation. Because MNA is measured as a difference in cortical excitability between rest state and observation of goal-directed actions, the observed difference in MNA is unlikely to be due to this property of antipsychotics.
The oxytocin-enhancing action of antipsychotic medications may possibly explain the observed differences in MNA across the 2 patient groups. Together, human54 and animal55 studies have demonstrated that antipsychotic medications increase both central and peripheral oxytocin levels. Oxytocin and dopamine interact in the nucleus accumbens and the ventral tegmental areas to regulate social bonding.56 Interestingly, intranasal oxytocin administration enhances putative MNA as measured using EEG (mu wave suppression).57 Collectively, these findings suggest the role of antipsychotic medications in adaptively modulating MNA. This, however, needs to be demonstrated in longitudinal study designs.
We also found consistent and significant associations between MNA measured using 3 out of 4 stimulus paradigms and ToM index in the patient group. In addition, we found a significant association between MNA measured using SICI stimulus paradigm and emotion-processing abilities in patients. This is a replication of our preliminary demonstration58 of associations between MNA and SC abilities in schizophrenia patients. Consistent with this finding, antipsychotic-naive patients also had significantly lower ToM and emotion recognition indices than the medicated group.
We found that the SICI stimulus paradigm was significantly modulated by MNA in healthy comparison subjects and medicated patients, but not in antipsychotic-naive schizophrenia subjects. The LICI paradigm did not show such MNA influence in any of the groups. A previous study demonstrated that action observation attenuates SICI, but not LICI in healthy individuals, suggesting the role of GABAA receptor-mediated neurotransmission40 in this process. Indeed, there is increasing evidence for abnormal GABA activity in schizophrenia as demonstrated by gene expression, immunohistopathological, in vivo brain imaging, and electrophysiological studies.59 Our finding of impaired MNA mediation with SICI suggests the role of impaired GABAA neurotransmission underlying MNA deficits in schizophrenia patients.
ToM or mental state attribution involves the ability to infer intentions, dispositions, and beliefs of others.3 In a functional MRI experiment, premotor MNA was shown to underlie the ability to understand intentions of others actions, which includes inferring forthcoming new goals.10 Not surprisingly, we observed a consistent association of different measures of MNA with ToM abilities, when compared with the other SC abilities examined. It is however important to understand that there are additional neural mechanisms underlying SC, and that the mirror system is one of the components of this complex social brain network, perhaps responsible for low-level mental state inference.60 Such an association was not found in the healthy control group possibly due to a ceiling effect in their SC performance.
Investigational TMS paradigms similar to the ones used in this experiment have been extensively employed to measure putative MNA in humans.15,17,42 However, a few limitations of TMS as a measure of MNA need to be noted. It is an indirect quantification of mirror mechanisms. While intracranial depth electrodes give the most definitive evidence of MNA, their use in humans, especially in those with psychiatric disorders, is very challenging. Further, TMS experiments provide poorer spatial resolution than functional neuroimaging methods for eliciting MNA. Nevertheless, they have excellent temporal resolution, with millisecond precision61 and offer reliable functional resolution. During the TMS experiment, there was no objective attention task (eg, continuous performance test)12 embedded during action observation to ensure optimal attention allocation. This raises the possibility that antipsychotic-naive schizophrenia patients had poorer MNA because they did not attend to the stimuli. However, efforts were made to ensure the subjects attended to the visual stimuli, by instructing them to do so and by having an experimenter monitor their behavior; all subjects who completed the TMS experiment were attentive. Finally, MNA recorded using TMS presumably reflects mirror properties of the premotor cortex, which through corticocortical connections to the motor cortex or corticospinal connections to the spinal cord15 result in greater MEPs from the hand muscle during action observation when compared with rest states. Measuring MNA from the motor cortex limits the generalizability of these experiments to possible premotor/inferior frontal gyrus mirror properties. MNA in other parts of the core mirror system (inferior parietal lobule) and the extended mirror system (insular cortex, superior temporal sulcus, primary and secondary motor, and somatosensory cortices), which also underlie emotion processing and self-awareness are left unmeasured.16 Yet, the significant associations between ToM and different measures of premotor MNA in this experiment may support the neural exploitation hypothesis of mirror neuron-driven embodied simulation.
In conclusion, we provide evidence for deficient MEP enhancement during action observation relative to rest states, in antipsychotic-naive schizophrenia patients as assessed using TMS. This indirectly indicates a dysfunction in the premotor mirror mechanisms of antipsychotic-naive schizophrenia. Lower MNA in the patient group was associated with poorer ToM abilities, and perhaps poorer emotion processing. Given the emerging evidence for the role of SC deficits (ToM in particular, Fett et al4) in determining socio-occupational dysfunction, future studies should aim to study MNA in patients remitted from their active positive symptoms (but having persistent negative symptoms), and examine its association with ToM and a measure of their socio-occupational functioning. These findings contribute to our understanding of the neurophysiology of SC deficits in schizophrenia patients, thus providing novel treatment targets to be explored in future research.
Supplementary Material
Supplementary material is available at http:// schizophreniabulletin.oxfordjournals.org.
Funding
Department of Biotechnology, Ministry of Science & Technology, Government of India (BT/PR14311/Med/30/470/2010 to U.M.M.); National Institutes of Health—Harvard Clinical and Translational Science Center/Harvard Catalyst (UL1 RR025758) and the Sidney-Baer Foundation to P.-L.
Supplementary Material
Acknowledgment
Dr Pascual-Leone serves on the scientific advisory boards for Nexstim, Neuronix, Starlab Neuroscience, Neuroelectrics, and Neosync; and is listed as an inventor on several issued and pending patents on the real-time integration of transcranial magnetic stimulation (TMS) with electroencephalography (EEG), and magnetic resonance imaging (MRI). The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Green MF, Bearden CE, Cannon TD., et al. Social cognition in schizophrenia, part 1: performance across phase of illness. Schizophr Bull. June, 2011; 38:854–864. 10.1093/schbul/sbq171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mehta UM, Thirthalli J, Naveen Kumar C, Keshav Kumar J, Keshavan MS, Gangadhar BN. Schizophrenia patients experience substantial social cognition deficits across multiple domains in remission. Asian J Psychiatr. 2013;6:324–329 [DOI] [PubMed] [Google Scholar]
- 3. Green MF, Penn DL, Bentall R., et al. Social cognition in schizophrenia: an NIMH workshop on definitions, assessment, and research opportunities. Schizophr Bull. 2008;34:1211–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fett AK, Viechtbauer W, Dominguez MD, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Biobehav Rev. 2011;35:573–588 [DOI] [PubMed] [Google Scholar]
- 5. Ventura J, Wood RC, Hellemann GS. Symptom domains and neurocognitive functioning can help differentiate social cognitive processes in schizophrenia: a meta-analysis. Schizophr Bull. 2013;39:102–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iacoboni M, Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nat Rev Neurosci. 2006;7:942–951 [DOI] [PubMed] [Google Scholar]
- 7. Gallese V, Sinigaglia C. What is so special about embodied simulation? Trends Cogn Sci. 2011;15:512–519 [DOI] [PubMed] [Google Scholar]
- 8. Dimaggio G, Lysaker PH, Carcione A, Nicolò G, Semerari A. Know yourself and you shall know the other. to a certain extent: multiple paths of influence of self-reflection on mindreading. Conscious Cogn. 2008;17:778–789 [DOI] [PubMed] [Google Scholar]
- 9. Mukamel R, Ekstrom AD, Kaplan J, Iacoboni M, Fried I. Single-neuron responses in humans during execution and observation of actions. Curr Biol. 2010;20:750–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iacoboni M, Molnar-Szakacs I, Gallese V, Buccino G, Mazziotta JC, Rizzolatti G. Grasping the intentions of others with one’s own mirror neuron system. PLoS Biol. 2005;3:e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rizzolatti G, Fadiga L, Matelli M., et al. Localization of grasp representations in humans by PET: 1. Observation versus execution. Exp Brain Res. 1996;111:246–252 [DOI] [PubMed] [Google Scholar]
- 12. McCormick LM, Brumm MC, Beadle JN, Paradiso S, Yamada T, Andreasen N. Mirror neuron function, psychosis, and empathy in schizophrenia. Psychiatry Res. 2012;201:233–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schürmann M, Järveläinen J, Avikainen S., et al. Manifest disease and motor cortex reactivity in twins discordant for schizophrenia. Br J Psychiatry. 2007;191:178–179 [DOI] [PubMed] [Google Scholar]
- 14. Kato Y, Muramatsu T, Kato M, Shibukawa Y, Shintani M, Mimura M. Magnetoencephalography study of right parietal lobe dysfunction of the evoked mirror neuron system in antipsychotic-free schizophrenia. PLoS One. 2011;6:e28087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fadiga L, Fogassi L, Pavesi G, Rizzolatti G. Motor facilitation during action observation: a magnetic stimulation study. J Neurophysiol. 1995;73:2608–2611 [DOI] [PubMed] [Google Scholar]
- 16. Molenberghs P, Cunnington R, Mattingley JB. Brain regions with mirror properties: a meta-analysis of 125 human fMRI studies. Neurosci Biobehav Rev. 2012;36:341–349 [DOI] [PubMed] [Google Scholar]
- 17. Enticott PG, Hoy KE, Herring SE, Johnston PJ, Daskalakis ZJ, Fitzgerald PB. Reduced motor facilitation during action observation in schizophrenia: a mirror neuron deficit? Schizophr Res. 2008;102:116–121 [DOI] [PubMed] [Google Scholar]
- 18. Mehta UM, Agarwal SM, Kalmady SV., et al. Enhancing putative mirror neuron activity with magnetic stimulation: a single-case functional neuroimaging study. Biol Psychiatry. 2013;74(3):e1–2 [DOI] [PubMed] [Google Scholar]
- 19. Matthews N, Gold BJ, Sekuler R, Park S. Gesture imitation in schizophrenia. Schizophr Bull. 2013;39:94–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fahim C, Stip E, Mancini-Marïe A, Boualem M, Malaspina D, Beauregard M. Negative socio-emotional resonance in schizophrenia: a functional magnetic resonance imaging hypothesis. Med Hypotheses. 2004;63:467–475 [DOI] [PubMed] [Google Scholar]
- 21. Enticott PG, Kennedy HA, Rinehart NJ., et al. Mirror neuron activity associated with social impairments but not age in autism spectrum disorder. Biol Psychiatry. 2012;71:427–433 [DOI] [PubMed] [Google Scholar]
- 22. McClintock SM, Freitas C, Oberman L, Lisanby SH, Pascual-Leone A. Transcranial magnetic stimulation: a neuroscientific probe of cortical function in schizophrenia. Biol Psychiatry. 2011;70:19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fadiga L, Craighero L, Olivier E. Human motor cortex excitability during the perception of others’ action. Curr Opin Neurobiol. 2005;15:213–218 [DOI] [PubMed] [Google Scholar]
- 24. Association AP. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Press; 1994 [Google Scholar]
- 25. Sheehan DV, Lecrubier Y, Sheehan KH., et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(s uppl 20):22–33;quiz 34–57. [PubMed] [Google Scholar]
- 26. Ganguli M, Ratcliff G, Chandra V., et al. A Hindi version of the MMSE: the development of a cognitive screening instrument for a largely illiterate rural elderly population in India. Int J Geriatr Psychiatry. 1995:367–377 [Google Scholar]
- 27. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113 [DOI] [PubMed] [Google Scholar]
- 28. Keel JC, Smith MJ, Wassermann EM. A safety screening questionnaire for transcranial magnetic stimulation. Clin Neurophysiol. 2001;112:720. [DOI] [PubMed] [Google Scholar]
- 29. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276 [DOI] [PubMed] [Google Scholar]
- 30. Mehta UM, Thirthalli J, Naveen Kumar C., et al. Validation of Social Cognition Rating Tools in Indian Setting (SOCRATIS): A new test-battery to assess social cognition. Asian J Psychiatr. 2011;4:203–209 [DOI] [PubMed] [Google Scholar]
- 31. Behere RV, Raghunandan V, Venkatasubramanian G, Subbakrishna DK, Jayakumar PN, Gangadhar BN. Trends - a tool for recognition of emotions in neuropsychiatric disorders. Indian J Psychol Med. 2008;30:32–38 [Google Scholar]
- 32. Wimmer H, Perner J. Beliefs about beliefs: representation and constraining function of wrong beliefs in young children’s understanding of deception. Cognition. 1983;13:103–128 [DOI] [PubMed] [Google Scholar]
- 33. Perner J, Leekam SR, Wimmer H. Three-year-olds’ difficulty with false belief: the case for a conceptual deficit. Br J Dev Psychol. 1987:125–137 [Google Scholar]
- 34. Perner J, Wimmer H. ‘John thinks that Mary thinks…’: Attribution of second-order beliefs by 5–10 year old children. J Exp Child Psychol. 1985:437–471 [Google Scholar]
- 35. Stone VE, Baron-Cohen S, Knight RT. Frontal lobe contributions to theory of mind. J Cogn Neurosci. 1998;10:640–656 [DOI] [PubMed] [Google Scholar]
- 36. Drury VM, Robinson EJ, Birchwood M. ‘Theory of mind’ skills during an acute episode of psychosis and following recovery. Psychol Med. 1998;28:1101–1112 [DOI] [PubMed] [Google Scholar]
- 37. Corrigan PW, Green MF. Schizophrenic patients’ sensitivity to social cues: the role of abstraction. Am J Psychiatry. 1993;150:589–594 [DOI] [PubMed] [Google Scholar]
- 38. Brüne M. “Theory of mind” in schizophrenia: a review of the literature. Schizophr Bull. 2005;31:21–42 [DOI] [PubMed] [Google Scholar]
- 39. Freedman M, Stuss DT. Theory of Mind in Parkinson’s disease. J Neurol Sci. 2011;310:225–227 [DOI] [PubMed] [Google Scholar]
- 40. Strafella AP, Paus T. Modulation of cortical excitability during action observation: a transcranial magnetic stimulation study. Neuroreport. 2000;11:2289–2292 [DOI] [PubMed] [Google Scholar]
- 41. Daskalakis ZJ, Christensen BK, Chen R, Fitzgerald PB, Zipursky RB, Kapur S. Evidence for impaired cortical inhibition in schizophrenia using transcranial magnetic stimulation. Arch Gen Psychiatry. 2002;59:347–354 [DOI] [PubMed] [Google Scholar]
- 42. Maeda F, Kleiner-Fisman G, Pascual-Leone A. Motor facilitation while observing hand actions: specificity of the effect and role of observer’s orientation. J Neurophysiol. 2002;87:1329–1335 [DOI] [PubMed] [Google Scholar]
- 43. Wasserman E. Inter- and intra-individual variation in the responses to TMS. In: Oxford Handbook of Transcranial Magnetic Stimulation. Oxford: Oxford University Press; 2008 [Google Scholar]
- 44. Trevillion L, Howells J, Bostock H, Burke D. Properties of low-threshold motor axons in the human median nerve. J Physiol. 2010;588:2503–2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kujirai T, Caramia MD, Rothwell JC., et al. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol. 1996;40:367–378 [DOI] [PubMed] [Google Scholar]
- 47. Valls-Solé J, Pascual-Leone A, Wassermann EM, Hallett M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol. 1992;85:355–364 [DOI] [PubMed] [Google Scholar]
- 48. McDonnell MN, Orekhov Y, Ziemann U. Suppression of LTP-like plasticity in human motor cortex by the GABAB receptor agonist baclofen. Exp Brain Res. 2007;180:181–186 [DOI] [PubMed] [Google Scholar]
- 49. Patuzzo S, Fiaschi A, Manganotti P. Modulation of motor cortex excitability in the left hemisphere during action observation: a single- and paired-pulse transcranial magnetic stimulation study of self- and non-self-action observation. Neuropsychologia. 2003;41:1272–1278 [DOI] [PubMed] [Google Scholar]
- 50. Geere J, Chester R, Kale S, Jerosch-Herold C. Power grip, pinch grip, manual muscle testing or thenar atrophy - which should be assessed as a motor outcome after carpal tunnel decompression? A systematic review. BMC Musculoskelet Disord. 2007;8:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Maeda F, Chang VY, Mazziotta J, Iacoboni M. Experience-dependent modulation of motor corticospinal excitability during action observation. Exp Brain Res. 2001;140:241–244 [DOI] [PubMed] [Google Scholar]
- 52. Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67:255–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Liu SK, Fitzgerald PB, Daigle M, Chen R, Daskalakis ZJ. The relationship between cortical inhibition, antipsychotic treatment, and the symptoms of schizophrenia. Biol Psychiatry. 2009;65:503–509 [DOI] [PubMed] [Google Scholar]
- 54. Beckmann H, Lang RE, Gattaz WF. Vasopressin–oxytocin in cerebrospinal fluid of schizophrenic patients and normal controls. Psychoneuroendocrinology. 1985;10:187–191 [DOI] [PubMed] [Google Scholar]
- 55. Uvnäs-Moberg K, Alster P, Svensson TH. Amperozide and clozapine but not haloperidol or raclopride increase the secretion of oxytocin in rats. Psychopharmacology (Berl). 1992;109:473–476 [DOI] [PubMed] [Google Scholar]
- 56. Skuse DH, Gallagher L. Dopaminergic-neuropeptide interactions in the social brain. Trends Cogn Sci. 2009;13:27–35 [DOI] [PubMed] [Google Scholar]
- 57. Perry A, Bentin S, Shalev I., et al. Intranasal oxytocin modulates EEG mu/alpha and beta rhythms during perception of biological motion. Psychoneuroendocrinology. 2010;35:1446–1453 [DOI] [PubMed] [Google Scholar]
- 58. Mehta UM, Basavaraju R, Thirthalli J, Gangadhar BN. Mirror neuron dysfunction-a neuro-marker for social cognition deficits in drug naïve schizophrenia. Schizophr Res. 2012;141:281–283 [DOI] [PubMed] [Google Scholar]
- 59. Daskalakis ZJ. On a quest for the elusive schizophrenia biomarker. Biol Psychiatry. 2012;72:714–715 [DOI] [PubMed] [Google Scholar]
- 60. Ochsner KN. The social-emotional processing stream: five core constructs and their translational potential for schizophrenia and beyond. Biol Psychiatry. 2008;64:48–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gangitano M, Mottaghy FM, Pascual-Leone A. Modulation of premotor mirror neuron activity during observation of unpredictable grasping movements. Eur J Neurosci. 2004;20:2193–2202 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.