Abstract
The task-positive network (TPN) is anticorrelated with activity in the default mode network (DMN), and possibly reflects competition between the processing of external and internal information, while the salience network (SN) is pivotal in regulating TPN and DMN activity. Because abnormal functional connectivity in these networks has been related to schizophrenia, we tested whether alterations are also evident in subjects at risk for psychosis. Resting-state functional magnetic resonance imaging was tested in 28 subjects with basic symptoms reporting subjective cognitive-perceptive symptoms; 19 with attenuated or brief, limited psychotic symptoms; and 29 matched healthy controls. We characterized spatial differences in connectivity patterns, as well as internetwork connectivity. Right anterior insula (rAI) was selected as seed region for identifying the SN; medioprefrontal cortex (MPFC) for the DMN and TPN. The 3 groups differed in connectivity patterns between the MPFC and right dorsolateral prefrontal cortex (rDLPFC), and between the rAI and posterior cingulate cortex (PCC). In particular, the typically observed antagonistic relationship in MPFC-rDLPFC, rAI-PCC, and internetwork connectivity of DMN-TPN was absent in both at-risk groups. Notably, those connectivity patterns were associated with symptoms related to reality distortions, whereas enhanced connectivity strengths of MPFC-rDLPFC and TPN-DMN were related to poor performance in cognitive functions. We propose that the loss of a TPN-DMN anticorrelation, accompanied by an aberrant spatial extent in the DMN, TPN, and SN in the psychosis risk state, reflects the confusion of internally and externally focused states and disturbance of cognition, as seen in psychotic disorders.
Key words: resting-state fMRI, schizophrenia, anticorrelated networks, functional connectivity, central executive network, anterior insula, prodrome, intrinsic connectivity, brain
Introduction
Resting-state functional magnetic resonance imaging (rs-fMRI) has revealed that spontaneous blood oxygen level-dependent (BOLD) signal activation is organized into spatially segregated functional networks.1–4 rs-fMRI studies applying intrinsic functional connectivity (iFC) analysis have shown that coordinated activity of dynamically configured large-scale brain networks is crucial for cognitive and executive functions.5–9 Disruption of these dynamics possibly leads to various pathological states, such as psychotic or cognitive symptoms as seen in schizophrenia.8,10,11 Indeed, these iFC abnormalities may play an important role in the pathogenesis of psychotic disorders. Particularly, research has focused on disturbances of the default mode network (DMN), the task-positive network (TPN), and the salience network (SN).12–18 The DMN, also known as the task-negative network, involves the posterior cingulate cortex (PCC), medial prefrontal cortex (MPFC), and lateral inferior parietal cortex. Activity in those regions is greater in individuals at rest than when engaged in goal-directed tasks and, correspondingly, has been associated with internally guided, perceptually decoupled thoughts, such as mental simulation or episodic retrieval.1,2,19–23 The DMN is anticorrelated with activity in the dorsolateral prefrontal cortex (DLPFC) and posterior parietal cortex that form the TPN (also referred to as central-executive network), a set of regions induced during goal-oriented activity.1,24–26 This suggests that the DMN-TPN antagonistic relationship is a fundamental property of the brain, possibly reflecting a shift between 2 distinct modes of information processing: the DMN, serving untargeted inner thought in one mode; and the TPN, serving focused, stimulus-dependent attention in the second mode.1,7,27
Evidence is also increasing that the functional competition between DMN and TPN is regulated by SN, which comprises the right anterior insula (rAI), ventrolateral prefrontal cortex, and anterior cingulate cortex.24,25,28 The rAI has a central role for bottom-up processing, assisting target brain regions in generating appropriate behavioral responses to salient stimuli.29 Moreover, the rAI is involved in the representation of current and predictive salience, particularly in the context of interoception.30
In schizophrenia, anomalies can occur in the coordination between DMN and TPN,14,31,32 at times weakening the reciprocity between those networks.15,33,34 Individuals with schizophrenia have reduced connectivity both within SN and between SN and DMN.18,35 Dynamic suppression of DMN is generally associated with better performance of attention-demanding tasks.36 Hence, the DMN hyperconnectivity, as seen in schizophrenia, may be related to impairments in attention and working memory and overly intensive self-referential and introspective processing.15,37 Emerging evidence has attributed those cognitive deficits in schizophrenia to dysfunctions in proper DMN-TPN coordination, whereas SN anomalies have been associated with reality distortion.30,35
Early in their prodromal phase of illness, most schizophrenia patients already exhibit attenuated or brief, limited psychotic symptoms and subtle, self-experienced disturbances in perception, thoughts, and cognition.38,39 Considering the changes in connectivity observed in schizophrenia, examining iFC in individuals at increased risk of developing psychosis may provide further insight into illness susceptibility and its underlying neuropathophysiological mechanisms. A reduced iFC between Broca’s area and the lateral and medial frontal cortices,40 plus increased TPN-DMN coupling, have been found in individuals at ultra-high risk (UHR) for psychosis.41 However, whether the risk state for psychosis is additionally associated with SN disturbances has not yet been investigated. Furthermore, to our knowledge, this is the first study exploring both, within and between iFC, and its association to symptoms related to reality distortions and cognitive processing in subjects at risk for psychosis.
Based on these previous findings, we hypothesized that clinical symptoms and disturbances of cognition seen in at-risk subjects are reflected by an aberrant spatial extent in DMN, TPN, and SN, accompanied by a loss of anticorrelation between those 3 networks. To test this, we evaluated rs-fMRI in 3 groups of subjects: 28 at risk for psychosis with basic symptoms (HR criteria), who described subtle, often only self-perceivable deficits42; 19 with attenuated and/or brief, limited intermittent psychotic (positive) symptoms43 (UHR criteria); and 29 healthy controls (CTRL). With this group separation, we also investigated whether iFC differs in the 2 clinical stages of risk, because it is presumed that HR criteria characterize the early prodromal phase, whereas UHR reflects the late prodromal phase.44,45 Using a seed-based approach, we first examined iFC to identify variations in spatiotemporal connectivity in SN, DMN, and the TPN among the 3 groups. We then tested group differences in internetwork connectivity via Pearson’s correlations between the first eigenvariate of each network. Finally, we explored possible relationships among significant aberrant iFC strength with severity of clinical symptoms, and cognitive variables.
Methods
Participants
This study consisted of 76 participants (29 CTRL, 28 HR, and 19 UHR) and was approved by the local ethics committee of Zurich. The risk groups were recruited in the Swiss region of Zurich within the context of a larger study on early recognition of psychosis (www.zinep.ch, accessed October 29, 2013). Following an initial screening, in-person diagnostic interviews were administered. After the subjects received complete project descriptions, we obtained their written, informed consent.
Participants reporting at least 1 cognitive-perceptive basic symptom or at least 2 cognitive disturbances, as assessed by the adult version of the Schizophrenia Proneness Interview (SPI-A), fulfilled the inclusion criterion for the HR status for psychosis.46 Those describing at least 1 attenuated psychotic symptom or brief, limited intermittent psychotic symptom, as assessed by the Structured Interview for Prodromal Syndromes (SIPS), fulfilled the criterion for UHR status.47 Four HR subjects and 6 in the UHR cohort were taking second-generation (atypical) antipsychotic medication at the time of scanning. Chlorpromazine (CPZ) equivalents were calculated for them.48 Five subjects each in the HR and UHR groups were being treated with an antidepressant.
Our healthy CTRLs were recruited through advertisement. Screening with the Mini-International Neuropsychiatric Interview49 was conducted to ensure that none had any current or prior history of psychiatric illness. Those receiving any medications were excluded.
Persons in HR, UHR, and CTRL were matched for handedness, sex, age, and IQ (table 1). The groups had a mean of estimated premorbid intelligence slightly above average, as assessed using a German test for fluid, nonverbal intelligence (LPS-3).50 Handedness was examined by the Edinburgh Handedness Inventory.51 Exclusion criteria were contraindications against MRI, pregnancy, history of neurological illness, drug, or alcohol dependence. Structural MRI scans were neurologically screened by an experienced neuroradiologist, and participants with structural brain abnormalities such as hyperintensities on fluid-attenuated inversion recovery sequences and other incidental lesions were excluded.
Table 1.
Demographic Characteristics and Symptom Rating
| CTRL | HR | UHR | Statistical Evaluation | |
|---|---|---|---|---|
| N | 29 | 28 | 19 | |
| Gender (female:male) | 13:16 | 13:15 | 6:13 | χ 2 = 1.17, n.s.a |
| Handedness (r:l:b) | 25:2:2 | 25:1:2 | 18:1:0 | χ 2 = 1.71, n.s.a |
| Age (years) | 22.8±5.0 | 24.01±5.6 | 20.3±3.9 | F = 3.1, n.s.b |
| Estimated intelligence (LPS-3) | 119.82±8.5 | 113.33±2.7 | 117.05±2.1 | F = 1.4, n.s.b |
| SIPS | ||||
| Positive | — | 4.32±2.52 | 10.11±3.31 | U = 45.00, P < .000c |
| Negative | — | 8.75±5.58 | 12.37±6.28 | U = 164.00, P = .03c |
| General | — | 6.61±3.38 | 7.47±3.76 | U = 215.00, n.s.c |
| Disorganization | — | 2.61±1.50 | 5.30±2.34 | U = 86.50, P < .000c |
| GAF | — | 64.04±13.6 | 59.1±11.26 | U = 1.27, n.s.c |
| CPZ equivalents | — | 5.1±14.1 | 128.2±380.3 | U = 208.5, n.s.c |
Notes: CPZ equivalents, chlorpromazin equivalents; CTRL, healthy controls; GAF, Global Assessment of Functioning scale mean; HR, subjects at risk for psychosis; r:l:b, r=right l=left b=both/bimanual; SIPS, symptoms according to Structured Interview for Prodromal Syndromes; UHR, subjects at ultra-high risk for psychosis.
aPearson’s chi-square test.
bThree-level ANOVA test.
cMann-Whitney U test; n.s., not significant (P > .05). ±SD where appropriate.
Imaging Data Acquisition
rs-fMRI data were acquired at the Zurich University Hospital of Psychiatry, Switzerland, using a Philips Achieva TX 3-T whole-body MR unit with an 8-channel head coil. Functional scans (6-min runs) involved a sensitivity-encoded single-shot echoplanar (factor 1) T2*-weighted echoplanar imaging (EPI) sequence (repetition time [TR] = 2000ms; echo time [TE] = 35ms; field of view [FOV] = 220 × 220mm2; acquisition matrix = 88 × 87, interpolated to 128 × 128; 32 contiguous slices with a spatial resolution of 2.5 × 2.5 × 4mm3 [reconstructed 1.72 × 1.72 × 4mm3]; flip angle θ = 78°; and sensitivity-encoded acceleration factor R = 1.8). Using a midsagittal scout image, we placed the contiguous axial slices along the anterior-posterior commissural plane, which covered the entire brain, and were acquired in ascending order. We also acquired 3-dimensional T1-weighted anatomical images (160 slices; TR = 1900ms; TE = 2.2ms; inversion time = 900ms; θ = 78°; spatial resolution, 1 × 1 × 1mm3 [reconstructed 0.94 × 0.94 × 1mm3]; FOV = 240 × 240mm2). The EPI sequences were conducted in darkness and participants were asked to keep their eyes closed during the session, lie as quietly as possible, and avoid falling asleep. Accuracy of compliance to these instructions was controlled by verbal confirmation immediately after the scan sequence. To minimize potential arousal and anxiety effects, we started rs-fMRI data acquisition 10 minutes after subjects were moved to their final positioning in the MR bore.
Data Analysis
Postprocessing of the rs-fMRI data was conducted using SPM8 (Wellcome Department of Imaging Neuroscience, London, UK; http://www.fil.ion.ucl.ac.uk/spm, accessed October 29, 2013), running in Matlab (Mathworks Inc, Sherbon, MA). The steps included realignment, slice-timing correction, coregistration to structural T1 scan, spatial normalization to Montreal Neurological Institute coordinates (MNI) space, and spatial smoothing (8-mm Gaussian kernel). The structural scans were segmented into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) tissue classes, per the unified segmentation approach.52 Using the SPM8 default values, the number of Gaussians were 2 for each; GM, WM, CSF, and 4 for everything else. None of the participants had to be excluded due to excessive head motion (linear shift <2mm across and, on a frame-to-frame basis, rotation <1°). Head motions in any direction did not differ significantly among the 3 groups (3-level ANOVA; all F <1.6, P > .14).
The CONN-fMRI functional connectivity toolbox v13 (http://www.nitrc.org/projects/conn, accessed October 29, 2013) was used to apply band-pass filtering (0.008 Hz < f < 0.09 Hz) and to create individual seed-to-voxel connectivity maps.53 Spurious sources of noise, such as heart rate and respiration signals, were first estimated by the anatomical component base noise reduction strategy (aCompCor),54 and then included with the head movement parameters as nuisance regressors in a general linear model. The aCompCor algorithm efficiently removes principal components from WM and CSF regions, and therefore does not rely on global signal regression, which can artificially introduce negative correlations.55,56 Based on experimental data from a schizophrenic population17,32–34 and recently proposed theoretical models for disturbance in the triple network,57 we tested spatiotemporal differences in interactions within and across DMN, TPN, and SN. To compare our results with existing iFC findings, we centered 2 a priori-determined seed regions of interest (ROI) on MNI coordinates, based on previous studies.1,13,14,24,32 The SN consisted of the correlation with the rAI (MNI coordinates: x = 38, y = 22, z = −10). In accordance with results reported by Fox et al. 1 and Fransson58 we defined DMN by regions showing positive correlations with the MPFC seed (MNI coordinates: x = −1, y = 49, z = −2); the TPN was then defined by regions showing negative correlations with that seed. The seed ROIs of 8-mm radius spheres were created with the MARSBAR toolbox (http://marsbar.sourceforge.net/, accessed October 29, 2013). Intrinsic connectivity networks were estimated on the basis of fMRI time series, and a bivariate Pearson’s correlation analysis was performed between the seed ROIs ascribed above and all other voxels in the brain. Cortical surface projection was performed for visualization using the (PALS)-B12 atlas59 and Caret software.60
To test for spatial differences among HR, UHR, and CTRL groups in each network, we entered the connectivity maps from all participants into a 3-level ANOVA to identify regions with different iFC among groups. Regions from the ANOVA that survived a voxel-level height threshold of P < .001 and an familywise error rate-corrected cluster-level extent threshold of P < .05 subsequently served as ROIs for post hoc analysis. Fisher z-transformed Pearson correlation values were used to compute the strength of iFC between either the MPFC or rAI seed and each ROI, and to examine the directionality of connectivity for each group.
We then aimed to determine whether, in addition to spatial differences within the networks, also the internetwork coupling varied among groups by assessing the internetwork connectivity of DMN-TPN, DMN-SN, and TPN-SN. To test whether connectivity differences are not only driven by an aberrant spatial extent of the networks, we conducted the analysis by using the same connectivity maps for all 3 groups. To estimate the extent to which between-network coupling differed from the normal internetwork coupling, we followed similar analysis of former studies13,14,32 and extracted the networks from our healthy CTRL group. Visual inspection of the networks indicated that the connectivity maps were consistent with prior findings25 (see figure 2A and supplementary figure S1). For the DMN, a single mask of within group thresholded map (whole-brain cluster corrected α .05 for voxel-wise P value of .001) was created, containing voxels that positively correlated with the MPFC seed ROI; for the TPN containing voxels that negatively correlated with the same ROI. Similarly, a single mask for the SN contained voxels that positively correlated with the rAI. We then calculated the first component from a single-value decomposition of the noncentral second moment of the BOLD time series within each mask. These components correspond to the first eigenvariate, which is a summary of the responses within a ROI and, unlike the average, does not assume homogenous responses within a ROI.61 These were extracted for each subject. To calculate coupling strengths, we performed pair-wise Pearson’s correlations between each first eigenvariate of the rs-fMRI time series within each network. Those z-transformed correlation values were then entered into a 3-level ANOVA (P < .05, corrected for multiple comparisons) to examine putative differences among groups. In correspondence to the analysis described above, first we aimed to identify the main effect of significant group differences by a 3-level ANOVA. Subsequently, post hoc we performed an unpaired 2-sample t test, to learn which group drives the effect. As a subsidiary analysis, we computed 1-sample t test of the Fisher z-transformed Pearson correlation values, which served as an index of coupling strength for each group.
Fig. 2.
Absence of anticorrelation between default mode network (DMN) and task-positive network (TPN) in subjects at risk for psychosis. (A) Seed-based functional connectivity for medioprefrontal cortex in healthy controls (CTRL) was used to derive spatial masks for DMN (yellow) and TPN (blue). Single-subject time series are illustrated for DMN (red) and TPN (blue) seen in CTRLs (B1) and persons meeting high-risk (HR) criteria (B2). Note increased TPN-DMN coupling in HR subject. (C) Group-level analysis showed no significant anticorrelations between TPN and DMN in HR and ultra-HR (UHR) compared with CTRL. Bars represent average DMN-TPN coupling among subjects within each group. Error bars indicate standard error of the means (SEM). ***P < .0001; n.s., P > .05.
The z values of aberrant iFC were used to determine how iFC strength and severity of clinical symptoms were related in at-risk subjects. As the HR and UHR groups were selected based on criteria that were also used to calculate the correlations, we avoided reporting any significance values and thus took account of the issue of circular analysis.62 Spearman’s correlation analysis was performed to test for relationships between altered iFCs of the rAI-PCC, MPFC-rDLPFC, DMN-TPN, and clinical symptom sum scores, as assessed by the SIPS and SPI-A clinical interviews. Additional Pearson’s correlations were examined between altered iFCs and cognitive domains known to be associated with early psychotic symptoms, we report the Bonferroni-corrected significance level at α < .003. Selective attention was measured using the Frankfurter Aufmerksamkeits-Inventar (FAIR).63 Executive function was assessed by the Rey-Osterrieth Complex Figure test (ROCF)64,65 and the computer-administered Tower of Hanoi (TOH),66 using age-standardized z scores. The normality of the score distributions was verified by the Kolmogorov-Smirnov test.
Results
Seed-Based Analysis
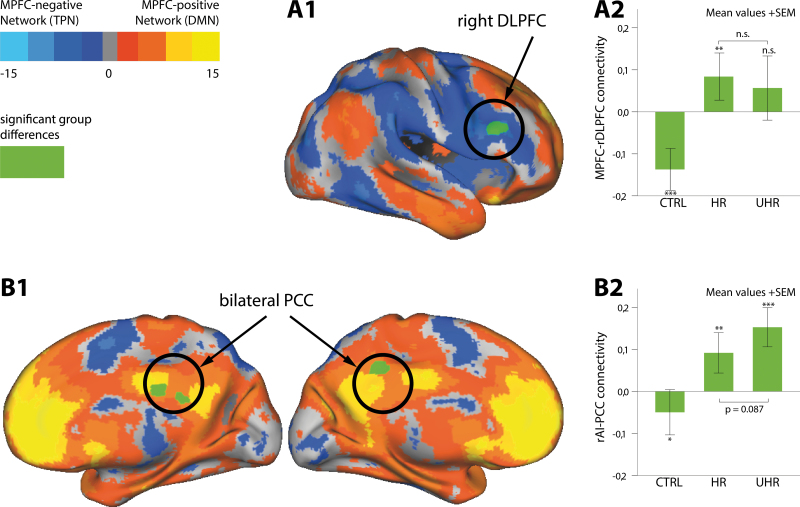
In order to explore differences in spatial extent, ie, to identify regions with aberrant iFC to the seeds, the connectivity maps from all participants were entered into a 3-level ANOVA. Our results showed that, among subject groups, differences were significant between the iFC of the MPFC seed and a single cluster in the right DLPFC (rDLPFC) (Brodmann Area [BA] 9/46, MNI peak coordinates: x = 56, y = 26, z = 28, cluster size = 222 voxels). For the SN, differences were significant between the rAI seed and bilateral PCC (BA 31/23, MNI peak coordinates: x = 6, y = −26, z = 30, cluster size = 201 voxels). The significant clusters were plotted onto areas that were anticorrelated with MPFC in CTRL, thereby revealing an overlap of DLPFC with the so-defined TPN. Similarly, PCC was an integral part of the DMN, as shown by areas with positive correlation to the MPFC (figure 1A1 and 1B1).
Fig. 1.
Spatial differences in intrinsic functional connectivity (iFC) networks among healthy controls (CTRL), subjects at high risk (HR), and ultra-HR for psychosis (UHR). Three-level ANOVA revealed significant differences (in green) in iFC between medioprefrontal cortex (MPFC) seed and right dorsolateral prefrontal cortex (rDLPFC) (A1), and between right anterior insular (rAI) cortex seed and posterior cingulate cortex (PCC) (B1). Projection of significant clusters onto areas anticorrelated with MPFC from CTRL (blue) indicated overlaps of rDLPFC with so-defined task-positive network (TPN), as well as PCC with the default mode network (DMN), ie, areas positively correlated with MPFC (orange). (A2) MPFC-rDLPFC was anticorrelated in CTRL, positively coupled in HR, and not coupled in UHR. (B2) CTRL revealed inverse coupling in rAI-PCC, while HR and UHR showed positive couplings with the seed. Bars represent average iFC of MPFC-rDLPFC and rAI-PCC among subjects within each group. Error bars indicate standard error of the means (SEM). ***P < .0001; **P < .01; *P < .05; n.s., P > .05.
Aberrant coupling between the seeds and corresponding clusters in HR and UHR groups compared with CTRL was manifested by post hoc ROI-level t tests (figure 1A2 and 1B2). CTRL showed a significant anticorrelation between the MPFC and rDLPFC connectivity (mean z = −0.14, t = −5.5, P < .0001, Cohen’s d = 1.08). By contrast, HR, but not UHR, had a positive coupling (mean z = 0.08, t = 3.0, P < .005, Cohen’s d = 0.53) (see also supplementary figure S2). Furthermore, CTRL had a significant inverse coupling between rAI and PCC (mean z = −0.05, t = −2.1, P < .05, Cohen’s d = 0.33), while the rAI seed was positively coupled with the PCC in both HR (mean z = 0.09, t = 3.8, P < .0005, Cohen’s d = 0.69) and UHR (mean z = 0.15, t = 5.2, P < .0001, Cohen’s d = 1.5) (see also supplementary figure S3). Whereas HR and UHR showed no significant differences in MPFC-rDLPFC connectivity (P = .56), the difference regarding rAI and PCC connectivity trended toward significance (P = .09).
Internetwork Functional Connectivity
In order to determine if connectivity differences are not only driven by an aberrant spatial extent within the networks, we assessed if the internetwork FC varied among groups. Significant group differences for internetwork connectivity were found between the DMN and TPN (F = 6.3, P = .003), and coupling in DMN-SN also trended toward significance (F = 2.6, P = .08). Significant anticorrelations were shown between the DMN and TPN in CTRL by post hoc 1-sample t tests (mean z = −0.21, t = −5.2, P < .0001, Cohen’s d = 0.96). In contrast, neither HR (mean z = 0.04, t = −0.74, P = .46, Cohen’s d = 0.14) nor UHR (mean z = 0.05, t = −0.69, P = .50, Cohen’s d = 0.16) exhibited significant coupling for DMN-TPN (figure 2C and supplementary figure S4).
Correlations Among Functional Connectivity Strength, Clinical Symptoms, and Cognitive Functions
The iFC values were used to determine how iFC strength and severity of clinical symptoms as well as cognitive performance were related in at-risk subjects. For rAI-PCC connectivity strength, positive correlation was found with the severity of reported positive symptom sum score (comprising symptoms such as unusual thought content, persecutory delusions, grandiosity, perceptional abnormalities, and disorganized communication) (ρ = 0.32). Both the rAI-PCC and MPFC-rDLPFC connectivities were correlated with the SPI-A subscore of body perception disturbances. These included migrating and electric bodily sensations, sensations of movement, pulling or pressure, sensations of body or parts of it extending, diminishing, shrinking, enlarging, growing, or constricting47 (rAI-PCC, ρ = 0.30; MPFC-rDLPFC, ρ = 0.32).
With regard to cognitive functions, all correlations indicated negative associations, meaning that a high coupling was related to poor performance on cognitive tests. The quality of performance on the FAIR was inversely associated with the DMN-TPN connectivity strength (r = −.38, P = .01). The time required to solve the problem in the TOH task (r = −.47, P = .002) as well as number of moves needed (r = −.50, P = .001) were significantly correlated with DMN-TPN coupling strength. Finally, performance on ROCF recall tasks was correlated with the iFC between MPFC and rDLPFC (r = −.63, P = .001). None of the measured cognitive functions was correlated with the rAI-PCC iFC. Furthermore, neither age nor CPZ equivalents was correlated with any connectivity values (P’s > .2).
Discussion
Our study demonstrated aberrant spatial connectivity patterns for subjects meeting HR or UHR criteria in all 3 networks. Intriguingly, the iFC for MPFC-rDLPFC and rAI-PCC, as well as the internetwork connectivity of DMN-TPN, were increased in both at-risk groups compared with CTRL, inferring that the typically observed anticorrelation is absent in the risk state for psychosis. Notably, those aberrant patterns were associated with symptoms related to reality distortions, and enhanced connectivity strengths of MPFC-rDLPFC and DMN-TPN were related to poor performance in cognitive functions.
Significant differences among the groups for iFC were identified between the MPFC and a single cluster in the rDLPFC (figure 1A1). Post hoc analysis revealed an anticorrelation between the MPFC and the rDLPFC only for CTRL. By contrast, HR showed positive coupling while UHR exhibited no coupling (figure 1A2). The DLPFC is consistently activated during demands for external attention and executive control, eg, in working memory tasks, whereas MPFC activation is suppressed.21,67 Associated therewith, the DLPFC forms a core region of the TPN, which, in healthy subjects, is anticorrelated with MPFC activity, ie, the seed region for the DMN.1,58 The rDLPFC overlapped with the TPN in our CTRL group (figure 1A1), which is in accordance with prior studies of schizophrenia showing that regions demonstrating greater connectivity with the DMN seed ROIs in patients overlap with TPN in healthy CTRL subjects.13,14,32 Additionally, our internetwork connectivity measurements showed that risk groups did not reveal the normal anticorrelation in DMN-TPN (figure 2). This analysis was restricted to areas that were the same for all 3 groups. Therefore, we could confirm that this result was not driven by the aberrant spatial extent of the DMN to the rDLPFC. This finding is consistent with reduced DMN-TPN anticorrelation in subjects at UHR for psychosis42 or schizophrenia.15,33,34
Whereas Shim et al. 42 examined averaged iFCs only within DMN and TPN, we additionally demonstrated that the rDLPFC was aberrantly coupled to the DMN in our risk groups. Furthermore, we extended this finding, demonstrating that not only subjects with brief or attenuated psychotic symptoms (UHR) lacked this negative coupling, but also subjects showing only basic symptoms (HR). We therefore inferred that the absence of TPN-DMN orthogonality also underlies subtle disturbances, eg, subclinical self-experienced disturbances in thought, speech, and perception processes, which are clearly distinct from attenuated or frank psychotic symptoms. This TPN-DMN antagonism is believed to reflect the competition between external and internal information processing, with the former suggested to serve goal-directed mental processing and the latter, perceptually decoupled thought.22 Therefore, a proper functional coordination between these normally anticorrelated networks is considered crucial for cognitive performance.1,23 Correspondingly, increased MPFC-rDLPFC coupling was associated with poor performance in a recall task, in line with a former report showing that increased DMN connectivity is associated with abnormal working memory-related activity in DLPFC.32 Furthermore, we found that connectivity strength between the DMN and the TPN was correlated with selective attention and measures of executive functions. However, the association to selective association does not reach significance, when corrected for multiple comparisons. Interestingly, Carhart-Harris et al. 27 described an increased DMN-TPN iFC in a drug-induced psychedelic state, which may serve as a model of psychosis. Those authors hypothesized that this aberrant coupling underlies “disturbed ego boundaries,” as seen in early psychosis, and, therefore, could explain the inability to distinguish between one’s internal world and the external environment. Likewise, we found a positive correlation between MPFC-rDLPFC coupling strength and the presence of body perception disturbances, which also occur in drug-induced models of psychosis.68
Using the rAI as a seed for the SN,13,24 we found significant spatial differences among the groups for a single cluster in the PCC (figure 1B1). The CTRL revealed an anticorrelation between the rAI and PCC, while HR and UHR showed positive couplings between those regions (figure 1B2). Noteworthy, the PCC forms an integral part of the DMN1 (see also figure 1B1 and supplementary figure S1). This suggested an aberrant overlap between DMN and SN. Interconnectivity measurements showed that differences among groups only trended toward significance, indicating that the SN in the risk groups is characterized by an overlap to their DMN rather than by abnormal internetwork connectivity of the whole SN with the DMN or TPN. Interestingly, the rAI-PCC connectivity strength was not correlated with any cognitive functions but instead with clinical features related to reality distortions, ie, to positive symptom scores and symptoms in body perception disturbances. Such symptoms have previously been speculated to be associated with rAI disturbances.69 This is further supported by recent data from schizophrenic patients that provide evidence for an association between rAI activity with both, reduced coupling between DMN/TPN and hallucinations.33 Beyond that, our results suggest that this association is present even in the subclinical psychosis state. Structural deficits in the insular cortex have been repeatedly reported in subjects at risk for psychosis.70 In particular, abnormalities in GM volumes in the right insula have been linked to higher risks for transition to psychosis.71 Therefore, it is notable that, even if only trending toward significance, the positive coupling between the rAI and PCC was higher for UHR than for HR (figure 1B2). Because HR criteria are presumed to characterize the early, and UHR, the late, prodromal phase,45,46 this finding might indicate the risk of developing psychotic symptoms. As we did not find a significant group difference in MPFC-rDLPFC coupling, it can be speculated that those changes occur early in the disease course (at the HR state) without a later increase of the coupling (at the UHR state). In the rAI-PCC connectivity, an increasing coupling occurs during disease progression.
Our findings strongly support the recent theory that SN plays a cardinal role in the development of psychotic symptoms.30,69 Functional deficits in SN potentially lead to excessive salience attribution to internal experiences, which consequently may be responsible for delusions and hallucinations in schizophrenia.57,72 A limitation in our study is that, only the correlation between iFC and cognitive variables; however, not with clinical variables, survived correction for multiple comparisons. Furthermore, some subjects were medicated, and (antipsychotic) medication contributes to changes in iFC.73
In summary, we have identified an association between impaired iFC and cognitive processing as well as symptoms related to reality distortions in the preclinical psychosis risk state. This suggests that abnormal network interactions are involved in disrupting one’s capacity to distinguish between the internal world and external environment, eventually leading to a rise in psychotic perceptions. Our findings imply that an aberrant spatial extent in the 3 networks, and decreased TPN-DMN orthogonality, are important features in the risk state. This strongly supports the existence of a triple network model (DMN, TPN, and SN)57 for the development of psychotic disorders.
Supplementary Material
Supplementary material is available at http://schizophre niabulletin.oxfordjournals.org.
Funding
Zurich Program for Sustainable Development of Mental Health Services (ZInEP).
Supplementary Material
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gusnard DA, Raichle ME, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694 [DOI] [PubMed] [Google Scholar]
- 3. Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Damoiseaux JS, Rombouts SA, Barkhof F, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA. 2006;103:13848–13853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Uhlhaas PJ, Singer W. Neuronal dynamics and neuropsychiatric disorders: toward a translational paradigm for dysfunctional large-scale networks. Neuron. 2012;75:963–980 [DOI] [PubMed] [Google Scholar]
- 6. Bressler SL, Kelso JA. Cortical coordination dynamics and cognition. Trends Cogn Sci. 2001;5:26–36 [DOI] [PubMed] [Google Scholar]
- 7. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38 [DOI] [PubMed] [Google Scholar]
- 8. Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci. 2010;4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37:1083–1090; discussion 1097. [DOI] [PubMed] [Google Scholar]
- 10. Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJ. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33:279–296 [DOI] [PubMed] [Google Scholar]
- 11. Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21:424–430 [DOI] [PubMed] [Google Scholar]
- 12. Williamson P. Are anticorrelated networks in the brain relevant to schizophrenia? Schizophr Bull. 2007;33:994–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Woodward ND, Rogers B, Heckers S. Functional resting-state networks are differentially affected in schizophrenia. Schizophr Res. 2011;130:86–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chai XJ, Whitfield-Gabrieli S, Shinn AK, et al. Abnormal medial prefrontal cortex resting-state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacology. 2011;36:2009–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49–76 [DOI] [PubMed] [Google Scholar]
- 16. Bluhm RL, Miller J, Lanius RA, et al. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull. 2007;33:1004–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164:450–457 [DOI] [PubMed] [Google Scholar]
- 18. White TP, Joseph V, Francis ST, Liddle PF. Aberrant salience network (bilateral insula and anterior cingulate cortex) connectivity during information processing in schizophrenia. Schizophr Res. 2010;123:105–115 [DOI] [PubMed] [Google Scholar]
- 19. Whitfield-Gabrieli S, Moran JM, Nieto-Castañón A, Triantafyllou C, Saxe R, Gabrieli JD. Associations and dissociations between default and self-reference networks in the human brain. Neuroimage. 2011;55:225–232 [DOI] [PubMed] [Google Scholar]
- 20. Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smallwood J, Tipper C, Brown K, et al. Escaping the here and now: evidence for a role of the default mode network in perceptually decoupled thought. Neuroimage. 2013;69:120–125 [DOI] [PubMed] [Google Scholar]
- 23. Anticevic A, Cole MW, Murray JD, Corlett PR, Wang XJ, Krystal JH. The role of default network deactivation in cognition and disease. Trends Cogn Sci. 2012;16:584–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA. 2008;105:12569–12574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carhart-Harris RL, Leech R, Erritzoe D, et al. Functional connectivity measures after psilocybin inform a novel hypothesis of early psychosis. Schizophr Bull. 2013;39:1343–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Deshpande G, Santhanam P, Hu X. Instantaneous and causal connectivity in resting state brain networks derived from functional MRI data. Neuroimage. 2011;54:1043–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Palaniyappan L, Liddle PF. Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. J Psychiatry Neurosci. 2012;37:17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shen H, Wang L, Liu Y, Hu D. Discriminative analysis of resting-state functional connectivity patterns of schizophrenia using low dimensional embedding of fMRI. Neuroimage. 2010;49:3110–3121 [DOI] [PubMed] [Google Scholar]
- 32. Gabrieli JDE, Seidman LJ, Whitfield-Gabrieli S, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA. 2009;106:1279–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Manoliu A, Riedl V, Zherdin A, et al. Aberrant dependence of default mode/central executive network interactions on anterior insular salience network activity in schizophrenia. Schizophr Bull. 2013:sbt037v1–sbt037 http://schizophreniabulletin.oxfordjournals.org/content/early/2013/03/21/schbul.sbt037.long Accessed November 9, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hasenkamp W, James GA, Boshoven W, Duncan E. Altered engagement of attention and default networks during target detection in schizophrenia. Schizophr Res. 2011;125:169–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pu W, Li L, Zhang H, et al. Morphological and functional abnormalities of salience network in the early-stage of paranoid schizophrenia. Schizophr Res. 2012;141:15–21 [DOI] [PubMed] [Google Scholar]
- 36. Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537 [DOI] [PubMed] [Google Scholar]
- 37. van Buuren M, Vink M, Kahn RS. Default-mode network dysfunction and self-referential processing in healthy siblings of schizophrenia patients. Schizophr Res. 2012;142:237–243 [DOI] [PubMed] [Google Scholar]
- 38. Addington J, Heinssen R. Prediction and prevention of psychosis in youth at clinical high risk. Annu Rev Clin Psychol. 2012;8:269–289 [DOI] [PubMed] [Google Scholar]
- 39. Salokangas RK, McGlashan TH. Early detection and intervention of psychosis. A review. Nord J Psychiatry. 2008;62:92–105 [DOI] [PubMed] [Google Scholar]
- 40. Jung WH, Jang JH, Shin NY, et al. Regional brain atrophy and functional disconnection in Broca’s area in individuals at ultra-high risk for psychosis and schizophrenia. PLoS One. 2012;7:e51975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shim G, Oh JS, Jung WH, et al. Altered resting-state connectivity in subjects at ultra-high risk for psychosis: an fMRI study. Behav Brain Funct. 2010;6:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Klosterkötter J, Hellmich M, Steinmeyer EM, Schultze-Lutter F. Diagnosing schizophrenia in the initial prodromal phase. Arch Gen Psychiatry. 2001;58:158–164 [DOI] [PubMed] [Google Scholar]
- 43. Yung AR, McGorry PD. Prediction of psychosis: setting the stage. Br J Psychiatry Suppl. 2007;51:s1–s8 [DOI] [PubMed] [Google Scholar]
- 44. Klosterkötter J, Schultze-Lutter F, Bechdolf A, Ruhrmann S. Prediction and prevention of schizophrenia: what has been achieved and where to go next? World Psychiatry. 2011;10:165–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fusar-Poli P, Borgwardt S, Bechdolf A, et al. The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA Psychiatry. 2013;70:107–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schultze-Lutter F, Addington J, Ruhrmann S, Klosterkötter J. Schizophrenia Proneness Instrument, Adult Version (SPI-A). Rome, Italy: Giovanni Fioriti Editore s.r.l.; 2007 [Google Scholar]
- 47. Miller TJ, McGlashan TH, Rosen JL, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29:703–715 [DOI] [PubMed] [Google Scholar]
- 48. Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67:255–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33;quiz 34. [PubMed] [Google Scholar]
- 50. Horn W. L-P-S Leistungsprüfsystem. 2nd ed Göttingen: Verlag für Psychologie, Hogrefe; 1983 [Google Scholar]
- 51. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113 [DOI] [PubMed] [Google Scholar]
- 52. Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851 [DOI] [PubMed] [Google Scholar]
- 53. Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141 [DOI] [PubMed] [Google Scholar]
- 54. Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chai XJ, Castañón AN, Ongür D, Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. Neuroimage. 2012;59:1420–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506 [DOI] [PubMed] [Google Scholar]
- 58. Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Van Essen DC. A Population-Average, Landmark- and Surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28:635–662 [DOI] [PubMed] [Google Scholar]
- 60. Van Essen DC, Lewis JW, Drury HA, et al. Mapping visual cortex in monkeys and humans using surface-based atlases. Vision Res. 2001;41:1359–1378 [DOI] [PubMed] [Google Scholar]
- 61. Friston KJ, Rotshtein P, Geng JJ, Sterzer P, Henson RN. A critique of functional localisers. Neuroimage. 2006;30:1077–1087 [DOI] [PubMed] [Google Scholar]
- 62. Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12:535–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Moosburger H, Oelschlägel J. FAIR - Frankfurter Aufmerksamkeitsinventar. Bern: Huber; 1996 [Google Scholar]
- 64. Rey A. L’examen psychologique dans le cas d’encephalopathie traumatique (Les problems.). Archives de Psychologie. 1941;28:215–285 [Google Scholar]
- 65. Osterrieth P. Filetest de copie d’une figure complex: contribution a l’etude de la perception et de la memoire [The test of copying a complex figure. A contribution to the study of perception and memory]. Archives de Psychologie. 1944;30:286–356 [Google Scholar]
- 66. Gediga G, Schöttke H. Die Türme von Hanoi oder computersimulierte Problemlöseszenarien. Göttingen: Hogrefe; 2006 [Google Scholar]
- 67. McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15:394–408 [DOI] [PubMed] [Google Scholar]
- 68. Gouzoulis-Mayfrank E, Heekeren K, Neukirch A, et al. Psychological effects of (S)-ketamine and N,N-dimethyltryptamine (DMT): a double-blind, cross-over study in healthy volunteers. Pharmacopsychiatry. 2005;38:301–311 [DOI] [PubMed] [Google Scholar]
- 69. Palaniyappan L, White TP, Liddle PF. The concept of salience network dysfunction in schizophrenia: from neuroimaging observations to therapeutic opportunities. Curr Top Med Chem. 2012;12:2324–2338 [DOI] [PubMed] [Google Scholar]
- 70. Fusar-Poli P, Radua J, McGuire P, Borgwardt S. Neuroanatomical maps of psychosis onset: voxel-wise meta-analysis of antipsychotic-naive VBM studies. Schizophr Bull. 2012;38:1297–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Smieskova R, Fusar-Poli P, Aston J, et al. Insular volume abnormalities associated with different transition probabilities to psychosis. Psychol Med. 2012;42:1613–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci. 2010;14:277–290 [DOI] [PubMed] [Google Scholar]
- 73. Sambataro F, Blasi G, Fazio L, et al. Treatment with olanzapine is associated with modulation of the default mode network in patients with Schizophrenia. Neuropsychopharmacology. 2010;35:904–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.