Abstract
Objective
Xpert MTB/RIF (‘Xpert’) and urinary lateral-flow lipoarabinomannan (LF-LAM) assays offer rapid tuberculosis (TB) diagnosis. This study evaluated the cost-effectiveness of novel diagnostic algorithms utilizing combinations of Xpert and LF-LAM for the detection of active TB among people living with HIV.
Design
Cost-effectiveness analysis using data from a comparative study of LF-LAM and Xpert, with a target population of HIV-infected individuals with signs/symptoms of TB in Uganda.
Methods
A decision-analysis model compared multiple strategies for rapid TB diagnosis:sputum smear-microscopy; sputum Xpert; smear-microscopy combined with LF-LAM; and Xpert combined with LF-LAM. Primary outcomes were the costs and DALY’s averted for each algorithm. Cost-effectiveness was represented using incremental cost-effectiveness ratios (ICER).
Results
Compared with an algorithm of Xpert testing alone, the combination of Xpert with LF-LAM was considered highly cost-effective (ICER $57/DALY-averted) at a willingness to pay threshold of Ugandan GDP per capita. Addition of urine LF-LAM testing to smear-microscopy was a less effective strategy than Xpert replacement of smear-microscopy, but was less costly and also considered highly cost-effective (ICER $33 per DALY-averted) compared with continued usage of smear-microscopy alone. Cost-effectiveness of the Xpert plus LF-LAM algorithm was most influenced by HIV/ART costs and life-expectancy of patients after TB treatment.
Conclusion
The addition of urinary LF-LAM to TB diagnostic algorithms for HIV-infected individuals is highly cost-effective compared with usage of either sputum smear-microscopy or Xpert alone.
Keywords: cost-effectiveness, HIV, lipoarabinomannan, Xpert
Introduction
Current strategies for the diagnosis of tuberculosis (TB) in HIV-infected persons remain suboptimal and TB is a leading cause of death among people living with HIV in Uganda and other endemic settings. Smear microscopy is widely available but detects less than one-half of HIV-related TB cases [1]. Mycobacterial culture remains the reference standard for TB diagnosis, but is not routinely available, is costly and slow. Emerging tools for rapid TB diagnosis in HIV-infected individuals with signs/symptoms of TB include the Xpert MTB/Rif (‘Xpert,’ Cepheid, Sunnyvale, California, USA) and the urinary tests for lipoarabinomannan (LAM) antigen detection. Xpert is an automated molecular assay for detection of TB and rifampin resistance from sputum samples, provides results in approximately 2–3 h, and is currently WHO endorsed for rapid TB diagnosis. Alternatively, the Determine TB-LAM test (Alere, Waltham, Massachusetts, USA) is a low-cost urinary lateral flow assay (LF-LAM) that requires no equipment, yields results in approximately 30 min, and can be implemented at the point-of-care [2]. Despite emergence of these tests, both Xpert and LF-LAM have trade-offs with regards to costs and performance in people living with HIV. The cost-effectiveness of novel diagnostic algorithms that incorporate these new tools alone or in combination with each other and with conventional diagnostic modalities is unknown.
Prior studies have suggested that Xpert replacement of smear-microscopy is cost-effective for diagnosis of pulmonary TB in the general population [3]. However, sputum Xpert testing has limitations in sensitivity when used in people living with HIV [4–7]. HIV-infected individuals have higher proportions of smear-negative pulmonary TB as well as disseminated or extrapulmonary forms of disease in which the yield of sputum testing by Xpert is reduced [8].
By contrast, newly emerging urine tests for LAM may enhance diagnostic algorithms by offering additional diagnostic yield for HIV-associated TB [2,9–11]. Urinary LAM detection offers the benefit of evaluating non-respiratory samples and has additive value when combined with sputum smear microscopy in HIV-infected individuals with signs/symptoms of TB [2,9,10,12]. In HIV-positive adults, the LF-LAM test has the highest sensitivity in those most severely immunocompromized and with disseminated forms of disease – a group with higher rates of smear-negative TB in whom Xpert testing may be insufficient, and in whom prompt ART initiation confers a survival benefit [2,9,12,13].
Sun et al. [14] recently showed that usage of LF-LAM for hospitalized patients with low CD4+ cell count in South Africa was considered highly cost-effective compared with smear-microscopy alone. The cost-effectiveness of incorporating LF-LAM testing as part of diagnostic algorithms with or without Xpert for a broader population of HIV-infected individuals, including outpatients and those with less immunosuppression, is unknown. We conducted an economic evaluation to determine the cost-effectiveness of a rapid algorithm combining sputum Xpert testing with urinary LF-LAM testing for symptomatic HIV-infected individuals in Uganda. We compared this rapid algorithm with current TB diagnostic approaches, which rely upon sputum examination by smear-microscopy or Xpert alone [15].
Methods
Ethics statement
The study was approved by the institutional review board (IRB) at the Johns Hopkins University School of Medicine (Baltimore, Maryland, USA), as well as in Uganda by the scientific review committee of the Infectious Diseases Institute, the Research Ethics Committees of the Ugandan Joint Clinical Research Centre and Mulago National Referral Hospital, the Uganda National Council for Science and Technology, and Boston University Medical Center IRB. Witnessed written informed consent was provided by all study participants in the parent study.
Study site, population, and diagnostic parameters
This economic evaluation was conducted from a health-system perspective with a target population of HIV-infected individuals presenting with signs/symptoms of active TB disease in Uganda, including pulmonary, extrapulmonary, and disseminated forms of TB [15]. An analytic time frame of 1 year was used for estimation of costs and immediate effects and the time horizon extended to the life expectancy of the cohort. Model development and analysis utilized TreeAge Software.
Key parameters including disease prevalence and diagnostic test performance are summarized in Table 1 and Supplemental Digital Content 1, http://links.lww.com/QAD/A400 [3,9,10,12,14,16–37]. Data were collected during a prospective study comparing the sensitivity and specificity (stratified by CD4+ cell count) of the urine LF-LAM assay, Xpert MTB/Rif, and combinations of tests among HIV-infected patients presenting with signs or symptoms of TB [12] [NCT01525134]. In brief, HIV-infected adults in the outpatient and inpatient setting at the Infectious Disease Institute (IDI) and Mulago Hospital in Uganda were enrolled on the basis of WHO TB screening criteria having at least one of cough, fever, night sweats, or weight loss [15]. Patients were evaluated using LF-LAM (grade 2 cut-off for positivity), sputum smear-microscopy, sputum culture on solid and liquid platforms, mycobacterial blood cultures, and sputum Xpert MTB/Rif. Patients were categorized as culture-confirmed TB (based on mycobacterial culture from any site) or without TB on the basis of no positive conventional microbiologic result and clinical improvement without TB therapy [12]. Individuals with isolated mycobacteremia without pulmonary TB were included/categorized as ‘smear-negative TB.’ Parameter estimates of diagnostic accuracy were varied in sensitivity analysis based on published literature.
Table 1.
Key parameter estimates.
| Cost Item | Cost | Value (lower, upper) | Source |
|---|---|---|---|
| Laboratory costs | |||
| AFB smear | $1.99 | $1.33–$3.50 | Calculated |
| Mycobacterial culture | $16.06 | $12.59–$29.80 | Calculated |
| Conventional culture-based DST | $22.73 | $16.78–$41.72 | Calculated |
| Urine LF-LAM | $4.19 | $2.01–$10.94 | Calculated |
| Xpert MTB/Rif | $17.42 | $11.36–$40.47 | Calculated |
| Treatment costs | |||
| TB treatment | $195 | $100–$500 | [3,16,17] |
| TB treatment category 2 | $302 | $130–$600 | [3,16,17] |
| MDR-TB treatment | $1790 | $1000–$5000 | [3,16,17] |
| Annual HIV care costsb | $0 ($470b) | $0–$2000 | [18,19] |
| Epidemiology and diagnostic and treatment parameters | |||
| Prevalence of TB among symptomatic HIV patients (CD4+<100) | 0.3 | 0.03–0.5 | [9,12,14] |
| Prevalence of TB among symptomatic HIV patients (CD4+>100) | 0.1 | 0.03–0.3 | [3,12] |
| Proportion of TB cases with prior TB treatment | 0.073 | 0.05–0.15 | [3] |
| Prevalence of MDR-TB, among new TB cases | 0.014 | 0.005–0.10 | [17,20] |
| Prevalence of MDR-TB, among previously treated TB cases | 0.12 | 0.03–0.19 | [17,20] |
| Urine LF-LAM sensitivity (specificity)a,c | 0.49 (0.97)a | 0.39–0.59 (.8–1) | [2,9,10,12,14], Study data |
| Sputum ZN Smear sensitivity (specificity)a | .32 (.99)a | 0.30–0.51(.9–1) | [9,12,14], Study data |
| Sputum mycobacterial culture sensitivity (specificity)a | .93 (1)a | 0.85–1 (0.9–1) | Study data |
| Xpert sensitivity (specificity)a | 0.76a (0.98) | 0.41–1 (0.93–1) | [2,3,8,12,21], Study data |
| Xpert Sensitivity for Rifampin Resistance (specificity) | 0.94 (0.98) | 0.9–1 (0.9–1) | [3,8] |
| LF-LAM +ZN smear sensitivity (specificity)c | 0.56a (0.97) | 0.53–0.76 (0.80–0.99) | [2,9,12], Study data |
| LF-LAM +Xpert sensitivity (specificity)c | 0.85a (95%) | 0.77–0.92 (0.80–0.99) | [2,10,12], Study data |
| Sensitivity of clinical diagnosis (Proportion empiric TB treatment without initial positive test)e | 0.30e | 0–0.75 | [3,21,22] |
| Specificity of clinical diagnosis | 0.89 | 0.5–1 | [3,22,23] |
| Mortality of untreated smear positive TB (smear-negative/disseminated TB)d | 1 (1) | 0.75–1 (0.5–1) | [3] |
| Treatment success new drug sensitive TB cased | 0.77 | .62–0.95 | [3,16,17,24,25] |
| Mortality – treated new drug sensitive TB cased | 0.105 | 0.04–0.30 | [3,16,17,24,25] |
| Life-Expectancy after TB recovery (assumes provisions for ART) | 12.9 years | 1.5–33.5 | [3,14,26,27] |
| Disability weight TB with HIV infection | .399 | 0.267–0.547 | [3,28] |
| Disability weight TB treatment (MDR treatment) | 0.1 (0.2) | 0.085–0.115 | [14] |
AFB, acid-fast bacilli; DST, drug-sensitivity testing; LF-LAM, lateral-flow lipoarabinomannan test; MDR, multidrug resistant; TB, tuberculosis; ZN, Ziehl-Neelsen.
Base-case parameters are based on study data and includes all HIV-associated TB including pulmonary TB and disseminated TB without pulmonary TB[12]. For the model, sensitivity of diagnostic tests were stratified by both CD4+ cell count and smear-status based on study data and are shown in online supplemental content [12]. Individuals with mycobacteremia/disseminated TB without pulmonary TB were included as part of smear-negative TB.
In the base-case, HIV/ART costs were not included. In secondary analysis, $470 of annual HIV/ART costs per year was used for the base-case analysis with future costs discounted at 3%.
Sensitivity and specificity of LF-LAM is based on using the grade-2 threshold to determine test positivity.
Untreated TB refers to individuals who remain without any treatment after repeat evaluations and diagnostic testing. Additional treatment outcomes for drug-sensitive and MDR-TB are shown in Supplemental Content 1, http://links.lww.com/QAD/A400.
Individuals were eligible for clinical diagnosis at initial presentation and during repeat evaluations.
Study model
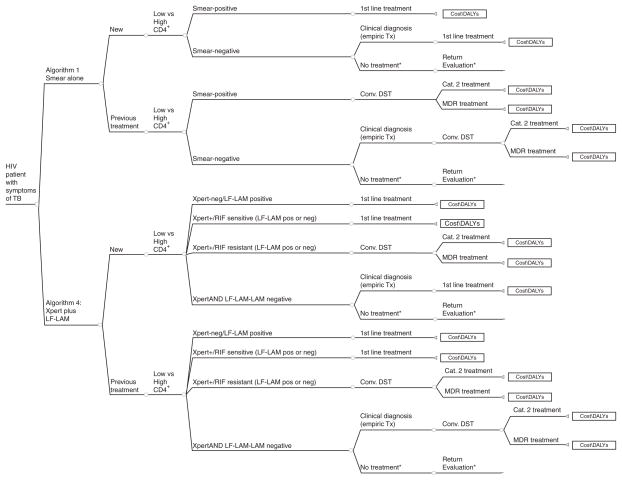
A decision-analysis model was constructed to determine if TB diagnostic algorithms that incorporate urine LF-LAM in combination with smear-microscopy or Xpert are cost-effective compared with strategies using smear-microscopy or sputum Xpert testing alone among symptomatic HIV-infected individuals evaluated for TB in Uganda (Fig. 1). In all model arms, patients were stratified according to CD4+ cell count to allow consideration of differential test performance based on degree of immunosuppression. We assumed individuals with active TB disease with negative initial diagnostic evaluation were eligible to return for repeat evaluations with 10% progressing to smear-positivity (Supplemental Content 1, http://links.lww.com/QAD/A400) [3]. Individuals with negative TB diagnostic testing were eligible for clinical diagnosis and empiric treatment in all model arms. Individuals with positive test results or empiric diagnosis of TB were assumed to be started on TB treatment according to WHO recommended regimens (Supplemental Content 1, http://links.lww.com/QAD/A400).
Fig. 1. Schematic of algorithm utilizing smear-microscopy alone versus algorithm utilizing Xpert with LF-LAM.
Schematic diagram of decision analysis model for TB diagnostic algorithms with and without Xpert and/or LF-LAM. Not all branches are shown. Schematic for algorithm 2 (Smear/LF-LAM) and algorithm 3 (Xpert alone) are shown in Supplemental content 1. *Individuals are eligible to return to clinic/hospital for repeat evaluation, with incremental increases in diagnosis. We assumed increased TB treatment mortality for individuals with delayed diagnoses (Supplemental Content for additional model and parameters). DALYs, disability-adjusted life years; DST, drug sensitivity testing.
We compared four strategies for rapid diagnosis of HIV-associated TB.
Algorithm 1: ‘Smear-microscopy’ algorithm in which all patients submit two sputa for direct Ziehl-Neelsen smear-microscopy testing.
Algorithm 2: ‘Smear plus LF-LAM (Smear/LF-LAM)’ algorithm in which all patients submit two sputa for direct Ziehl-Neelsen smear-microscopy testing and one urine sample for point-of-care LF-LAM testing.
Algorithm 3: ‘Xpert as replacement for smear-microscopy (Xpert)’ algorithm in which all patients submit one sputum for Xpert testing. Xpert detection of Rifampin resistance is confirmed with conventional culture and DST for all patients.
Algorithm 4: ‘Xpert plus LF-LAM (Xpert/LF-LAM) ‘ algorithm in which all patients with symptoms of TB submit one sputum for Xpert testing and urine for point-of-care LF-LAM testing. Xpert detection of Rifampin resistance is confirmed with conventional culture and DST for all patients.
Estimation of costs
Costs for TB diagnostics are shown in Table 1 and were based on a cost analysis conducted during the parent study along with published literature [38] (supplemental content 1, http://links.lww.com/QAD/A400). The amount of staff time, consumable supplies, and equipment utilized for each test were determined through direct observation of testing procedures. Costs of key consumables and equipment were obtained from laboratory invoices. Overhead costs were incorporated based on estimated laboratory resources utilized by each diagnostic system. Uganda is eligible for negotiated discounts for Xpert testing and the costs utilized in this analysis reflect this price structure.
TB treatment costs were based on published literature and are shown in Table 1. Consistent with other economic evaluations of TB diagnostics, we did not include the costs of ART or HIV care in the base-case [3,14]. However, we conducted additional secondary analyses that also incorporated HIV/ART costs. All costs are presented in 2013 US dollars. Future costs were discounted at 3% [39].
Outcome parameters
The primary outcomes were the expected costs per patient with suspected TB, disability-adjusted life years (DALYs) accrued per patient, and cost-effectiveness of the proposed diagnostic algorithms expressed as the incremental cost-effectiveness ratios (ICER). We compared ICERs to WHO’s suggested country-specific willingness-to-pay (WTP) threshold to determine cost-effectiveness, defined in our analysis as per-capita Ugandan gross domestic product ($487) per DALY-averted [40–42]. Parameter estimates for treatment outcomes are shown in Table 1 and assumes initiation of ART according to current guidelines; future DALY’s were discounted at 3%. Our model incorporated the potential impact of CD4+ cell count (i.e. at the time of evaluation) and diagnostic delays on TB treatment outcomes; in the base-case we assumed relative increases in TB treatment mortality for those with CD4+ cell counts less than 100 as well as individuals with diagnostic/therapeutic delays (i.e. diagnosed only on repeat presentation to care). The impact of all parameters on expected costs and outcomes were explored in sensitivity analysis. Probabilistic sensitivity analysis using Monte-Carlo simulation methods were conducted to further explore parameter uncertainty and to generate uncertainty ranges.
Results
Impact of rapid diagnostic algorithms
In the base-case analysis, our model estimated that case-detection of HIV-associated TB cases increases from 66% (95% uncertainty range 41–80%) with the reference ‘Smear-microscopy’ algorithm to 80% (62–91%) with the ‘Smear/LF-LAM algorithm,’ to 87% (73–92%) with the ‘Xpert’ algorithm, and 93% (81–96%) with the ‘Xpert/LF-LAM’ algorithm (Table 2).
Table 2.
Expected outcomes and cost-effectiveness of rapid diagnostic algorithms in the base-case analysis for a cohort of 10 000 patients.
| Algorithm | Total costs per patient (95% UR) | % TB cases detectedb (95%UR) | TB deaths per cohort | TB deaths averted (95% UR) | DALY’s per patient (95% UR) | DALY’s averted per cohort (95% UR) | ICER cost per DALY averted (95% UR)c |
|---|---|---|---|---|---|---|---|
| ‘Smear’ | $70.76 ($42–$133) | 66% (0.41–0.80) | 1039 (423–1809) | REF | 14.24 (9.2–19.0) | REF | REF |
| ‘Smear/LF-LAM’ | $81.32 ($52–$157) | 81% (0.62–0.91) | 768 (342–1352) | 271 (67–570) | 13.92 (8.84–18.85) | 3191 (782–9446) | $33 ($24–$175) |
| ‘Xpert’ | $98.31 ($66–$181) | 87% (0.73–0.92) | 635 (277–1202) | 404 (137–715) | 13.76 (8.74–18.78) | 4757 (1471–10964) | $58 ($39–$289) |
| ‘Xpert/LF-LAM’ | $104.69 ($69–$187) | 93% (0.81–0.96) | 530 (237–1051) | 508 (170–885) 104 (30–202)a |
13.64 (8.57–18.75) | 5982 (1794–13933) 1225 (311–3088)a |
$57 ($37–$262) $52 ($12–$175)a |
DALY, disability adjusted life year; UR, uncertainty range.
Result when compared with the ‘Xpert’ algorithm.
Includes clinical diagnosis and empiric TB treatment.
In secondary analysis inclusive of annual HIV/ART costs, ICERs comparing to reference ‘Smear’ algorithm were $421.37 per DALY-averted ($200–$752), $446.29 per DALY-averted ($247–$777), $445.09 per DALY-averted ($244–$766) for the ‘Smear/LF-LAM’, ‘Xpert’, and ‘Xpert/LF-LAM’ algorithms, respectively. Comparing ‘Xpert/LF-LAM’ to ‘Xpert’, the ICER was $440.41 per DALY-averted ($187–$742).
For a cohort of 10 000 HIV patients in Uganda, the ‘Smear/LF-LAM’ algorithm was estimated to lead to 3191 DALY’s-averted (782–9446) and 271 (67–570) TB deaths averted compared with the ‘Smear’ algorihm. By contrast, ‘Xpert’ implementation was estimated to avert 4757 DALY’s (1471–10964) compared with the ‘Smear’ algorithm. LF-LAM in combination with Xpert (‘Xpert/LF-LAM’ algorithm) could reduce TB mortality further compared with ‘Xpert’ algorithm (16% relative risk reduction) and would avert a further 104 deaths (30–202) and an additional 1225 (311–3088) DALY’s (Table 2).
Costs of rapid tuberculosis diagnostic algorithms
Rapid diagnostic algorithms incorporating Xpert and LF-LAM were associated with increased diagnostic costs compared with the ‘Smear’ algorithm and are shown in Table 3. Compared with the reference ‘Smear’ algorithm, addition of urinary LF-LAM (‘Smear/LF-LAM’ algorithm) increases diagnostic costs per person from $17.42 to $21.34 (incremental diagnostic cost $3.92). Implementation of the ‘Xpert’ algorithm would nearly double diagnostic costs to $32.81 per person (incremental diagnostic cost $15.39). Compared with the ‘Xpert’ algorithm, ‘Xpert plus LF-LAM’ would increase diagnostic costs by $3.87 to $36.68 per patient
Table 3.
Expected diagnostic yield and costs of rapid diagnostic algorithms for a cohort of 10 000 symptomatic individuals evaluated for Tuberculosis.
| Algorithm | Cohort | N | Total TB detectedc | % of TB cases detected | Total MDR detected | % of MDR cases detected | Diagnostic cost per patienta | Diagnostic cost per TB case detected | Diagnostic costs: % of total costs | Total diagnostic costs per cohort | Total treatment costs |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ‘Smear’ | Total | 10000 | 1465 | 66% | 9 | 27% | $17.42 | $119 | 25%b | $174 156 | $533 346 |
| Not TB | 7780 | $15.83 | $123 131 | $187 605 | |||||||
| TB | 2172 | 1434 | 66% | $22.79 | $49 507 | $315 933 | |||||
| MDR-TB | 48 | 32 | 66% | 9 | 27% | $31.62 | $1518 | $29 808 | |||
| ‘Smear/LF-LAM’ | Total | 10000 | 1798 | 81% | 13 | 33% | $21.34 | $119 | 26%b | $213 339 | $599 940 |
| Not TB | 7780 | $20.00 | $155 588 | $177 433 | |||||||
| TB | 2172 | 1759 | 81% | $25.78 | $55 996 | $386 085 | |||||
| MDR-TB | 48 | 39 | 81% | 13 | 33% | $36.55 | $1755 | $36 422 | |||
| ‘Xpert’ | Total | 10000 | 1931 | 87% | 34 | 81% | $32.81 | $170 | 33%b | $328 143 | $655 047 |
| Not TB | 7780 | $31.21 | $242 850 | $158 000 | |||||||
| TB | 2172 | 1890 | 87% | $37.79 | $82 077 | $423 993 | |||||
| MDR-TB | 48 | 42 | 87% | 34 | 81% | $67.01 | $3216 | $73 054 | |||
| ‘Xpert/LF-LAM’ | Total | 10000 | 2065 | 93% | 37 | 82% | $36.68 | $177 | 35%b | $366 830 | $679 843 |
| Not TB | 7780 | $35.40 | $275 398 | $154 368 | |||||||
| TB | 2172 | 2020 | 93% | $40.55 | $88 072 | $450 870 | |||||
| MDR-TB | 48 | 45 | 93% | 37 | 82% | $70.01 | $3360 | $74 605 |
Inclusive of costs associated with clinical evaluation, chest radiograph, CD4+ testing, and specimen transport in addition to TB diagnostic testing
When HIV/ART care is included in total costs, diagnostic costs as a percentage of total costs are 0.006, 0.006%, 0.007, and 0.008% for ‘Smear’, ‘Smear/LF-LAM’, ‘Xpert’, ‘Xpert/LF-LAM’, respectively.
Includes clinical diagnosis and empiric TB treatment, and is inclusive of repeat evaluations.
Total costs associated with diagnostic algorithms are shown in Tables 2 and 3, inclusive of treatment costs. Net treatment costs increase for the ‘Smear/LAM’, ‘Xpert’, and ‘Xpert/LAM’ algorithm compared with the ‘Smear’ algorithm due to increased case detection, but was moderated by reductions in costs associated with false positive empiric treatment of individuals without TB (Table 3). Overall, diagnostic costs represented only a small percentage of total costs per patient (25, 26, 33, and 35% of total costs for ‘Smear’, ‘Smear/LF-LAM’, ‘Xpert’, ‘Xpert/LF-LAM,’ respectively; Table 3). Inclusion of HIV/ART care costs increased the total program costs of each diagnostic approach further. Overall, the cost of TB diagnostic testing comprised less than 0.01% of total costs per person when HIV/ART care was considered for all diagnostic algorithms.
Cost-effectiveness of tuberculosis diagnostic algorithms
Compared with the reference ‘Smear’ algorithm, all algorithms incorporating rapid diagnostic testing with Xpert and/or LF-LAM were considered highly cost-effective at a willingness to pay threshold of per-capita GDP in Uganda ($487) [41]. Compared with ‘Smear’, the ICER for the ‘Smear/LF-LAM’ algorithm was $33 per DALY-averted ($24–$175 per DALY-averted). ‘Xpert’ replacement of ‘Smear’ was partially dominated [ICER $58 per DALY-averted ($39–$289 per DALY-averted)] by the ‘Xpert plus LF-LAM’ algorithm [ICER $57 per DALY-averted ($37–$262 per DALY-averted)].
The ‘Xpert plus LF-LAM’ was considered highly cost-effective compared with the ‘Xpert’ algorithm and associated with an ICER of $52 per DALY-averted ($12–$175 per DALY-averted). Alternatively, comparing ‘Xpert’ algorithm to ‘Smear/LF-LAM’, ‘Xpert’ was associated with incremental total costs of $16.99 per person, but would avert a further 1566 DALY’s per 10 000 patients and was highly cost-effective with an ICER of $108/DALY-averted.
Sensitivity analysis
Nearly all one-way sensitivity analyses did not increase the ICER to above the WTP when comparing Xpert and LF-LAM algorithms to ‘Smear’ (Supplemental Digital Content 2, http://links.lww.com/QAD/A400). Similarly, when comparing ‘Xpert plus LF-LAM’ with ‘Xpert’ testing alone, there were few circumstances in which ‘Xpert/LF-LAM’ algorithm was not considered highly cost-effective. The ICER comparing ‘Xpert plus LF-LAM’ algorithm with ‘Xpert’ was most influenced by the specificity of LF-LAM and life-expectancy after TB treatment, but remained cost-effective even at lowest estimates of LF-LAM specificity and life-expectancy.
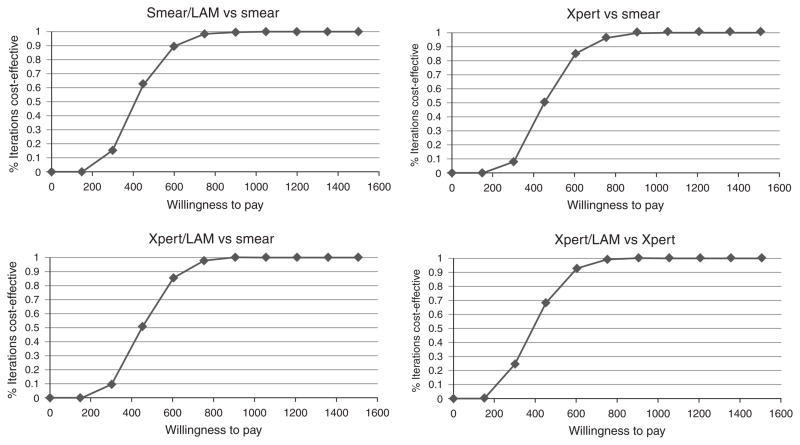
The ICERs for all diagnostic algorithms were significantly influenced by annual HIV/ART costs and effects and rise to $422 per DALY-averted, $446 per DALY-averted, and $445 per DALY-averted for the ‘Smear/LF-LAM’, ‘Xpert’, and ‘Xpert/LF-LAM’ algorithms, respectively, compared with ‘Smear’ when annual HIV costs are included (Table 2). All rapid diagnostic algorithms exceed the WTP threshold if annual HIV/ART costs increase beyond ~$600 per year (Supplemental Digital Content 2, http://links.lww.com/QAD/A400). Alternatively, if ART costs and benefits are eliminated altogether (i.e. reduced life-expectancy after TB treatment), the ICERs for algorithms incorporating LF-LAM and Xpert increase significantly compared with the reference Smear algorithm (assuming a low of 2 year survival after TB treatment, ICER $285/DALY-averted for ‘Smear/LF-LAM’ algorithm, $502/DALY-averted for ‘Xpert’ algorithm, $491/DALY-averted for ‘Xpert/LF-LAM’ algorithm; Supplemental Digital Content 2, http://links.lww.com/QAD/A400). Cost-effectiveness acceptability curves inclusive of HIV/ART care costs and effects are shown in Fig. 2. In probabalistic sensitivity analysis using Monte-Carlo simulation methods, the ‘Xpert/LF-LAM’ algorithm was considered cost-effective in 77% of iterations at a WTP threshold of GDP per capita, and was cost-effective 100% of the time at a willingness to pay threshold of three times GDP per capita.
Fig. 2. Cost-effectiveness acceptability curves inclusive of all health system costs including HIV and ART costs.
Compared with the Reference ‘Smear’ algorithm, at WTP threshold of GDP per capita ($487) per DALY-averted, ‘Smear/LF-LAM’ was cost-effective in 72% of simulations, ‘Xpert’ was cost-effective in 60% of simulations, ‘Xpert/LF-LAM’ was cost-effective in 64% of simulations. All algorithms were cost-effective in 100% of simulations if the WTP threshold of three times GDP per capita per DALY averted is used. Compared to ‘Xpert’ algorithm, ‘Xpert/LF-LAM’ was cost-effective in 77% of simulations at WTP of $487/DALY-averted, and 100% of simulations at WTP thresholds above three times GDP per capita per DALY averted. When HIV/ART care costs are excluded, all rapid diagnostic algorithms were considered cost-effective in 100% of simulations.
LF-LAM algorithms were more cost-effective if utilized for a cohort with CD4+ cell count less than 100. The incremental increase in case-detection for algorithms adding LF-LAM was predicted to be lower for individuals with CD4+ cell count greater than 100, but addition of LF-LAM remained cost-effective due to low incremental costs (Supplemental Digital Content 2 and 3, http://links.lww.com/QAD/A400). Compared with the ‘Xpert’ replacement of smear-microscopy algorithm, the ICER for ‘Xpert plus LF-LAM’ ranged from $41/DALY-averted for a cohort of patients with CD4+ cell count less than 100 to $343/DALY-averted for a cohort with CD4+ cell count greater than 100 and would be considered cost-effective regardless of the percentage of patients with advanced immunosuppression; compared with continued usage of ‘Smear’ algorithm, the ICER for ‘Smear/LF-LAM’ ranged from $29/DALY-averted, to $229/DALY-averted.
When comparing ‘Xpert’ directly to ‘Smear/LF-LAM’, the ICER was most impacted by the sensitivity of sputum Xpert testing for smear-negative TB; Xpert testing becomes more costly and less effective (dominated) than ‘Smear/LF-LAM’ if Xpert sensitivity for smear-negative TB is less than 46%. Alternatively, if the sensitivity of ‘Smear/LF-LAM’ increases beyond 84%, this strategy would be less costly and more effective and would dominate Xpert testing (Supplemental Digital Content 2, http://links.lww.com/QAD/A400).
We additionally examined the impact of increased TB treatment mortality among those with low CD4+ cell count less than 100 on algorithms using LF-LAM. We found that the ‘Xpert/LF-LAM’ algorithm was cost-effective compared with ‘Xpert,’ even under the circumstance that there is a three-fold increase in TB treatment mortality for those with CD4+ cell counts less than 100 (ICER $65 per DALY-averted). Similarly, we found that the ICER for the ‘Smear/LF-LAM’ algorithm compared with ‘Smear’ alone ranged from $32/DALY-averted in the base-case to $42/DALY-averted for a threefold increase in TB treatment mortality for individuals with CD4+ cell count less than 100.
Discussion
TB case detection among people living with HIV in Uganda remains low, with many health centers relying upon smear-microscopy as the primary diagnostic tool. Our study suggests that for HIV-infected individuals meeting WHO symptom screening criteria for TB evaluation, a diagnostic algorithm utilizing the combination of rapid point-of-care urine LF-LAM and sputum Xpert testing would be considered highly cost-effective compared with usage of either smear-microscopy or Xpert alone. Overall, addition of urinary LF-LAM testing to sputum evaluation by the Xpert assay would be expected to improve case-detection of HIV-associated TB compared with Xpert testing alone while increasing diagnostic costs by less than $5 per patient. In particular, for individuals with low CD4+ cell counts at risk for smear-negative and disseminated forms of TB, implementation of an ‘Xpert plus LF-LAM’ algorithm in place of continued reliance on smear-microscopy and clinical diagnoses may be preferable to a strategy of Xpert replacement of smear-microscopy.
Despite the potential benefits of Xpert testing, with or without LF-LAM, scale-up of Xpert in Uganda has challenges. Logistical barriers include the need for consistent power supply, as well as mechanisms for specimen transport and results reporting when implemented in peripheral or reference laboratories. Moreover, some programs may find that Xpert implementation remains unaffordable; for a cohort of 10 000 patients, usage of Xpert as a replacement of smear-microscopy with or without LF-LAM would increase annual diagnostic costs by more than $150 000 and total HIV/TB program costs by more than $275 000. For settings in which Xpert testing remains unavailable, we found that an algorithm of smear-microscopy plus LF-LAM may be a less costly but less effective alternative. Importantly, our results suggest this strategy would detect fewer cases of TB and MDR-TB than Xpert replacement of smear-microscopy, but has lower expense and was considered highly cost-effective compared with continued usage of smear-microscopy alone for evaluation of HIV-patients with signs/symptoms of TB.
Overall, we found that incorporation of both LF-LAM and Xpert into TB case-finding algorithms represented excellent value for money for HIV and TB programs. The ICER for this rapid ‘Xpert plus LF-LAM’ diagnostic intervention ($57/DALY-averted) compares favorably to other HIV-care interventions in Uganda such as combination ART for prevention of mother-to-child transmission ($46/DALY-averted) [19]. Importantly for policy makers, we showed that diagnostic costs associated with algorithms utilizing either LF-LAM or Xpert represent only a minority of total program costs for HIV patients with suspected TB. As case detection increases through the usage of emerging diagnostics tools, a greater number of individuals will require TB and MDR-TB treatment; the majority of the costs associated with rapid case-finding algorithms (>65%) are attributable to TB treatment costs rather than diagnostic tests. When HIV/ART costs are included in the economic evaluation, we found that the diagnostic costs associated with algorithms using Xpert and/or LF-LAM represented less than 0.01% of total health system expenses for each patient.
Our study has several limitations. Our model structure did not incorporate the impact of rapid TB diagnostics on averted TB transmission, for which there is little published literature. Nonetheless, we found that novel rapid TB diagnostic algorithms are highly cost-effective compared with current approaches; incorporation of averted transmission would be expected to lead to even further health benefits and add to the value of using Xpert and/or LF-LAM for HIV-infected individuals with symptoms of TB. To allow generalizability our model included individuals with varying degrees of immunosuppression based on site and study data. Given the improved diagnostic performance of urinary LAM testing in individuals with low CD4+ cell counts and disseminated disease, this approach could overestimate the potential benefits of LF-LAM testing if applied to populations with less immunosuppression. Nonetheless, we present detailed sensitivity analysis around this parameter and show that while cost-effectiveness of LF-LAM usage is optimized for HIV-patients with CD4+ cell count less than 100, given the low-cost of LF-LAM it remained cost-effective for individuals with higher CD4+ cell counts. Finally, our study evaluated the current LF-LAM platform whose diagnostic sensitivity remains suboptimal; future platforms and assay modifications that have better accuracy are likely possible. Nevertheless, our economic evaluation demonstrates that the incorporation of low cost diagnostic tests that identify smear-negative and disseminated forms of TB in HIV-infected individuals is highly cost-effective and complementary to sputum evaluation by microscopy or Xpert.
Our study has several important strengths. Our results examining replacement of smear-microscopy with Xpert in Uganda (ICER $58/DALY-averted) are consistent with prior published economic evaluations of Xpert testing (e.g. Vassall et al. ICER $52/DALY-averted) [3]. Furthermore, we are the first to examine and compare the expected costs, effects, and cost-effectiveness of algorithms that combine Xpert testing and urinary LF-LAM testing for HIV-infected individuals screened using the current WHO symptom screening guidelines for intensified TB case-finding [15]. We additionally included all forms of HIV-associated TB and did not restrict the analysis to pulmonary TB, and conducted extensive analysis around the impact of CD4+ cell count on diagnostic performance of new algorithms. Individuals with advanced immunosuppression, in whom LF-LAM sensitivity is optimized, may also have higher mortality despite TB treatment and therefore derive fewer health benefits from rapid diagnosis [8,11,12]. Our analysis is unique in incorporating this potential increase in mortality for individuals with advanced immunosuppression, and provides important insight into usage of rapid TB diagnostics. We found that the Xpert/LF-LAM algorithm was considered highly cost-effective compared with an algorithm of sputum smear-microscopy or Xpert testing alone even at the highest estimates of TB treatment mortality for patients with low CD4+ cell counts. Finally, we additionally show the importance and impact of incorporating downstream TB treatment and HIV/ART care costs and effects in economic evaluations of TB case-finding algorithms. Reductions in HIV/TB mortality expected from increased TB case-finding are likely to have significant budgetary implications for HIV programs, with a need for expanded and extended HIV/ART care. Without provisions for long-term ART, potential health benefits made possible through averted TB mortality may be compromised, and the cost-effectiveness of rapid TB diagnostic algorithms will be reduced. These data stress the importance of continued efforts to integrate TB and HIV care to increase the timely initiation of ART.
Supplementary Material
Acknowledgments
M.S. conceived the study, conducted data analysis, and led article writing. D.D. assisted in data analysis and study design. M.J. and W.S. conducted data collection. Y.M., J.E., S.D. provided technical support for study conduct and data analysis and assisted with article writing.
This project was funded with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract no. HHSN2722000900050C (‘TB Clinical Diagnostics Research Consortium’), and under grant # K23AI089259.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kivihya-Ndugga LE, van Cleeff MR, Githui WA, Nganga LW, Kibuga DK, Odhiambo JA, Klatser PR. A comprehensive comparison of Ziehl-Neelsen and fluorescence microscopy for the diagnosis of tuberculosis in a resource-poor urban setting. Int J Tuberc Lung Dis. 2003;7:1163. [PubMed] [Google Scholar]
- 2.Lawn SD. Point-of-care detection of lipoarabinomannan (LAM) in urine for diagnosis of HIV-associated tuberculosis: a state of the art review. BMC Infect Dis. 2012;12:103. doi: 10.1186/1471-2334-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vassall A, van Kampen S, Sohn H, Michael JS, John KR, den Boon S, et al. Rapid diagnosis of tuberculosis with the Xpert MTB/RIF assay in high burden countries: a cost-effectiveness analysis. PLoS Med. 2012;8:e1001120. doi: 10.1371/journal.pmed.1001120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helb D, Jones M, Story E, Boehme C, Wallace E, Ho K, et al. Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. J Clin Microbiol. 2010;48:229–237. doi: 10.1128/JCM.01463-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005–1015. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Theron G, Peter J, van Zyl-Smit R, Mishra H, Streicher E, Murray S, et al. Evaluation of the Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in a high HIV prevalence setting. Am J Respir Crit Care Med. 2011;184:132–140. doi: 10.1164/rccm.201101-0056OC. [DOI] [PubMed] [Google Scholar]
- 7.WHO. [Accessed 21 July 2012];Automated Real-Time Nucleic Acid Amplification Technology for Rapid and Simultaneous Detection of Tuberculosis and Drug Resistant Tuberculosis: Policy Statement. http://whqlibdoc.who.int/publications/2011/9789241501545_eng.pdf. [PubMed]
- 8.Chang K, Lu W, Wang J, Zhang K, Jia S, Li F, et al. Rapid and effective diagnosis of tuberculosis and rifampicin resistance with Xpert MTB/RIF assay: a meta-analysis. J Infect. 2012;64:580–588. doi: 10.1016/j.jinf.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Peter JG, Theron G, van Zyl-Smit R, Haripersad A, Mottay L, Kraus S, et al. Diagnostic accuracy of a urine LAM strip-test for TB detection in HIV-infected hospitalised patients. Eur Respir J. 2012;40:1211–1220. doi: 10.1183/09031936.00201711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah M, Variava E, Holmes CB, Coppin A, Golub JE, McCallum J, et al. Diagnostic accuracy of a urine lipoarabinomannan test for tuberculosis in hospitalized patients in a High HIV prevalence setting. J Acquir Immune Defic Syndr. 2009;52:145–151. doi: 10.1097/QAI.0b013e3181b98430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tessema TA, Hamasur B, Bjun G, Svenson S, Bjorvatn B. Diagnostic evaluation of urinary lipoarabinomannan at an Ethiopian tuberculosis centre. Scand J Infect Dis. 2001;33:279–284. doi: 10.1080/003655401300077306. [DOI] [PubMed] [Google Scholar]
- 12.Shah M, Ssengooba W, Armstrong D, Nakiyingi L, Holshouser M, Ellner J, et al. Comparative Performance of Rapid Urinary Lipoarabinomannan Assays and Xpert MTB/RIF if TB Suspects: Uganda. CROI; Atlanta. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawn SD, Kerkhoff AD, Vogt M, Wood R. Diagnostic accuracy of a low-cost, urine antigen, point-of-care screening assay for HIV-associated pulmonary tuberculosis before antiretroviral therapy: a descriptive study. Lancet Infect Dis. 2012;12:201–209. doi: 10.1016/S1473-3099(11)70251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun D, Dorman S, Shah M, Manabe YC, Moodley VM, Nicol MP, Dowdy DW. Cost utility of lateral-flow urine lipoarabinomannan for tuberculosis diagnosis in HIV-infected African adults. Int J Tuberc Lung Dis. 2013;17:552–558. doi: 10.5588/ijtld.12.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO. [Accessed 10 April 2013];Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. http://www.who.int/hiv/pub/tb/9789241500708/en/index.html.
- 16.Manabe YC, Hermans SM, Lamorde M, Castelnuovo B, Mullins CD, Kuznik A. Rifampicin for continuation phase tuberculosis treatment in Uganda: a cost-effectiveness analysis. PLoS One. 2012;7:e39187. doi: 10.1371/journal.pone.0039187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO. [Accessed 3 September 2012];Tuberculosis Country and Finance Profile-Uganda. http://www.who.int/tb/country/data/profiles/en/index.html.
- 18.Menzies NA, Berruti AA, Berzon R, Filler S, Ferris R, Ellerbrock TV, Blandford JM. The cost of providing comprehensive HIV treatment in PEPFAR-supported programs. AIDS (London, England) 2011;25:1753–1760. doi: 10.1097/QAD.0b013e3283463eec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuznik A, Lamorde M, Hermans S, Castelnuovo B, Auerbach B, Semeere A, et al. Evaluating the cost-effectiveness of combination antiretroviral therapy for the prevention of mother-to-child transmission of HIV in Uganda. Bull World Health Organ. 2012;90:595–603. doi: 10.2471/BLT.11.095430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.USAID. Uganda Tuberculosis Country Profile. http://www1.usaid.gov/our_work/global_health/id/tuberculosis/countries/africa/uganda.pdf.
- 21.Yoon C, Cattamanchi A, Davis JL, Worodria W, den Boon S, Kalema N, et al. Impact of Xpert MTB/RIF testing on tuberculosis management and outcomes in hospitalized patients in Uganda. PLoS One. 2012;7:e48599. doi: 10.1371/journal.pone.0048599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Field N, Murray J, Wong ML, Dowdeswell R, Dudumayo N, Rametsi L, et al. Missed opportunities in TB diagnosis: a TB process-based performance review tool to evaluate and improve clinical care. BMC Public Health. 2011;11:127. doi: 10.1186/1471-2458-11-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinson NA, Karstaedt A, Venter WD, Omar T, King P, Mbengo T, et al. Causes of death in hospitalized adults with a premortem diagnosis of tuberculosis: an autopsy study. AIDS. 2007;21:2043–2050. doi: 10.1097/QAD.0b013e3282eea47f. [DOI] [PubMed] [Google Scholar]
- 24.Khan FA, Minion J, Pai M, Royce S, Burman W, Harries AD, Menzies D. Treatment of active tuberculosis in HIV-coinfected patients: a systematic review and meta-analysis. Clin Infect Dis. 2010;50:1288–1299. doi: 10.1086/651686. [DOI] [PubMed] [Google Scholar]
- 25.WHO. Global Tuberculosis Report 2012. 2012. [Google Scholar]
- 26.Mills EJ, Bakanda C, Birungi J, Chan K, Ford N, Cooper CL, et al. Life expectancy of persons receiving combination antiretroviral therapy in low-income countries: a cohort analysis from Uganda. Ann Intern Med. 2011;155:209–216. doi: 10.7326/0003-4819-155-4-201108160-00358. [DOI] [PubMed] [Google Scholar]
- 27.Mills EJ, Bakanda C, Birungi J, Mwesigwa R, Chan K, Ford N, et al. Mortality by baseline CD4 cell count among HIV patients initiating antiretroviral therapy: evidence from a large cohort in Uganda. AIDS. 2011;25:851–855. doi: 10.1097/QAD.0b013e32834564e9. [DOI] [PubMed] [Google Scholar]
- 28.Salomon JA, Vos T, Hogan DR, Gagnon M, Naghavi M, Mokdad A, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380:2129–2143. doi: 10.1016/S0140-6736(12)61680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362:697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meintjes G, Schoeman H, Morroni C, Wilson D, Maartens G. Patient and provider delay in tuberculosis suspects from communities with a high HIV prevalence in South Africa: a cross-sectional study. BMC Infect Dis. 2008;8:72. doi: 10.1186/1471-2334-8-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lienhardt C, Rowley J, Manneh K, Lahai G, Needham D, Milligan P, McAdam KP. Factors affecting time delay to treatment in a tuberculosis control programme in a sub-Saharan African country: the experience of The Gambia. Int J Tuberc Lung Dis. 2001;5:233–239. [PubMed] [Google Scholar]
- 32.Kahn JG, Marseille E, Moore D, Bunnell R, Were W, Degerman R, et al. CD4 cell count and viral load monitoring in patients undergoing antiretroviral therapy in Uganda: cost effectiveness study. BMJ. 2011;343:d6884. doi: 10.1136/bmj.d6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samandari T, Bishai D, Luteijn M, Mosimaneotsile B, Motsamai O, Postma M, Hubben G. Costs and consequences of additional chest x-ray in a tuberculosis prevention program in Botswana. Am J Respir Crit Care Med. 2011;183:1103–1111. doi: 10.1164/rccm.201004-0620OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahuja SD, Ashkin D, Avendano M, Banerjee R, Bauer M, Bayona JN, et al. Multidrug resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9,153 patients. PLoS Med. 2012;9:e1001300. doi: 10.1371/journal.pmed.1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seung KJ, Omatayo DB, Keshavjee S, Furin JJ, Farmer PE, Satti H. Early outcomes of MDR-TB treatment in a high HIV-prevalence setting in Southern Africa. PLoS One. 2009;4:e7186. doi: 10.1371/journal.pone.0007186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.WHO. [Accessed 7 March 2013];Treatment of Tuberculosis: Guidelines. (4). http://whqlibdoc.who.int/publications/2010/9789241547833_eng.pdf.
- 37.WHO. [Accessed 24 April 2012];Global Tuberculosis Control. 2011 http://www.who.int/tb/publications/global_report/en/
- 38.Sohn H, Minion J, Albert H, Dheda K, Pai M. TB diagnostic tests: how do we figure out their costs? Expert Rev Anti Infect Ther. 2009;7:723–733. doi: 10.1586/eri.09.52. [DOI] [PubMed] [Google Scholar]
- 39.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the panel on cost-effectiveness in health and medicine. JAMA. 1996;276:1253–1258. [PubMed] [Google Scholar]
- 40.Hirth RA, Chernew ME, Miller E, Fendrick AM, Weissert WG. Willingness to pay for a quality-adjusted life year: in search of a standard. Med Decis Making. 2000;20:332–342. doi: 10.1177/0272989X0002000310. [DOI] [PubMed] [Google Scholar]
- 41.World_Bank. [Accessed 10 April 2013];GDP per Capita By Country. http://data.worldbank.org/indicator/NY.GDP.PCAP.CD.
- 42.WHO. [Accessed 10 April 2013];Cost-Effectiveness Thresholds. http://www.who.int/choice/costs/CER_thresholds/en/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.