Individual neurons in the mammalian central nervous system communicate with their downstream targets via subcellular specializations in their axon. Arranged like pearls on a necklace, these presynaptic terminals are self-contained entities that possess all of the machinery to make them competent to rapidly release neurotransmitter in response to an electrical action potential wavefront that travels from the cell body to the far reaches of the entire axon. A single axon may make contact with hundreds of downstream targets that often includes numerous distinct cell types. Importantly, despite being separated by only a few microns, the properties of each of these presynaptic release sites is often tuned to the particular cell type it innervates such that transmission may be robust onto one particular cell type yet weak at another, despite all terminals sensing the same action potential waveform (Koester and Johnston 2005). This arrangement allows different terminals in the axon to behave independently and “translate” presynaptic action potentials into its own unique language to establish appropriate mechanisms of both short and long term synaptic transmission and plasticity (Toth and McBain 2000; Pelkey and McBain 2008). What dictates this differential synaptic processing has been unclear, with elements in both the presynaptic terminal (e.g. SNARE components, voltage-gated Ca2+ channels and autoreceptors), postsynaptic membrane (retrograde transmitters and gases, eg cannabinoids and NO) or transynaptic proteins (e.g. Ephrins) that “handshake” across the synaptic cleft being posited. Sylwestrak and Ghosh (this issue) now show that postsynaptic expression of the Extracellular Leucine-rich repeat Fibronectin containing 1 (Elfn1) plays an important role in establishing such target specific differential transmission at synapses between CA1 pyramidal neurons (PN) of the hippocampus and two of its downstream inhibitory interneuron targets, the parvalbumin-containing fast spiking basket cell (BC) and the somatostatin-positive oriens-lacunosum/moleculare neuron (O-LM).
Under normal conditions, a train of presynaptic action potentials in CA1 pyramidal neurons triggers robust synaptic transmission onto BCs (such synapses are referred to as having a high initial release probability, or Pr), such that larger synaptic events are triggered early in the train, which then rapidly wane as the train progresses (i.e. short term depression). In contrast, synaptic events onto O-LM cells start small and grow as the train of action potentials progresses, (a process termed short term facilitation, and indicative of synapses with a low initial transmitter Pr). Sylwestrak and Ghosh (2012) demonstrate that Elfn1 is selectively expressed in O-LM inhibitory interneurons and its punctate expression on dendrites reveals a strong enrichment at glutamatergic but not GABAergic synapses. Targeted elimination of Elfn1 from O-LM neurons using a lentiviral/shRNA approach increased the evoked EPSC amplitude and strongly reduced the degree of short-term facilitation observed across a range of frequencies. Interestingly, elimination of Elfn1 in early postnatal neurons had the greatest impact on transmission, suggesting an instructive role in the development and maturation of synaptic function. Elfn1 loss of function was not accompanied by changes in postsynaptic properties, consistent with a role for Elfn1 in establishing low presynaptic release probability synapses onto O-LM cells (Figure 1). Surprisingly, despite this apparent Elfn1 control over Pr, the absence of Elfn1 did not covert PN-O-LM synaptic activity to match those of PN- BC connections, which have an extremely high initial Pr. Rather loss of Elfn1 normalized transmission across the train of stimuli with only weak facilitation remaining. This suggests that other factors must be involved in establishing the high Pr at PN-BC targets. Consistent with a role for Elfn1 in establishing low Pr synapses, overexpression of Elfn1 in BCs converted their hallmark short-term synaptic depression into facilitation. From the available data one could speculate that the default setting for excitatory synapses onto interneurons may occupy the middle ground of release probability with Elfn1 acting to lower Pr at O-LM synapses and an as yet unidentified element acting to elevate the Pr of PN-BC synapses.
Figure 1. Postsynaptic Elfn1 establishes a low transmitter release probability at pyramidal neuron to somatostatin-containing O-LM interneurons.
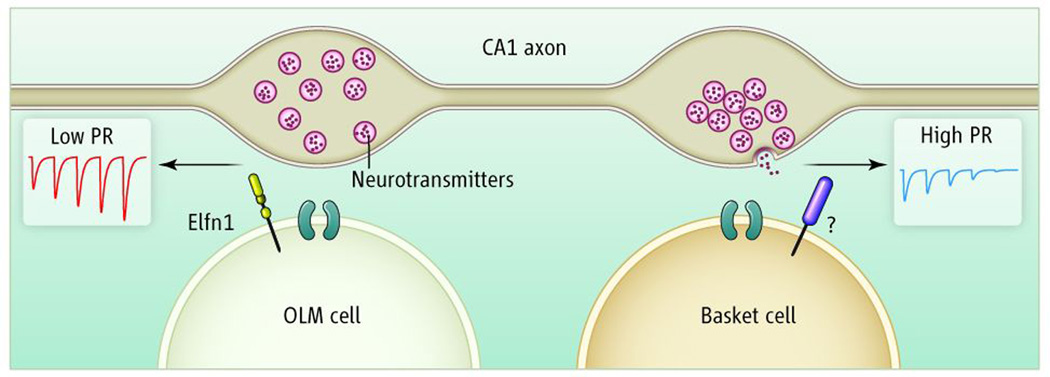
In hippocampus somatostatin-containing O-LM and parvalbumin-containing basket cells receive common afferent input from CA1 pyramidal neurons. The postsynaptic expression of the leucine rich repeat protein Elfn1 in O-LM cells acts to set the presynaptic initial transmitter release probability low, ensuring that a train of presynaptic action potentials yields an epoch of short term facilitation of synaptic transmission. In contrast, the absence or Elfn1, or the presence of an as yet undiscovered trans-synaptic protein, endows CA1 pyramidal neuron synapses onto basket cells with a high initial Pr and resulting depressing synapses.
Although structural roles for proteins containing leucine rich repeat (LRR) motifs in axon guidance, synapse target selection, maturation and myelination are well established (for review see de Wit et al. 2011), functional roles for LRR proteins in regulating synaptic transmission are not without precedent. Neurotrophins and their Trk receptors have both structural and functional roles to play in cortical circuits. Similarly, leucine rich glioma inactivated 1 (LGI1) and ADAM22 interact with PSD95 to regulated postsynaptic glutamate receptor subunit availability. In addition, interaction of LGI1 with the presynaptic voltage-gated potassium channel Kv1.1 influences presynaptic release probability in perforant path-granule cell synapses. How Elfn1 acts to influence Pr is currently unknown. Sylwestrak and Ghosh (2012) indicate an interplay between Elfn1 and GluK2 receptor mediate short term plasticity but the underlying mechanism is not fully explored. The available data suggest that Elfn1 must have downstream diffusible or transynaptic partners that can act to regulate the availability of synaptic glutamate for presynaptic kainate receptors.
The two Elfn genes have highly complimentary expression patterns in mammalian central neurons. Elfn2 is largely confined to cortical and hippocampal principal glutamatergic neurons (Dolan et al 2007). Although Sylwestrak and Ghosh show high enrichment of Elfn1 in somatostatin-containing O-LM cells, inspection of the Allen Brain Atlas and elsewhere (Dolan et al 2009) suggests a wider pattern of expression among other unidentified inhibitory interneuron subtypes. Whether such expression coincides with other excitatory inputs with low Prs remains to be tested. Thus the first step to decode target specific synaptic transmission has been taken, with undoubtedly many more to follow.
References
- De Wit J, et al. Annu. Rev. Cell Dev. Biol. 2011;27:697–729. doi: 10.1146/annurev-cellbio-092910-154111. [DOI] [PubMed] [Google Scholar]
- Dolan J, et al. BMC Genetics. 2007;8:320. [Google Scholar]
- Koester HJ, Johnston D. Science. 2005;308:863–866. doi: 10.1126/science.1100815. 2005. [DOI] [PubMed] [Google Scholar]
- Pelkey KA, McBain CJ. J Physiol. 2008;586:1495–1502. doi: 10.1113/jphysiol.2007.148635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylwestrak EL, Ghosh A. Science. 2012 doi: 10.1126/science.1222482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth K, McBain CJ. 2000;525:41–51. doi: 10.1111/j.1469-7793.2000.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


