Abstract
Because increasing evidence point to the convergence of environmental and genetic risk factors to drive redox dysregulation in schizophrenia, we aim to clarify whether the metabolic anomalies associated with early psychosis reflect an adaptation to oxidative stress. Metabolomic profiling was performed to characterize the response to oxidative stress in fibroblasts from control individuals (n = 20) and early psychosis patients (n = 30), and in all, 282 metabolites were identified. In addition to the expected redox/antioxidant response, oxidative stress induced a decrease of lysolipid levels in fibroblasts from healthy controls that were largely muted in fibroblasts from patients. Most notably, fibroblasts from patients showed disrupted extracellular matrix- and arginine-related metabolism after oxidative stress, indicating impairments beyond the redox system. Plasma membrane and extracellular matrix, 2 regulators of neuronal activity and plasticity, appeared as particularly susceptible to oxidative stress and thus provide novel mechanistic insights for pathophysiological understanding of early stages of psychosis. Statistically, antipsychotic medication at the time of biopsy was not accounting for these anomalies in the metabolism of patients’ fibroblasts, indicating that they might be intrinsic to the disease. Although these results are preliminary and should be confirmed in a larger group of patients, they nevertheless indicate that the metabolic signature of reactivity to oxidative stress may provide reliable early markers of psychosis. Developing protective measures aimed at normalizing the disrupted pathways should prevent the pathological consequences of environmental stressors.
Key words: mental disorder, glutathione, arginine, extracellular matrix, phospholipid, metabolism
Introduction
The development of metabolomics fostered search for the identification of biomarkers for complex disorders such as psychosis. However, it remains unclear whether these markers provide actual hints of pathological processes. Challenging candidate biomarkers with the current hypotheses in schizophrenia etiology is a required step to understand whether the molecular mechanisms underlying the physiopathology translate into a characteristic metabolic signature.
Redox dysregulation is regarded as a key point of convergence for environmental and genetic factors in schizophrenia.1 Lipid peroxidation and decreased total antioxidant defenses in the blood of schizophrenia patients are persistent findings,2–5 providing further evidence for a systemic component of the disease. In the central nervous system, decreased antioxidant defenses have been reported,6–8 as well as markers of oxidative stress in postmortem prefrontal cortex.9 Genetics further supports the role of the redox system in schizophrenia pathology because the 2 genes coding the rate-limiting enzyme for glutathione (GSH) synthesis (GCLC, GCLM, the catalytic and modulatory subunits of the glutamate-cysteine ligase [GCL]) are linked to disease.10,11 In particular, GAG tri-nucleotide repeats in the in the 5′ noncoding region of GCLC present a polymorphism with 7, 8, or 9 repetitions. Genotypes with 7–8, 8–8, 8–9, 9–9 repetitions (GCLC high-risk genotypes) are more frequent in patients than controls10 and are associated with altered blood redox status,12 defects in GCL regulation and lowered GSH content after an oxidative stress compared with GCLC low-risk genotypes.10 Moreover, the critical role of oxidative stress has been validated in redox dysregulated animal models reproducing numerous schizophrenia phenotypes, reversed by the antioxidant N-acetyl-cysteine.13,14 Increasing evidence points to altered stress reactivity as a vulnerability marker for psychosis, with increase of negative affects in patients challenged with daily life stress.15,16 This psychotic reactivity to stress may be related to hyperreactivity of dopamine neurons to environmental stimuli and excess of dopamine,17–22 thus leading to oxidative stress.23
Available metabolomic studies were limited to the profiling of steady-state basal levels of metabolites. To date, 6 metabolomic analyses based on blood samples from schizophrenia or psychotic patients have been published,24–29 including 4 characterizing the lipidome.24–27 Pathways involved in these studies are arginine and proline metabolism,27,28 glucoregulatory processes,27,29 and fatty acids and lipid metabolism.24–27,29 The largest study (He et al,28 265 patients), suggests that a defect in nitrogen compound biosynthesis may reflect the genetic susceptibility of schizophrenia. Yet, there is no clear consensus on the pathways that might be dysregulated in patients. Bias linked to ethnic background, diet, and lifestyle may account for the discrepancies and limit the use of the results for diagnosis and monitoring of treatment.
This study was undertaken to shed light on the systems vulnerability to the disease: oxidative challenge reveals pathways that would be masked under basal conditions. For this purpose, skin-derived fibroblasts present 3 main assets compared with blood samples. First, cells can be challenged with stressors to potentially reveal a phenotype that is expressed only in oxidative condition. We used tert-butylhydroquinone (t-BHQ), an inducer of reactive oxygen species30 and of the antioxidant response.31,32 Second, cultures grown in a defined medium minimize heterogeneity due to differences in lifestyle, environmental, or dietary background of the donors. Third, fibroblasts have been successfully used to reveal anomalies that are relevant for brain physiopathology in other neurological diseases. Indeed, mitochondrial and bioenergetic dysfuntions, which are key pathological processes in Parkinson’s disease, were shown first in fibroblasts from patients.33–35
We used fibroblasts from control individuals and patients with early psychosis (EP) to avoid confounding effects of long-term medication and illness. Moreover, analyses were stratified based on the patients’ genotype in GCLC GAG tri-nucleotide repeats to better determine the anomalies associated with an altered redox control. We used metabolomics to draw a detailed picture of the response to oxidative stress in cells from control individuals and determine which metabolic pathways are dysfunctional in EP patients. This study highlighted aberrations in the metabolic response to oxidative stress in fibroblasts from patients. Besides altered redox regulation, the metabolism of lipids, arginine, and collagen were affected, indicating impairments beyond the redox system.
Materials/Subjects and Methods
Recruitment of Patients and Control Individuals
Patients from 18 to 30 years old who crossed the threshold of psychosis (according to the Comprehensive Assessment of At-Risk Mental States criteria)36 were recruited from the Treatment and Early Intervention in Psychosis Program (TIPP Program, University Hospital Lausanne, Switzerland).37 Mean duration of illness at the time of biopsy was 2 years (743 days ± 647; range: 116–3171 days). Patients were diagnosed according to DSM-IV criteria after a 3-year follow-up in the TIPP program: 87% of diagnoses were in the schizophrenia spectrum (n = 26), 2 patients had a bipolar disorder, and 2 a major depression with psychotic features. Symptom severity at the time of biopsy was assessed using the 30 items of the Positive and Negative Syndrom scale (PANSS) with a 1–7 rating scale. PANSS was administered by trained psychologists in 1-hour interview. Control subjects were assessed and selected with the Diagnostic Interview for Genetic Studies.38 Major mood, psychotic, or substance-use disorder and having a first-degree relative with a psychotic disorder were exclusion criteria for controls. All enrolled subjects provided a fully informed written consent as required by the ethical guidelines of the Lausanne University Hospital. Patients and controls were matched for age and sex (table 1). The GAG tri-nucleotide repeat polymorphism in GCLC was genotyped as previously described.10 Classification into GCLC high-risk or GCLC low-risk genotype is based on the number of GAG repeats as defined in Gysin et al.10 All controls in this study had GCLC low-risk genotypes.
Table 1.
Demographic Details
| Disease Status | Controls (n = 20) | Patients (n = 30) | ||
|---|---|---|---|---|
| GCLC genotype | GCLC low risk | All | GCLC high risk (n = 15) | GCLC low risk (n = 15) |
| Age, mean ± SD | 24.7±3.3 | 23.2±3.3 | 23.1±3.3 | 23.3±3.4 |
| Sex | 20 men | 30 men | 15 men | 15 men |
| Ethnicity | 19 C, 1 As | 18 C, 1 Afr, 1 As | 13 C, 1 Afr, 1 As | 15 C |
| BMI, mean ± SD a; (n) | 22.9±2.9; (n = 18) | 24.8±3.7; (n = 26) | 25.6±3.9; (n = 11) | 24.2±3.5; (n = 15) |
| Smokers (users/non users)a | 16.7% (3/15) | 60.7% (17/11) | 50.0% (7/7) | 71.4% (10/4) |
| Cigarettes/day, mean ± SD | 2.8±6 | 10±10.7 | 10±12.7 | 10.1±8.9 |
| Cannabis (users/nonusers) | 33.3% (6/13) | 33.3% (10/20) | 46.7% (7/8) | 20% (3/12) |
| Illness duration, mean ± SD; (n) | n.a. | 744±647; (n = 29) | 603±329; (n = 15) | 895±859; (n = 14) |
| PANSS score, mean ± SD; (n) | n.a. | 67.7±26.1; (n = 30) | 64.1±21.3; (n = 15) | 71.5±30.9; (n = 15) |
| CPZ eq., mean ± SD a; (n) | n.a. | 307±192; (n = 28) | 240±168; (n = 14) | 373±197; (n = 14) |
| Samples | ||||
| Basal condition | n = 20 | n = 30 | n = 15 | n = 15 |
| Treated condition | n = 20 | n = 30 | n = 15 | n = 15 |
Note: SD, standard deviation; BMI, body mass index; PANSS, Positive and Negative Syndrom scale; CPZ eq., chlorpromazine equivalent; Afr, African; As, Asian; C, Caucasian; n.a., not applicable. Age in years, BMI (kg/m2), percentage of smokers, cigarette consumption per day and percentage of cannabis users at the time of biopsy are reported for each group. For patients, illness duration in days, disease severity (PANSS score), and the antipsychotic medication (converted to chlorpromazine equivalent, in milligrams) at the time of biopsy are reported. Cells of treated conditions have been incubated with tert-butylhydroquinone, 50 uM for 18 hours prior to harvesting; basal conditions correspond to vehicle (dimethyl sulfoxide) treated cells.
aDifferent in patients vs controls with P value < .05.
Cell Culture
Fibroblast cultures established from skin biopsies were grown mainly as previously described10 and detailed in supplementary methods. For experiments, fibroblasts were treated for 18 hours either with t-BHQ (50 uM) to induce an oxidative stress or with vehicle alone (dimethyl sulfoxide, 0.05% final).
Sample Preparation and Liquid and Gas Chromatography/Mass Spectrometry
All mass spectrometry data were collected at Metabolon Inc by combining 3 independent platforms: ultrahigh-performance liquid chromatography/tandem mass spectrometry optimized for basic or acidic species, and gas chromatography/mass spectrometry. Samples were processed essentially as described previously.39,40 For details, see the supplementary methods.
Statistics
All statistical tests were conducted using Array Studio v 5 or R v 2.14.1 (http://cran.r-project.org/).
For compound analyses, Welch’s t-tests were performed to compare log-transformed data between experimental groups. This test is based on the assumption of log-normal distribution of the metabolic data, which is well supported by literature and internal investigations at Metabolon.41 Multiple comparisons were accounted for by estimating the false discovery rate using q values.42
For pathways analysis, individual metabolites were classified based on current knowledge as being in one of the following pathways: neurotransmitter-related metabolism, redox homeostasis and oxidative stress, arginine metabolism, extracellular matrix (EMC) and collagen metabolism, glucose metabolism, free fatty acids, fatty acid oxidation, or phospholipid degradation and remodeling (supplementary table 2). Overall comparisons of these pathways were then made between the groups using Hotelling’s T 2 test, for basal and treated conditions, as well as for the biochemical ratios. The same procedure was applied to data from only schizophrenia patients (n = 19) and controls (n = 20).
To evaluate the impact of confounding factors, we first compared their distribution in patients vs controls using Student’s t-test or Chi square test (see table 1). We further studied only the factors that were significantly different between the 2 groups, ie, body mass index (BMI), smoking, and medication. To analyze the effect of medication on the metabolic pathways, the chlorpromazine equivalent of the antipsychotic treatment was calculated for each patient.43,44 We first performed a series of exploratory factor analyses to extract 3 factors per pathway, both on data from patients and controls and used linear mixed effect models to determine if the antipsychotic treatment affects one of the factors (supplementary table 3). We proceeded similarly to analyze the effect of BMI and smoking status, except that factor analyses were performed on data from patients and controls together. P values were adjusted using Holm’s method.
Results
Abnormal Metabolic Response to Oxidative Stress in EP Patients
We cultured human skin-derived fibroblasts and treated them either with vehicle or with t-BHQ, in conditions previously used to reveal differences between controls and schizophrenia patients.10 We compared global metabolic responses of fibroblasts from controls (GCLC low risk) and EP patients, who were classified between GCLC low-risk and GCLC high-risk genotypes. The number of controls with GCLC high-risk genotypes was not sufficient to be included in this study. Nevertheless, controls were age- and sex-matched with the patients (table 1).
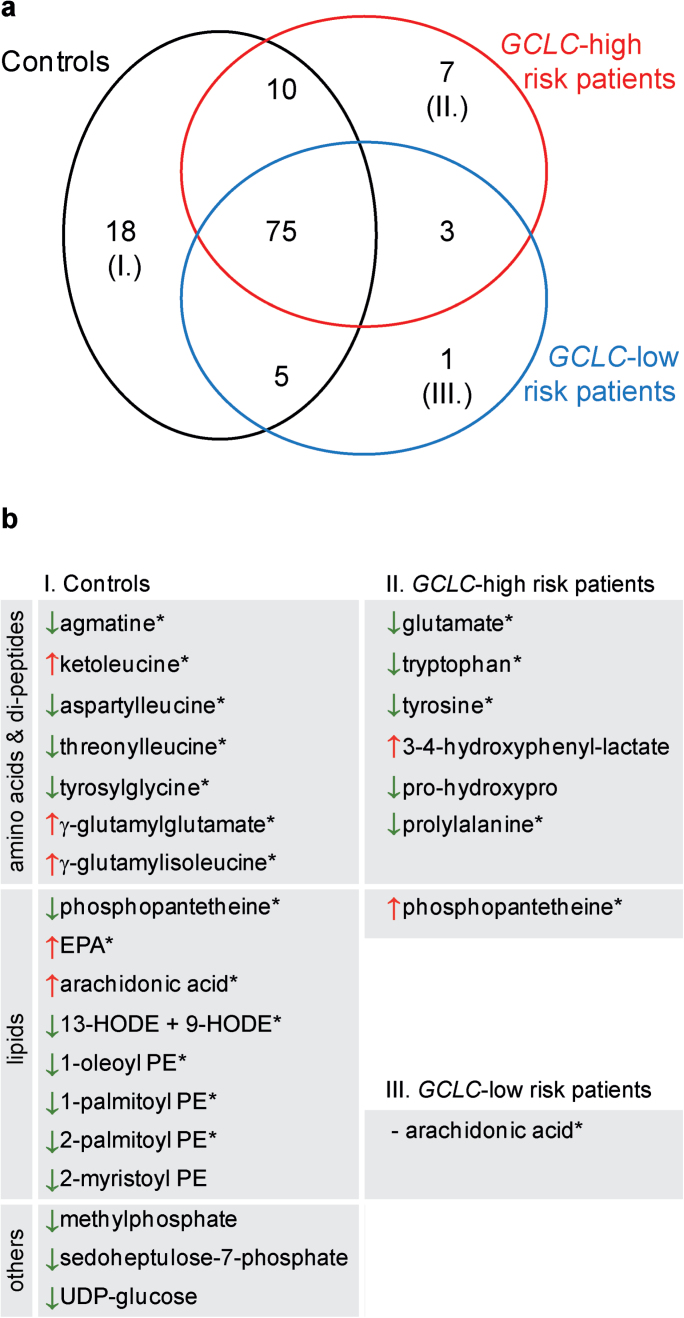
After correction for multiple comparisons, none but one of the 282 metabolites (creatine) revealed differences between patients and controls in basal and treated conditions. To bypass multiple testing, we studied classes of metabolites to identify metabolic pathways with an altered response to oxidative stress in patients. Within each of the 3 groups of individuals, we selected the biochemicals whose levels were significantly affected by t-BHQ compared with the baseline vehicle treatment (see supplementary table 1), using a stringent cutoff of <1 theoritical false positive hit. Short-listed compounds spanned the 5 families of biochemical as follows: amino acids, carbohydrates, lipids, nucleotides, and cofactors. In all, 118 metabolites changed significantly between t-BHQ and vehicle treatment (figure 1a). Seventy-five changes were shared among controls, GCLC low-risk patients, and GCLC high-risk patients; 19 were shared by 2 of the groups; and 26 were unique to a specific group (figure 1b).
Fig. 1.
Response to oxidative stress in control individuals (black), GCLC high-risk patients (red) and GCLC low-risk patients (blue). (a) For each group, a short list (<1 false discovery hit) of metabolites significantly affected by tert-butylhydroquinone (t-BHQ) treatment was determined among a total of 282 chemicals identified. Venn diagram illustrates the overlap of these lists between groups. Numbers of corresponding biochemical are indicated. Note that the biochemicals that are specific to each group are listed in the panel b. Arrows indicate the direction of the fold change (FC) induced by t-BHQ: red arrow: FC > 1.1; green arrow: FC < 0.9; (−): FC = 0.97. *Robust signature; ie, no P < .1 in the other groups. PE, phosphatidylethanolamine; EPA, eicosapentaenoate; HODE, hydroxyoctadecadienoic acid.
As conservative statistic criteria were applied to establish the short list of biochemicals shown in figure 1, the results might be biased by false negative hits. Therefore, we elaborated on this preliminary screening, to raise hypothesis on pathways that might be abnormally regulated in patients (table 2 and supplementary table 2). We then performed a pathway analysis (Hotelling’s T 2 test) to compare patients and controls in basal conditions (vehicle), after treatment with t-BHQ and their change in response to t-BHQ (ratio t-BHQ/vehicle). Hotelling’s test is an equivalent of the t-test, adapted when there is >1 outcome variable (multivariate analysis) and thus was used to compare groups of metabolites (ie, pathways) between patient and controls. Resulting P values are given in table 2. Patients (both GCLC high risk and GCLC low risk) and controls present differences in (1) the collagen and ECM-related metabolism after oxidative stress (P = .034); (2) the regulation of arginine metabolism in response to oxidative stress (P = .029); (3) at baseline, the regulation of amino acids known to modulate neurotransmission in neurons (neurotransmitter-related pathway, P = .028). Additionally, patients with the GCLC high-risk genotypes displayed a specific profile, compared with controls, with (4) a trend for abnormal regulation of the redox system in response to oxidative stress (P = .063) and (5) alterations of lysolipid metabolism in basal conditions (P = .043).
Table 2.
Pathway Analysis
| Controls vs Patients | Controls vs GCLC Low-Risk Patients | Controls vs GCLC High-Risk Patients | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Vehicle | t-BHQ | t-BHQ Vehicle | Vehicle | t-BHQ | t-BHQ Vehicle | Vehicle | t-BHQ | t-BHQ Vehicle | |
| Transmethylation pathway | 0.067 | — | — | — | — | — | — | — | — |
| Redox homeostasis | — | — | — | — | — | — | — | — | 0.063 |
| Glucose metabolism | — | — | — | — | — | — | — | — | — |
| Long chain fatty acids | — | — | — | — | — | — | — | — | — |
| Fatty acid oxidation | — | — | — | — | — | — | 0.085 | — | — |
| Lysolipids | — | — | — | — | — | — | 0.043 | 0.066 | — |
| Neurotransmitter-related metabolism | 0.028 | — | — | 0.085 | — | — | — | — | — |
| ECM and collagen metabolism | — | 0.034 | — | — | — | 0.085 | — | 0.051 | — |
| Arginine metabolism | — | — | 0.029 | 0.074 | 0.089 | — | — | — | 0.064 |
Note: ECM, extracellular matrix; t-BHQ, tert-butylhydroquinone. Pathway regulations are compared between fibroblasts from controls (n = 20) and early psychosis patients (n = 30), from controls (n = 20) and GCLC high-risk patients (n = 15), from controls (n = 20) and GCLC low-risk patients (n = 15). Fibroblast metabolism was studied either after treatment with vehicle, or after treatment with t-BHQ or in response to t-BHQ (ratio t-BHQ/vehicle). P values resulting from Hotelling’s T 2 test are indicated when <.1. Bold, P < .05.
Because patients were included in this study at early stages of psychosis, definite diagnoses were established only after a 3-year follow-up according to the DSM-IV criteria. We tested if pathway anomalies remained significant in a smaller but more homogeneous group of patients subsequently diagnosed with schizophrenia. We therefore performed pathway analyses on control (n = 20) vs schizophrenia individuals (n = 19), and excluded other forms of psychosis (n = 11). The metabolism of arginine and ECM and collagen remained differentially regulated between schizophrenia patients and controls (P = .044 and P = .040 respectively) indicating that they might be early markers of schizophrenia. However, neurotransmitter-related metabolism was not significantly differentially regulated between this subgroup of patients and controls (P = .070) and thus is not further discussed.
In conclusion, regulation of arginine metabolism and ECM and collagen metabolism in response to oxidative stress differs between EP patients and control individuals. In addition, the subgroup of patients with GCLC high-risk genotypes presents anomalies in lysolipid metabolism. These pathways of interest are further detailed in the following paragraphs.
Higher Levels of ECM-Related Compounds in Fibroblasts From EP Patients After Oxidative Stress
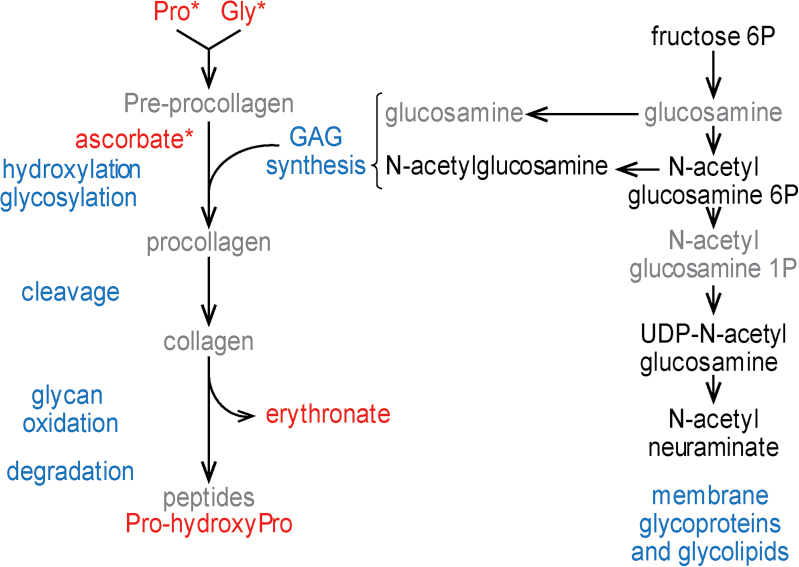
After the t-BHQ treatment, levels of proline and glycine, the two most abundant amino acid in collagen, were higher in patients of both genotypes compared with controls (patients vs controls: fold change [FC] = 1.14, P = .033; FC = 1.2, P = .038). The same effect was observed for ascorbate, which is required for collagen folding (patients vs controls: FC = 2.13, P = .024; figure 2). On the contrary, the sugars involved in proteoglycan maturation had similar levels in patients and controls (see for instance N-acetylglucosamine 6-phosphate or N-acetylneuraminate). Levels of 2 degradation products, proline-hydroxyproline (patients vs controls: FC = 1.19, P = .070), a dipeptide present in collagen, and erythronate (patients vs controls: FC = 1.4, P = .091), which is an oxidation product from sugars, tended to be higher in EP patients than controls.
Fig. 2.
Differences between patients and controls in ECM and collagen metabolism after t-BHQ treatment. Differences in metabolite level between patients and controls (P < .1) are color coded; black: no difference; red: levels higher in patients (FC > 1.1); Compounds written in grey: not detected. *P < .05. Indications written in blue correspond to processes. Note that intermediates of collagen metabolism have higher levels in patients than controls, while compounds for glycosaminoglycan synthesis are similar in both groups.
Following oxidative stress, the increased availability of collagen precursors may favor ECM synthesis in fibroblasts from patients of both groups of genotypes, compared with controls, but increased levels of catabolic products suggest that extracellular components are more degraded.
Abnormal Regulation of Arginine Metabolism in Response to Oxidative Stress in EP Patients
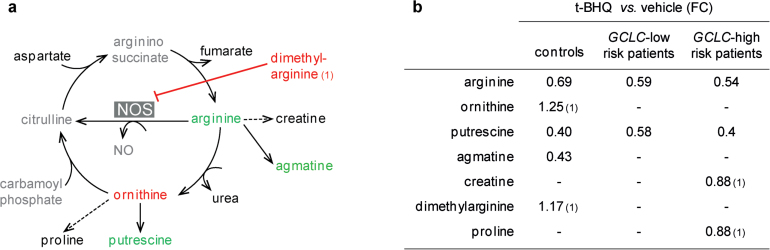
In cells from control individuals, t-BHQ induced a decrease of arginine levels (FC = 0.69, P = 5.10−4). Dimethylarginine, which inhibits the nitric oxide synthase (NOS) enzymes and may regulate arginine levels (figure 3a), accumulated upon t-BHQ treatment (FC = 1.17, P = .012). Arginine derivatives presented varied responses. Creatine had stable levels, while ornithine increased after t-BHQ (FC = 1.25, P = .017). Levels of the polyamines putrescine (FC = 0.4, P = .002) and agmatine (FC = 0.43, P = 2.10− 5) were decreased in response to stress, and proline levels tended to be lowered (FC = 0.89, P = .076).
Fig. 3.
Regulation of arginine metabolism in response to oxidative stress, in fibroblasts from control individuals (a), from GCLC high-risk and from GCLC low-risk patients (b). (a) Differences in metabolite levels induced by t-BHQ (P < .05) are illustrated for fibroblasts from control individuals with a color code; black: levels not affected; green: levels lowered with FC < 0.9; red: levels increased with FC > 1.1. Compounds written in grey: not detected. (b) Summary of the fold change for the metabolites regulated by t-BHQ with a P < .05. (1): compound not short-listed.
In patients (both GCLC high risk and GCLC low risk) at baseline, creatine levels were higher than in controls (patients vs controls: FC = 1.26, P = .016). In response to oxidative stress, creatine levels decreased in fibroblasts from the GCLC high-risk patients (FC = 0.88, P = .017) but not in GCLC low-risk patients (FC = 1.04, P = .86, figure 3b). Levels of dimethylarginine did not increase in cells from patients as observed for controls after t-BHQ treatment (GCLC high-risk patients: FC = 1.07, P = .46; for GCLC low-risk patients: FC = 1.11, P = .57). Ornithine was not increased following oxidative stress or only moderately for the cells with GCLC high-risk genotypes (GCLC low-risk patients: FC = 1.01, P = .402; GCLC high-risk patients: FC = 1.14, P = .089). Among the polyamines, putrescine was lowered in response to stress (GCLC low risk: FC = 0.58, P = 6.10− 6; GCLC high risk: FC = 0.4, P = 2.10–4) but not agmatine (GCLC low risk: FC = 0.83, P = .574; GCLC high risk: FC = 0.56, P = .061), while proline was increased and reached higher levels in cells from patient than controls after treatment (patients vs controls: FC = 1.14, P = .033).
As summarized in figure 3b, levels of arginine-derivate compounds are not properly regulated in EP patients’ fibroblasts compared with controls’ fibroblasts.
Pathway Dysregulation in EP Patients Is Statistically Independent From Antipsychotic Medication
As fibroblasts are cultured for 5 passages before assessment, it is unlikely that patients’ treatment at the time of biopsy accounts for the differences between patients and controls in pathway regulation. Nevertheless, medication may lead to epigenetic imprinting, so we tested if the dose of antipsychotic treatment impacts the levels of metabolites in the pathways of ECM and collagen or arginine metabolism. Similarly, other confounding factors such as smoking, cannabis use, and BMI may affect fibroblasts’ metabolism. First, we tested if these factors are different between patients and controls (table 1). The use of cannabis was not more frequent in patients than in controls and thus only treatment at the time of biopsy, BMI, and smoking were further analyzed. Second, we set a model with 3 factors describing metabolite levels within each pathway. Third, we used a linear mixed model to study if one of the factors was dependent of antipsychotic treatment, BMI, or smoking at the time of biopsy. Resulting P values are summarized in the supplemental table 3. Smoking tends to modulate ECM and collagen metabolism, but overall none of the tested factors had an effect on the metabolic pathways of interest.
Altered Regulation of Antioxidant Response in Fibroblasts From GCLC High-Risk Patients
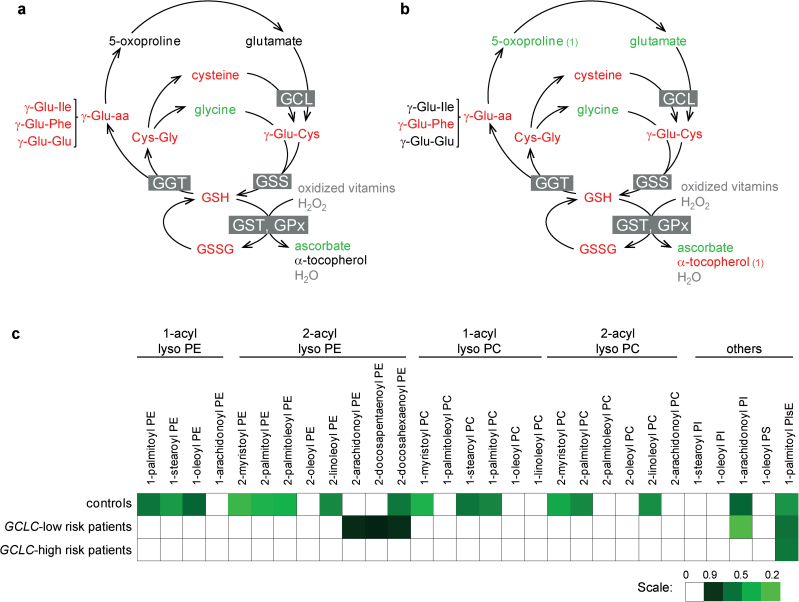
In cells from controls, the 5 compounds that displayed the highest increase following t-BHQ treatment are involved in glutathione (GSH) metabolism (γ-Glu-Cys, cysteine, cysteine sulfinic acid, Cys-Gly, oxidized glutathione), and the antioxidant ascorbate (vitamin C) was among the most downregulated biochemicals (supplementary table 1). Evidence of increased GSH consumption was indicated by the increase of γ-glutamyl-amino acid: γ-Glu-Ile (FC = 1.30, P = .005), γ-Glu-Glu (FC = 1.31, P = .006), and γ-Glu-Phe (FC = 1.58, P = .002, see figure 4a). In parallel, the levels of reduced GSH (FC = 2.2, P = 3.10–12) and of its precursor γ-Glu-Cys (FC = 5.97, P = 7.10− 7) were increased in response to oxidative stress, thus reflecting the high activity of the rate limiting enzyme GCL to replenish the antioxidant defenses.
Fig. 4.
Response to oxidative stress in GCLC high-risk patients. Scheme illustrating the response of GSH metabolism to t-BHQ treatment, in fibroblasts from control individuals (a) and from GCLC high-risk patients (b). Differences in metabolite level (P < .05) induced by t-BHQ are color coded; black: not affected; green: lowered with FC < 0.9; red: levels increased with FC > 1.1. Compounds written in grey: not detected. γ-Glu-aa: γ-glutamyl-amino acids. Boxed names are enzymes, GCL: Glutamate-cysteine ligase; GSS: GSH synthetase; GGT: γ-glutamyl-transpeptidase; GST: GSH transferase. (1): compound not short-listed. (c) Heat map showing differences in lysolipid levels induced by t-BHQ in fibroblasts of control individuals, GCLC high-risk and GCLC low-risk patients. Increased levels are shown in red and decreased levels in green when P < .05. GSSG: oxidized glutathione; Lyso PC: lyso-phosphatidylcholine; Lyso PE: lyso-phosphatidylethanolamine; Lyso PI: lyso-phosphatidylinositol; Lyso PlsE: lyso-plasmenylethanolamine.
In GCLC high-risk patients, levels of GSH, oxidized glutathione, and ascorbate, following t-BHQ treatment, varied similarly as in controls. Levels of GSH precursors glutamate and 5-oxoproline decreased in response to oxidative stress in the GCLC high-risk patients (glutamate: FC = 0.83, P = .003; 5-oxoproline: FC = 0.87, P = .012) while remaining steady in cells from controls (figure 4b). At baseline, cells with GCLC high-risk genotypes tended to present higher levels of glutamate and 5-oxoproline, compared with control cells (GCLC high-risk patients vs controls, FC = 1.16, P = .089; FC = 1.26, P = .082). Therefore, the decrease of these 2 metabolites in GCLC high-risk patients in response to oxidative stress may reflect a blockage at the level of GCL in basal condition, which was released in oxidative condition.
In conclusion, the known effect of GCLC high-risk polymorphisms on redox metabolism was detected by the global metabolic profiling, validating its use to characterize the stress response of cells from EP.
Altered Regulation of Lysolipid Levels in Fibroblasts From GCLC High-Risk Patients
In fibroblasts from controls, the largest family of compounds regulated in response to oxidative stress was the lipid and in particular the lysolipids, products of phospholipid deacylation. In the short list, 6 different lysolipids were diminished after t-BHQ treatment, compared with baseline, and grew to 16 if the threshold of significance was relaxed to P values < .05 (figure 4c). Among these 6 lysolipids, 4 were lyso-phosphatidylethanolamines (lysoPE).
Fibroblasts from GCLC high-risk patients displayed lower levels of lysolipids than those from controls at baseline (P = .043, see table 2) although none was individually significant. Response to oxidative stress did not affect the levels of most lysolipids in GCLC high-risk patients, while GCLC low-risk patients presented an intermediate phenotype with only 3 lysoPE decreasing after t-BHQ treatment (figure 3c). Of note, α-tocopherol (vitamin E), a fat-soluble antioxidant that protects lipid membranes, was increased by t-BHQ in cells from GCLC high-risk patients (FC = 1.3, P = .049) but not in fibroblasts of controls.
To summarize, the regulation of lysolipids was impaired, suggesting anomalies in phospholipid breakdown/remodeling in patients with GCLC high-risk genotypes.
Discussion
This global metabolic-profiling study examined the metabolic response to oxidative stress in culture of fibroblasts isolated from healthy individuals and EP patients with GCLC low-risk or GCLC high-risk genotypes. The fibroblasts from GCLC high-risk patients showed abnormalities in GSH metabolism in response to oxidative stress and alterations of the lysolipids levels compared with those from control individuals. When both groups of genotypes were combined, fibroblasts from patients showed impaired regulation of collagen- and arginine-related metabolism in response to oxidative stress. Importantly, these pathways were statistically independent of the antipsychotic treatment and thus medication is unlikely to account for the differences between patients and controls. Therefore, fibroblasts represent a valuable model compared with blood to assess metabolic anomalies intrinsic to psychosis by avoiding effects of medication. In addition, oxidative stress appeared as a powerful tool to reveal differences between EP patients and controls. Indeed, most of the differences highlighted in this study are associated with oxidative conditions.
In GCLC high-risk fibroblasts, 5-oxoproline and glutamate, which are intermediates of GSH synthesis, were abnormally regulated. It reflects the defect in GCL inducibility by oxidative stress that was previously associated with the GCLC high-risk polymorphisms.10,12 However, we do not report abnormal levels of GSH associated with GCLC high-risk genotypes as initially described.10 This discrepancy might result from technical differences in the methods used to assess GSH levels (fluorescence vs chromatography coupled to mass spectrometry). Alternatively, it may reflect biological differences in GSH regulation linked to age (mean age was 36.7 years, SD = 11.6 in the first study vs 23.7 years, SD = 3.2 in the present one). Nevertheless, these results on 5-oxoproline and glutamate indicate that we can detect the impact of genomic variations on the metabolome and hence support the validity of our approach to assess metabolic consequences of schizophrenia risk factors. Fibroblasts from GCLC high-risk patients also displayed lower levels of lysolipid at baseline than those from controls. As oxidative stress diminished the levels of lysolipids in cells from control individuals, abnormally low lysolipid levels in cells from GCLC high-risk patients may indicate the existence of a mild oxidative damage in basal conditions. Changes in lysolipid levels may modify membrane properties and affect neurotransmission as hypothesized in the membrane theory of schizophrenia.45 Alterations of lipid levels in blood of patients are a common finding to all the metabolomic studies on schizophrenia available so far. Changes in membrane fatty acid composition,25,46–48 enhancement of phospholipid breakdown,49–55 and low polyunsaturated fatty acids levels56–62 are reported in fibroblasts, blood, or brain of first-episode and chronic schizophrenic patients.
Moreover, data in our study suggest that patients from both genotypes (ie, GCLC low and high risk) may experience more significant oxidative stress than those from controls and thus present signs of impaired redox control: (1) Level of Cys-GSH mixed bisulfide moiety was lower in patients than controls after oxidative stress, which may reflect a decreased bioavailability of GSH. (2) Fibroblasts from patients presented elevated levels of the antioxidant ascorbate, compared with controls. (3) Lipid peroxidation (9-hydroxyoctadecadienoic acid [9-HODE], 13-HODE) did not decrease in fibroblasts from patients as it does in controls in response to oxidative stress. As detailed in the introduction, many reports revealed marks of oxidation in samples from schizophrenia patients. A postmortem study that combined data from transcriptomics, metabolomics, and proteomics, reports altered regulation of the antioxidant response in the prefrontal cortex of patients.9 Lipid peroxidation was detected in the blood of patients by many studies, results strengthened by 2 meta-analyses that report increased levels of malondialdehyde levels.63,64 Additionally, a recent study of olfactory cells with neuronal phenotype revealed a specific epigenetic regulation of genes that code for enzymes of phase II detoxification, which includes the antioxidant response, in cells from patients with schizophrenia.65 All together, these results indicate that oxidation might be a shared trait across tissues from schizophrenia patients and might lead to epigenetic imprinting and profound changes in cell physiology.
Most notably, we identified abnormal response to oxidative challenge for the regulation of EMC and arginine metabolism in EP patients. The degradation of collagen and oxidation of proteoglycan was enhanced in t-BHQ-treated fibroblasts from EP patients, which may indicate a greater instability of the ECM (figure 2). This hypothesis is in line with earlier work showing decreased adhesiveness and lower fibronectin levels in fibroblasts from patients with schizophrenia.66 Immunoassays on serum from schizophrenia patients highlighted matrix metalloproteinases as part of the molecular signature associated with symptom severity and short time to relapse.67 It is worth mentioning that our assay was not designed to study the extracellular compartment, and these results deserve further validation. Additionally, the metabolism of arginine was hampered in cells from EP patients as revealed by anomalies in the regulation of creatine, polyamine, and proline levels in response to oxidative stress (figure 3). We also report higher levels of proline in patients compared with controls, particularly after the stress. Among 5 potential biomarkers of schizophrenia identified by He et al28 in a large cohort of patients, 2 relate to arginine: a plasma increase of ornithine and decrease of arginine. In addition, elevated levels of proline have been found specifically in sera of patients with schizophrenia, compared with other psychosis.27 Of course, comparison of our data set with previous studies is hampered by the lack of information on metabolite regulation in fibroblasts vs blood or vs brain.
Potential consequences of these findings for brain function in patients are numerous. Altered levels of certain metabolites in response to oxidative stress may directly affect neurotransmission. In the metabolism of arginine, polyamines such as spermidine and agmatine regulate astrocytic gap junctions68 and inhibit N-methyl-d-aspartate receptors activity,69–72 and putrescine serves as one of many potential precursors for gamma-aminobutyric acid [GABA]. We found anomalies in regulation of dimethylarginines, which are competitive inhibitors of the NO synthase and may thus participate in regulating NO signaling.73 Deregulation of arginine metabolism may lead to defects in NO signaling and nitrosative stress, with major impact on neuronal survival and functioning,74 as hypothesized to occur in schizophrenia patients.75 Collagen and proteoglycan are essential components of basement membrane and of ECM in the central nervous system. In the brain, redox dysregulation may particularly affect the ECM: mice with impaired synthesis of glutathione present a delayed formation of the perineuronal net, a specialized type of ECM wrapping GABAergic parvalbumin immunoreactive interneurons (PVI).14 According to this study, the perineuronal net protects the PVI interneurons from oxidative stress.14,76 Therefore, oxidative conditions might be associated with changes in neurotransmission and defects of ECM stability, deficits which may impair specific neuronal populations.
Altogether, this study highlights that reactivity to oxidative stress is abnormal in EP patients, besides the redox system. Indeed other pathways are involved, as arginine, ECM, and collagen metabolism, regardless of GCLC genotype. The anomalies were statistically independent from the medication and therefore represent good candidate markers of the disease. It would be of great interest to further study the impact of antioxidant molecules (as omega-3 or N-acetyl-cysteine) on the regulation of the identified pathways to shed light on the role of oxidative stress and the illness itself. Deregulation of the response to stress was also present in the subgroup of patients with schizophrenia diagnosis at clinical follow-up. Because of the relatively limited number of enrolled subjects in our study, further evaluations are needed to clarify if these anomalies may predict disease outcome. Plasma membrane and ECM, 2 regulators of neuronal activity and plasticity, appeared as particularly susceptible to oxidative stress and thus provide novel mechanistic insights for further pathophysiological understanding of the early stage of schizophrenia. The metabolic reactivity to oxidative stress challenges in fibroblasts may therefore pave the way for the search of early biomarkers in psychosis. A metabolic signature allowing detection of at risk individuals before overt manifestation of clinical symptoms would open the prospect of developing protective measures to normalize the disrupted pathways, thus avoiding the pathological consequences of environmental insults.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
The Swiss National Science Foundation (#320030_122419 to P.C. and K.Q.D.), the National Center of Competence in Research “SYNAPSY—The Synaptic Bases of Mental Diseases” financed by the Swiss National Science Foundation (#51AU40_125759), the Brazilian Swiss Joint Research Program, the “Loterie Romande,” Fondation Damm-Etienne, Fondation Avina, Alamaya Foundation.
Supplementary Material
Acknowledgments
We are grateful for technical assistance of Hélène Moser. We thank Mehdi Gholam for support with the statistical analyses. Most of all, we express our gratitude to all patients and healthy volunteers for their enduring participation. J.W. and K.L.P. have affiliations and financial involvement with Metabolon Inc. through their employment by Metabolon, Inc.
References
- 1. Do KQ, Cabungcal JH, Frank A, Steullet P, Cuenod M. Redox dysregulation, neurodevelopment, and schizophrenia. Curr Opin Neurobiol. 2009;19:220–230 [DOI] [PubMed] [Google Scholar]
- 2. Ben Othmen L, Mechri A, Fendri C, et al. Altered antioxidant defense system in clinically stable patients with schizophrenia and their unaffected siblings. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:155–159 [DOI] [PubMed] [Google Scholar]
- 3. Pedrini M, Massuda R, Fries GR, et al. Similarities in serum oxidative stress markers and inflammatory cytokines in patients with overt schizophrenia at early and late stages of chronicity. J Psychiatr Res. 2012;46:819–824 [DOI] [PubMed] [Google Scholar]
- 4. Yao JK, Reddy R, McElhinny LG, van Kammen DP. Reduced status of plasma total antioxidant capacity in schizophrenia. Schizophr Res. 1998;32:1–8 [DOI] [PubMed] [Google Scholar]
- 5. Zhang XY, Tan YL, Cao LY, et al. Antioxidant enzymes and lipid peroxidation in different forms of schizophrenia treated with typical and atypical antipsychotics. Schizophr Res. 2006;81:291–300 [DOI] [PubMed] [Google Scholar]
- 6. Do KQ, Trabesinger AH, Kirsten-Krüger M, et al. Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur J Neurosci. 2000;12:3721–3728 [DOI] [PubMed] [Google Scholar]
- 7. Gawryluk JW, Wang JF, Andreazza AC, Shao L, Young LT. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol. 2011;14:123–130 [DOI] [PubMed] [Google Scholar]
- 8. Yao JK, Leonard S, Reddy R. Altered glutathione redox state in schizophrenia. Dis Markers. 2006;22:83–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prabakaran S, Swatton JE, Ryan MM, et al. Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. Mol Psychiatry. 2004;9:684–97, 643 [DOI] [PubMed] [Google Scholar]
- 10. Gysin R, Kraftsik R, Sandell J, et al. Impaired glutathione synthesis in schizophrenia: convergent genetic and functional evidence. Proc Natl Acad Sci U S A. 2007;104:16621–16626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tosic M, Ott J, Barral S, et al. Schizophrenia and oxidative stress: glutamate cysteine ligase modifier as a susceptibility gene. Am J Hum Genet. 2006;79:586–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gysin R, Kraftsik R, Boulat O, et al. Genetic dysregulation of glutathione synthesis predicts alteration of plasma thiol redox status in schizophrenia. Antioxid Redox Signal. 2011;15:2003–2010 [DOI] [PubMed] [Google Scholar]
- 13. Kulak A, Steullet P, Cabungcal JH, et al. Redox dsregulation in the pathophysiology of schizophrenia and bipolar disorder: insights from animal models. Antiox redox signal. 2013;18:1423–1443 [DOI] [PubMed] [Google Scholar]
- 14. Cabungcal JH, Steullet P, Kraftsik R, Cuenod M, Do KQ. Early-life insults impair parvalbumin interneurons via oxidative stress: reversal by N-acetylcysteine. Biol Psychiatry. 2013;73:574–582 [DOI] [PubMed] [Google Scholar]
- 15. Lataster T, Valmaggia L, Lardinois M, van Os J, Myin-Germeys I. Increased stress reactivity: a mechanism specifically associated with the positive symptoms of psychotic disorder. Psychol med. 2013;43:1389–1400 [DOI] [PubMed] [Google Scholar]
- 16. Myin-Germeys I, Peeters F, Havermans R, et al. Emotional reactivity to daily life stress in psychosis and affective disorder: an experience sampling study. Acta Psychiatr Scand. 2003;107:124–131 [DOI] [PubMed] [Google Scholar]
- 17. Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148:1474–1486 [DOI] [PubMed] [Google Scholar]
- 18. Glenthøj BY. The brain dopaminergic system. Pharmacological, behavioural and electrophysiological studies. Dan Med Bull. 1995;42:1–21 [PubMed] [Google Scholar]
- 19. Laruelle M. The role of endogenous sensitization in the pathophysiology of schizophrenia: implications from recent brain imaging studies. Brain Res Brain Res Rev. 2000;31:371–384 [DOI] [PubMed] [Google Scholar]
- 20. Laruelle M, Abi-Dargham A. Dopamine as the wind of the psychotic fire: new evidence from brain imaging studies. J Psychopharmacol. 1999;13:358–371 [DOI] [PubMed] [Google Scholar]
- 21. Lataster J, Collip D, Ceccarini J, et al. Psychosocial stress is associated with in vivo dopamine release in human ventromedial prefrontal cortex: a positron emission tomography study using [¹⁸F]fallypride. Neuroimage. 2011;58:1081–1089 [DOI] [PubMed] [Google Scholar]
- 22. Myin-Germeys I, Marcelis M, Krabbendam L, Delespaul P, van Os J. Subtle fluctuations in psychotic phenomena as functional states of abnormal dopamine reactivity in individuals at risk. Biol Psychiatry. 2005;58:105–110 [DOI] [PubMed] [Google Scholar]
- 23. Hastings TG. The role of dopamine oxidation in mitochondrial dysfunction: implications for Parkinson’s disease. J Bioenerg Biomembr. 2009;41:469–472 [DOI] [PubMed] [Google Scholar]
- 24. Kaddurah-Daouk R, McEvoy J, Baillie R, et al. Impaired plasmalogens in patients with schizophrenia. Psychiatry Res. 2012;198:347–352 [DOI] [PubMed] [Google Scholar]
- 25. Kaddurah-Daouk R, McEvoy J, Baillie RA, et al. Metabolomic mapping of atypical antipsychotic effects in schizophrenia. Mol Psychiatry. 2007;12:934–945 [DOI] [PubMed] [Google Scholar]
- 26. Orešič M, Seppänen-Laakso T, Sun D, et al. Phospholipids and insulin resistance in psychosis: a lipidomics study of twin pairs discordant for schizophrenia. Genome Med. 2012;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Orešič M, Tang J, Seppänen-Laakso T, et al. Metabolome in schizophrenia and other psychotic disorders: a general population-based study. Genome Med. 2011;3:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. He Y, Yu Z, Giegling I, et al. Schizophrenia shows a unique metabolomics signature in plasma. Transl Psychiatry. 2012;2:e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang J, Chen T, Sun L, et al. Potential metabolite markers of schizophrenia. Mol Psychiatry. 2013;18:67–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pinkus R, Weiner LM, Daniel V. Role of oxidants and antioxidants in the induction of AP-1, NF-kappaB, and glutathione S-transferase gene expression. J Biol Chem. 1996;271:13422–13429 [DOI] [PubMed] [Google Scholar]
- 31. Prestera T, Holtzclaw WD, Zhang Y, Talalay P. Chemical and molecular regulation of enzymes that detoxify carcinogens. Proc Natl Acad Sci U S A. 1993;90:2965–2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schilderman PA, van Maanen JM, ten Vaarwerk FJ, et al. The role of prostaglandin H synthase-mediated metabolism in the induction of oxidative DNA damage by BHA metabolites. Carcinogenesis. 1993;14:1297–1302 [DOI] [PubMed] [Google Scholar]
- 33. Auburger G, Klinkenberg M, Drost J, et al. Primary skin fibroblasts as a model of Parkinson’s disease. Mol Neurobiol. 2012;46:20–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mytilineou C, Werner P, Molinari S, Di Rocco A, Cohen G, Yahr MD. Impaired oxidative decarboxylation of pyruvate in fibroblasts from patients with Parkinson’s disease. J Neural Transm Park Dis Dement Sect. 1994;8:223–228 [DOI] [PubMed] [Google Scholar]
- 35. Wiedemann FR, Winkler K, Lins H, Wallesch CW, Kunz WS. Detection of respiratory chain defects in cultivated skin fibroblasts and skeletal muscle of patients with Parkinson’s disease. Ann N Y Acad Sci. 1999;893:426–429 [DOI] [PubMed] [Google Scholar]
- 36. Yung AR, Yuen HP, McGorry PD, et al. Mapping the onset of psychosis: the Comprehensive Assessment of At-Risk Mental States. Aust N Z J Psychiatry. 2005;39:964–971 [DOI] [PubMed] [Google Scholar]
- 37. Baumann PS, Crespi S, Marion-Veyron R, et al. Treatment and early intervention in psychosis program (TIPP-Lausanne): implementation of an early intervention programme for psychosis in Switzerland. Early Interv Psychiatry. 2013;7:322–328 [DOI] [PubMed] [Google Scholar]
- 38. Preisig M, Fenton BT, Matthey ML, Berney A, Ferrero F. Diagnostic interview for genetic studies (DIGS): inter-rater and test-retest reliability of the French version. Eur Arch Psychiatry Clin Neurosci. 1999;249:174–179 [DOI] [PubMed] [Google Scholar]
- 39. Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem. 2009;81:6656–6667 [DOI] [PubMed] [Google Scholar]
- 40. Ohta T, Masutomi N, Tsutsui N, et al. Untargeted metabolomic profiling as an evaluative tool of fenofibrate-induced toxicology in Fischer 344 male rats. Toxicol Pathol. 2009;37:521–535 [DOI] [PubMed] [Google Scholar]
- 41. Lu C, King RD. An investigation into the population abundance distribution of mRNAs, proteins, and metabolites in biological systems. Bioinformatics. 2009;25:2020–2027 [DOI] [PubMed] [Google Scholar]
- 42. Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A. 2003;100:9440–9445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67:255–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Davis JM, Chen N. Dose response and dose equivalence of antipsychotics. J Clin Psychopharmacol. 2004;24:192–208 [DOI] [PubMed] [Google Scholar]
- 45. Horrobin DF. Schizophrenia as a membrane lipid disorder which is expressed throughout the body. Prostaglandins Leukot Essent Fatty Acids. 1996;55:3–7 [DOI] [PubMed] [Google Scholar]
- 46. Keshavan MS, Mallinger AG, Pettegrew JW, Dippold C. Erythrocyte membrane phospholipids in psychotic patients. Psychiatry Res. 1993;49:89–95 [DOI] [PubMed] [Google Scholar]
- 47. Mahadik SP, Mukherjee S, Correnti EE, et al. Plasma membrane phospholipid and cholesterol distribution of skin fibroblasts from drug-naive patients at the onset of psychosis. Schizophr Res. 1994;13:239–247 [DOI] [PubMed] [Google Scholar]
- 48. Yao JK, Leonard S, Reddy RD. Membrane phospholipid abnormalities in postmortem brains from schizophrenic patients. Schizophr Res. 2000;42:7–17 [DOI] [PubMed] [Google Scholar]
- 49. Kato T, Shioiri T, Murashita J, Hamakawa H, Inubushi T, Takahashi S. Lateralized abnormality of high-energy phosphate and bilateral reduction of phosphomonoester measured by phosphorus-31 magnetic resonance spectroscopy of the frontal lobes in schizophrenia. Psychiatry Res. 1995;61:151–160 [DOI] [PubMed] [Google Scholar]
- 50. Pettegrew JW, Keshavan MS, Panchalingam K, et al. Alterations in brain high-energy phosphate and membrane phospholipid metabolism in first-episode, drug-naive schizophrenics. A pilot study of the dorsal prefrontal cortex by in vivo phosphorus 31 nuclear magnetic resonance spectroscopy. Arch Gen Psychiatry. 1991;48:563–568 [DOI] [PubMed] [Google Scholar]
- 51. Potwarka JJ, Drost DJ, Williamson PC, et al. A 1H-decoupled 31P chemical shift imaging study of medicated schizophrenic patients and healthy controls. Biol Psychiatry. 1999;45:687–693 [DOI] [PubMed] [Google Scholar]
- 52. Shioiri T, Someya T, Murashita J, et al. Multiple regression analysis of relationship between frontal lobe phosphorus metabolism and clinical symptoms in patients with schizophrenia. Psychiatry Res. 1997;76:113–122 [DOI] [PubMed] [Google Scholar]
- 53. Stanley JA, Williamson PC, Drost DJ, et al. Membrane phospholipid metabolism and schizophrenia: an in vivo 31P-MR spectroscopy study. Schizophr Res. 1994;13:209–215 [DOI] [PubMed] [Google Scholar]
- 54. Williamson PC, Brauer M, Leonard S, Thompson T, Drost D. 31P magnetic resonance spectroscopy studies in schizophrenia. Prostaglandins Leukot Essent Fatty Acids. 1996;55:115–118 [DOI] [PubMed] [Google Scholar]
- 55. Stanley JA, Williamson PC, Drost DJ, et al. An in vivo study of the prefrontal cortex of schizophrenic patients at different stages of illness via phosphorus magnetic resonance spectroscopy. Arch Gen Psychiatry. 1995;52:399–406 [DOI] [PubMed] [Google Scholar]
- 56. Arvindakshan M, Ghate M, Ranjekar PK, Evans DR, Mahadik SP. Supplementation with a combination of omega-3 fatty acids and antioxidants (vitamins E and C) improves the outcome of schizophrenia. Schizophr Res. 2003;62:195–204 [DOI] [PubMed] [Google Scholar]
- 57. Bentsen H, Solberg DK, Refsum H, et al. Bimodal distribution of polyunsaturated fatty acids in schizophrenia suggests two endophenotypes of the disorder. Biol Psychiatry. 2011;70:97–105 [DOI] [PubMed] [Google Scholar]
- 58. Glen AI, Glen EM, Horrobin DF, et al. A red cell membrane abnormality in a subgroup of schizophrenic patients: evidence for two diseases. Schizophr Res. 1994;12:53–61 [DOI] [PubMed] [Google Scholar]
- 59. Hoen WP, Lijmer JG, Duran M, Wanders RJ, van Beveren NJ, de Haan L. Red blood cell polyunsaturated fatty acids measured in red blood cells and schizophrenia: a meta-analysis. Psychiatry Res. 2013;207:1–12 [DOI] [PubMed] [Google Scholar]
- 60. Khan MM, Evans DR, Gunna V, Scheffer RE, Parikh VV, Mahadik SP. Reduced erythrocyte membrane essential fatty acids and increased lipid peroxides in schizophrenia at the never-medicated first-episode of psychosis and after years of treatment with antipsychotics. Schizophr Res. 2002;58:1–10 [DOI] [PubMed] [Google Scholar]
- 61. Peet M, Laugharne J, Rangarajan N, Horrobin D, Reynolds G. Depleted red cell membrane essential fatty acids in drug-treated schizophrenic patients. J Psychiatr Res. 1995;29:227–232 [DOI] [PubMed] [Google Scholar]
- 62. van der Kemp WJ, Klomp DW, Kahn RS, Luijten PR, Hulshoff Pol HE. A meta-analysis of the polyunsaturated fatty acid composition of erythrocyte membranes in schizophrenia. Schizophr Res. 2012;141:153–161 [DOI] [PubMed] [Google Scholar]
- 63. Grignon S, Chianetta JM. Assessment of malondialdehyde levels in schizophrenia: a meta-analysis and some methodological considerations. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:365–369 [DOI] [PubMed] [Google Scholar]
- 64. Zhang M, Zhao Z, He L, Wan C. A meta-analysis of oxidative stress markers in schizophrenia. Sci China Life Sci. 2010;53:112–124 [DOI] [PubMed] [Google Scholar]
- 65. Kano S, Colantuoni C, Han F, et al. Genome-wide profiling of multiple histone methylations in olfactory cells: further implications for cellular susceptibility to oxidative stress in schizophrenia. Mol Psychiatry. 2013;18:740–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mahadik SP, Mukherjee S, Wakade CG, Laev H, Reddy RR, Schnur DB. Decreased adhesiveness and altered cellular distribution of fibronectin in fibroblasts from schizophrenic patients. Psychiatry Res. 1994;53:87–97 [DOI] [PubMed] [Google Scholar]
- 67. Schwarz E, Guest PC, Steiner J, Bogerts B, Bahn S. Identification of blood-based molecular signatures for prediction of response and relapse in schizophrenia patients. Transl Psychiatry. 2012;2:e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Benedikt J, Inyushin M, Kucheryavykh YV, et al. Intracellular polyamines enhance astrocytic coupling. Neuroreport. 2012;23:1021–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Aricioglu F, Altunbas H. Is agmatine an endogenous anxiolytic/antidepressant agent? Ann N Y Acad Sci. 2003;1009:136–140 [DOI] [PubMed] [Google Scholar]
- 70. Williams K. Modulation and block of ion channels: a new biology of polyamines. Cell Signal. 1997;9:1–13 [DOI] [PubMed] [Google Scholar]
- 71. Zeidan MP, Zomkowski AD, Rosa AO, Rodrigues AL, Gabilan NH. Evidence for imidazoline receptors involvement in the agmatine antidepressant-like effect in the forced swimming test. Eur J Pharmacol. 2007;565:125–131 [DOI] [PubMed] [Google Scholar]
- 72. Zomkowski AD, Santos AR, Rodrigues AL. Putrescine produces antidepressant-like effects in the forced swimming test and in the tail suspension test in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1419–1425 [DOI] [PubMed] [Google Scholar]
- 73. MacAllister RJ, Parry H, Kimoto M, et al. Regulation of nitric oxide synthesis by dimethylarginine dimethylaminohydrolase. Br J Pharmacol. 1996;119:1533–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Shahani N, Sawa A. Nitric oxide signaling and nitrosative stress in neurons: role for S-nitrosylation. Antioxid Redox Signal. 2011;14:1493–1504 [DOI] [PubMed] [Google Scholar]
- 75. Yao JK, Leonard S, Reddy RD. Increased nitric oxide radicals in postmortem brain from patients with schizophrenia. Schizophr Bull. 2004;30:923–934 [DOI] [PubMed] [Google Scholar]
- 76. Cabungcal JH, Steullet P, Morishita H, et al. Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc Natl Acad Sci U S A. 2013;110:9130–9135 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.