Abstract
Overcoming apresumed differentiation block in the childhood muscle cancer embryonalrhabdomyosarcomais often thought to hold promise as an approach to replace cytotoxic chemotherapy with molecularly-targeted differentiation therapies. In this issue of Cancer Cell, Tremblay and colleagues implicate YAP1 and the Hippo signaling pathway in the maintenance of differentiation-arrested and proliferative phenotypesforembryonalrhabdomyosarcoma.
Differentiation therapy for the muscle cancer rhabdomyosarcoma has been thought to hold promise for replacing cytotoxic chemotherapy with molecularly-targeted therapies. Such a targeted therapy mightrestore the terminal myogenic differentiation program to the rhabdomyosarcoma cells and (potentially)reduce life-long chemotherapy related sequelaefor the patient. Indeed, differentiation therapy has been used successfully in the treatment of acute promyelocyticleukemiaandneuroblastoma(Reynolds and Lemons, 2001). Embryonalrhabdomyosarcoma (eRMS), an RMS subtype thought to have an activated satellite cell phenotype and an arrested myogenic differentiation program, displays the greatest tendency towards myodifferentiation and may be amenable to differentiation therapy.However, no successful differentiation therapies for RMS have entered the clinic. Recently, there has been renewed interest in differentiation therapy for solid tumors, thedevelopment of whichwill depend on understanding the molecular mechanisms involved in suppressing differentiation and identifying targets for therapeutics. In work presented in this issue of Cancer Cell, Tremblay and colleagues (Tremblay et al., 2014)implicate YAP1 and the Hippo signaling pathwayin the differentiation-arrestedand proliferative phenotypesofeRMS.
Tremblay at al. first exploredthe expression and cellular compartment localization of YAP1 in human RMS samples and found that YAP1 was overexpressed in eRMS tumorsand was predominately nuclear-localized. YAP1 immunostaining correlated with Ki-67positivity. These results are in accord with a recent report in which the YAP1oncoproteinwasfound to be overexpressed in both the cytoplasmic and nuclear compartments in aRMSand eRMS tumor samples (Crose et al., 2014). Furthermore, a number of patient-derived eRMS samples also exhibited arecurrentYAP1locus copy number gain.
To examinethe functional relevance of these findings, Tremblay et al. conditionally activated adoxycycline(DOX)-induciblehYAP1 S127Atransgene to drive YAP1 overexpression in specific lineages: Pax7-creERT2 (activated and quiescent satellite cells), Myf5-Cre (prenatal and post-natal lineages of very early myogenic progenitors/activated satellite cells and early myoblasts), and Myod1-iCre (early myogenic progenitors/activated satellite cells andearly & late myoblasts). Myf5-Cre also marks an adipose lineage. Myf5-Creand Myod1-iCremice developed eRMS-like tumors in the interstitial compartment of all muscles.These tumorsdemonstrated positivedesmin and myogenin immunostaining, although no tumors developed in the brown fat pads of Myf5-Cre mice. Pax7 mice whose limbs were cardiotoxin-injured developed tumors arising from the Pax7-creERT2 lineage:no tumors developed in the contralateral uninjured limbs of these micesuggestingthat activated satellite cells and their progeny, not the quiescent population,maybe the cell-of-origin in this YAP1-driven model of eRMS.
In this genetic system, the tumors were transplantable – and yet thistumorigencity was reversible. Primary cell cultures established from explant secondary tumors were able to proliferate in the presence of DOX but spontaneously differentiated when withdrawn from DOX and subjected to low-serum culture conditions.In vivo, mice bearing secondary tumors experienced spontaneous regression and differentiation of their tumors when withdrawn from DOX demonstrating that YAP1 overexpression drives proliferation and may have a role in arresting the terminal differentiation program.It is perhaps not surprising then thatthe genes preferentially downregulated following YAP1 normalization included the early myogenic lineage markers Pax7 and Myf5 with concomitant upregulation of the differentiation markers Myod1 and Myh4.Tremblay et al. also found that YAP1 globally regulatesgene expression maintaining the pro-proliferation phenotype through direct transcriptional repressionof myogenic regulatory factors and gene expression upregulation of known inhibitors of Myod1 and Mef2 (i.e., Id2, Twist1, Snai1/2). Correlatively, YAP1 expression declines in differentiating mouse and human fetal myoblasts.
It should be noted that murine primary tumors in this model have only one genetic lesion -- and YAP1 overexpression is linked to not only the Rosa26 promoter but also a tetracycline-responsive element, resulting in a perhaps non-physiological level of (over)expression.While Tremblay et al. demonstrate that activated YAP1 expression can be a sufficient transformational event in the murine myoblast cell line C2C12, human eRMS is more heterogeneous with a mutational landscape known to be considerably more complex with multiple copy number variants, a non-modest background mutation rate, and recurrent activating RAS mutations (Shern et al., 2014). The exact role of YAP1 in the context of oncogenic RAS signaling for eRMS is as of now unexplored. However, recent reports suggest that YAP1and KRAS converge in other forms of cancer. The same may be true in eRMS, for whichTremblay et al. provide evidence thata YAP1 overexpression signature is associated with higher stage tumorsandworsened prognosis.
The most poignantresult of these studies was the attempt to translate from a murine genetic proof-of-concept system to a human tumor system as measured by the differentiation effect on thehumaneRMS cell line RDin axenograft system. Knockdown of YAP1 in overexpressingRDcells resulted in a reduced tumorigencity-- but only a 1.7% increase in differentiation ability (and overall, no more than 3% differentiationof tumor cells was seen). Thus, the reversibility of YAP1 driven tumors was less impressive in human RD tumor cells. Unfortunately, too, only one human eRMS cell culture was tested.The results presented by Tremblay et al., while novel and exciting, raise an important question about the feasibility of differentiation therapy: is completedifferentiation of nearly all eRMS cells within a tumor really possible (Figure 1), if not only in the setting of microscopic residual disease? The authors suggest in their Highlight that, “YAP1 inhibition is a promising strategy for differentiation therapy of ERMS”. We ask for caution on this point. In the context of the mouse model studies, their approach is interesting; however, their experimental evidence is insufficient and inadequate in the context of a therapeutic strategy for human patients. The same concern raised in recent commentaries on the rigorousness of preclinical studies (Macleod, 2014) should be embraced here, so that unjustified clinical trials are not initiated - and so that families of children affected by eRMS are not given false hope.Nonetheless, one might say this approach is worthy of deeper study – potentially by means of targeting several pathways simultaneously. We have known since the earliest chemotherapy clinical trials that combination therapies are more effective than single agents. In RMS, differentiation therapy may be no different.
Figure 1. Benchmarking myogenic differentiation in human eRMS.
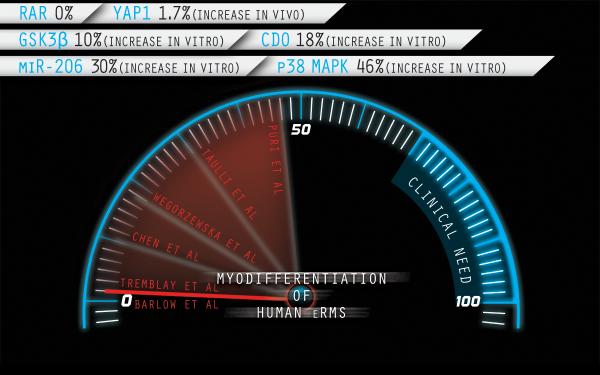
Representative interventions reported as percentage increase of MHC positive cells*in vitro or in vivo(Barlow et al., 2006; Chen et al., 2014; Puri et al., 2000; Taulli et al., 2009; Wegorzewska et al., 2003). Corresponding targets are noted. For consistency, only studies of the prototypic RD cell line (generated in 1968) are included. Some of these pathways may be interlinked (e.g., GSK3β and YAP1 have been reported to be co-associated on the Axin scaffold, regulating β-catenin and YAP1 signaling in parallel).*In the case of RAR, MHC was not done but the authors reported no differentiation by morphology or by Troponin-T immunocytochemistry in response to retinoic acid. Illustration by Nick Escobar.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barlow JW, Wiley JC, Mous M, Narendran A, Gee MF, Goldberg M, Sexsmith E, Malkin D. Differentiation of rhabdomyosarcoma cell lines using retinoic acid. Pediatric blood & cancer. 2006;47:773–784. doi: 10.1002/pbc.20650. [DOI] [PubMed] [Google Scholar]
- Chen EY, DeRan MT, Ignatius MS, Grandinetti KB, Clagg R, McCarthy KM, Lobbardi RM, Brockmann J, Keller C, Wu X, Langenau DM. Glycogen synthase kinase 3 inhibitors induce the canonical WNT/beta-catenin pathway to suppress growth and self-renewal in embryonal rhabdomyosarcoma. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:5349–5354. doi: 10.1073/pnas.1317731111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crose LE, Galindo KA, Kephart JG, Chen C, Fitamant J, Bardeesy N, Bentley RC, Galindo RL, Chi JT, Linardic CM. Alveolar rhabdomyosarcoma-associated PAX3- FOXO1 promotes tumorigenesis via Hippo pathway suppression. The Journal of clinical investigation. 2014;124:285–296. doi: 10.1172/JCI67087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod MR. Preclinical research: Design animal studies better. Nature. 2014;510:35. doi: 10.1038/510035a. [DOI] [PubMed] [Google Scholar]
- Puri PL, Wu Z, Zhang P, Wood LD, Bhakta KS, Han J, Feramisco JR, Karin M, Wang JY. Induction of terminal differentiation by constitutive activation of p38 MAP kinase in human rhabdomyosarcoma cells. Genes & development. 2000;14:574–584. [PMC free article] [PubMed] [Google Scholar]
- Reynolds CP, Lemons RS. Retinoid therapy of childhood cancer. Hematology/oncology clinics of North America. 2001;15:867–910. doi: 10.1016/s0889-8588(05)70256-2. [DOI] [PubMed] [Google Scholar]
- Shern JF, Chen L, Chmielecki J, Wei JS, Patidar R, Rosenberg M, Ambrogio L, Auclair D, Wang J, Song YK, et al. Comprehensive genomic analysis of rhabdomyosarcoma reveals a landscape of alterations affecting a common genetic axis in fusion-positive and fusion-negative tumors. Cancer discovery. 2014;4:216–231. doi: 10.1158/2159-8290.CD-13-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taulli R, Bersani F, Foglizzo V, Linari A, Vigna E, Ladanyi M, Tuschl T, Ponzetto C. The muscle-specific microRNA miR-206 blocks human rhabdomyosarcoma growth in xenotransplanted mice by promoting myogenic differentiation. The Journal of clinical investigation. 2009;119:2366–2378. doi: 10.1172/JCI38075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay, Missiaglia E, Galli GG, Hettmer S, Urcia R, Carrara M, Judson RN, Thway K, Nadal G, Selfe JL, et al. The Hippo Transducer YAP1 Transforms Activated Satellite Cells and is a Potent Effector of Embryonal Rhabdomyosarcoma Formation. Cancer Cell. 2014 doi: 10.1016/j.ccr.2014.05.029. in press. [DOI] [PubMed] [Google Scholar]
- Wegorzewska M, Krauss RS, Kang JS. Overexpression of the immunoglobulin superfamily members CDO and BOC enhances differentiation of the human rhabdomyosarcoma cell line RD. Molecular carcinogenesis. 2003;37:1–4. doi: 10.1002/mc.10121. [DOI] [PubMed] [Google Scholar]


