Abstract
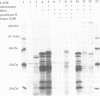
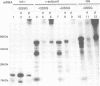
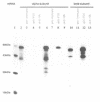
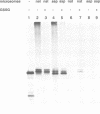
Prolyl 4-hydroxylase (P4-H) catalyses a vital post-translational modification in the biosynthesis of collagen. The enzyme consists of two distinct polypeptides forming an alpha 2 beta 2 tetramer (alpha = 64 kDa, beta = 60 kDa), the beta-subunit being identical to the multifunctional enzyme protein disulfide isomerase (PDI). By studying the cell-free synthesis of the rat alpha-subunit of P4-H we have shown that the alpha-subunit can be translocated, glycosylated and the signal peptide cleaved by dog pancreatic microsomal membranes to yield both singly and doubly glycosylated forms. When translations were carried out under conditions which prevent disulfide bond formation, the product synthesized formed aggregates which were associated with the immunoglobulin heavy chain binding protein (BiP). Translations carried out under conditions that promote disulfide bond formation yielded a product that was not associated with BiP but formed a complex with the endogenous beta-subunit (PDI). Complex formation was detected by co-precipitation of the newly synthesized alpha-subunit with antibodies raised against PDI, by sucrose gradient centrifugation and by chemical cross-linking. When microsomal vesicles were depleted of PDI, BiP and other soluble endoplasmic reticulum proteins, no complex formation was observed and the alpha-subunit aggregated even under conditions that promote disulfide bond formation. We have therefore demonstrated that the enzyme P4-H can be assembled at synthesis in a cell-free system and that the solubility of the alpha-subunit is dependent upon its association with PDI.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austen B. M., Hermon-Taylor J., Kaderbhai M. A., Ridd D. H. Design and synthesis of a consensus signal sequence that inhibits protein translocation into rough microsomal vesicles. Biochem J. 1984 Nov 15;224(1):317–325. doi: 10.1042/bj2240317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassuk J. A., Kao W. W., Herzer P., Kedersha N. L., Seyer J., DeMartino J. A., Daugherty B. L., Mark G. E., 3rd, Berg R. A. Prolyl 4-hydroxylase: molecular cloning and the primary structure of the alpha subunit from chicken embryo. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7382–7386. doi: 10.1073/pnas.86.19.7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. A., Kao W. W., Kedersha N. L. The assembly of tetrameric prolyl hydroxylase in tendon fibroblasts from newly synthesized alpha-subunits and from preformed cross-reacting protein. Biochem J. 1980 Sep 1;189(3):491–499. doi: 10.1042/bj1890491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. A., Prockop D. J. The thermal transition of a non-hydroxylated form of collagen. Evidence for a role for hydroxyproline in stabilizing the triple-helix of collagen. Biochem Biophys Res Commun. 1973 May 1;52(1):115–120. doi: 10.1016/0006-291x(73)90961-3. [DOI] [PubMed] [Google Scholar]
- Bickel M., Baader E., Brocks D. G., Engelbart K., Günzler V., Schmidts H. L., Vogel G. H. Beneficial effects of inhibitors of prolyl 4-hydroxylase in CCl4-induced fibrosis of the liver in rats. J Hepatol. 1991;13 (Suppl 3):S26–S34. doi: 10.1016/0168-8278(91)90005-v. [DOI] [PubMed] [Google Scholar]
- Blount P., Merlie J. P. BIP associates with newly synthesized subunits of the mouse muscle nicotinic receptor. J Cell Biol. 1991 Jun;113(5):1125–1132. doi: 10.1083/jcb.113.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Braakman I., Helenius J., Helenius A. Manipulating disulfide bond formation and protein folding in the endoplasmic reticulum. EMBO J. 1992 May;11(5):1717–1722. doi: 10.1002/j.1460-2075.1992.tb05223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulleid N. J., Bassel-Duby R. S., Freedman R. B., Sambrook J. F., Gething M. J. Cell-free synthesis of enzymically active tissue-type plasminogen activator. Protein folding determines the extent of N-linked glycosylation. Biochem J. 1992 Aug 15;286(Pt 1):275–280. doi: 10.1042/bj2860275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulleid N. J., Curling E., Freedman R. B., Jenkins N. Source of heterogeneity in secreted interferon-gamma. A study on products of translation in vitro. Biochem J. 1990 Jun 15;268(3):777–781. doi: 10.1042/bj2680777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulleid N. J., Freedman R. B. Cotranslational glycosylation of proteins in systems depleted of protein disulphide isomerase. EMBO J. 1990 Nov;9(11):3527–3532. doi: 10.1002/j.1460-2075.1990.tb07561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulleid N. J., Freedman R. B. The transcription and translation in vitro of individual cereal storage-protein genes from wheat (Triticum aestivum, cv. Chinese Spring). Evidence for translocation of the translation products and disulphide-bond formation. Biochem J. 1988 Sep 15;254(3):805–810. doi: 10.1042/bj2540805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dottavio-Martin D., Ravel J. M. Radiolabeling of proteins by reductive alkylation with [14C]formaldehyde and sodium cyanoborohydride. Anal Biochem. 1978 Jul 1;87(2):562–565. doi: 10.1016/0003-2697(78)90706-6. [DOI] [PubMed] [Google Scholar]
- Fujiki Y., Hubbard A. L., Fowler S., Lazarow P. B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982 Apr;93(1):97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich V. V., Pokrovskaya I. D., Obukhova T. A., Zozulya S. A. Preparative in vitro mRNA synthesis using SP6 and T7 RNA polymerases. Anal Biochem. 1991 Jun;195(2):207–213. doi: 10.1016/0003-2697(91)90318-n. [DOI] [PubMed] [Google Scholar]
- Hanauske-Abel H. M., Günzler V. A stereochemical concept for the catalytic mechanism of prolylhydroxylase: applicability to classification and design of inhibitors. J Theor Biol. 1982 Jan 21;94(2):421–455. doi: 10.1016/0022-5193(82)90320-4. [DOI] [PubMed] [Google Scholar]
- Hawkins H. C., Freedman R. B. The reactivities and ionization properties of the active-site dithiol groups of mammalian protein disulphide-isomerase. Biochem J. 1991 Apr 15;275(Pt 2):335–339. doi: 10.1042/bj2750335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helaakoski T., Vuori K., Myllylä R., Kivirikko K. I., Pihlajaniemi T. Molecular cloning of the alpha-subunit of human prolyl 4-hydroxylase: the complete cDNA-derived amino acid sequence and evidence for alternative splicing of RNA transcripts. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4392–4396. doi: 10.1073/pnas.86.12.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassenbrock C. K., Garcia P. D., Walter P., Kelly R. B. Heavy-chain binding protein recognizes aberrant polypeptides translocated in vitro. Nature. 1988 May 5;333(6168):90–93. doi: 10.1038/333090a0. [DOI] [PubMed] [Google Scholar]
- Kedersha N. L., Tkacz J. S., Berg R. A. Biosynthesis of prolyl hydroxylase: evidence for two separate dolichol-media pathways of glycosylation. Biochemistry. 1985 Oct 8;24(21):5960–5967. doi: 10.1021/bi00342a041. [DOI] [PubMed] [Google Scholar]
- Kedersha N. L., Tkacz J. S., Berg R. A. Characterization of the oligosaccharides of prolyl hydroxylase, a microsomal glycoprotein. Biochemistry. 1985 Oct 8;24(21):5952–5960. doi: 10.1021/bi00342a040. [DOI] [PubMed] [Google Scholar]
- Knittler M. R., Haas I. G. Interaction of BiP with newly synthesized immunoglobulin light chain molecules: cycles of sequential binding and release. EMBO J. 1992 Apr;11(4):1573–1581. doi: 10.1002/j.1460-2075.1992.tb05202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivu J., Myllylä R., Helaakoski T., Pihlajaniemi T., Tasanen K., Kivirikko K. I. A single polypeptide acts both as the beta subunit of prolyl 4-hydroxylase and as a protein disulfide-isomerase. J Biol Chem. 1987 May 15;262(14):6447–6449. [PubMed] [Google Scholar]
- Koivu J., Myllylä R. Protein disulfide-isomerase retains procollagen prolyl 4-hydroxylase structure in its native conformation. Biochemistry. 1986 Oct 7;25(20):5982–5986. doi: 10.1021/bi00368a022. [DOI] [PubMed] [Google Scholar]
- Kozak M. Evaluation of the fidelity of initiation of translation in reticulocyte lysates from commercial sources. Nucleic Acids Res. 1990 May 11;18(9):2828–2828. doi: 10.1093/nar/18.9.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuutti E. R., Tuderman L., Kivirikko K. I. Human prolyl hydroxylase. Purification, partial characterization and preparation of antiserum to the enzyme. Eur J Biochem. 1975 Sep 1;57(1):181–188. doi: 10.1111/j.1432-1033.1975.tb02289.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambert N., Freedman R. B. Structural properties of homogeneous protein disulphide-isomerase from bovine liver purified by a rapid high-yielding procedure. Biochem J. 1983 Jul 1;213(1):225–234. doi: 10.1042/bj2130225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987 Mar 13;48(5):899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- Nietfeld J. J., Van der Kraan I., Kemp A. Dissociation and reassociation of prolyl 4-hydroxylase subunits after cross-linking of monomers. Biochim Biophys Acta. 1981 Sep 15;661(1):21–27. doi: 10.1016/0005-2744(81)90078-4. [DOI] [PubMed] [Google Scholar]
- Pihlajaniemi T., Helaakoski T., Tasanen K., Myllylä R., Huhtala M. L., Koivu J., Kivirikko K. I. Molecular cloning of the beta-subunit of human prolyl 4-hydroxylase. This subunit and protein disulphide isomerase are products of the same gene. EMBO J. 1987 Mar;6(3):643–649. doi: 10.1002/j.1460-2075.1987.tb04803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele G., Jacoby R. Conformational changes associated with proteolytic processing of presecretory proteins allow glutathione-catalyzed formation of native disulfide bonds. J Biol Chem. 1982 Oct 25;257(20):12277–12282. [PubMed] [Google Scholar]
- Segal M. S., Bye J. M., Sambrook J. F., Gething M. J. Disulfide bond formation during the folding of influenza virus hemagglutinin. J Cell Biol. 1992 Jul;118(2):227–244. doi: 10.1083/jcb.118.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaney J. B., O'Brien K. Cross-linking studies of the self-association properties of apo-A-I and apo-A-II from human high density lipoprotein. J Biol Chem. 1978 Oct 10;253(19):7069–7077. [PubMed] [Google Scholar]
- Tiruppathi C., Alpers D. H., Seetharam B. Phase separation of rat intestinal brush border membrane proteins using Triton X-114. Anal Biochem. 1986 Mar;153(2):330–335. doi: 10.1016/0003-2697(86)90100-4. [DOI] [PubMed] [Google Scholar]
- Tuderman L., Kuutti E. R., Kivirikko K. I. Radiommunoassay for human and chick prolyl hydroxylases. Eur J Biochem. 1975 Dec 15;60(2):399–405. doi: 10.1111/j.1432-1033.1975.tb21016.x. [DOI] [PubMed] [Google Scholar]
- Vuori K., Pihlajaniemi T., Myllylä R., Kivirikko K. I. Site-directed mutagenesis of human protein disulphide isomerase: effect on the assembly, activity and endoplasmic reticulum retention of human prolyl 4-hydroxylase in Spodoptera frugiperda insect cells. EMBO J. 1992 Nov;11(11):4213–4217. doi: 10.1002/j.1460-2075.1992.tb05515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetterau J. R., Combs K. A., McLean L. R., Spinner S. N., Aggerbeck L. P. Protein disulfide isomerase appears necessary to maintain the catalytically active structure of the microsomal triglyceride transfer protein. Biochemistry. 1991 Oct 8;30(40):9728–9735. doi: 10.1021/bi00104a023. [DOI] [PubMed] [Google Scholar]
- Yilla M., Doyle D., Sawyer J. T. Early disulfide bond formation prevents heterotypic aggregation of membrane proteins in a cell-free translation system. J Cell Biol. 1992 Jul;118(2):245–252. doi: 10.1083/jcb.118.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]