Abstract
With the advent of effective antiretroviral therapy (ART), HIV is becoming a chronic disease. HIV seropositive (+) individuals on ART can expect to live longer and, as a result, they are at risk of developing chronic non-communicable diseases related to factors such as aging, lifestyle, long-term HIV infection, and the potential adverse effects of ART. Though data are incomplete, evidence suggests that even in low- and middle-income countries (LMICs), chronic cardiovascular and pulmonary diseases are increasing in HIV+ individuals. This review summarizes evidence linking HIV infection to the most commonly cited chronic cardiovascular and pulmonary conditions in LMICs: heart failure, hypertension, coronary artery disease/myocardial infarction, stroke, obstructive lung diseases, and pulmonary arterial hypertension. We describe the observed epidemiology of these conditions, factors affecting expression in LMICs, and key populations that may be at higher risk (i.e., illicit drug users and children), and finally, we suggest strategic areas of research and training intended to counter these conditions effectively. As access to ART in LMICs increases, long-term outcomes among HIV+ persons will increasingly be determined by a range of associated chronic cardiovascular and pulmonary complications. Actions taken now to identify those conditions that contribute to long-term morbidity and mortality, optimize early recognition and diagnosis, and implement effective prevention strategies and/or disease interventions are likely to have the greatest impact on limiting cardiovascular and pulmonary disease comorbidity and improving population health among HIV+ individuals in LMICs.
INTRODUCTION
The risk of developing chronic cardiovascular and pulmonary diseases is increasingly recognized as a major public health problem in individuals infected with HIV [1-3], perhaps related to long-term exposure to the virus, the effects of ongoing inflammatory responses, progressive immunologic dysfunction, and/or long-term adverse effects associated with antiretroviral therapy (ART). The inevitable consequences of aging, the development of age-related chronic conditions, and other factors affecting cardio-pulmonary health are likely to compound any HIV viral or treatment effect. The overall impact of chronic non-communicable cardiovascular and pulmonary diseases occurring among HIV+ people in low- and middle-income countries (LMICs) (defined according to World Bank Country and Lending Group designation) is profound given that the majority (>70%) of HIV+ people reside in these areas and may experience cardio-pulmonary risk factors unique to this setting [4]. We review available data on chronic non-communicable cardiovascular and pulmonary diseases among HIV+ persons in LMICs and suggest directions for future research to stimulate improvements in diagnosis, prevention, and treatment of HIV-associated chronic cardiovascular and pulmonary diseases in LMICs.
METHODS AND EPIDEMIOLOGY
The most frequently reported chronic non-communicable cardiovascular and pulmonary diseases linked to HIV in LMICs are heart failure, hypertension, coronary artery disease/myocardial infarction, stroke, obstructive lung disease, and pulmonary hypertension (Table 1). This list was derived from a search of the published literature using PubMed to identify articles using subject headings or MeSH terms related to cardiovascular disease, pulmonary disease, HIV, developing countries, and LMICs. The search was not a systematic review. Reference lists were also reviewed for additional articles. Articles were selected based on their relevance to the subject matter and availability of data within the article. Although the epidemiology of these diseases varies by region of the world, we focus on Sub-Saharan Africa (SSA) in this review due to the large proportion of the HIV+ population living there and because the bulk of the published literature emanates from this region. As many of these conditions have not been extensively studied in LMICs (Table 1), we first discuss these diseases in HIV+ persons in HICs and then summarize findings in LMICs. Table 1 is organized according to the most frequently reported conditions, yet due to variation in patient population, method of screening and selection of patients, and definitions of outcomes, there is wide variation in findings.
Table 1.
Key defining studiesa from LMICs of HIV and non-communicable cardiovascular and pulmonary diseases
| Disease | Reference | Country | Sample | HIV+ (n) | HIV− (n) | Study type | Brief Study Description | Key findings |
|---|---|---|---|---|---|---|---|---|
| Heart Failure | Hakim, et al. 1996 [10] | Zimbabwe | 157 | 157 | 0 | P,O | Echocardiographic study | 50% of patients had dilated cardiomyopathy, left ventricular systolic dysfunction, isolated right ventricular dilatation, or pericardial disease |
| Niakara, et al. 2002 [11] | Burkina Faso | 79 | 79 | 0 | R | Prevalence of cardiac diseases found among HIV patients | Common cardiac diseases in HIV+ patients were systolic HF, myocarditis, pericarditis, PAH, pulmonary embolism, and MI. | |
| Longo-Mbenza, et al. 1997 [12] | Zaire | 332 | 166 | 166 | P,O | Echocardiographic study of HIV+ and HIV− patients | Higher incidence of echocardiographic abnormalities in HIV+ patients. Worse systolic function in HIV+ patients. | |
| Longo-Mbenza, et al. 1998 [13] | Congo | 157 | 157 | 0 | P,O | Longitudinal echocardiographic study of risk factors for developing cardiac pathologies | Low socio-economic status and pericardial effusion were independent predictors of death. | |
| Nzuobontane, et al. 2002 [15] | Cameroon | 75 | 54 | 21 | P,O | Echocardiographic study of HIV+ and HIV− patients | Low CD4 cell count was associated with dilated cardiomyopathy. | |
| Twagirumukiza, et al. 2007 [16] | Rwanda | 416 | 416 | 0 | P,O multicenter | Echocardiographic study of patients not receiving HAART | Predictors of dilated cardiomyopathy were low socioeconomic status, duration of HIV infection, CD4 count, HIV viral load, advanced stage of HIV and low plasma level of selenium. | |
| Sliwa, et al. 2012 [18] | South Africa | 5328 | 518 | 4810 | P clinical registry | Description of all cases where HIV was concurrently diagnosed among patients admitted with de novo heart disease | HIV-related cardiac disease a minor contributor to overall disease burden (<4% prevalence). Cardiomyopathy (systolic and diastolic dysfunction) was the most common HIV-related cardiac disease. | |
| Damasceno, et al. 2012 [20] | Multiple | 1006 (500 HIV tested) | 65 | 435 | P,O multicenter | Registry of patients admitted with acute heart failure. A subset of the population was tested for HIV | HIV-associated cardiomyopathy (systolic dysfunction) was rare (<3% prevalence). | |
| Tantchou Tchoumi, et al. 2011 [21] | Cameroon | 462 | 7 | 455 | P,O | Description of patients admitted with heart failure | 1.6% of cases with HIV-associated cardiomyopathy (systolic dysfunction). | |
| Chillo, et al. 2012 [22] | Tanzania | 102 | 102 | 0 | P,O | Echocardiographic study to diagnose cardiac abnormalities in HIV+ patients | 10% of HIV+ patients with cardiomyopathy (systolic dysfunction), 34% with hypertensive heart disease. | |
| Longenecker, et al. 2011 [23] | Uganda | 82 | 41 | 41 | P,O | Echocardiographic study of pregnant women with and without HIV to look for signs of cardiomyopathy and pulmonary hypertension and to examine outcomes. | HIV was not associated with any echocardiographic signs of cardiomyopathy (systolic dysfunction). Unexpectedly, HIV was associated with a slightly lower RVSP, but the number of observations was small. Maternal and fetal outcomes were similar for HIV+ and HIV− patients. | |
| Sliwa, et al. 2011 [24] | South Africa | 80 | 27 | 53 | P,O | Description of clinical outcomes and mortality among patients with a first time diagnosis of peripartum cardiomyopathy stratified by HIV status | No statistically significant difference in LVEF and mortality was observed between patients with peripartum cardiomyopathy with and without HIV infection. These patients had continuous high mortality occurring beyond 6 months independent of HIV infection and subsequent pregnancy. | |
| HTN | Bloomfield, et al. 2011 [32] | Kenya | 12,194 | 12,194 | 0 | R | Description of patterns of hypertension and obesity among HIV+ adults | Overweight/obesity was more strongly associated with hypertension among HIV+ men than a higher successive age category. Among women, higher age category and overweight/obesity were most strongly associated with hypertension. Length of time on protease inhibitors was not found to be related to hypertension for men or women after adjustment. |
| Mateen, et al. 2013 [33] | Sub-Saharan Africa | 5563 | 5563 | 0 | P,O | Assessment of blood pressure in HIV+ patients over the first year following initiation of HAART | HTN was diagnosed in 28% of patients. Almost all women were in the 10% or less 10-year Framingham Risk Score category, but 20% of men were at least 10% or more. | |
| Nyabera, et al. 2011 [34] | Kenya | 5786 | 4629 | 1157 | P | Implemention of a project for integration of cardiovascular risk factors and disease evaluation and management into HIV care and treatment programs | High blood pressure was present in 19% of the HIV− and 32% of the HIV+ patients. Authors note cardiovascular disease can be identified early among HIV infected patietns through routine integrated activities. | |
| Schwartz, et al. 2011 [35] | Botswana | 179 | 179 | 0 | P,O | Description of cardiac abnormalities among patients with HIV | HIV infection was strongly associated with pericarditis and cardiomyopathy. 18% of HIV+ patients with hypertensive heart disease (20% in HIV−). | |
| Sani, et al. 2011 [36] | Nigeria | 200 | 200 | 0 | P,O | Descriptive study of cardiovascular risk factors in treated and treatment-naïve HIV+ patients | 17% prevalence of HTN in patients on HAART, 2% in HAART-naïve. Higher prevalence of dyslipidemia in treated vs. HAART-naïve patients. | |
| Adewole, et al. 2010 [37] | Nigeria | 174 | 174 (130 on ART) | 0 | P,O | Comparison of serum lipid profiles among HIV+ patients based on whether or not they were receiving HAART | HIV+ patients who were not treated with HAART had higher LDL and lower HDL levels compared to patients who did receive HAART. No relationship between hypertension and lipid parameters. | |
| CAD/MI/Atherosclerosis | Becker, et al. 2010 [47] | South Africa | 60 | 30 | 30 | P,O | Comparison of clinical and angiographic features of treatment-naïve HIV+ and HIV− patients with acute coronary syndrome | HIV+, treatment-naïve patients presenting with ACS were younger and were more likely to be smokers compared to HIV− patients. However, these HIV+ patients had fewer risk factors than the control group, including less hypertension, diabetes, hyperlipidemia, and other coronary risk factors. HIV+ patients had less atherosclerosis but higher degree of large thrombus burden. Stents were used to a similar degree in the HIV+ and HIV− groups. |
| Becker, et al. 2011 [48] | South Africa | 60 | 30 | 30 | P,O | Comparison of thrombotic profiles of treatment-naïve HIV+ patients and HIV− patients with acute coronary syndrome | Treatment-naïve HIV+ patients with ACS were younger with fewer traditional risk factors but a higher degree of thrombophilia compared to HIV− patients. | |
| Lazar, et al. 2009 [50] | Rwanda | 343 | 276 | 67 | P,O | Assessment of differences in arterial wave reflection, a marker of atherosclerosis, in HIV+ vs. HIV− women | HIV infection not associated with increased arterial wave reflection in women with little exposure to antiretroviral therapy and without cardiovascular risk factors. | |
| Stroke | Hoffmann, et al. 2000 [58] | South Africa | 1298 | 24 | NR | R, case-control study | Comparison of stroke characteristics of young, black, HIV+ patients in a stroke registry compared to historical HIV− controls | Large vessel cryptogenic stroke 2.5 times more common in HIV+ compared to HIV−. |
| Patel, et al. 2005 [59] | South Africa | 293 | 56 | 154 | R | Comparison of etiologies of stroke among young (<44 years) HIV+ and HIV− patients | No difference in stroke etiologies between young HIV+ and HIV− patients without AIDS. | |
| Tipping, et al. 2007 [60] | South Africa | 1087 | 67 | 1020 | P,O | Comparison of etiologies of stroke among HIV+ and HIV− patients | HIV+ young stroke patients did not demonstrate hypertension, diabetes, hyperlipidemia, or smoking as significant risk factors for ischemic stroke. Primary etiologies of ischemic stroke included infectious meningitis/ vasculitides, coagulopathy, cardioembolism. | |
| Heikinheimo, et al. 2012 [61] | Malawi | 147 | 50 | 97 | P,O | Comparison of outcomes after stroke among HIV+ and HIV− patients | Poor outcomes after stroke were related to stroke severity and female gender but not to presence of HIV infection. HIV+ patients were younger and did not have many of the common risk factors for stroke. HIV+ patients more often suffered from ischemic stroke than HIV− patients. | |
| OLD | Hnizdo, et al. 2000 [77] | South Africa | 1343 | 305 | 1038 | R | Examination of the chronic effect of initial and recurrent TB on lung function impairment with a subanalysis examining HIV+ vs. HIV− patients | TB infection caused chronic impairment of lunch function which increased incrementally with the number of episodes of TB and was not affected by HIV status. |
| Ramin, et al. 2008 [80] | Ethiopia | 153 | 43 | 110 | P,O | Comparison of the outcomes in patients with and without TB who smoked with a subanalysis examining HIV status | Cigarette smoking and HIV status were the 2 key risk factors for TB infection. | |
| PAH | Stewart, et al. 2011 [88] | South Africa | 697 (141 with PAH) | 43 | 98 | P clinical registry | Examination of the characteristics and pathways to right heart failure | PAH due to HIV in 33% of women and 23% of men. |
Abbreviations: LMIC, low- and middle-income country; HIV+, human immunodeficiency seropositive; HIV−, human immunodeficiency virus seronegative; HF, heart failure; PAH, pulmonary arterial hypertension; MI, myocardial infarction; P, prospective; O, observational; R, retrospective; HAART, highly active antiretroviral therapy; CDC, Centers for Disease Control; AIDS, acquired immune deficiency syndrome; NR, not reported; HTN, hypertension; LDL, low-density lipoprotein; HDL, high density lipoprotein; CAD, coronary artery disease; ACS, acute coronary syndrome; OLD, obstructive lung disease; TB, tuberculosis;
Observational or intervention studies conducted in LMICs that examined the relationship between HIV status or therapy and selected non-communicable cardiovascular and pulmonary diseases based on published data between 1996 and present
Heart failure
The link between HIV and heart failure in the ART era has been described in HICs in the last decade [5, 6]. A population-based, retrospective cohort study from the U.S. found that HIV infection was associated with a 1.8-fold risk of heart failure in HIV+ persons compared to age and ethnicity matched HIV− controls [7]. A meta-analysis of 11 studies of heart failure in HIV+ individuals showed a prevalence of 8.3% for systolic dysfunction and 43.4% for diastolic dysfunction [8]. In terms of potential pathophysiologic mechanisms, systolic dysfunction was significantly associated with chronic inflammation, tobacco smoking and history of myocardial infarction, while diastolic dysfunction was associated with hypertension and age in this meta-analysis [8].
Most of the LMIC data implicating HIV as a cause of heart failure were generated before the widespread availability of ART in LMICs and document an overall prevalence between 9 and 57%, which, in some instances, was higher than in the general population [9-13]. During this era, systolic heart failure was commonly reported in association with HIV, however diastolic dysfunction was documented in a large proportion (86%) of asymptomatic HIV+ patients [14]. HIV-associated cardiomyopathy occurs most often among young persons with low CD4 counts (usually <100), higher viral load, and advanced HIV stage [15, 16]. Possible causes include opportunistic infections, nutrient deficiency (e.g., selenium), HIV viremia, and auto-immunity [7, 16-18].
Studies in the ART era demonstrate that cardiomyopathy is still a relevant form of HIV-associated cardiovascular disease [19]. Between 5% and 29% of HIV+ individuals have cardiomyopathy depending on the methods and definitions used and populations studied (Table 1)[18, 20-22]. Systolic dysfunction remains the most commonly investigated form, however diastolic dysfunction is concurrent in 21-46% of HIV+ patients with cardiomyopathy depending on the clinical presentation [18]. The relationship between peripartum cardiomyopathy and HIV remains unclear [23, 24]. Whether heart failure occurs disproportionately among HIV+ persons in LMICs in the ART era is not yet known (owing to lack of information on rates of heart failure in the general or HIV− population), nor is how common diastolic function is in HIV+ patients as a function of degree of HIV disease control. Moreover, specific ART drug classes are associated with a higher prevalence of cardiomyopathy [25]. Further work is required to characterize the frequency and causes of heart failure and the role of ART in both HIV+ and HIV− LMIC populations.
Hypertension
A relationship between higher systolic blood pressure and duration of HIV has been reported in HICs. In the Multicenter AIDS Cohort, Seaberg et al. noted that the odds of developing systolic hypertension among men followed between 1984 and 2003 was greatest after 5 years or more of ART (OR 1.7, 95% CI 1.34-2.16) [26]. The effect is greatest for protease inhibitor (PI) and non-nucleoside reverse transcriptase inhibitor (NNRTI) based regimens, but it may also be mediated through metabolic derangements [27-29]. Hypertension and pre-hypertension are associated with increased risk of acute myocardial infarction in the U.S. Veterans Aging Cohort, which highlights the importance of treating this risk factor at the appropriate level in HIV+ patients [30]. Failure of blood pressure to normally decrease by >10% overnight is also more common in HIV+ individuals in HICs and is a risk factor for cardiovascular events [31].
The prevalence and severity of hypertension among HIV+ individuals in LMICs is only recently gaining attention. Studies from Kenya and Uganda describe hypertension prevalence rates between 11% and 28% among HIV+ individuals, and men are disproportionately affected [32, 33]. CD4 count is positively correlated with hypertension, especially among younger individuals [32]. Some studies suggest HIV+ individuals have a higher prevalence of hypertension than those who are HIV− [34], while others report lower blood pressure in HIV+ individuals or no difference in blood pressure between HIV+ and HIV− individuals (Table 1) [22, 35-37]. Importantly, many of these studies were not designed to compare blood pressure among patients, were focused on specific sub-groups, included individuals with and without AIDS, and often specifically excluded individuals with hypertension or other cardiac diseases. Kalyesubula et al. also describe the evidence linking HIV status, hypertension, and chronic kidney disease in SSA [38]. Clearly, there is a great need for prospective data to establish the relationship between hypertension, HIV infection, and ART in LMICs.
Coronary artery disease and myocardial infarction
In HICs, the linkage between HIV infection and coronary artery disease (CAD) has been established by observational data, prospective and retrospective registries, and pathology studies [1,2,39, 40]. In addition, surrogate endpoints related to atherosclerosis (e.g., endothelial dysfunction, carotid intima-media thickness, flow mediated dilatation) are also more common among HIV+ individuals compared to HIV− [41, 42]. The overall increase in risk for CAD/myocardial infarction (MI) in HIV+ persons is approximately 1-2 fold and is attenuated slightly by adjusting for tobacco use [2, 39,42]. Data suggest the use of protease inhibitors and recent/current abacavir use may increase MI risk by ~2-fold [43]; however, the risk with abacavir has not been noted in other cohorts [44]. The role of HIV infection as a factor in CAD was identified in observations of accelerated atherosclerosis in HIV+ individuals prior to the use of ART [45].
In general, CAD appears to be relatively uncommon among HIV+ individuals in LMICs, but this low frequency is possibly related to diagnostic limitations. A 2012 meta-analysis of 11 studies of acute coronary syndromes in HIV+ individuals included one study from an LMIC representing only 60 of the 2442 patients reported [46]. HIV+ individuals in this group were all ART-naïve, were younger than HIV− controls (43 vs. 54 years), had higher rates of smoking (73% vs. 33%), and lacked other traditional cardiovascular risk factors [47]. HIV+ individuals more commonly presented with large thrombus burden rather than having substantial atherosclerotic plaques, suggesting a unique pathophysiology that is incompletely understood but may be related to thrombophilia [47, 48]. Some antiretroviral drugs (PIs and NNRTIs) are known to adversely affect atherogenic lipid profiles and may thereby enhance the likelihood of developing CAD [49]. Future studies of HIV+ individuals at risk for CAD/MI should include investigation of a potentially unique CAD pathophysiology and the role of surrogate markers for CAD [50], in addition to describing the roles of age, ART use, and other potentially relevant HIV characteristics.
Stroke
Stroke is a leading cause of death and disability worldwide and is now common in LMICs [51, 52]. Compelling evidence linking HIV infection with stroke in the ART era comes from a large European-based retrospective study showing HIV+ individuals had an increased risk of stroke relative to the comparison cohorts (adjusted incidence rate ratio 1.60, 95% CI1.32-1.94)[53]. Ischemic stroke is the predominant type in HICs, and the age at presentation varies from 35-48 years, a substantially younger group than the HIV-stroke population [53, 54].
Proposed mechanisms for HIV-related stroke include HIV-associated vasculopathy, thrombophilia, cardioembolism, and opportunistic infection [55]. Suppression of HIV by ART may be incomplete, and ART does not eradicate the virus. As a result, ART may delay rather than extinguish HIV-associated factors such as ongoing inflammation and the pro-inflammatory state [56, 57]. As with CAD/MI, some types of ART may increase rates of dyslipidemia and accelerate atherosclerosis [49].
There are no data from LMICs prospectively assessing the role of HIV infection on stroke risk in the ART era. A single retrospective case-control study in South Africa showed a trend towards an increased risk of stroke in HIV+ individuals; however, another in the same location showed no association (Table 1) [58, 59]. Other studies have attempted to identify a link based on observations such as increased hospital admissions for young immunosuppressed HIV+ patients with ischemic stroke and without other obvious causes [60, 61]. Better data are essential to understanding the problem and its scope.
The phenotype of HIV+ individuals with stroke in LMICs appears to differ from those in HICs; in LMICs, this population is younger, there is no difference in prevalence by gender, and the comorbidity occurs most often among heterosexuals not receiving ART [55]. The difference is likely a consequence of the differing epidemiology of HIV infection in LMICs compared to HICs. The additional burden and interplay with HIV, hypertension, other vascular risk factors, and stroke risk in LMICs is yet to be fully explored.
Obstructive lung disease
Non-infectious pulmonary complications such as obstructive lung disease (OLD) have been reported in HIV+ populations starting in the pre-ART era [62]. The types of OLD that may occur with HIV include fixed airway obstruction, emphysema, diffusing capacity for carbon monoxide (DLco) impairment, chronic bronchitis, bronchial hyper-responsiveness, and asthma [63-66]. Obstructive lung disease is of particular interest in the current era because it is likely to increase as the HIV+ population ages.
Available data from HICs indicate that OLD occurs more frequently in HIV+ persons and that obstruction is worse and progresses more rapidly among persons with higher HIV viral levels. HIV infection is an independent predictor of DLco impairment [66-70]. Although smoking is strongly associated with OLD, it is not the only cause of OLD in HIV+ individuals, and lung function abnormalities may be present even in individuals who have never smoked [65]. The impact of ART on OLD is uncertain and may depend on underlying patient characteristics [65, 66, 71]. Asthma has also been reported to be highly prevalent in HIV+ individuals and may have different risk factors than in the HIV− population [64, 72]
HIV+ individuals in LMICs may have additional risk factors for OLD. Where mining is common, the associated exposures to dust (e.g., silica) may contribute to significant pulmonary morbidity [73]. Household air pollution (HAP) from burning solid fuels is another important risk factor for OLD, disproportionately affecting women and children [74, 75]. For example, the Burden of Obstructive Lung Disease (BOLD) study demonstrated that the highest prevalence of OLD worldwide was in South Africa (men 22%, women 17%)—a country that has a high HIV prevalence and uses a significant amount of biomass fuels [76]. Other factors such as infectious lung diseases (e.g., tuberculosis) may also contribute to greater risk of OLD [77]. Improved understanding of OLD in HIV+ LMIC populations requires better data on relationships between HIV infection, tuberculosis, tobacco smoking, environmental exposures, and overall pulmonary heath [78-80].
Pulmonary arterial hypertension
Pulmonary arterial hypertension (PAH) is one of the most severe complications of HIV infection, carrying high mortality [81]. Its prevalence in developed countries is low (<1%) [72, 82, 83]. HIV−associated PAH has a slight female predominance and an average age at presentation of 33 years [84]. Knowledge derived from studies in HICs suggests that PAH is more common in HIV+ persons compared to HIV−; the prevalence of HIV-associated PAH has not changed substantially following the introduction of ART; the severity of PAH does not correlate consistently with the degree of immunosuppression, and the impact of ART on the severity of PAH is uncertain [3, 22, 67, 81, 83, 85, 86]. Illicit drug use may potentiate the impact of HIV on pulmonary endothelial dysfunction and is associated with increased prevalence of PAH [86, 87].
Although data are limited, the prevalence of HIV-associated PAH may be higher in LMICs than in HICs. Two studies in the pre-ART era showed a prevalence of 5-6% in SSA among hospitalized patients [10, 11]. In the ART era, estimates range from 8-13% in HIV+ populations [18, 22]. The second most common form of PAH in South African women and men is PAH related to concurrent HIV infection (Table 1) [88]. Studies of the prevalence and severity of HIV-associated PAH from LMICs suffer from limitations of diagnostic tools, leading to inaccurate estimates of the frequency and magnitude of the problem [89, 90]. The considerable disparity in prevalence of illicit drug use in LMICs versus many HICs would seem to favor lower HIV−associated PAH rates in LMICs, the opposite of what appears to be the case. Thus, there is clearly a need to describe more completely the risk factors for HIV-associated PAH from HIV cohorts around the world.
FACTORS AFFECTING EXPRESSION OF NCDS IN LMICS
Environmental exposures in LMICs, such as tobacco smoking and HAP, are associated with increased risk for cardiopulmonary diseases [91], and it is possible that HAP might trigger pulmonary inflammation and/or other mechanisms increasing susceptibility of HIV+ individuals to pulmonary diseases [92]. For some diseases, such as CAD, the increasing trend seen in HICs is yet to be realized in LMICs. The difference in expression may result from low baseline incidence of coronary artery disease or dyslipidemia, differences in ART regimens, degree of HIV disease control, poor access to diagnostic tools or low awareness among clinicians, differences in rates of illicit drug use, comorbidities, genetic variation, tobacco use, and other cardiopulmonary risk factors, including lower average age [93].
KEY POPULATIONS AT HIGHER RISK
Children may be particularly susceptible to chronic NCDs, and vulnerability to NCDs may start in utero according to the “Developmental Origins of Health and Disease” hypothesis [94, 95]. Birth cohorts, while potentially logistically challenging in low resource settings, offer an opportunity to characterize the relationships between in utero exposures (such as maternal ART use), maternal health, and HIV [96]. Concerted efforts to bring together birth cohorts in LMICs are ongoing [97].
Sickle cell disease is not uncommon in LMICs and occurs in HIV endemic regions [98]. Stroke and PAH are especially prevalent in children and young adults with this disease [99, 100]. Although HIV may pose an additional burden to this vulnerable population, its interplay with sickle cell disease and NCDs is unknown.
Use of substances that increase risk for NCDs is greater among HIV+ individuals. Alcohol use accentuates the risk for hypertension, stroke, heart failure, and OLD [101-105], while tobacco smoking predisposes to a number of cardiopulmonary conditions (e.g., OLD, CAD) [106]. Methamphetamine use is associated with PAH [107]. While its use is generally low in LMICS, in countries where methamphetamine use is more prevalent, it is associated with HIV risk behaviors [108], and these patients may be at higher risk of developing PAH. Attention is needed to understand the burden of substance use among HIV+ individuals in LMICs and its effects on cardiopulmonary morbidity.
RESEARCH PRIORITIES AND CHALLENGES
Strategies to improve understanding of the relationship between HIV and non-communicable cardiovascular and pulmonary disorders are essential in order to maximize the impact of interventions and optimize resource allocation. Epidemiological, clinical, and mechanistic studies employing standard methods of disease definition, patient selection, and outcomes are needed (Table 2). Specific research priorities and examples of potential existing resources, research designs, and major challenges to implementation are discussed below and in Table 2.
Table 2.
Research priorities for non-communicable cardiovascular and pulmonary disease comorbidities in HIV in LMICs
| Recommendation | Expected outcome | Potential existing resources | Potential research design | Major challenges |
|---|---|---|---|---|
| A. Epidemiological Research | ||||
| 1. Analysis of existing and novel epidemiological data | a. Identification of NCDs where HIV or ART plays a role in presentation, expression or severity of disease | Internationally-funded HIV treatment programs in LMICs NHLBI Centers of Excellence (COE) Program (see text for reference) Global Alliance for Chronic Diseases (GACD) (see text for reference) IeDEA networka |
Longitudinal cohort studies | Lack of HIV- comparators |
| b. Identification of NCDs for which HIV-infected persons represent a significant portion of the affected population | Existing disease specific cohorts (e.g., Heart of Soweto, THESUS-HF, BOLD) (see text for references) COE program, GACD network |
Case-control studies identifying HIV- associations | HIV testing not a routine aspect of data collection | |
| 2. Incorporation of HIV and NCD data capture into existing population surveilance | a. Population-based estimates of disease comorbidity burden | National demographic surveillance systems INDEPTH networkb |
Population-based surveillance | Cost Surveillance infrastructre |
| B. Mechanistic Studies | ||||
| 1. Collaborations to elucidate pathways by which HIV and ART impact NCDs | a. Selection of disease targets for which HIV-linked interventions (e.g., timing of ART initiation) can be expected to have significant favorable impact | LMIC centers with access to infrastructure to perform such studies | Pathologic inspection of target organs or target organ tissues accessible ex vivo (e.g., bronchoalveolar cells) Animal studies |
Resources Infrastructure Access to tissues Appropriate animal models |
| C. Clinical Research | ||||
| 1. Health outcomes research | a. Optimized management of HIV/NCD comorbidities | IeDEA network Internationally-funded HIV treatment programs in LMICs(e.g. PEPFAR, Global Fund for AIDS, TB and Malaria) Monitoring and evaluation programs for HIV care |
Randomized (cluster) trials of treatment strategies to establish best practices for specific regions | Monitoring and evaluation Poor health information systems |
| b. Appropriate allocation of resources | (as above) | Embed cost-effectiveness studies into therapeutic and management strategy trials | (as above) | |
| 2. Close examination of the effect of ART on the condition | a. Ensuring the potential negative effects of ART on NCDs are minimized. | IeDEA network AIDS clinical trial group HIV/AIDS Clinical Trials Network (NIAID)(see text for reference) Birth cohorts in LMICs |
Randomized trials and observational studies of ART regimens and NCD outcomes with close attention to ART regimens, viral load, markers of immune activation and inflammation. | Cost Poor health information systems |
Abbreviations: NCD, non-communicable diseases, LMIC, low- and middle-income countries; ART, antiretroviral therapy; NHLBI, National Heart, Lung and Blood Institute; NIAID, National Institute of Allergy and Infectious Diseases
International Epidemiologic Databases to Evaluate AIDS, www.iedea.org
International Network of field sites for continuous Demographic Evaluation of Populations and Their Health in developing countries, www.indepthnetwork.org
Epidemiological research
1. Careful analysis of existing and novel epidemiologic data. The expected outcomes of these analyses are to identify those areas in which the presence of HIV infection clearly plays a role in the pathogenesis, expression, or severity of disease, and to identify diseases in which HIV+ persons are a significant proportion of the overall affected population. These efforts will also allow focus on diseases of relatively high prevalence so that successful interventions will impact a substantial population. One example of a research approach is leveraging the National Heart Lung and Blood Institute/UnitedHealth Group Global Centers of Excellence (COE) network in LMICs to pool existing data and compare the prevalence of hypertension in comparable HIV+ and HIV− cohorts.
2. Incorporate HIV and NCD data capture into existing population surveillance. The goal of these studies is to collect population-based estimates of the diseases’ comorbidity burden. Health and Demographic Surveillance Systems (HDSSs) offer an opportunity, for example, to identify the longitudinal association between the very long-term complications of ART and stroke risk [109]. HDSSs or birth cohorts also offer an opportunity to understand the comorbidity impact over the lifespan and plan meaningful interventions.
Mechanistic studies
1. Collaborations to elucidate pathways by which HIV and ART impact NCDs. Identification of disorders with high morbidity or mortality, or for which a plausible mechanism is postulated, should be a priority. Mechanistic studies will provide guidance for selection of disease targets for which HIV-linked interventions (e.g., initiation of ART) can be expected to have favorable impact (Figure). When possible, use of animal models or pilot studies in small numbers of patients should be pursued initially. HIV-associated PAH, owing to its great morbidity and lack of therapies, is one disorder for which identification of molecular targets for therapy could reap substantial benefits. The advances in the molecular causes of other cardiovascular diseases that have emanated from institutions in South Africa, for example, could theoretically be extended into the realm of HIV-associated PAH [110].
Figure. Determinants of Non-Communicable Cardiovascular and Pulmonary Diseases in HIV+ Individuals.
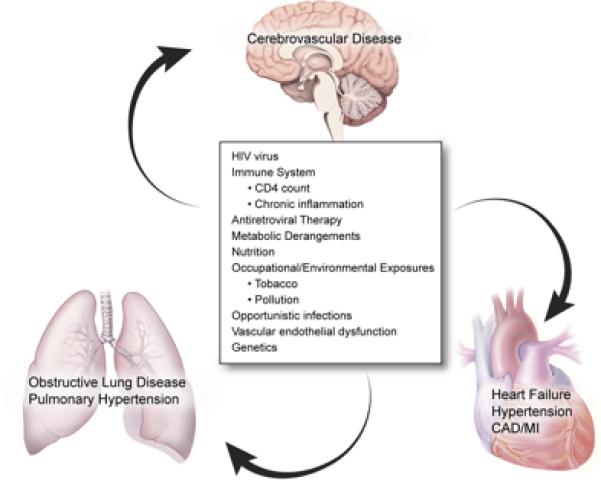
Illustration of the many potential factors related to chronic non-communicable cardiovascular and pulmonary diseases in HIV+ individuals. The HIV virus, antiretroviral therapy, host factors (immune system, metabolic derangements, nutrition, vascular endothelial dysfunction or genetics), occupational/environmental exposures and infections influence the development and progression of a variety of chronic cardiopulmonary conditions. Figure by NIH Medical Arts/Kibiuk.
Clinical research
1. Health outcomes research. Outcomes research offers an opportunity to optimize management of HIV/NCD comorbidities and inform appropriate allocation of resources. There are examples of ongoing monitoring and evaluation efforts in HIV treatment programs (e.g., www.iedea.org) that could be expanded to other disease states or linked with data from health information systems in LMICs. A variety of research designs are potentially feasible where the informatics capabilities exist. For example, establishing an accurate and timely diagnosis of heart failure is challenging owing to limitations in access to echocardiography. The extent to which different screening strategies (e.g., questionnaire- vs. laboratory- vs. imaging-based) in the general population are effective at reducing morbidity or mortality from HIV-associated heart failure is an unanswered question. Applying these strategies to the specifics of the region for which the intervention is intended will optimize the allocation of resources.
2. Close examination of the effect of ART on the condition. The effect of ART on different NCDs may be independent of the type of antiretroviral agent used or class specific. Within the AIDS Clinical Trial Group (ACTG), for example, measures of forced expiratory volume in 1 second or other spirometric data could be collected to understand and predict how OLD responds to ART exposure in LMICs. Clinical trial networks that are primarily investigating ART regimens could include electrocardiograms, carotid intima-media thickness, wall motion abnormalities on echocardiogram, or other surrogate measures of atherosclerosis as endpoints. It is also essential to consider drug-drug interactions as patients become increasingly exposed to HIV and NCD therapies simultaneously. Ensuring that the intervention proposed has a favorable effect without a concomitant potential negative effect needs to be accomplished when determining the way forward.
TRAINING
Even if the data gaps identified were to be addressed, the problems of a limited number of health care providers and inadequate access to health care facilities would remain. Solutions to these problems might require a restructuring of health systems and an investment in capacity building, both great challenges to LMICs [111]. Several models that address the needs of LMICs have already been developed and are being tested for effectiveness (e.g., “The PIH Guide to Chronic Care Integrations for Endemic Non-Communicable Diseases” [http://www.pih.org] and “Primary Care 101” [http://www.knowledgetranslation.co.za]). These efforts require training and supporting a cadre of health care providers with the tools and knowledge to work in those environments.
The new generation of NCD researchers is multidisciplinary, eager to work in global health, and uses social media platforms to discuss and seek opportunities for collaboration (e.g., http://ncdaction.org/). It is necessary to create opportunities for cross learning among disciplines, from fundamental discovery to clinical research and care, that bring together the expertise in HIV/AIDS and different NCDs. Platforms on HIV/AIDS, like the HIV/AIDS Clinical Trials Networks from the NIAID (http://www.niaid.nih.gov/about/organization/daids/networks/pages/daidsnetworks.aspx), and on chronic diseases, like the National Heart, Lung and Blood Institute/United Health Group Global Centers of Excellence [112], and the global research networks developed by the Global Alliance for Chronic Diseases (http://www.gacd.org/projects), are excellent infrastructures to train researchers and integrate research programs.
Conclusions
The landscape of cardiovascular and pulmonary diseases in HIV+ individuals in LMICs is dynamic. Factors that may impact the changing landscape include increasing access to ART, increasing life expectancy, more exposure to HIV viremia and ongoing inflammation, lifestyle pattern changes, and increasing awareness among practitioners and patients. A shift in the epidemiology of cardiovascular and pulmonary diseases has been seen in HICs, and data are slowly emerging from LMICs as well.
While the types of research and training needed are myriad and may differ in specifics, similarities exist (Figure). Carefully collected epidemiologic data comparing HIV+ to HIV− individuals in LMICs are critical to improved understanding. The answers to many important clinical questions will lead to improved outcomes for HIV+ patients. Where infrastructure and capabilities exist, researchers in LMICs may discover important mechanistic pathways and therapeutic targets. Only with thoughtful and meticulous collaborations will there be appreciation of the true burden of cardiovascular and pulmonary comorbidities in HIV+ persons living in LMICs and the development of sound strategies to optimally manage and prevent these chronic conditions as a way to improve the world’s health.
Acknowledgement
GSB is supported by the Fogarty International Center of the National Institutes of Health under award number K01TW008407. AM is supported by the National Heart, Lung and Blood Institute of the National Institutes of Health under award numbers R01HL090330 and P01HL103455. LAB is supported by the Wellcome Trust Foundation under grant 089672/Z/09/A. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding: Figure Office of AIDS Research, NIH
Footnotes
Conflicts of Interest: None
References
- 1.Currier JS, Taylor A, Boyd F, Dezii CM, Kawabata H, Burtcel B, et al. Coronary heart disease in HIV-infected individuals. J Acquir Immune Defic Syndr. 2003;33:506–512. doi: 10.1097/00126334-200308010-00012. [DOI] [PubMed] [Google Scholar]
- 2.Friis-Møller N, Reiss P, Sabin CA, Weber R, Monforte ADA, El-Sadr W, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 3.Morris A, Gingo MR, George MP, Lucht L, Kessinger C, Singh V, et al. Cardiopulmonary function in individuals with HIV infection in the antiretroviral therapy era. AIDS (London, England) 2012 doi: 10.1097/QAD.0b013e32835099ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joint United Nations Programme on HIV/AIDS (UNAIDS) Global Report on the Gobal AIDS Epidemic. Geneva, Switzerland: 2012. pp. 1–110. [Google Scholar]
- 5.Lorgis L, Cottenet J, Molins G, Benzenine E, Zeller M, Aube H, et al. Outcomes after acute myocardial infarction in HIV-infected patients: analysis of data from a French nationwide hospital medical information database. Circulation. 2013;127:1767–1774. doi: 10.1161/CIRCULATIONAHA.113.001874. [DOI] [PubMed] [Google Scholar]
- 6.Hsue PY, Hunt PW, Ho JE, Farah HH, Schnell A, Hoh R, et al. Impact of HIV infection on diastolic function and left ventricular mass. Circulation Heart Fail. 2010;3:132–139. doi: 10.1161/CIRCHEARTFAILURE.109.854943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butt AA, Chang CC, Kuller L, Goetz MB, Leaf D, Rimland D, et al. Risk of heart failure with human immunodeficiency virus in the absence of prior diagnosis of coronary heart disease. Arch Intern Med. 2011;171:737–743. doi: 10.1001/archinternmed.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cerrato E, D'Ascenzo F, Biondi-Zoccai G, Calcagno A, Frea S, Grosso Marra W, et al. Cardiac dysfunction in pauci symptomatic human immunodeficiency virus patients: a meta-analysis in the highly active antiretroviral therapy era. Eur Heart J. 2013;34:1432–1436. doi: 10.1093/eurheartj/ehs471. [DOI] [PubMed] [Google Scholar]
- 9.Ntsekhe M, Hakim J. Impact of human immunodeficiency virus infection on cardiovascular disease in Africa. Circulation. 2005;112:3602–3607. doi: 10.1161/CIRCULATIONAHA.105.549220. [DOI] [PubMed] [Google Scholar]
- 10.Hakim JG, Matenga JA, Siziya S. Myocardial dysfunction in human immunodeficiency virus infection: an echocardiographic study of 157 patients in hospital in Zimbabwe. Heart. 1996;76:161–165. doi: 10.1136/hrt.76.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niakara A, Drabo YJ, Kambire Y, Nebie LV, Kabore NJ, Simon F. [Cardiovascular diseases and HIV infection: study of 79 cases at the National Hospital of Ouagadougou (Burkina Faso)]. Bull Soc Pathol Exot. 2002;95:23–26. [PubMed] [Google Scholar]
- 12.Longo-Mbenza B, Tonduangu K, Kintonki Vita E, Seghers KV. [The effect of HIV infection on high incidence of heart diseases in Kinshasa (Zaire). Echocardiographic study]. Ann Cardiol Angeiol (Paris) 1997;46:81–87. [PubMed] [Google Scholar]
- 13.Longo-Mbenza B, Seghers KV, Phuati M, Bikangi FN, Mubagwa K. Heart involvement and HIV infection in African patients: determinants of survival. Int J Cardiol. 1998;64:63–73. doi: 10.1016/s0167-5273(97)00321-5. [DOI] [PubMed] [Google Scholar]
- 14.Longo-Mbenza B, Seghers LV, Vita EK, Tonduangu K, Bayekula M. Assessment of ventricular diastolic function in AIDS patients from Congo: a Doppler echocardiographic study. Heart. 1998;80:184–189. doi: 10.1136/hrt.80.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nzuobontane D, Blackett KN, Kuaban C. Cardiac involvement in HIV infected people in Yaounde, Cameroon. Postgrad Med J. 2002;78:678–681. doi: 10.1136/pmj.78.925.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Twagirumukiza M, Nkeramihigo E, Seminega B, Gasakure E, Boccara F, Barbaro G. Prevalence of dilated cardiomyopathy in HIV-infected African patients not receiving HAART: a multicenter, observational, prospective, cohort study in Rwanda. Curr HIV Res. 2007;5:129–137. doi: 10.2174/157016207779316288. [DOI] [PubMed] [Google Scholar]
- 17.Currie PF, Goldman JH, Caforio AL, Jacob AJ, Baig MK, Brettle RP, et al. Cardiac autoimmunity in HIV related heart muscle disease. Heart. 1998;79:599–604. doi: 10.1136/hrt.79.6.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sliwa K, Carrington MJ, Becker A, Thienemann F, Ntsekhe M, Stewart S. Contribution of the human immunodeficiency virus/acquired immunodeficiency syndrome epidemic to de novo presentations of heart disease in the Heart of Soweto Study cohort. Eur Heart J. 2012;33:866–874. doi: 10.1093/eurheartj/ehr398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Syed FF, Sani MU. Recent advances in HIV-associated cardiovascular diseases in Africa. Heart. 2013;99:1146–1153. doi: 10.1136/heartjnl-2012-303177. [DOI] [PubMed] [Google Scholar]
- 20.Damasceno A, Mayosi BM, Sani M, Ogah OS, Mondo C, Ojji D, et al. The Causes, Treatment, and Outcome of Acute Heart Failure in 1006 Africans From 9 Countries: Results of the Sub-Saharan Africa Survey of Heart Failure. Arch Intern Med. 2012:1–9. doi: 10.1001/archinternmed.2012.3310. [DOI] [PubMed] [Google Scholar]
- 21.Tantchou Tchoumi JC, Ambassa JC, Kingue S, Giamberti A, Cirri S, Frigiola A, et al. Occurrence, aetiology and challenges in the management of congestive heart failure in sub-Saharan Africa: experience of the Cardiac Centre in Shisong, Cameroon. Pan Afr Med J. 2011;8:11. doi: 10.4314/pamj.v8i1.71059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chillo P, Bakari M, Lwakatare J. Echocardiographic diagnoses in HIV-infected patients presenting with cardiac symptoms at Muhimbili National Hospital in Dar es Salaam, Tanzania. Cardiovasc J Afr. 2012;23:90–97. doi: 10.5830/CVJA-2011-060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Longenecker CT, Mondo C, Le VV, Jensen TP, Foster E. HIV infection is not associated with echocardiographic signs of cardiomyopathy or pulmonary hypertension among pregnant Ugandan women. Int J Cardiol. 2011;147:300–302. doi: 10.1016/j.ijcard.2010.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sliwa K, Forster O, Tibazarwa K, Libhaber E, Becker A, Yip A, et al. Long-term outcome of peripartum cardiomyopathy in a population with high seropositivity for human immunodeficiency virus. Int J Cardiol. 2011;147:202–208. doi: 10.1016/j.ijcard.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 25.Tanuma J, Ishizaki A, Gatanaga H, Kikuchi Y, Kimura S, Hiroe M, et al. Dilated cardiomyopathy in an adult human immunodeficiency virus type 1-positive patient treated with a zidovudine-containing antiretroviral regimen. Clin Infect Dis. 2003;37:e109–e111. doi: 10.1086/377609. [DOI] [PubMed] [Google Scholar]
- 26.Seaberg EC, Muñoz A, Lu M, Detels R, Margolick JB, Riddler SA, et al. Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. AIDS. 2005;19:953–960. doi: 10.1097/01.aids.0000171410.76607.f8. [DOI] [PubMed] [Google Scholar]
- 27.Palacios R, Santos J, García A, Castells E, González M, Ruiz J, et al. Impact of highly active antiretroviral therapy on blood pressure in HIV-infected patients. A prospective study in a cohort of naive patients. HIV Medicine. 2006;7:10–15. doi: 10.1111/j.1468-1293.2005.00333.x. [DOI] [PubMed] [Google Scholar]
- 28.Friis-Møller N, Weber R, Reiss P, Thiébaut R, Kirk O, d'Arminio Monforte A, et al. Cardiovascular disease risk factors in HIV patients--association with antiretroviral therapy. Results from the DAD study. AIDS. 2003;17:1179–1193. doi: 10.1097/01.aids.0000060358.78202.c1. [DOI] [PubMed] [Google Scholar]
- 29.Bergersen BM, Sandvik L, Dunlop O, Birkeland K, Bruun JN. Prevalence of hypertension in HIV-positive patients on highly active retroviral therapy (HAART) compared with HAART-naïve and HIV-negative controls: results from a Norwegian study of 721 patients. Eur J Clin Microbiol Infect Dis. 2003;22:731–736. doi: 10.1007/s10096-003-1034-z. [DOI] [PubMed] [Google Scholar]
- 30.Armah KA, Chang CCH, Baker JV, Ramachandran VS, Budoff MJ, Crane HM, et al. Prehypertension, hypertension, and the risk of acute myocardial infarction in HIV-infected and -uninfected veterans. Clin Infect Dis. 2014;58:121–129. doi: 10.1093/cid/cit652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Socio GV, Bonfanti P, Martinelli C, Ricci E, Pucci G, Marinoni M, et al. Negative influence of HIV infection on day-night blood pressure variability. J Acquir Immune Defic Syndr. 2010;55:356–360. doi: 10.1097/QAI.0b013e3181e46456. [DOI] [PubMed] [Google Scholar]
- 32.Bloomfield GS, Hogan JW, Keter A, Sang E, Carter EJ, Velazquez EJ, et al. Hypertension and Obesity as Cardiovascular Risk Factors among HIV Seropositive Patients in Western Kenya. PLoS ONE. 2011;6:e22288. doi: 10.1371/journal.pone.0022288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mateen FJ, Kanters S, Kalyesubula R, Mukasa B, Kawuma E, Kengne AP, et al. Hypertension prevalence and Framingham risk score stratification in a large HIV-positive cohort in Uganda. J Hypertens. 2013;31:1372–1378. doi: 10.1097/HJH.0b013e328360de1c. discussion 1378. [DOI] [PubMed] [Google Scholar]
- 34.Nyabera R, Yonga G, Mwangemi F, Bukachi F. Evaluation of a project integrating cardiovascular care into HIV programmes. Cardiovascular Journal of Africa. 2011;22:S17. [Google Scholar]
- 35.Schwartz T, Girgis M, Steen TW, Sjaasatd I. HIV as a risk factor for cardiac disease in Botswana: A cross-sectional study. Cardiovascular Journal of Africa. 2011;22:S23. doi: 10.1016/j.inhe.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 36.Sani MU, Muhammad S, Okeahialam BN. Effects of HAART on cardiovascular risk profile of HIV/AIDS patients in Aminu Kano Teaching Hospital, Kano, Nigeria. Cardiovascular Journal of Africa. 2011;22:S22. [Google Scholar]
- 37.Adewole OO, Eze S, Betiku Y, Anteyi E, Wada I, Ajuwon Z, et al. Lipid profile in HIV/AIDS patients in Nigeria. Afr Health Sci. 2010;10:144–149. [PMC free article] [PubMed] [Google Scholar]
- 38.Kalyesubula R, et al. JAIDS Supplementary Issue. 2014 [Google Scholar]
- 39.Freiberg MS, Chang CCH, Kuller LH, Skanderson M, Lowy E, Kraemer KL, et al. HIV Infection and the Risk of Acute Myocardial Infarction. JAMA Int Med. 2013;173:614–622. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morgello S, Mahboob R, Yakoushina T, Khan S, Hague K. Autopsy findings in a human immunodeficiency virus-infected population over 2 decades: influences of gender, ethnicity, risk factors, and time. Arch Pathol Lab Med. 2002;126:182–190. doi: 10.5858/2002-126-0182-AFIAHI. [DOI] [PubMed] [Google Scholar]
- 41.Ho JE, Hsue PY. Cardiovascular manifestations of HIV infection. Heart. 2009;95:1193–1202. doi: 10.1136/hrt.2008.161463. [DOI] [PubMed] [Google Scholar]
- 42.Hakeem A, Bhatti S, Cilingiroglu M. The spectrum of atherosclerotic coronary artery disease in HIV patients. Curr Atheroscler Rep. 2010;12:119–124. doi: 10.1007/s11883-010-0089-4. [DOI] [PubMed] [Google Scholar]
- 43.Bavinger C, Bendavid E, Niehaus K, Olshen RA, Olkin I, Sundaram V, et al. Risk of Cardiovascular Disease from Antiretroviral Therapy for HIV: A Systematic Review. PLoS One. 2013;8:e59551. doi: 10.1371/journal.pone.0059551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ding X, Andraca-Carrera E, Cooper C, Miele P, Kornegay C, Soukup M, et al. No association of abacavir use with myocardial infarction: findings of an FDA meta-analysis. J Acquir Immune Defic Syndr. 2012;61:441–447. doi: 10.1097/QAI.0b013e31826f993c. [DOI] [PubMed] [Google Scholar]
- 45.Paton P, Tabib A, Loire R, Tete R. Coronary artery lesions and human immunodeficiency virus infection. Res Virol. 1993;144:225–231. doi: 10.1016/s0923-2516(06)80033-6. [DOI] [PubMed] [Google Scholar]
- 46.D'Ascenzo F, Cerrato E, Biondi-Zoccai G, Moretti C, Omedè P, Sciuto F, et al. Acute coronary syndromes in human immunodeficiency virus patients: a meta-analysis investigating adverse event rates and the role of antiretroviral therapy. Eur Heart J. 2012;33:875–880. doi: 10.1093/eurheartj/ehr456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Becker AC, Sliwa K, Stewart S, Libhaber E, Essop AR, Zambakides CA, et al. Acute coronary syndromes in treatment-naïve black South africans with human immunodeficiency virus infection. Journal Interv Cardiol. 2010;23:70–77. doi: 10.1111/j.1540-8183.2009.00520.x. [DOI] [PubMed] [Google Scholar]
- 48.Becker AC, Jacobson B, Singh S, Sliwa K, Stewart S, Libhaber E, et al. The thrombotic profile of treatment-naive HIV-positive Black South Africans with acute coronary syndromes. Clin Appl Thromb Hemost. 2011;17:264–272. doi: 10.1177/1076029609358883. [DOI] [PubMed] [Google Scholar]
- 49.Ali MK, et al. JAIDS Supplementary Issue. 2014 [Google Scholar]
- 50.Lazar JM, Wu X, Shi Q, Kagame A, Cohen M, Binagwaho A, et al. Arterial wave reflection in HIV-infected and HIV-uninfected Rwandan women. AIDS Res Hum Retroviruses. 2009;25:877–882. doi: 10.1089/aid.2008.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012. 380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 53.Rasmussen LD, Engsig FN, Christensen H, Gerstoft J, Kronborg G, Pedersen C, et al. Risk of cerebrovascular events in persons with and without HIV: a Danish nationwide population-based cohort study. AIDS. 2011;25:1637–1646. doi: 10.1097/QAD.0b013e3283493fb0. [DOI] [PubMed] [Google Scholar]
- 54.Ovbiagele B, Nath A. Increasing incidence of ischemic stroke in patients with HIV infection. Neurology. 2011;76:444–450. doi: 10.1212/WNL.0b013e31820a0cfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Benjamin LA, Bryer A, Emsley HC, Khoo S, Solomon T, Connor MD. HIV infection and stroke: current perspectives and future directions. Lancet Neurol. 2012;11:878–890. doi: 10.1016/S1474-4422(12)70205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Corral I, Quereda C, Moreno A, Pérez-Elías MJ, Dronda F, Casado JL, et al. Cerebrovascular ischemic events in HIV-1-infected patients receiving highly active antiretroviral therapy: incidence and risk factors. Cerebrovasc Dis. 2009;27:559–563. doi: 10.1159/000214219. [DOI] [PubMed] [Google Scholar]
- 57.Lo J, Plutzky J. The biology of atherosclerosis: general paradigms and distinct pathogenic mechanisms among HIV-infected patients. J Infect Dis. 2012;205(Suppl 3):S368–374. doi: 10.1093/infdis/jis201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoffmann M, Berger JR, Nath A, Rayens M. Cerebrovascular disease in young, HIV-infected, black Africans in the KwaZulu Natal province of South Africa. J Neurovirol. 2000;6:229–236. doi: 10.3109/13550280009015825. [DOI] [PubMed] [Google Scholar]
- 59.Patel VB, Sacoor Z, Francis P, Bill PL, Bhigjee AI, Connolly C. Ischemic stroke in young HIV-positive patients in Kwazulu-Natal, South Africa. Neurology. 2005;65:759–761. doi: 10.1212/01.wnl.0000174434.00402.b5. [DOI] [PubMed] [Google Scholar]
- 60.Tipping B, de Villiers L, Wainwright H, Candy S, Bryer A. Stroke in patients with human immunodeficiency virus infection. J Neurol Neurosurg Psychiatry. 2007;78:1320–1324. doi: 10.1136/jnnp.2007.116103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heikinheimo T, Chimbayo D, Kumwenda JJ, Kampondeni S, Allain TJ. Stroke outcomes in Malawi, a country with high prevalence of HIV: a prospective follow-up study. PLoS One. 2012;7:e33765. doi: 10.1371/journal.pone.0033765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murray JF, Felton CP, Garay SM, Gottlieb MS, Hopewell PC, Stover DE, et al. Pulmonary complications of the acquired immunodeficiency syndrome. Report of a National Heart, Lung, and Blood Institute workshop. N Engl J Med. 1984;310:1682–1688. doi: 10.1056/NEJM198406213102529. [DOI] [PubMed] [Google Scholar]
- 63.Diaz PT, Wewers MD, Pacht E, Drake J, Nagaraja HN, Clanton TL. Respiratory symptoms among HIV-seropositive individuals. Chest. 2003;123:1977–1982. doi: 10.1378/chest.123.6.1977. [DOI] [PubMed] [Google Scholar]
- 64.Gingo MR, Wenzel SE, Steele C, Kessinger CJ, Lucht L, Lawther T, et al. Asthma diagnosis and airway bronchodilator response in HIV-infected patients. J Allergy Clin Immunol. 2012;129:708–714. e708. doi: 10.1016/j.jaci.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gingo MR, George MP, Kessinger CJ, Lucht L, Rissler B, Weinman R, et al. Pulmonary function abnormalities in HIV-infected patients during the current antiretroviral therapy era. Am J Respir Crit Care Med. 2010;182:790–796. doi: 10.1164/rccm.200912-1858OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Drummond MB, Kirk GD, Astemborski J, Marshall MM, Mehta SH, McDyer JF, et al. Association between obstructive lung disease and markers of HIV infection in a high-risk cohort. Thorax. 2012;67:309–314. doi: 10.1136/thoraxjnl-2011-200702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crothers K, Huang L, Goulet JL, Goetz MB, Brown ST, Rodriguez-Barradas MC, et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med. 2011;183:388–395. doi: 10.1164/rccm.201006-0836OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Drummond MB, Merlo CA, Astemborski J, Marshall MM, Kisalu A, McDyer JF, et al. The effect of HIV infection on longitudinal lung function decline among injection drug users: a prospective cohort. AIDS. 2013 doi: 10.1097/QAD.0b013e32835e395d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Crothers K, McGinnis K, Kleerup E, Wongtrakool C, Soo Hoo G, Kim J, et al. HIV infection is associated with reduced pulmonary diffusing capacity. J Acquir Immune Defic Syndr. 2013;64:271–278. doi: 10.1097/QAI.0b013e3182a9215a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fitzpatrick M, Gingo M, Kessinger C, Lucht L, Kleerup E, Greenblatt R, et al. HIV infection is associated with diffusing capacity impairment in women. J Acquir Immune Defic Syndr. 2013;64:284–288. doi: 10.1097/QAI.0b013e3182a9213a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.George MP, Kannass M, Huang L, Sciurba FC, Morris A. Respiratory symptoms and airway obstruction in HIV-infected subjects in the HAART era. PLoS One. 2009;4:e6328. doi: 10.1371/journal.pone.0006328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gingo MR, Morris A. Pathogenesis of HIV and the lung. Curr HIV/AIDS Rep. 2013;10:42–50. doi: 10.1007/s11904-012-0140-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Murray J, Davies T, Rees D. Occupational lung disease in the South African mining industry: research and policy implementation. J Public Health Policy. 2011;32(Suppl 1):S65–79. doi: 10.1057/jphp.2011.25. [DOI] [PubMed] [Google Scholar]
- 74.Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374:733–743. doi: 10.1016/S0140-6736(09)61303-9. [DOI] [PubMed] [Google Scholar]
- 75.Lamprecht B, McBurnie MA, Vollmer WM, Gudmundsson G, Welte T, Nizankowska-Mogilnicka E, et al. COPD in never smokers: results from the population-based burden of obstructive lung disease study. Chest. 2011;139:752–763. doi: 10.1378/chest.10-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370:741–750. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 77.Hnizdo E, Singh T, Churchyard G. Chronic pulmonary function impairment caused by initial and recurrent pulmonary tuberculosis following treatment. Thorax. 2000;55:32–38. doi: 10.1136/thorax.55.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bloomfield GS, Lagat DK, Akwanalo OC, Carter EJ, Lugogo N, Vedanthan R, et al. Conditions that predispose to pulmonary hypertension and right heart failure in persons exposed to household air pollution in LMIC. Glob Heart. 2012;7:249–259. doi: 10.1016/j.gheart.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Zyl Smit RN, Pai M, Yew WW, Leung CC, Zumla A, Bateman ED, et al. Global lung health: the colliding epidemics of tuberculosis, tobacco smoking, HIV and COPD. Eur Respir J. 2010;35:27–33. doi: 10.1183/09031936.00072909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ramin B, Kam D, Feleke B, Jacob B, Jha P. Smoking, HIV and non-fatal tuberculosis in an urban African population. Int J Tuberc Lung Dis. 2008;12:695–697. [PubMed] [Google Scholar]
- 81.Nunes H, Humbert M, Sitbon O, Morse JH, Deng Z, Knowles JA, et al. Prognostic factors for survival in human immunodeficiency virus-associated pulmonary arterial hypertension. Am J Respir Crit Care Med. 2003;167:1433–1439. doi: 10.1164/rccm.200204-330OC. [DOI] [PubMed] [Google Scholar]
- 82.Sitbon O, Lascoux-Combe C, Delfraissy JF, Yeni PG, Raffi F, De Zuttere D, et al. Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med. 2008;177:108–113. doi: 10.1164/rccm.200704-541OC. [DOI] [PubMed] [Google Scholar]
- 83.Morris A, Crothers K, Beck JM, Huang L, Disease ATSCoHP An official ATS workshop report: Emerging issues and current controversies in HIV-associated pulmonary diseases. Proc Am Thorac Soc. 2011;8:17–26. doi: 10.1513/pats.2009-047WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reinsch N, Buhr C, Krings P, Kaelsch H, Kahlert P, Konorza T, et al. Effect of gender and highly active antiretroviral therapy on HIV-related pulmonary arterial hypertension: results of the HIV-HEART Study. HIV Med. 2008;9:550–556. doi: 10.1111/j.1468-1293.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 85.Zuber J-P, Calmy A, Evison JM, Hasse B, Schiffer V, Wagels T, et al. Pulmonary arterial hypertension related to HIV infection: improved hemodynamics and survival associated with antiretroviral therapy. Clin Infect Dis. 2004;38:1178–1185. doi: 10.1086/383037. [DOI] [PubMed] [Google Scholar]
- 86.Opravil M, Sereni D. Natural history of HIV-associated pulmonary arterial hypertension: trends in the HAART era. AIDS. 2008;22(Suppl 3):S35–40. doi: 10.1097/01.aids.0000327514.60879.47. [DOI] [PubMed] [Google Scholar]
- 87.George MP, Champion HC, Gladwin MT, Norris KA, Morris A. Injection drug use as a “second hit” in the pathogenesis of HIV-associated pulmonary hypertension. Am J Respir Crit Care Med. 2012;185:1144–1146. doi: 10.1164/rccm.201204-0609ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stewart S, Mocumbi AO, Carrington MJ, Pretorius S, Burton R, Sliwa K. A not-so-rare form of heart failure in urban black Africans: pathways to right heart failure in the Heart of Soweto Study cohort. Eur J Heart Fail. 2011;13:1070–1077. doi: 10.1093/eurjhf/hfr108. [DOI] [PubMed] [Google Scholar]
- 89.Fisher MR, Forfia PR, Chamera E, Housten-Harris T, Champion HC, Girgis RE, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179:615–621. doi: 10.1164/rccm.200811-1691OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Selby VN, Scherzer R, Barnett CF, MacGregor JS, Morelli J, Donovan C, et al. Doppler echocardiography does not accurately estimate pulmonary artery systolic pressure in HIV-infected patients. AIDS. 2012;26:1967–1969. doi: 10.1097/QAD.0b013e3283579653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fullerton DG, Bruce N, Gordon SB. Indoor air pollution from biomass fuel smoke is a major health concern in the developing world. Trans R Soc Trop Med Hyg. 2008;102:843–851. doi: 10.1016/j.trstmh.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lagat DK, DeLong AK, Wellenius GA, Carter EJ, Bloomfield GS, Velazquez EJ, et al. Factors Associated with Isolated Right Heart Failure (IRHF) in Women of Western Kenya: A Pilot Study. Glob Heart. 2014 doi: 10.1016/j.gheart.2014.04.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ntsekhe M, Mayosi BM. Cardiac manifestations of HIV infection: an African perspective. Nature clinical practice Cardiovascular medicine. 2009;6:120–127. doi: 10.1038/ncpcardio1437. [DOI] [PubMed] [Google Scholar]
- 94.Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341:938–941. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- 95.Harding R, Maritz G. Maternal and fetal origins of lung disease in adulthood. Semin Fetal Neonatal Med. 2012;17:67–72. doi: 10.1016/j.siny.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 96.Lipshultz SE, Shearer WT, Thompson B, Rich KC, Cheng I, Orav EJ, et al. Cardiac effects of antiretroviral therapy in HIV-negative infants born to HIV-positive mothers: NHLBI CHAART-1 (National Heart, Lung, and Blood Institute Cardiovascular Status of HAART Therapy in HIV-Exposed Infants and Children cohort study). J Am Coll Cardiol. 2011;57:76–85. doi: 10.1016/j.jacc.2010.08.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Richter LM, Victora CG, Hallal PC, Adair LS, Bhargava SK, Fall CH, et al. Cohort profile: the consortium of health-orientated research in transitioning societies. Int J Epidemiol. 2012;41:621–626. doi: 10.1093/ije/dyq251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Piel FB, Patil AP, Howes RE, Nyangiri OA, Gething PW, Dewi M, et al. Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model-based map and population estimates. Lancet. 2013;381:142–151. doi: 10.1016/S0140-6736(12)61229-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Aliyu ZY, Kato GJ, Taylor J, Babadoko A, Mamman AI, Gordeuk VR, et al. Sickle cell disease and pulmonary hypertension in Africa: a global perspective and review of epidemiology, pathophysiology, and management. Am J Hematol. 2008;83:63–70. doi: 10.1002/ajh.21057. [DOI] [PubMed] [Google Scholar]
- 100.Verduzco LA, Nathan DG. Sickle cell disease and stroke. Blood. 2009;114:5117–5125. doi: 10.1182/blood-2009-05-220921. [DOI] [PubMed] [Google Scholar]
- 101.Fisher JC, Bang H, Kapiga SH. The association between HIV infection and alcohol use: a systematic review and meta-analysis of African studies. Sex Transm Dis. 2007;34:856–863. doi: 10.1097/OLQ.0b013e318067b4fd. [DOI] [PubMed] [Google Scholar]
- 102.Bloomfield GS, Barasa FA, Doll JA, Velazquez EJ. Heart Failure in Sub-Saharan Africa. Curr Cardiol Rev. 2013;9:157–173. doi: 10.2174/1573403X11309020008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thorogood M, Connor MD, Lewando-Hundt G, Tollman S, Ngoma B, Team SP. Secondary prevention of stroke--results from the Southern Africa Stroke Prevention Initiative (SASPI) study. Bull World Health Organ. 2004;82:503–508. [PMC free article] [PubMed] [Google Scholar]
- 104.Vogel RA. Requested Editorial: Binge Drinking and Vascular Function: A Sober Look at the Data. J Am Coll Cardiol. 2013;62:208–209. doi: 10.1016/j.jacc.2013.03.047. [DOI] [PubMed] [Google Scholar]
- 105.Sisson JH, Stoner JA, Romberger DJ, Spurzem JR, Wyatt TA, Owens-Ream J, et al. Alcohol intake is associated with altered pulmonary function. Alcohol. 2005;36:19–30. doi: 10.1016/j.alcohol.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 106.Lifson AR, Neuhaus J, Arribas JR, van den Berg-Wolf M, Labriola AM, Read TR, et al. Smoking-related health risks among persons with HIV in the Strategies for Management of Antiretroviral Therapy clinical trial. Am J Public Health. 2010;100:1896–1903. doi: 10.2105/AJPH.2009.188664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chin KM, Channick RN, Rubin LJ. Is methamphetamine use associated with idiopathic pulmonary arterieal hypertension? Chest. 2006;130:1657–1663. doi: 10.1378/chest.130.6.1657. [DOI] [PubMed] [Google Scholar]
- 108.Meade CS, Watt MH, Sikkema KJ, Ranby KW, Skinner D, Pieterse D, et al. Methamphetamine use is associated with childhood sexual abuse and HIV sexual risk behaviors among patrons of alcohol-servine venues in Cape Town, South Africa. Drug Alcohol Depend. 2012;126:232, 239. doi: 10.1016/j.drugalcdep.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Phillips-Howard PA, Odhiambo FO, Hamel M, Adazu K, Ackers M, van Eijk AM, et al. Mortality trends from 2003 to 2009 among adolescents and young adults in rural Western Kenya using a health and demographic surveillance system. PLoS One. 2012;7:e47017. doi: 10.1371/journal.pone.0047017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Walenta K, Schwarz V, Schirmer SH, Kindermann I, Friedrich EB, Solomayer EF, et al. Circulating microparticles as indicators of peripartum cardiomyopathy. Eur Heart J. 2012;33:1469–1479. doi: 10.1093/eurheartj/ehr485. [DOI] [PubMed] [Google Scholar]
- 111.Ali MK, Rabadán-Diehl C, Flanigan J, Blanchard C, Narayan KM, Engelgau M. Systems and capacity to address noncommunicable diseases in low- and middle- income countries. Sci Transl Med. 2013;5:181cm184. doi: 10.1126/scitranslmed.3005121. [DOI] [PubMed] [Google Scholar]
- 112.UnitedHealth Group/National Heart Lung, and Blood Institute Centres of Excellence A global research network for non-communicable diseases. Lancet. 2013:S0140–6736. 61808–5. [Google Scholar]


