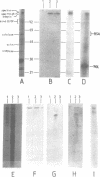
Abstract
The major surface protein MSP-1 of Plasmodium falciparum blood-stage malaria parasites contains notably conserved sequence blocks with unknown function. The recombinant protein 190L, which represents such a block, exhibits a high affinity for red blood cell membranes. We demonstrate that both 190L and native MSP-1 protein bind to the inner red blood cell membrane skeleton protein spectrin. By using overlapping peptides covering the 190L molecule, we show that the spectrin contact site of 190L is included in a linear sequence of 30 amino acid residues. Association of 190L with naturally occurring spectrin deficient red blood cells is drastically reduced. In the same cells parasite invasion is normal, but the intracellular parasite development arrests late in the trophozoite stage. A similar situation arises when synthetic peptides covering the spectrin recognition sequence of 190L are added to P.falciparum cultures. These data and the cellular localization of MSP-1 suggest the possibility that MSP-1 associates with spectrin under natural conditions.
Full text
PDF
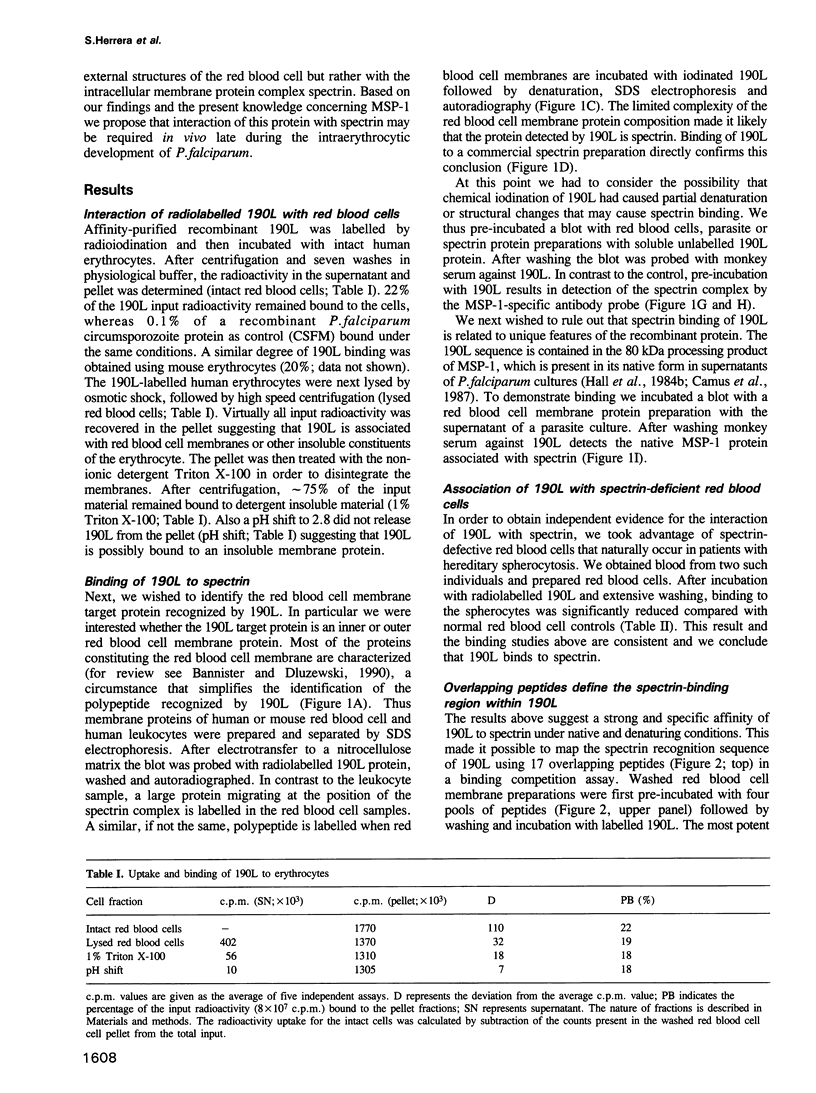

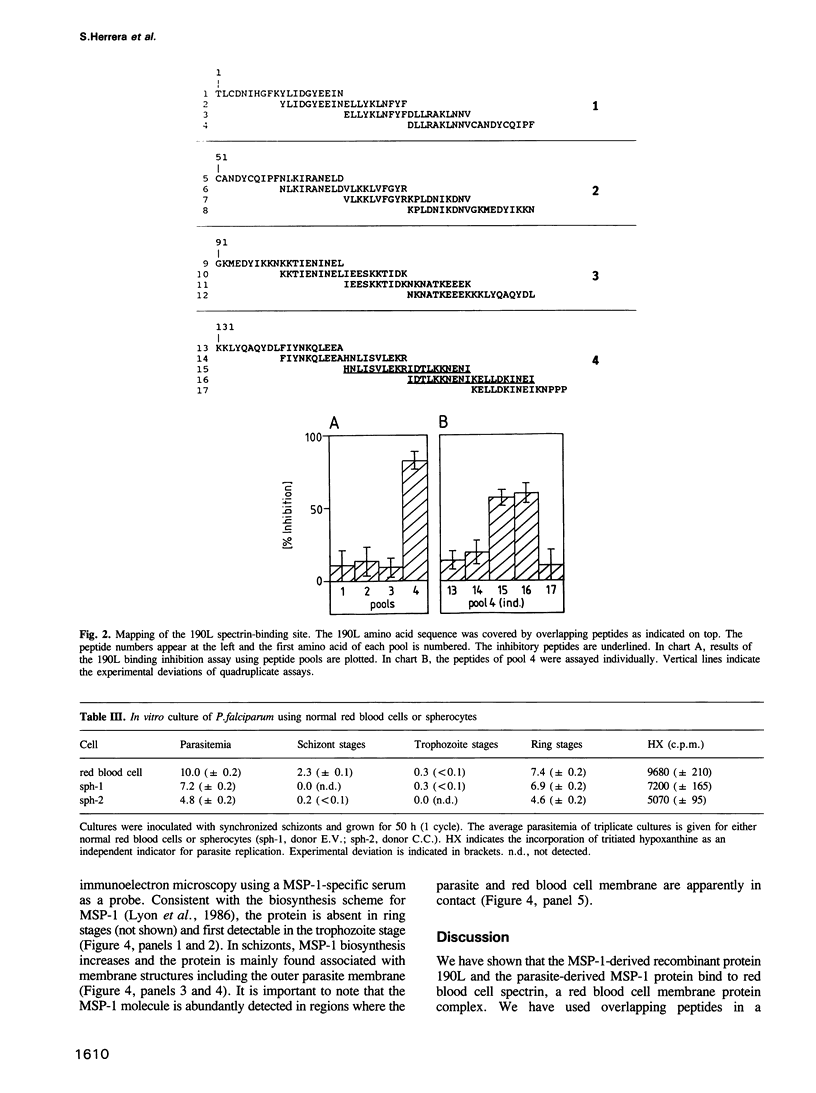

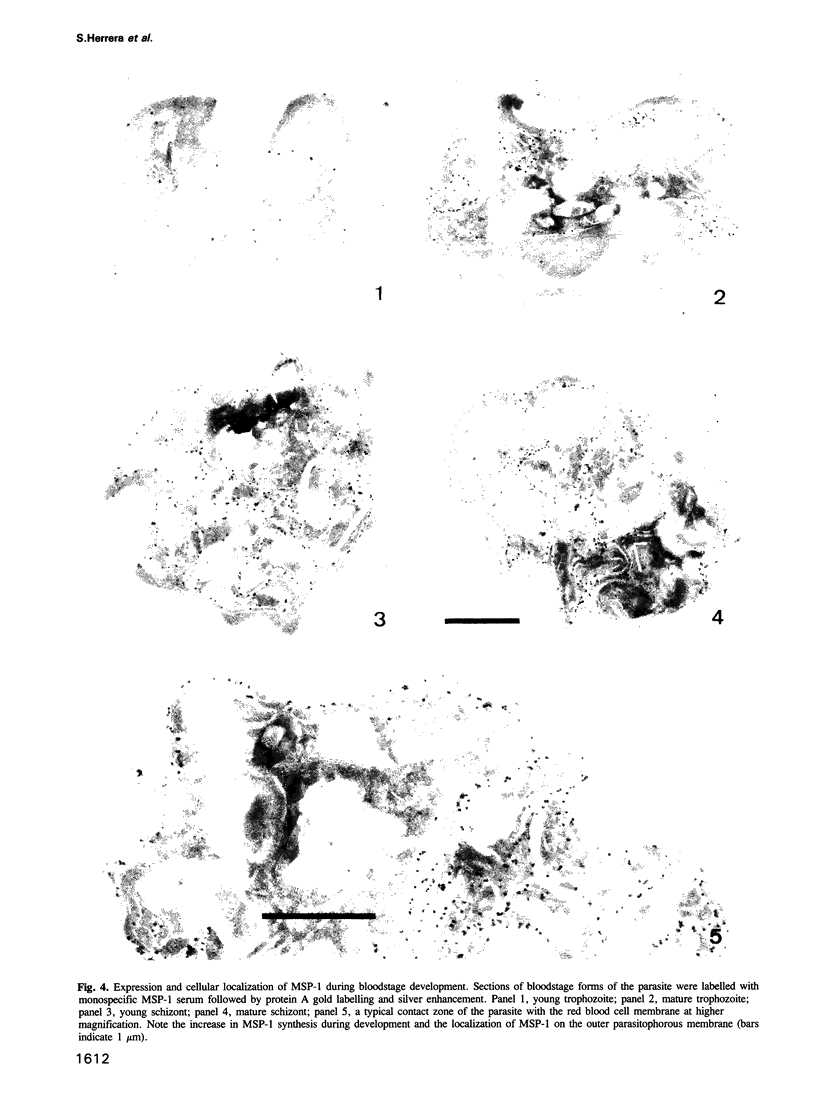


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aikawa M., Torii M., Sjölander A., Berzins K., Perlmann P., Miller L. H. Pf155/RESA antigen is localized in dense granules of Plasmodium falciparum merozoites. Exp Parasitol. 1990 Oct;71(3):326–329. doi: 10.1016/0014-4894(90)90037-d. [DOI] [PubMed] [Google Scholar]
- Bannister L. H., Dluzewski A. R. The ultrastructure of red cell invasion in malaria infections: a review. Blood Cells. 1990;16(2-3):257–297. [PubMed] [Google Scholar]
- Blackman M. J., Ling I. T., Nicholls S. C., Holder A. A. Proteolytic processing of the Plasmodium falciparum merozoite surface protein-1 produces a membrane-bound fragment containing two epidermal growth factor-like domains. Mol Biochem Parasitol. 1991 Nov;49(1):29–33. doi: 10.1016/0166-6851(91)90127-r. [DOI] [PubMed] [Google Scholar]
- Blackman M. J., Whittle H., Holder A. A. Processing of the Plasmodium falciparum major merozoite surface protein-1: identification of a 33-kilodalton secondary processing product which is shed prior to erythrocyte invasion. Mol Biochem Parasitol. 1991 Nov;49(1):35–44. doi: 10.1016/0166-6851(91)90128-s. [DOI] [PubMed] [Google Scholar]
- Calvo M., Guzman F., Perez E., Segura C. H., Molano A., Patarroyo M. E. Specific interactions of synthetic peptides derived from P. falciparum merozoite proteins with human red blood cells. Pept Res. 1991 Nov-Dec;4(6):324–333. [PubMed] [Google Scholar]
- Camus D., Lyon J. A., Reaud-Jareed T., Haynes J. D., Diggs C. L. Characterization of gp195 processed products purified from Plasmodium falciparum culture supernates. Mol Biochem Parasitol. 1987 Nov;26(1-2):21–27. doi: 10.1016/0166-6851(87)90126-5. [DOI] [PubMed] [Google Scholar]
- Caspers P., Gentz R., Matile H., Pink J. R., Sinigaglia F. The circumsporozoite protein gene from NF54, a Plasmodium falciparum isolate used in malaria vaccine trials. Mol Biochem Parasitol. 1989 Jun 15;35(2):185–189. doi: 10.1016/0166-6851(89)90121-7. [DOI] [PubMed] [Google Scholar]
- Crisanti A., Müller H. M., Hilbich C., Sinigaglia F., Matile H., McKay M., Scaife J., Beyreuther K., Bujard H. Epitopes recognized by human T cells map within the conserved part of the GP190 of P. falciparum. Science. 1988 Jun 3;240(4857):1324–1326. doi: 10.1126/science.2453924. [DOI] [PubMed] [Google Scholar]
- Deguercy A., Hommel M., Schrével J. Purification and characterization of 37-kilodalton proteases from Plasmodium falciparum and Plasmodium berghei which cleave erythrocyte cytoskeletal components. Mol Biochem Parasitol. 1990 Jan 15;38(2):233–244. doi: 10.1016/0166-6851(90)90026-i. [DOI] [PubMed] [Google Scholar]
- Etlinger H. M., Caspers P., Matile H., Schoenfeld H. J., Stueber D., Takacs B. Ability of recombinant or native proteins to protect monkeys against heterologous challenge with Plasmodium falciparum. Infect Immun. 1991 Oct;59(10):3498–3503. doi: 10.1128/iai.59.10.3498-3503.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaloro J. M., Coppel R. L., Corcoran L. M., Foote S. J., Brown G. V., Anders R. F., Kemp D. J. Structure of the RESA gene of Plasmodium falciparum. Nucleic Acids Res. 1986 Nov 11;14(21):8265–8277. doi: 10.1093/nar/14.21.8265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley M., Tilley L., Sawyer W. H., Anders R. F. The ring-infected erythrocyte surface antigen of Plasmodium falciparum associates with spectrin in the erythrocyte membrane. Mol Biochem Parasitol. 1991 May;46(1):137–147. doi: 10.1016/0166-6851(91)90207-m. [DOI] [PubMed] [Google Scholar]
- Freeman R. R., Holder A. A. Surface antigens of malaria merozoites. A high molecular weight precursor is processed to an 83,000 mol wt form expressed on the surface of Plasmodium falciparum merozoites. J Exp Med. 1983 Nov 1;158(5):1647–1653. doi: 10.1084/jem.158.5.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentz R., Certa U., Takacs B., Matile H., Döbeli H., Pink R., Mackay M., Bone N., Scaife J. G. Major surface antigen p190 of Plasmodium falciparum: detection of common epitopes present in a variety of plasmodia isolates. EMBO J. 1988 Jan;7(1):225–230. doi: 10.1002/j.1460-2075.1988.tb02803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttinger M., Romagnoli P., Vandel L., Meloen R., Takacs B., Pink J. R., Sinigaglia F. HLA polymorphism and T cell recognition of a conserved region of p190, a malaria vaccine candidate. Int Immunol. 1991 Sep;3(9):899–906. doi: 10.1093/intimm/3.9.899. [DOI] [PubMed] [Google Scholar]
- Hall R., Hyde J. E., Goman M., Simmons D. L., Hope I. A., Mackay M., Scaife J., Merkli B., Richle R., Stocker J. Major surface antigen gene of a human malaria parasite cloned and expressed in bacteria. 1984 Sep 27-Oct 3Nature. 311(5984):379–382. doi: 10.1038/311379a0. [DOI] [PubMed] [Google Scholar]
- Hall R., McBride J., Morgan G., Tait A., Zolg J. W., Walliker D., Scaife J. Antigens of the erythrocytes stages of the human malaria parasite Plasmodium falciparum detected by monoclonal antibodies. Mol Biochem Parasitol. 1983 Mar;7(3):247–265. doi: 10.1016/0166-6851(83)90025-7. [DOI] [PubMed] [Google Scholar]
- Hall R., Osland A., Hyde J. E., Simmons D. L., Hope I. A., Scaife J. G. Processing, polymorphism, and biological significance of P190, a major surface antigen of the erythrocytic forms of Plasmodium falciparum. Mol Biochem Parasitol. 1984 Apr;11:61–80. doi: 10.1016/0166-6851(84)90055-0. [DOI] [PubMed] [Google Scholar]
- Herrera M. A., Rosero F., Herrera S., Caspers P., Rotmann D., Sinigaglia F., Certa U. Protection against malaria in Aotus monkeys immunized with a recombinant blood-stage antigen fused to a universal T-cell epitope: correlation of serum gamma interferon levels with protection. Infect Immun. 1992 Jan;60(1):154–158. doi: 10.1128/iai.60.1.154-158.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera S., Herrera M. A., Perlaza B. L., Burki Y., Caspers P., Döbeli H., Rotmann D., Certa U. Immunization of Aotus monkeys with Plasmodium falciparum blood-stage recombinant proteins. Proc Natl Acad Sci U S A. 1990 May;87(10):4017–4021. doi: 10.1073/pnas.87.10.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarolim P., Palek J., Amato D., Hassan K., Sapak P., Nurse G. T., Rubin H. L., Zhai S., Sahr K. E., Liu S. C. Deletion in erythrocyte band 3 gene in malaria-resistant Southeast Asian ovalocytosis. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11022–11026. doi: 10.1073/pnas.88.24.11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilejian A., Rashid M. A., Aikawa M., Aji T., Yang Y. F. Selective association of a fragment of the knob protein with spectrin, actin and the red cell membrane. Mol Biochem Parasitol. 1991 Feb;44(2):175–181. doi: 10.1016/0166-6851(91)90003-o. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambros C., Vanderberg J. P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979 Jun;65(3):418–420. [PubMed] [Google Scholar]
- Leech J. H., Barnwell J. W., Aikawa M., Miller L. H., Howard R. J. Plasmodium falciparum malaria: association of knobs on the surface of infected erythrocytes with a histidine-rich protein and the erythrocyte skeleton. J Cell Biol. 1984 Apr;98(4):1256–1264. doi: 10.1083/jcb.98.4.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A. P. Cloning and analysis of the gene encoding the 230-kilodalton merozoite surface antigen of Plasmodium yoelii. Mol Biochem Parasitol. 1989 Oct;36(3):271–282. doi: 10.1016/0166-6851(89)90175-8. [DOI] [PubMed] [Google Scholar]
- Lyon J. A., Geller R. H., Haynes J. D., Chulay J. D., Weber J. L. Epitope map and processing scheme for the 195,000-dalton surface glycoprotein of Plasmodium falciparum merozoites deduced from cloned overlapping segments of the gene. Proc Natl Acad Sci U S A. 1986 May;83(9):2989–2993. doi: 10.1073/pnas.83.9.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride J. S., Heidrich H. G. Fragments of the polymorphic Mr 185,000 glycoprotein from the surface of isolated Plasmodium falciparum merozoites form an antigenic complex. Mol Biochem Parasitol. 1987 Feb;23(1):71–84. doi: 10.1016/0166-6851(87)90189-7. [DOI] [PubMed] [Google Scholar]
- McBride J. S., Newbold C. I., Anand R. Polymorphism of a high molecular weight schizont antigen of the human malaria parasite Plasmodium falciparum. J Exp Med. 1985 Jan 1;161(1):160–180. doi: 10.1084/jem.161.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molano A., Segura C., Guzman F., Lozada D., Patarroyo M. E. In human malaria protective antibodies are directed mainly against the Lys-Glu ion pair within the Lys-Glu-Lys motif of the synthetic vaccine SPf 66. Parasite Immunol. 1992 Jan;14(1):111–124. doi: 10.1111/j.1365-3024.1992.tb00010.x. [DOI] [PubMed] [Google Scholar]
- Palek J., Lambert S. Genetics of the red cell membrane skeleton. Semin Hematol. 1990 Oct;27(4):290–332. [PubMed] [Google Scholar]
- Perkins M. E., Rocco L. J. Sialic acid-dependent binding of Plasmodium falciparum merozoite surface antigen, Pf200, to human erythrocytes. J Immunol. 1988 Nov 1;141(9):3190–3196. [PubMed] [Google Scholar]
- Perkins M. E. Surface proteins of Plasmodium falciparum merozoites binding to the erythrocyte receptor, glycophorin. J Exp Med. 1984 Sep 1;160(3):788–798. doi: 10.1084/jem.160.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruangjirachuporn W., Afzelius B. A., Paulie S., Wahlgren M., Berzins K., Perlmann P. Cytoadherence of knobby and knobless Plasmodium falciparum-infected erythrocytes. Parasitology. 1991 Jun;102(Pt 3):325–334. doi: 10.1017/s003118200006426x. [DOI] [PubMed] [Google Scholar]
- Schulman S., Roth E. F., Jr, Cheng B., Rybicki A. C., Sussman I. I., Wong M., Wang W., Ranney H. M., Nagel R. L., Schwartz R. S. Growth of Plasmodium falciparum in human erythrocytes containing abnormal membrane proteins. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7339–7343. doi: 10.1073/pnas.87.18.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shear H. L., Roth E. F., Jr, Ng C., Nagel R. L. Resistance to malaria in ankyrin and spectrin deficient mice. Br J Haematol. 1991 Aug;78(4):555–560. doi: 10.1111/j.1365-2141.1991.tb04488.x. [DOI] [PubMed] [Google Scholar]
- Siddiqui W. A., Tam L. Q., Kramer K. J., Hui G. S., Case S. E., Yamaga K. M., Chang S. P., Chan E. B., Kan S. C. Merozoite surface coat precursor protein completely protects Aotus monkeys against Plasmodium falciparum malaria. Proc Natl Acad Sci U S A. 1987 May;84(9):3014–3018. doi: 10.1073/pnas.84.9.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinigaglia F., Takacs B., Jacot H., Matile H., Pink J. R., Crisanti A., Bujard H. Nonpolymorphic regions of p190, a protein of the Plasmodium falciparum erythrocytic stage, contain both T and B cell epitopes. J Immunol. 1988 May 15;140(10):3568–3572. [PubMed] [Google Scholar]
- Strych W., Miettinen-Baumann A., Lottspeich F., Heidrich H. G. Isolation and characterization of the 80,000 dalton Plasmodium falciparum merozoite surface antigen. Parasitol Res. 1987;73(5):435–441. doi: 10.1007/BF00538201. [DOI] [PubMed] [Google Scholar]
- Tanabe K., Mackay M., Goman M., Scaife J. G. Allelic dimorphism in a surface antigen gene of the malaria parasite Plasmodium falciparum. J Mol Biol. 1987 May 20;195(2):273–287. doi: 10.1016/0022-2836(87)90649-8. [DOI] [PubMed] [Google Scholar]
- del Portillo H. A., Longacre S., Khouri E., David P. H. Primary structure of the merozoite surface antigen 1 of Plasmodium vivax reveals sequences conserved between different Plasmodium species. Proc Natl Acad Sci U S A. 1991 May 1;88(9):4030–4034. doi: 10.1073/pnas.88.9.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]