Abstract
A disruption of dopaminergic transmission in the amygdala of subjects with schizophrenia was proposed as a main contributor to pathophysiological and clinical manifestations of this disorder. We tested the hypothesis that the expression of the dopamine transporter (DAT) is decreased in the amygdala of subjects with schizophrenia. In normal control, schizophrenic subjects and bipolar disorder subjects, we measured numerical density of axon varicosities immunoreactive (IR) for DAT in the lateral (LN), basal, accessory basal (ABN), and cortical (CO) nuclei and intercalated cell masses (ITCM) of the amygdala. Tyrosine hydroxylase (TH)-IR and dopamine beta-hydroxylase (DBH)-IR varicosities were measured to test for potential loss of varicosities and serotonin transporter (5HTT)-IR for involvement of the serotoninergic system. Among several potential confounding variables tested, particular emphasis was placed on exposure to therapeutic drugs. In schizophrenic subjects, DAT-IR varicosities were decreased in LN (P = .0002), ABN (P = .013), and CO (P = .0001) in comparison with controls, and in comparison with bipolar disorder subjects in LN (P = .004) and CO (P = .002). DBH-IR varicosities were decreased in ABN (P = .008) and ITCM (P = .017), compared with controls. TH- and 5HTT-IR varicosities were not altered. No changes were detected in bipolar disorder. Taken together with TH and DBH findings, reductions of DAT-IR varicosities point to decreased DAT expression in dopaminergic terminals in the amygdala of subjects with schizophrenia. This DAT decrease may disrupt dopamine uptake, leading to increased dopaminergic synaptic transmission and spillage into the extracellular space with activation of extrasynaptic dopamine receptors. Concurrent decrease of noradrenaline in the ABN may disrupt memory consolidation.
Key words: schizophrenia, bipolar disorder, amygdala, dopamine transporter, dopamine beta-hydroxylase
Introduction
Emerging views on schizophrenia (SZ) highlight a disruption of salience attribution and prediction error mechanisms as a key component of this disorder.1,2 Typical features of SZ, such as adherence to erroneous beliefs despite contradicting evidence, may be interpreted as a disruption of these mechanisms.3–5 Their regulation by the mesolimbic dopamine system supports the hypothesis that a disruption of dopaminergic inputs to the striatum and amygdala may underlie misattribution of salience, leading to mistakenly linking valence to otherwise neutral stimuli (delusion, inappropriate affect) or failure to attribute emotional valence (flat affect).2–7 Several studies reported a disruption of dopaminergic transmission in the striatum of subjects with SZ, thought to contribute to aspects of these mechanisms.8,9 However, as suggested by Pankow and coworkers, it may not account for the pervasive sense of ‘danger’, intense fear, and anxiety that typically accompany paranoid delusions.2 The possibility that altered dopaminergic transmission in the amygdala, impacting attribution of salience, may at least in part underlie psychotic symptoms has been suggested by several authors.2,7,10–12 Here, we tested the hypothesis that dopaminergic innervation of the amygdala may be altered in SZ.
Notably, the mesolimbic dopaminergic system controls several aspects of salience information processing in the amygdala. A particularly poignant example is the key role played by dopamine in modulating fear learning in this region,13 incidentally a type of learning recently shown to be altered in SZ.14 The amygdala’s orchestration of complex visceral and behavioral responses triggered by affective stimuli, including fear, is controlled by cortical and dopaminergic inputs.15–18 These latter tend to facilitate amygdalar responses under conditions of high emotional arousal, potentially counteracting cortical modulation.17,18 In addition to biasing affective valence processing by the amygdala, eventually integrated in neural circuits creating goal-directed strategies,15,19,20 dopamine may also affect the amygdala’s modulation on primary sensory cortices, modulating attention to salient stimuli, eg, ref. 21 and ref. 22 Abnormal dopaminergic transmission in the amygdala may impact on primary sensory cortices, potentially contributing to hallucinations, eg, ref.23
Together, these considerations support the hypothesis that altered dopaminergic transmission in the amygdala may play a key role in the pathogenesis and clinical manifestations of SZ.2,7,10–12 Surprisingly, dopaminergic abnormalities in the amygdala of subjects with this disorder have not been tested thus far, with the notable exception of a study by Reynolds,11 showing an increase of dopamine concentration in the amygdala. We postulated that decreased dopamine transporter (DAT) expression may contribute to increased dopamine levels and, with the present studies, tested the hypothesis that DAT expression may be reduced in the amygdala of subjects with SZ. Numerical densities (Nd) of axon varicosities (putative en passant presynaptic specializations) expressing DAT, tyrosine hydroxylase (TH), or dopamine beta-hydroxylase (DBH) were measured in the lateral (LN), basal (BN), accessory basal (ABN), and cortical (CO) nuclei and intercalated cell masses (ITCM) of the amygdala of normal control, SZ, and bipolar disorder (BD) subjects. BD subjects were included in this study to control for the potential effects of stress related to a chronic psychiatric disorder and to help account for exposure to antipsychotic drugs. Superficial nuclei of the amygdala, namely the central and medial nuclei, were not available for this study. TH is the rate-limiting enzyme in catecholamine synthesis. Thus, TH-immunoreactive (IR) varicosities represent a composite of dopaminergic (DAT-IR) and noradrenergic (DBH-IR) varicosities24 so that combined results from these 3 markers can be used to distinguish between expression changes vs loss of varicosities. DAT is expressed exclusively in dopaminergic fibers and terminals,25,26 where it mediates dopamine reuptake.25,27 DBH is the noradrenaline synthetic enzyme and is thus expressed in TH positive noradrenergic fibers. Finally, serotonin transporter (5HTT)-IR varicosities were measured to investigate the potential for concurrent involvement of the serotoninergic system.
Methods
Human Subjects
Tissue blocks containing the whole amygdala (1 hemisphere/subject) from 10 SZ, 12 BD, and 12 normal control donors, matched for age, gender, and postmortem interval (PMI), were obtained from the Harvard Brain Tissue Resource Center (HBTRC) (supplementary table 1). Diagnoses of SZ and BD were made by 2 psychiatrists on the basis of retrospective review of medical records and extensive questionnaires concerning social and medical history provided by family members. Several regions from each brain were examined by a neuropathologist. The cohort used for this study did not include subjects with evidence for gross and/or macroscopic brain changes, or clinical history, consistent with cerebrovascular accident or other neurological disorders. Subjects with Braak stages III or higher (modified Bielchowsky stain) were not included. None of these subjects had significant history of substance dependence within 10 or more years from death, as further corroborated by negative toxicology reports. Absence of recent substance abuse is typical for samples from the HBTRC, which receives exclusively community-based tissue donations.
Tissue Processing and Immunohistochemistry
Tissue processing, sectioning, and storage were carried out as described previously.28,29 In brief, tissue blocks containing the whole amygdala were dissected from fresh brains, postfixed for 2 weeks in 0.1mol/ml phosphate buffer (pH 7.4) containing 4% paraformaldehyde and 0.1% Na azide in 0.1M phosphate buffer (PB) at 4°C, cryoprotected for 3 weeks (30% glycerol, 30% ethylene glycol, 0.1% Na azide in 0.1M PB) and then exhaustively sectioned using a freezing microtome (American Optical 860). Using systematic random sampling criteria, sections through the amygdala were serially distributed in 26 compartments (40-µm thick sections; 10–12 sections/compartment; 1.04-mm section separation within each compartment).
Immunocytochemistry for each marker in this study was carried out on the same subject cohort and sections from all subjects for each marker were processed simultaneously, taking all precautions to avoid sequence effects.29 Antigen unmasking for DAT, TH, and DBH: 100°C PB with 1:100 Antigen Unmasking Solution (Vector Labs Inc) for 3 minutes. Antigen unmasking for 5HTT: citric buffer (pH = 4.5) for 12 hours at 4°C, then in the same buffer heated to 80°C for 30 minutes. Primary antibodies: polyclonal rabbit anti-DAT antibody (1:10.000; D6944; synthetic peptide corresponding to amino acids 42–59 of rat DAT; Sigma-Aldrich); polyclonal sheep anti-TH antibody (1:500; P60101-0; native rat TH purified from pheochromocytoma; Pel-Freez); polyclonal sheep anti-DBH (1:5,000; D217; synthetic peptide from the N-terminal region of human DBH; Sigma-Aldrich); and monoclonal mouse anti-5HTT antibody (1:6000; ST51-1; 16-aa peptide from the N-terminus of the human serotonin transporter; Mab Technologies). Biotinylated secondary serum (1:500; Vector Labs, Inc) was followed by streptavidin (1:5000; Vector Labs Inc.) and detection of the antigen/antibody complex using diaminobenzidine (0.02%; Sigma-Aldrich Inc), nickel sulfate (0.08%), and hydrogen peroxide (0.006%). All steps were followed by rinses with 0.1M PB 0.5% Triton-X. For all antibodies, immunolabeling results were consistent with published data,30–32 and western blotting (respective maker companies) showed a single band of the expected molecular weight; omission of primary or secondary antibodies did not result in immunolabeling.
Data Collection
Light microscopy interfaced with stereology software was used for quantitative analysis (Stereo-Investigator 6.0, MicroBrightField, Inc). Stereology-based quantitative microscopy was used to measure the volume of each nucleus and numerical densities (Nd) of IR varicosities as described previously (for detailed description, see supplementary figure 1).
Statistical Analyses
Logarithmic transformation was applied to all original values because the data were not normally distributed. Statistical analyses were performed using JMP v5.0.1a (SAS Institute Inc). Differences between groups relative to the main outcome measures were assessed for statistical significance using a stepwise linear regression process equivalent to an analysis of covariance (ANCOVA) model, testing for diagnostic effects and accounting simultaneously for potential effects of other covariates. Effect sizes were calculated according to Hedges’ g (supplementary table 3).
Age, gender, PMI, hemisphere, cause of death (acute, eg, myocardial infarction; chronic, eg, cancer), brain weight, exposure to alcohol, nicotine, electroconvulsive therapy, and lifetime and final 6 months’ exposure to antipsychotic drugs and lithium treatment (supplementary tables 1 and 2) were tested systematically for their effects on the main outcome measures and included in the model if they significantly improved the model goodness-of-fit. Estimated daily mg doses of antipsychotic drugs were converted to the approximate equivalent of chlorpromazine (CPZ) as a standard comparator and corrected on the basis of a qualitative assessment of treatment-adherence as indicated by antemortem clinical records and family questionnaires.29 These values are reported as lifetime, as well as last 6 months of life, CPZ grams per patient. Exposure to lithium salt was estimated and reported in the same manner. Nicotine and alcohol consumptions were rated subjectively from 0 to 4 (0 = no smoking, 4 = heavy smoking) on the basis of medical records and family questionnaires. Tissue pH, available for approximately half of the subjects included in this cohort, was tested for its overall effects on each outcome measure across diagnostic groups. In a second stage of analyses, reported separately, the ANCOVA model previously chosen was repeated separately for left and right hemispheres.
Results
Normal Human Amygdala
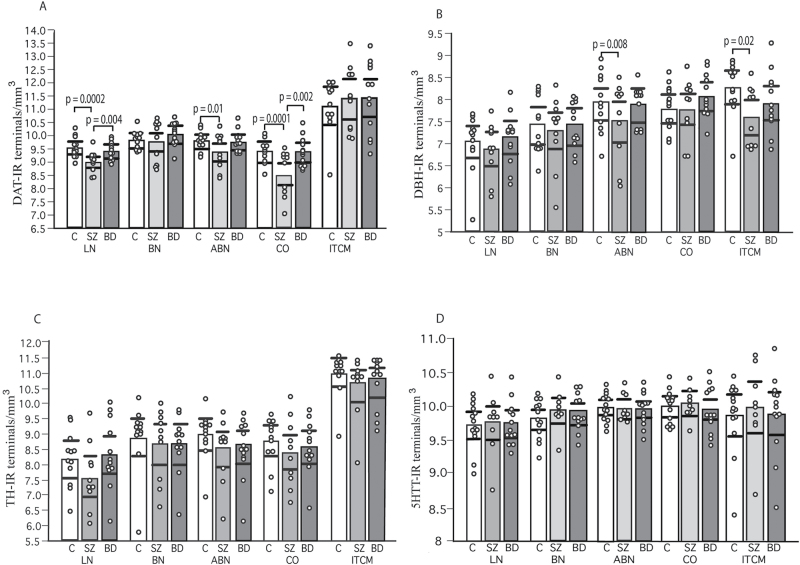
Consistent with previous reports in the normal primate amygdala, Nd of DAT-, TH-, and 5HTT-IR varicosities were comparable, while DBH-IR varicosities showed lower densities (figure 1, supplementary figure 2, supplementary table 3).32–35 Densities were highest in BN and ABN, followed closely by the LN (supplementary table 3), although variability within each of these nuclei was observed. ITCM showed the highest Nd of DAT-IR and TH-IR varicosities (eg, DAT-IR varicosities: 4.4 times higher than BN and 6.8 times higher than CO). Recent data showing that LN, BN, and ABN have the second highest DAT densities after the central nucleus30 (and ITCM) are consistent with our findings. DBH-IR and 5HTT-IR varicosities showed a more homogeneous distribution across nuclei (supplementary table 3).
Fig. 1.
Dopamine transporter immunoreactive (DAT-IR) and dopamine beta-hydroxylase (DBH)-IR varicosities are decreased in distinct nuclei of the amygdala of subjects with SZ. Nd (varicosities/mm2; logarithmic transformation) of DAT-, DBH-, tyrosine hydroxylase (TH)-, and 5HTT-IR varicosities in the amygdala of control (C; n = 12 in DAT, TH, DBH, and n = 14 in 5HTT studies), SZ subjects (n = 10 in DAT, TH, DBH, and n = 9 in 5HTT studies), and bipolar disorder subjects (n = 12 in DAT, TH, DBH, and n = 13 in 5HTT studies). Error bars: 95% confidence intervals.
Group Comparisons
DAT-IR Varicosities.
In SZ subjects, Nd of DAT-IR varicosities were significantly decreased in LN (P = .0002; effect size, g = −2.31), ABN (P = .013; effect size, g = −1.36), and CO (P = .0001; effect size, g = −2.48), compared with controls (figures 1 and 2, supplementary table 3). In these nuclei, significance values for DAT-IR varicosities were adjusted for lifetime CPZ (supplementary table 3). Linear correlation analyses showed positive correlations of lifetime CPZ with DAT-IR varicosities in LN (P = .05), ABN (P = .04), and CO (P = .004). Significance values for CO were also adjusted for the effects of age, and sex (supplementary table 3). No correlation with age at onset of the disease or duration of the illness was detected in the LN and ABN, while the latter was negatively correlated in CO (P = .0006). No significant changes were detected in BN and ITCM. SZ subjects also showed decreased Nd of DAT-IR varicosities with respect to BD in the LN (P = .004) and CO (P = .0025) (figures 1 and 2). In BD, no changes were observed in any of the nuclei examined.
Fig. 2.
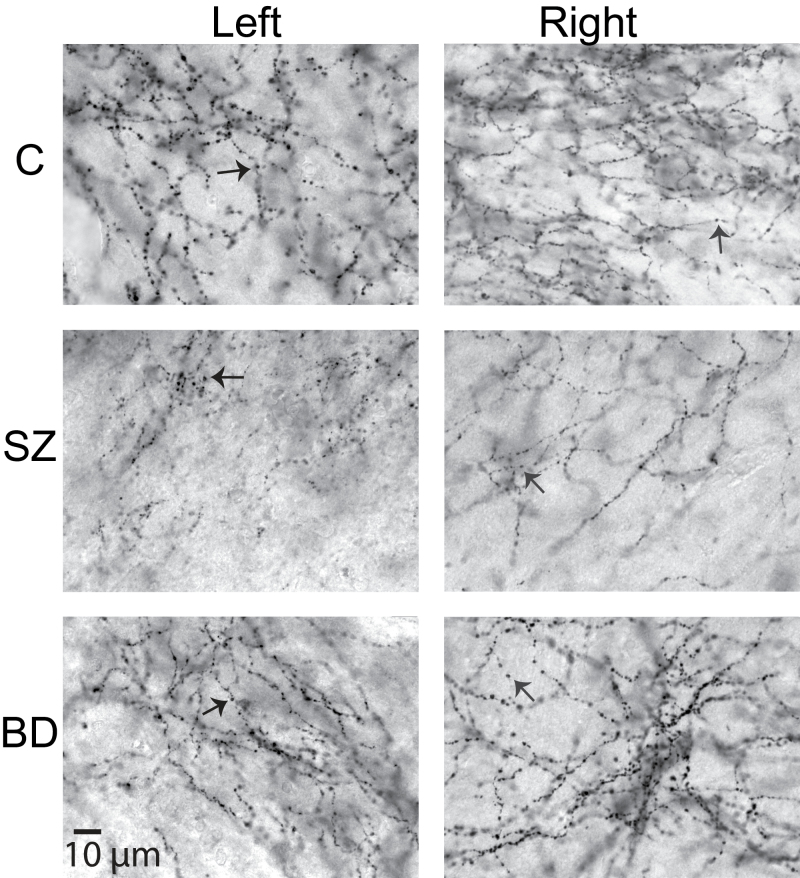
DAT-IR varicosities in the lateral nucleus. Photomicrographs of DAT-IR varicosities (arrows; ×63 magnification) in the ventral portion of the lateral nucleus of the right and left amygdala of healthy control, schizophrenic subjects and bipolar disorder subjects. Note decreased DAT-IR varicosities in subjects with SZ. Scale bar: 10 μm.
TH-IR Varicosities.
Nd of TH-IR varicosities were not significantly altered in SZ or BD in any of the nuclei tested (figure 1, supplementary table 3). None of the covariates tested, including lifetime CPZ and last 6 months of life CPZ, were found to have a significant effect on Nd of TH-IR varicosities.
DBH-IR Varicosities.
In SZ subjects, Nd of DBH-IR varicosities were decreased in ABN (P = .008; effect size, g = −1.52) and ITCM (P = .017; effect size, g = −1.21), compared with controls (figure 1, supplementary table 3). In ABN, significance values for DBH-IR varicosities were adjusted for last 6 months of lifetime CPZ (P = .05) and hemisphere (less in left, P = .008). No significant changes were detected in LN, BN, or CO (figure 1, supplementary table 3). In BD, Nd of DBH-IR varicosities were not altered in any of the nuclei examined.
5HTT-IR Varicosities.
Nd of 5HTT-IR varicosities were not altered in SZ or BD in any of the amygdala nuclei tested (figure 1, supplementary table 3).
Volume of Amygdala Nuclei.
Volumes of the amygdala nuclei included in this study, estimated from sections labeled with 5HTT, did not differ between diagnosis and control groups (supplementary figure 3).
Additional Analyses on Potential Confounding Factors
The effects of exposure to valproic acid, selective serotonin reuptake inhibitors, and electroconvulsive treatment could not be tested in a statistical model because the number of subjects exposed to each was too low (supplementary table 2). However, values for subjects exposed to these treatments were within their group’s standard deviation, making it unlikely that these factors contributed to group differences. Nicotine and ethanol use in SZ and BD subjects did not show significant correlations or effects in regression models with Nd of DAT-, TH-, DBH-, or 5HT-T-IR varicosities. Clinical and toxicological evidence for current or recent drug addiction was an exclusionary criteria for this study. In a small number of subjects (3 SZ subjects; 1 BD subject) our records suggest drug exposure, such as cocaine, heroin, and marijuana, during the early phases of the disease. These subjects were not outliers for any of the main outcome measures and fell close to the group mean.
Because a previous report showed dopamine increases predominantly in the left amygdala,11 we tested the 2 hemispheres separately using the same statistical model for each nucleus. In the left amygdala of subjects with SZ, Nd of DAT-IR varicosities were significantly decreased in LN (P = .02; effect size, g = −2.15; figure 2), BN (P = .004; effect size, g = −3.98), ABN (P = .005; effect size, g = −2.83), and CO (P = .01; effect size, g = −3.97). In the right amygdala, Nd of DAT-IR varicosities were decreased in LN (P = .015; effect size, g = −2.35; figure 2) and CO (P = .034; effect size, g = −2.40). Findings relative to TH-, DBH-, and 5HTT-IR varicosities were virtually identical in the 2 hemispheres.
To test whether cancer-related death and/or chemotherapy may affect TH expression, as shown peripherally,36 we repeated analyses explicitly classifying death as related or not to cancer. Results were virtually identical to those obtained with the acute vs chronic classification and showed no effect of these variables on outcome measures. Tissue pH, potentially affecting protein stability, was not correlated with any of the main outcome measures. Finally, stress effects of living with chronic disorder are not likely to contribute to DAT and DBH changes detected in subjects with SZ because subjects with BD did not show equivalent changes.
Discussion
In SZ, large effect size decreases of DAT-IR varicosities were detected in LN, ABN, and CO, and DBH-IR varicosities were decreased in the ABN and ITCM. Because DAT and DBH are expressed in TH-IR varicosities, which were not altered, these results point to a reduction of DAT and DBH expressions, rather than varicosities themselves, in distinct amygdala nuclei (see below). Analysis of potential confound variables suggest that these changes may be inherent to the pathophysiology of SZ. Lack of changes in BD subjects and significant differences between SZ and BD subjects point to divergence on this pathological aspect. We propose that decreased DAT expression may result in abnormally amplified dopaminergic transmission in the amygdala of subjects with SZ, potentially contributing to the clinical symptomatology of this disorder.
Technical Considerations
Potential effects of antipsychotic exposure are particularly relevant to these results. Notably, significant correlations between antipsychotic exposure and Nd of IR varicosities, where present, were consistently positive, thus opposite to changes associated with SZ. Furthermore, no changes were detected in subjects with BD although the majority of these subjects were exposed to moderate-to-high doses of antipsychotics over their lifetime (supplementary table 2). Together, these results suggest that antipsychotic treatment tends to increase densities of DAT- and DBH-IR varicosities, thus potentially offsetting the decreases inherent to SZ.
Nd of IR varicosities were used as the main outcome measure. A potential concern may be that decreased Nd observed for DAT-IR varicosities may reflect volumetric increases of the LN, ABN, and CO. Pointing to the contrary, no volumetric changes were detected in any of these nuclei in this study (supplementary figure 3), consistent with previous findings using a largely overlapping cohort of subjects.28 Furthermore, volumetric abnormalities would affect Nd of all varicosities across markers, which is not the case in this study.
It is well established that dopaminergic fibers reaching the amygdala contact their target through en passant synapses, morphologically corresponding to axon varicosities,31,37,38 consistent with our observation that within terminal fields of innervation, DAT-, TH-, and DBH-fibers are thin, tortuous, and form “bead-like,” densely spaced, varicosities (figure 2, supplementary figure 2). Synapses formed by nonvaricose axonal segments have not been shown in human amygdala; if present, they would not have been counted in this study. The validity of group differences relative to numbers of varicosities, shown to correspond to synapses,37,38 would not be affected by this eventuality.
Caution in interpreting these results is necessary given the small sample size (12 normal control, 10 SZ subjects, and 12 BD subjects). However, the magnitude of the changes observed (see effect sizes, supplementary table 3) and their remarkable consistency, both internally and with respect to previous independent findings,11 support their validity.
Result Interpretation
TH-IR varicosities represent a composite of dopaminergic (DAT-IR) and noradrenergic (DBH-IR) varicosities.24 In this study, the observed combination of normal densities of TH-IR varicosities and decreased DAT- and/or DBH-IR varicosities in selective amygdala nuclei is interpreted to reflect normal numbers of dopaminergic and noradrenergic varicosities in combination with decreased DAT and DBH expressions. For example, decreased DAT-IR varicosities are interpreted as reduced DAT expression, not decreased dopaminergic varicosities, because there was no parallel decrease of TH-IR varicosities. Reduced (dopaminergic) TH-IR varicosities might have been masked by increased (noradrenergic) TH-IR varicosities, but no DBH-IR varicosity increase was detected in this study. Normal TH expression is interpreted as normal dopamine synthesis.
Dopamine Transporter
Taken together, our results indicate that a decrease of DAT expression in the amygdala of subjects with SZ occurs in the context of normal numbers of dopaminergic varicosities and normal dopamine synthesis. DAT mediates dopamine uptake, representing the primary and most effective way of terminating dopamine actions at postsynaptic receptors.27 Reduced DAT expression in LN, ABN, and CO of SZ subjects may impair dopamine uptake, resulting in amplification and dysregulation of dopaminergic transmission and dopamine diffusion in the extrasynaptic space. In the amygdala, DAT decreases are likely to have particularly strong effects on dopamine diffusion because mechanisms controlling extracellular dopamine are considerably weaker in this region in comparison to the striatum and the cortex.39,40 These findings are consistent with increased dopamine concentration in this region described by G. Reynolds in SZ subjects.11 Remarkably, our findings and Reynolds are also consistent in suggesting predominant dopaminergic abnormalities in the left amygdala, supporting a left temporal lobe dysfunction in SZ.11
These findings also resonate with the hypothesis proposed by Carlsson et al,41 who postulated dopamine extrasynaptic spill and consequent activation of poorly regulated extrasynaptic D2 receptors, as a key pathophysiological mechanism in this disorder and the main target of antipsychotic treatment. Our findings suggest that DAT reductions in the amygdala may be the root of the neuropharmacological effects postulated by Carlsson. Consistent with this possibility is the hypothesis that enhanced dopaminergic transmission in the amygdala may significantly contribute to the emergence of positive symptoms,2,7,10–12 the clinical dimension most responsive to antipsychotic treatment. In this context, DAT decreases in the LN are particularly relevant because this nucleus plays a key role in stimulus valence processing.42–45 Similar mechanisms are not likely to occur in the striatum because striatal DAT expression has consistently been found to be normal in SZ subjects46,47; discordant information is available for the prefrontal cortex.48,49
Lack of DAT changes in the ITCM, the amygdala subregion most heavily innervated by DAergic projections, may seem surprising. However, several lines of evidence suggest that DAergic neurons represent a heterogeneous population, with significant differences in their phenotypes and DAT expression,50–52 and that the dopaminergic innervation of the deep amygdala nuclei differs from that of the ITCM in several important aspects, such as location of dopaminergic synapses on dendrites and convergence with cortical inputs.38,53 These differences may account for the discrepancy we observed with respect to DAT changes in SZ. Despite the lack of DAT changes in ITCM, our data suggest that these key amygdala cell islands are anomalous in SZ (see below).
Dopamine Beta-hydroxylase
A decrease of DBH varicosities in ITCM and ABN is likely to reflect reduced noradrenaline synthesis in these nuclei. Compelling evidence supports a role for noradrenaline effects on the amygdala in mediating memory consolidation.54,55 Through projections to the bed nucleus of the stria terminalis and ventral striatum, the ABN may impact on anxiety- and reward-related behaviors and learning.56–58 At the same time, noradrenaline decreases in the ITCM may alter their role as gateways to inhibitory cortical inputs to the amygdala.17
Tyrosine Hydroxylase and 5HTT
Lack of TH expression abnormalities in the amygdala contrasts with findings in the caudate nucleus.8 However, as mentioned above, heterogeneity among dopaminergic neurons includes DAT and TH expressions, perhaps accounting for the region specificity of dopamine abnormalities in SZ, emerging from our and other groups’ findings.8,46–49 Finally, our negative findings on 5HTT add to those of other groups, showing normal serotoninergic neurotransmission or modest changes in several brain regions of SZ subjects (for review see ref.59).
Conclusions
Our main results show a significant reduction of DAT expression in the amygdala of subjects with SZ. This reduction is postulated to result in enhanced dopaminergic synaptic activation and involvement of extrasynaptic dopaminergic receptors. We put forth the hypothesis that excess dopamine may disrupt dopamine-dependent amygdalar functions, such as attribution of stimulus salience and accurate prediction error signaling,2,5,6,13,60 which may in turn contribute to psychosis. Speculatively, these changes may simulate and/or enhance hyperdopaminergic effects of heightened emotional states, activating amygdalar-driven responses through direct activation of LN, ABN, and CO, while ITCM abnormalities may release these nuclei from the inhibitory control of the prefrontal cortex.17 In addition, decreased noradrenergic transmission in the BN may impact processing and consolidation of context-related information, perhaps also contributing to aspects of psychosis.54,55,61
Supplementary Material
Supplementary material is available at http://schizophre- niabulletin.oxfordjournals.org.
Funding
National Institute of Mental Health at National Institutes of Health (R01MH066955, R01MH066280 to S.B.).
Supplementary Material
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. van Os J. ‘Salience syndrome’ replaces ‘schizophrenia’ in DSM-V and ICD-11: psychiatry’s evidence-based entry into the 21st century? Acta Psychiatry Scand. 2009;120:363–372 [DOI] [PubMed] [Google Scholar]
- 2. Pankow A, Knobel A, Voss M, Heinz A. Neurobiological correlates of delusion: beyond the salience attribution hypothesis. Neuropsychobiology. 2012;66:33–43 [DOI] [PubMed] [Google Scholar]
- 3. Heinz A, Schlagenhauf F. Dopaminergic dysfunction in schizophrenia: salience attribution revisited. Schizophr Bull. 2010;36:472–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berridge KC. From prediction error to incentive salience: mesolimbic computation of reward motivation. Eur J Neurosci. 2012;35:1124–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morrison SE, Salzman CD. Re-valuing the amygdala. Curr Opin Neurobiol. 2010;20:221–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Paton JJ, Belova MA, Morrison SE, Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439:865–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aleman A, Kahn RS. Strange feelings: do amygdala abnormalities dysregulate the emotional brain in schizophrenia? Prog Neurobiol. 2005;77:283–298 [DOI] [PubMed] [Google Scholar]
- 8. Roberts RC, Roche JK, Conley RR, Lahti AC. Dopaminergic synapses in the caudate of subjects with schizophrenia: relationship to treatment response. Synapse. 2009;63:520–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abi-Dargham A, Rodenhiser J, Printz D, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci U S A. 2000;97:8104–8109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Haber SN, Fudge JL. The interface between dopamine neurons and the amygdala: implications for schizophrenia. Schizophr Bull. 1997;23:471–482 [DOI] [PubMed] [Google Scholar]
- 11. Reynolds GP. Increased concentrations and lateral asymmetry of amygdala dopamine in schizophrenia. Nature. 1983;305:527–529 [DOI] [PubMed] [Google Scholar]
- 12. Salgado-Pineda P, Delaveau P, Blin O, Nieoullon A. Dopaminergic contribution to the regulation of emotional perception. Clin Neuropharmacol. 2005;28:228–237 [DOI] [PubMed] [Google Scholar]
- 13. Darvas M, Fadok JP, Palmiter RD. Requirement of dopamine signaling in the amygdala and striatum for learning and maintenance of a conditioned avoidance response. Learn Mem. 2011;18:136–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Holt DJ, Coombs G, Zeidan MA, Goff DC, Milad MR. Failure of neural responses to safety cues in schizophrenia. Arch Gen Psychiatry. 2012;69:893–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Murray EA, Rudebeck PH. The drive to strive: goal generation based on current needs. Front Neurosci. 2013;7:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dolan RJ. The human amygdala and orbital prefrontal cortex in behavioural regulation. Philos Trans R Soc Lond B Biol Sci. 2007;362:787–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marowsky A, Yanagawa Y, Obata K, Vogt KE. A specialized subclass of interneurons mediates dopaminergic facilitation of amygdala function. Neuron. 2005;48:1025–1037 [DOI] [PubMed] [Google Scholar]
- 18. Inglis FM, Moghaddam B. Dopaminergic innervation of the amygdala is highly responsive to stress. J Neurochem. 1999;72:1088–1094 [DOI] [PubMed] [Google Scholar]
- 19. Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411:305–309 [DOI] [PubMed] [Google Scholar]
- 22. Peck CJ, Lau B, Salzman CD. The primate amygdala combines information about space and value. Nat Neurosci. 2013;16:340–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Escartí MJ, de la Iglesia-Vayá M, Martí-Bonmatí L, et al. Increased amygdala and parahippocampal gyrus activation in schizophrenic patients with auditory hallucinations: an fMRI study using independent component analysis. Schizophr Res. 2010;117:31–41 [DOI] [PubMed] [Google Scholar]
- 24. Melchitzky DS, Lewis DA. Tyrosine hydroxylase- and dopamine transporter-immunoreactive axons in the primate cerebellum. Evidence for a lobular- and laminar-specific dopamine innervation. Neuropsychopharmacology. 2000;22:466–472 [DOI] [PubMed] [Google Scholar]
- 25. Sesack SR, Hawrylak VA, Guido MA, Levey AI. Cellular and subcellular localization of the dopamine transporter in rat cortex. Adv Pharmacol. 1998;42:171–174 [DOI] [PubMed] [Google Scholar]
- 26. Ciliax BJ, Heilman C, Demchyshyn LL, et al. The dopamine transporter: immunochemical characterization and localization in brain. J Neurosci. 1995;15:1714–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612 [DOI] [PubMed] [Google Scholar]
- 28. Berretta S, Pantazopoulos H, Lange N. Neuron numbers and volume of the amygdala in subjects diagnosed with bipolar disorder or schizophrenia. Biol Psychiatry. 2007;62:884–893 [DOI] [PubMed] [Google Scholar]
- 29. Pantazopoulos H, Woo TU, Lim MP, Lange N, Berretta S. Extracellular matrix-glial abnormalities in the amygdala and entorhinal cortex of subjects diagnosed with schizophrenia. Arch Gen Psychiatry. 2010;67:155–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. García-Amado M, Prensa L. Distribution of dopamine transporter immunoreactive fibers in the human amygdaloid complex. Eur J Neurosci. 2013;38:3589–3601 [DOI] [PubMed] [Google Scholar]
- 31. Asan E. The catecholaminergic innervation of the rat amygdala. Adv Anat Embryol Cell Biol. 1998;142:1–118 [DOI] [PubMed] [Google Scholar]
- 32. Mash DC, Staley JK, Izenwasser S, Basile M, Ruttenber AJ. Serotonin transporters upregulate with chronic cocaine use. J Chem Neuroanat. 2000;20:271–280 [DOI] [PubMed] [Google Scholar]
- 33. Ciliax BJ, Drash GW, Staley JK, et al. Immunocytochemical localization of the dopamine transporter in human brain. J Comp Neurol. 1999;409:38–56 [DOI] [PubMed] [Google Scholar]
- 34. Klimek V, Schenck JE, Han H, Stockmeier CA, Ordway GA. Dopaminergic abnormalities in amygdaloid nuclei in major depression: a postmortem study. Biol Psychiatry. 2002;52:740–748 [DOI] [PubMed] [Google Scholar]
- 35. Sadikot AF, Parent A. The monoaminergic innervation of the amygdala in the squirrel monkey: an immunohistochemical study. Neuroscience. 1990;36:431–447 [DOI] [PubMed] [Google Scholar]
- 36. Dias SJ, Zhou X, Ivanovic M, et al. Nuclear MTA1 overexpression is associated with aggressive prostate cancer, recurrence and metastasis in African Americans. Sci Rep. 2013;3:2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Asan E. Ultrastructural features of tyrosine-hydroxylase-immunoreactive afferents and their targets in the rat amygdala. Cell Tissue Res. 1997;288:449–469 [DOI] [PubMed] [Google Scholar]
- 38. Pinto A, Sesack SR. Ultrastructural analysis of prefrontal cortical inputs to the rat amygdala: spatial relationships to presumed dopamine axons and D1 and D2 receptors. Brain Struct Funct. 2008;213:159–175 [DOI] [PubMed] [Google Scholar]
- 39. Garris PA, Wightman RM. Different kinetics govern dopaminergic transmission in the amygdala, prefrontal cortex, and striatum: an in vivo voltammetric study. J Neurosci. 1994;14:442–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Garris PA, Wightman RM. Distinct pharmacological regulation of evoked dopamine efflux in the amygdala and striatum of the rat in vivo. Synapse. 1995;20:269–279 [DOI] [PubMed] [Google Scholar]
- 41. Carlsson A, Carlsson ML. A dopaminergic deficit hypothesis of schizophrenia: the path to discovery. Dialogues Clin Neurosci. 2006;8:137–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184 [DOI] [PubMed] [Google Scholar]
- 43. Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Blair HT, Schafe GE, Bauer EP, Rodrigues SM, LeDoux JE. Synaptic plasticity in the lateral amygdala: a cellular hypothesis of fear conditioning. Learn Mem. 2001;8:229–242 [DOI] [PubMed] [Google Scholar]
- 45. Nieh EH, Kim SY, Namburi P, Tye KM. Optogenetic dissection of neural circuits underlying emotional valence and motivated behaviors. Brain Res. 2013;1511:73–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fusar-Poli P, Meyer-Lindenberg A. Striatal presynaptic dopamine in schizophrenia, Part I: meta-analysis of dopamine active transporter (DAT) density. Schizophr Bull. 2013;39:22–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Howes OD, Kambeitz J, Kim E, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69:776–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Seeman P, Niznik HB. Dopamine receptors and transporters in Parkinson’s disease and schizophrenia. FASEB J. 1990;4:2737–2744 [DOI] [PubMed] [Google Scholar]
- 49. Rao JS, Kellom M, Reese EA, Rapoport SI, Kim HW. Dysregulated glutamate and dopamine transporters in postmortem frontal cortex from bipolar and schizophrenic patients. J Affect Disord. 2012;136:63–71 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50. Simeone A, Di Salvio M, Di Giovannantonio LG, Acampora D, Omodei D, Tomasetti C. The role of otx2 in adult mesencephalic-diencephalic dopaminergic neurons. Mol Neurobiol. 2011;43:107–113 [DOI] [PubMed] [Google Scholar]
- 51. Perez-Costas E, Melendez-Ferro M, Rice MW, Conley RR, Roberts RC. Dopamine pathology in schizophrenia: analysis of total and phosphorylated tyrosine hydroxylase in the substantia nigra. Front Psychiatry. 2012;3:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cho YT, Fudge JL. Heterogeneous dopamine populations project to specific subregions of the primate amygdala. Neuroscience. 2010;165:1501–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fuxe K, Jacobsen KX, Höistad M, et al. The dopamine D1 receptor-rich main and paracapsular intercalated nerve cell groups of the rat amygdala: relationship to the dopamine innervation. Neuroscience. 2003;119:733–746 [DOI] [PubMed] [Google Scholar]
- 54. McGaugh JL. Making lasting memories: remembering the significant. Proc Natl Acad Sci U S A. 2013;110(suppl 2):10402–10407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McIntyre CK, Miyashita T, Setlow B, et al. Memory-influencing intra-basolateral amygdala drug infusions modulate expression of Arc protein in the hippocampus. Proc Natl Acad Sci U S A. 2005;102:10718–10723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. deCampo DM, Fudge JL. Amygdala projections to the lateral bed nucleus of the stria terminalis in the macaque: comparison with ventral striatal afferents. J Comp Neurol. 2013;521:3191–3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Somerville LH, Whalen PJ, Kelley WM. Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biol Psychiatry. 2010;68:416–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Everitt BJ, Parkinson JA, Olmstead MC, Arroyo M, Robledo P, Robbins TW. Associative processes in addiction and reward. The role of amygdala-ventral striatal subsystems. Ann N Y Acad Sci. 1999;877:412–438 [DOI] [PubMed] [Google Scholar]
- 59. Abi-Dargham A. Alterations of serotonin transmission in schizophrenia. Int Rev Neurobiol. 2007;78:133–164 [DOI] [PubMed] [Google Scholar]
- 60. Gradin VB, Kumar P, Waiter G, et al. Expected value and prediction error abnormalities in depression and schizophrenia. Brain. 2011;134:1751–1764 [DOI] [PubMed] [Google Scholar]
- 61. Li Z, Richter-Levin G. Priming stimulation of basal but not lateral amygdala affects long-term potentiation in the rat dentate gyrus in vivo. Neuroscience. 2013;246:13–21 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.