Abstract
INTRODUCTION
Focal Adhesion Kinase (FAK) plays a significant role in cancer cell survival signaling and is overexpressed in various malignancies, including lung cancer. Previous studies suggest that FAK overexpression is an independent factor predicting poor prognosis in NSCLC. The aim of this study is to confirm these findings specifically in stage I NSCLC.
MATERIALS AND METHODS
A retrospective tissue microarray (TMA) analysis of FAK protein expression by immunohistochemistry (IHC) was performed in 157 surgically resected stage I NSCLC specimen and in the corresponding matched normal lung tissue. The FAK 4.47 monoclonal antibody was used for FAK immunostaining. The scoring system of triplicate tumor cores included intensity of staining plus extent of staining for a composite score that ranged from 0–6. The association between FAK score and survival was evaluated.
RESULTS
There were 103 stage IA and 54 stage IB patients, with mean follow-up of 5.5 years. Normal lung alveoli and interstitial tissue had mean FAK score of 0 (median score 0, range 0–2). Tumor samples had mean FAK score 3.1 (median score 3.5, range 0–6), with 57% of the samples having FAK score ≥ 3. Continuous FAK score was not associated with demographic data, tumor histology or grade, nor survival in this cohort of stage I NSCLC patients.
CONCLUSIONS
FAK is expressed in more than 50% of stage I NSCLC lung cancer but not in normal lung alveoli and interstitial tissue. FAK expression is not associated with survival outcome in this North American cohort.
KEWORDS/PHRASES: Focal adhesion kinase, Prognosis, Non-small cell lung cancer, Tumor marker
INTRODUCTION
Lung cancer is the most common cause of death from cancer worldwide, with an estimated 1.4 million deaths in 2008.1 The overall five-year survival rate is less than 20%,2 reflecting the advanced stage of the lung cancer at diagnosis in majority of patients. However, even among pathologic stage I lung cancers, outcomes vary according to specific TNM(Tumor Node Metastasis) classification, with 5-year survival between 58%-83% at best.3 A five-gene signature has been shown to be an independent predictor of relapse-free and overall survival among NSCLC patients.4 However, rapid and widespread adoption of this assay platform is limited. It is thus critical to identify biomarkers using easily reproducible assays to improve risk-stratification in stage I NSCLC for future adjuvant studies.
Focal Adhesion Kinase (FAK) is a 125kDa protein tyrosine kinase that is overexpressed in a number of different types of solid tumors.5,6 FAK suppresses apoptosis in tumor cells and is critical for survival, invasion, and metastasis.7–9 FAK has been shown to be overexpressed in colon tumors compared to normal matched samples by both immunohistochemistry and RT-PCR.6 Recently, FAK was found to be overexpressed in lung tumors, and its higher expression correlated with nodal spread and advanced disease stages,10–12 suggesting its important role in lung cancer progression and metastasis. Moreover, activation of FAK, along with SRC, promote cell migration and adhesion in LKB1-deficient KRAS mutant lung tumors, a genotype associated with high propensity for nodal and distant metastases.13 As SRC is a substrate of activated FAK,5 there maybe a potential therapeutic role for SRC inhibitors such as dasatinib in FAK-activated NSCLC.We thus hypothesized that FAK expression may discriminate stage I NSCLC patients who are at high-risk for relapse and mortality from lung cancer. FAK expression was thus evaluated by immunohistochemistry in a retrospective cohort of stage I NSCLC patients who underwent curative surgical resection.
MATERIALS AND METHODS
Patients and tissue samples
The Roswell Park Cancer Institute Institutional Review Board (IRB) approved this retrospective project in compliance with federal, state and local requirements. All clinical and outcome patient data were de-identified. Tumor specimens and matched normal lung tissue were collected from patients who underwent surgical resection of lung cancer at Roswell Park Cancer Institute (RPCI), Buffalo, NY.
Patients with small cell or mixed histologies with small cell component were excluded from this study. 157 patients diagnosed with pathologic stage I NSCLC between December 1992 to October 2008 who had sufficient tissue for this project were included in the analysis. Median follow-up duration was 5.1 years (range 0.1 to 15.5 years). The AJCC staging criteria (6th edition) was used for all patients for uniformity of pathologic staging.
Tissue microarrays (TMA)
TMA’s were constructed from formalin-fixed paraffin-embedded tissues with tumors. TMA’s containing lung tumors and matched normal tissues from 161 samples were prepared with each tumor and normal core sample in triplicate. Three 0.6 millimeter tissue cores from formalin-fixed paraffin embedded donor blocks were precisely arrayed into a new recipient paraffin block. Each patient had three lung tumor tissue cores on a TMA slide.
Immunohistochemical staining
The immunohistochemical staining was performed with FAK 4.47 antibody (Millipore#05-537). For antigen retrieval, slides were heated in the microwave for 10 minutes in citrate buffer (pH 6.0), followed by a 15 minute cooling period. Endogenous peroxidase was quenched with aqueous 3% H2O2 for 10 minutes and washed with 1xPBS using 0.5% Tween 20 solution. Slides were loaded on a DAKO autostainer and blocked with serum-free protein block solution (Dako #X0909) for five minutes and then probed with FAK primary antibody (Millipore #05-537) for one hour, followed by the biotinylated goat anti-mouse IgG (Jackson Immuno Research Labs, #115-065-062) for 30 minutes, then by the Elite ABC Kit (Vectastain, #PK-6200) for 30 minutes, and the DAB chromagen (Dako, #K4007) for five minutes. The slides were counterstained with hematoxylin.
Scoring for FAK
Slides were analyzed by two of the co-authors (L.Y. and S.P.), board-certified pathologists in a blinded and independent manner. The scoring system of triplicate tumor cores included intensity of staining (0, none; 1+, weak; 2+, moderate; 3+, strong) plus extent of staining which represented the number of cores with a positive staining (extent 0, no staining in three cores; 1, only one core had a positive staining, mild; 2, only 2 cores had a positive staining, moderate; 3, all three cores had the positive staining, diffuse). The composite score ranged from 0 to 6, which was equal to the sum of the average intensity and the extent of staining in the three cores. The percentage of stained cells was not used in the scoring as FAK staining was diffuse within the tumors. Patients with less than two tissue sample cores were excluded from analysis.
Statistical Methods
The associations between continuous FAK scores and clinical factors were assessed using non-parametric Spearman correlation or Kruskal-Wallis tests as appropriate. The univariate and multivariate associations of clinicopathological patient data and FAK lung tumor staining were performed using ordinary least squares regression. For further analysis, patients were dichotomized into two groups: FAK tumor score ≥3 (a group with intensity of FAK staining ≥ 1 and extent ≥2) and FAK tumor <3 (a group with intensity <1 and extent < 2). Contingency tables were analyzed by Fisher’s Exact Test.
Overall survival was defined as time in months between enrollment and death or last followup, whichever occurred first. Multivariate effects on Overall Survival were assessed using multivariate Proportional Hazards models. Associations between overall survival and stage or FAK expression were also described by Kaplan Meier curves and Logrank tests.
Reproducibility of the scores was assessed using a set of validation scores from a second independent pathologist. Agreement between the two sets of continuous scores was assessed using the Concordance Correlation Coefficient (CCC), which ranges between −1 to +1. Higher values indicate better agreement.14 The CCC adjusts for the probability of observing good agreement by chance alone.
P-values < 0.05 were considered statistically significant, with no adjustment for multiplicity. All analyses were obtained using SAS/STAT software, version 9.4, copyright 2012 SAS Inc. (Cary, NC).
RESULTS
Clinicopathologic characteristics
Table 1 summarizes the clinical and pathologic characteristics of patients whose tumor specimen were utilized for this study. The median age of all patients was 68 (range 46 to 86 years). There were 103 stage IA and 54 stage IB patients. There was no difference in terms of gender, age, histology, smoking or alcohol use status between stage IA and stage IB patients. Stage IB patients received perioperative (neoadjvuant and/or adjuvant) systemic therapy more frequently than stage IA patients (26% vs 6%, p < 0.001). The male to female ratio was 0.91 (75 male, 82 female). Majority (90%) identified themselves as Caucasian. Current smokers comprised 33% of the group, whereas former smokers represented the majority (62%). Histologic subtype was divided into four groups: adenocarcinoma, squamous cell carcinoma, large cell or poorly differentiated NSCLC not otherwise specified (NOS). One case of adenosquamous carcinoma was grouped under the adenocarcinoma category.
Table 1.
Characteristics of NSCLC cases analyzed for FAK staining according to disease stage
| Stage 1A | Stage 1B | Overall | pvalue | ||
|---|---|---|---|---|---|
| Patients | N | 103 (65.6) | 54 (34.4) | 157 (100%) | |
| Age at Dx | Mean/StdErr | 67.4/1.0 | 67.9/1.2 | 67.6/0.7 | 0.827 |
| Median/Min/Max | 68.0/46.4/85.5 | 67.2/47.8/85.8 | 67.3/46.4/85.8 | ||
| Sex | Female | 59 (57.3%) | 23 (42.6%) | 82 (52.2%) | 0.080 |
| Male | 44 (42.7%) | 31 (57.4%) | 75 (47.8%) | ||
| Race | Caucasian | 94 (91.3%) | 47 (87.0%) | 141 (89.8%) | 0.074 |
| African American | 5 (4.9%) | 7 (13.0%) | 12 (7.6%) | ||
| Other | 4 (3.9%) | 4 (2.5%) | |||
| Smoking | Current | 36 (35.0%) | 15 (27.8%) | 51 (32.5%) | 0.453 |
| Previous | 62 (60.2%) | 35 (64.8%) | 97 (61.8%) | ||
| Never | 5 (4.9%) | 3 (5.6%) | 8 (5.1%) | ||
| Other* | 1 (1.9%) | 1 (0.6%) | |||
| Alcohol | Current | 19 (18.4%) | 8 (14.8%) | 27 (17.2%) | 0.636 |
| Previous | 6 (5.8%) | 5 (9.3%) | 11 (7.0%) | ||
| Never | 19 (18.4%) | 7 (13.0%) | 26 (16.6%) | ||
| Other* | 59 (57.3%) | 34 (63.0%) | 93 (59.2%) | ||
| Histology | Adenocarcinoma | 55 (53.4%) | 25 (46.3%) | 80 (51.0%) | 0.600 |
| Squamous | 38 (36.9%) | 20 (37.0%) | 58 (36.9%) | ||
| NSCLC,NOS | 4 (3.9%) | 3 (5.6%) | 7 (4.5%) | ||
| Large Cell | 6 (5.8%) | 6 (11.1%) | 12 (7.6%) | ||
| Topology | Upper Lobe | 68 (66.0%) | 36 (66.7%) | 104 (66.2%) | 0.843 |
| Lower Lobe | 29 (28.2%) | 16 (29.6%) | 45 (28.7%) | ||
| Middle Lobe | 6 (5.8%) | 2 (3.7%) | 8 (5.1%) | ||
| Differentiation | Poor | 53 (51.4%) | 31 (57.4%) | 84(53.5%) | 0.769 |
| Moderate | 42 (40.8%) | 19 (35.2%) | 61 (38.9%) | ||
| Well | 8 (7.8%) | 4 (7.4%) | 12 (7.6%) | ||
| Periop Chemo | Yes | 6 (5.8%) | 14 (25.9%) | 20 (12.7%) | <0.001 |
| No | 97 (94.2%) | 40 (74.1%) | 137 (87.3%) | ||
| Alive/Dead | Alive | 35 (34.0%) | 19 (35.2%) | 54 (34.4%) | 0.880 |
| Dead | 68 (66.0%) | 35 (64.8%) | 103 (65.6%) | ||
| Followup (Mo) | Mean/StdErr | 5.5/0.3 | 5.5/0.5 | 5.5/0.3 | 0.587 |
| Median/Min/Max | 5.3/0.3/14.8 | 4.9/0.1/15.5 | 5.2/0.1/15.5 | ||
| FAK(normal) | Mean/StdErr | 0.0/0.0 | 0.1/0.0 | 0.0/0.0 | 0.456 |
| Median/Min/Max | 0.0/0.0/1.7 | 0.0/0.0/2.0 | 0.0/0.0/2.0 |
Legends and abbrevations:
Dx-diagnosis
FAK(normal)- FAK score in normal lung specimen
FAK(tumor)- FAK score in tumor specimen
NOS- NSCLC, not otherwise specified
Periop Chemo- neoadjuvant and/or adjuvant chemotherapy
Information not reported, not available or not collected
FAK expression
The inter-pathologist agreement on the continuous patient-level FAK expression scores revealed a CCC of 0.977 (95% CI: 0.970 to 0.984) indicating low inter-observer variation. Our results showed that FAK expression was predominantly diffuse and cytoplasmic in distribution in tumor cells whereas its expression was absent in normal lung alveoli and interstitial tissue. Figure 1 shows a representative image of the range of FAK staining intensity. Most of the normal lung tissue did not express FAK, and the mean score was equal to 0 (range 0–2), as demonstrated in Figure 2. The mean FAK score in the entire group of lung tumors was 3.13 (median 3.5, range 0–6). As expected, there was no difference in the FAK score of normal lung tissue between pathologic stage IA and stage IB patients.
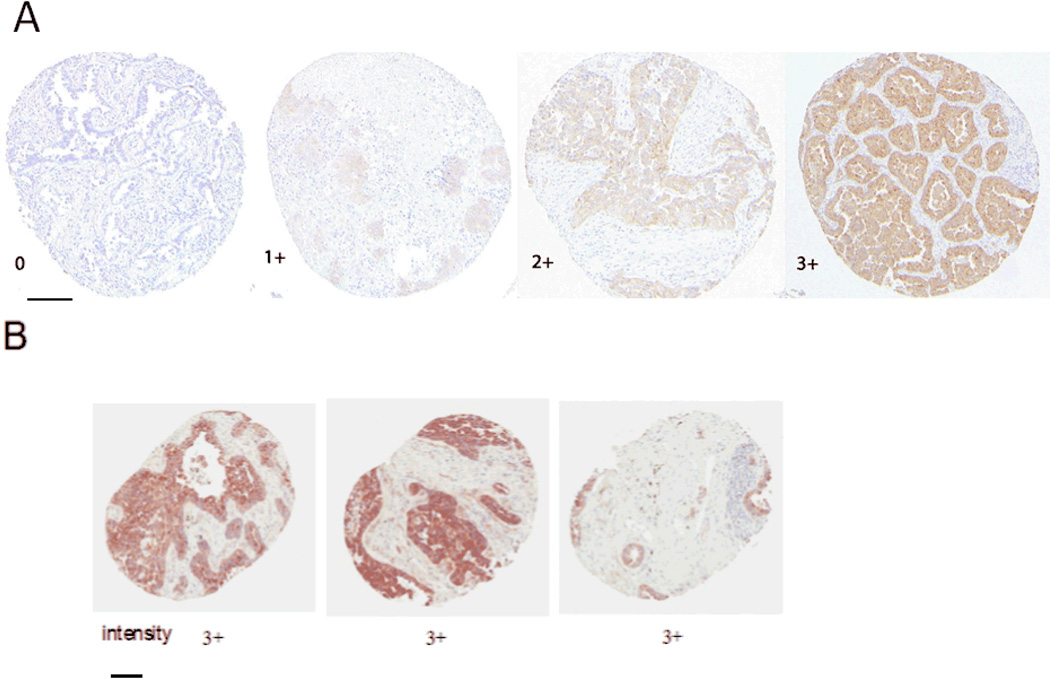
Figure 1. Immunohistochemical analysis of FAK expression in stage I NSCLC TMA samples.
FAK expression was detected by immunohistochemical staining with FAK 4.47 monoclonal antibody using TMA samples with triplicate tissue cores per tumor stained. Scale bars shown represent 150 micrometers in length. Images taken under x20 magnification. Figure 1 A shows representative images of scoring system that measured intensity (0, none; 1+, weak; 2+, moderate; 3+, strong. Figure 1B shows representative triplicate core showing extent of staining which represented the number of cores with a positive staining (extent 0, no staining in three cores; 1, only one core had a positive staining, mild; 2, only 2 cores had a positive staining, moderate; 3, all three cores had the positive staining, diffuse). In this example, the “extent” score is 3. The total FAK score including the measured intensity is 6.
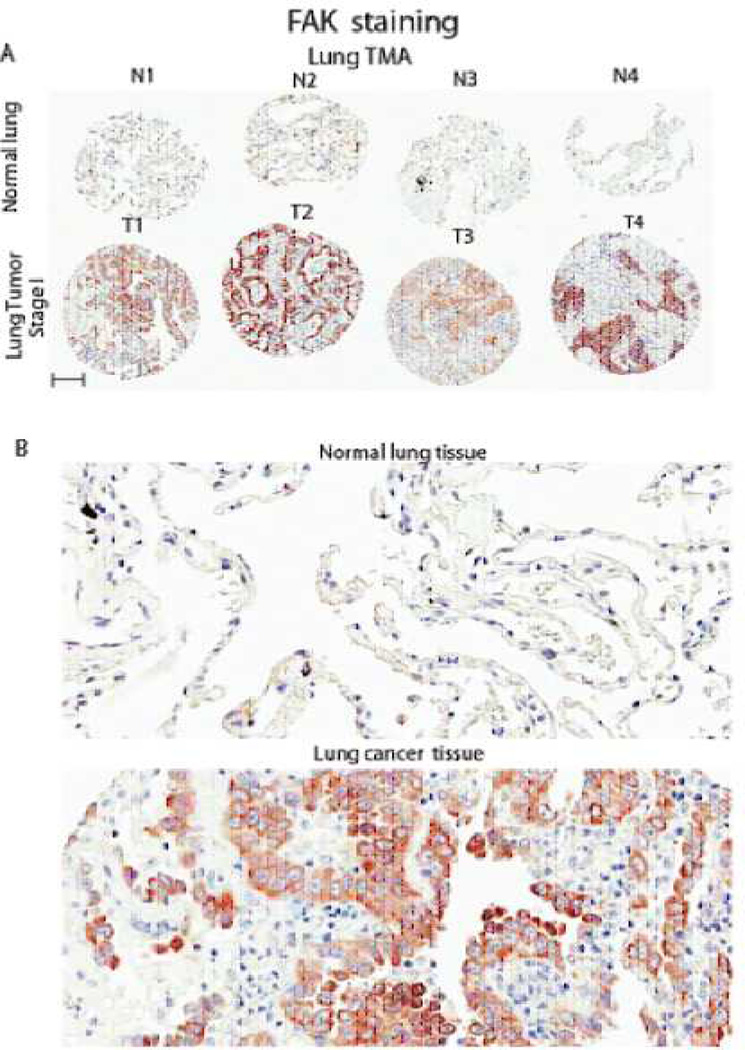
Figure 2. FAK staining in normal lung and lung cancer TMA.
2A: Four representative TMA cores with normal and tumor samples are shown and marked as N1-4 and T1-4, respectively. As seen in the upper row, most of the normal lung tissue did not express FAK. In comparison, FAK expression is seen in lung cancer cells (but not in surrounding stromal tissue). Scale bar shown represents 150 micrometers in length.
2B: Representative magnified (200X) images derived from N2 and T2 samples in Figure 2A of FAK staining in normal and tumor sample.
Univariate comparisons of tumor FAK score as continuous variable showed no association with any of the clinicopathologic variables, including overall survival. We then dichotomized the lung tumors according to the composite FAK score: ≥ 3 and < 3 as the maximum FAK score found in normal alveoli was 2 which may represent a transition zone with biological relevance. Fifty seven percent of the tumors had FAK scores ≥3. The remaining 43% of tumors, in the group with FAK score < 3, had a median score of 1.3. We also performed analysis according to a higher FAK score cut-off. Twenty five percent of tumors had FAK scores ≥ 5. The median FAK score of tumors in the FAK score < 5 group was 2.7.
FAK score and clinicopathologic features
As aforementioned, univariate comparisons of tumor FAK score as continuous variable showed no association with any of the clinicopathologic variables. Using the above dichotomy in FAK scoring, correlation between FAK expression and clinicopathologic features was analyzed. As shown in Table 2, there was no association between lung tumor FAK score of ≥ 3 and < 3 with age, gender, race, smoking status, pathologic stage, histologic subtype nor histologic grade. Statistically significant correlations were established between tumor FAK score ≥ 5 with histology (adenocarcinoma type), gender (women) and alcohol use (never-users).
Table 2.
Correlation between immunohistochemical FAK expression score and clinicopathological variables
| Overall | FAK (tumor)<3 | FAK (tumor)>=3 |
pvalue | FAK(tumor) <5 | FAK (tumor)>=5 |
pvalue | ||
|---|---|---|---|---|---|---|---|---|
| Patients | N | 157 (100%) | 68 (43.3) | 89 (56.7) | 118 (75.2) | 39 (24.8) | ||
| Age at Dx | Mean/StdErr | 67.6/0.7 | 67.2/1.1 | 67.9/1.0 | 0.576 | 67.4/0.8 | 68.3/1.5 | 0.689 |
| Median/Min/Max | 67.3/46.4/85.8 | 66.2/47.2/85.5 | 68.5/46.4/85.8 | 66.9/46.4/85.8 | 70.7/49.1/84.7 | |||
| Sex | Female | 82 (52.2%) | 34 (50.0%) | 48 (53.9%) | 0.625 | 56 (47.5%) | 26 (66.7%) | 0.037 |
| Male | 75 (47.8%) | 34 (50.0%) | 31 (46.1%) | 62 (52.5%) | 13 (33.3%) | |||
| Race | Caucasian | 141 (89.8%) | 63 (92.6%) | 78 (87.6%) | 0.565 | 108 (91.5%) | 33 (84.6%) | 0.372 |
| African-American | 12 (7.6%) | 4 (5.9%) | 8 (9.0%) | 7 (5.9%) | 5 (12.8%) | |||
| Other | 4 (2.5%) | 1 (1.5%) | 3 (3.4%) | 3 (2.5%) | 1 (2.6%) | |||
| Smoking | Current | 51 (32.5%) | 21 (30.9%) | 30 (33.7%) | 0.243 | 36 (30.5%) | 15 (38.5%) | 0.315 |
| Previous | 97 (61.8%) | 41 (60.3%) | 56 (62.9%) | 73 (61.9%) | 24 (61.5%) | |||
| Never | 8 (5.1%) | 6 (8.8%) | 2 (2.2%) | 8 (6.8%) | ||||
| Other* | 1 (0.6%) | 1 (1.1%) | 1 (0.8%) | |||||
| Alcohol | Current | 27 (17.2%) | 14 (20.6%) | 13 (14.6%) | 0.767 | 20 (16.9%) | 7 (17.9%) | 0.044 |
| Previous | 11 (7.0%) | 5 (7.4%) | 6 (6.7%) | 9 (7.6%) | 2 (5.1%) | |||
| Never | 26 (16.6%) | 10 (14.7%) | 16 (18.0%) | 14 (11.9%) | 12 (30.8%) | |||
| Other* | 93 (59.2%) | 39 (57.4%) | 54 (60.7%) | 75 (63.6%) | 18 (46.2%) | |||
| Histology | Adenocarcinoma | 80 (51.0%) | 33 (48.5%) | 47 (52.8%) | 0.747 | 54 (45.8%) | 26 (66.7%) | 0.044 |
| Squamous | 58 (36.9%) | 27 (39.7%) | 31 (34.8%) | 51 (43.2%) | 7 (17.9%) | |||
| NSCLC,NOS | 7 (4.5%) | 2 (2.9%) | 5 (5.6%) | 5 (4.2%) | 2 (5.1%) | |||
| Large Cell | 12 (7.6%) | 6 (8.8%) | 6 (6.7%) | 8 (6.8%) | 4 (10.3%) | |||
| Topology | Upper Lobe | 104 (66.2%) | 47 (69.1%) | 57 (64.0%) | 0.195 | 77 (65.3%) | 27 (69.2%) | 0.889 |
| Lower Lobe | 45 (28.7%) | 20 (29.4%) | 25 (28.1%) | 35 (29.7%) | 10 (25.6%) | |||
| Middle Lobe | 8 (5.1%) | 1 (1.5%) | 7 (7.9%) | 6 (5.1%) | 2 (5.1%) | |||
| Differentiation | Poor | 84 (53.5%) | 33 (48.5%) | 51 (57.3%) | 0.544 | 61 (51.7%) | 23 (59.0%) | 0.658 |
| Moderate | 61 (38.9%) | 29 (42.6%) | 32 (36.0%) | 47 (39.8%) | 14 (35.9%) | |||
| Well | 12 (7.6%) | 6 (8.8%) | 6 (6.7%) | 10 (8.5%) | 2 (5.1%) | |||
| Stage(Path) | 1A | 103 (65.6%) | 44 (64.7%) | 59 (66.3%) | 0.836 | 77 (65.3%) | 26 (66.7%) | 0.872 |
| 1B | 54 (34.4%) | 24 (35.3%) | 30 (33.7%) | 41 (34.7%) | 13 (33.3%) | |||
| Periop Chemo | Yes | 20 (12.7%) | 7 (10.3%) | 13 (14.6%) | 0.422 | 14 (11.9%) | 6 (15.4%) | 0.568 |
| No | 137 (87.3%) | 61 (89.7%) | 76 (85.4%) | 104 (88.1%) | 33 (84.6%) | |||
| Alive/Dead | Alive | 54 (34.4%) | 20 (29.4%) | 34 (38.2%) | 0.251 | 37 (31.4%) | 17 (43.6%) | 0.163 |
| Dead | 103 (65.6%) | 48 (70.6%) | 55 (61.8%) | 81 (68.6%) | 22 (56.4%) | |||
| Followup (Mo) | Mean/StdErr | 5.5/0.3 | 5.2/0.5 | 5.7/0.3 | 0.148 | 5.4/0.3 | 5.6/0.5 | 0.529 |
| Median/Min/Max | 5.2/0.1/15.5 | 4.9/0.1/15.5 | 5.5/0.4/14.8 | 5.2/0.1/15.5 | 5.7/0.7/12.8 | |||
| FAK(normal) | Mean/StdErr | 0.0/0.0 | 0.0/0.0 | 0.1/0.0 | 0.072 | 0.0/0.0 | 0.1/0.1 | 1.000 |
| Median/Min/Max | 0.0/0.0/2.0 | 0.0/0.0/0.0 | 0.0/0.0/2.0 | 0.0/0.0/1.7 | 0.0/0.0/2.0 |
Legends and abbrevations:
Dx-diagnosis
FAK(normal)- FAK score in normal lung specimen
FAK(tumor)- FAK score in tumor specimen
NOS- NSCLC, not otherwise specified
Periop Chemo- neoadjuvant and/or adjuvant chemotherapy
Information not reported, not available or not collected
FAK score and survival
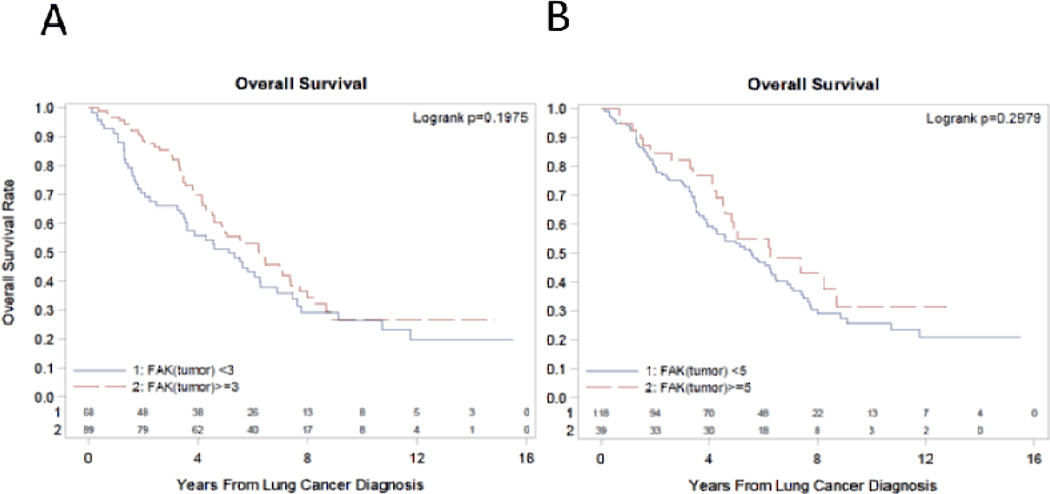
We subsequently analyzed the relationship between FAK score and survival outcomes. The median survival of patients with lung tumor FAK score ≥ 3 was 6.3 years (95% CI: 4.6 to 7.4) while those with lung tumor FAK score < 3 was 5.1 years (95% CI: 3.5 to 6.3). This difference was not statistically significant (logrank p= 0.2). Using a higher FAK score of 5 as cut-off also did not reveal any association as well with survival. Figure 3 shows the Logrank overall survival curve according to FAK score. In comparison, the difference in the median survival between stage IA and IB patients was not significant (5.8 years vs 5.7 years, p=0.9). Among other clinicopathologic variables in multivariate analysis, only age at diagnosis (p=0.02) was associated with survival.
Figure 3. Overall survival rate and FAK expression in stage I NSCLC patients.
There is no significant difference between overall survival and FAK expression in lung cancer. Logrank curve and p-values are shown for Figure 3A according to FAK score≥ 3 and <3 and for Figure 3B according to FAK score ≥ 5 and < 5.
DISCUSSION
This study analyzed FAK expression in stage I NSCLC and matched normal lung tissues using TMA. Our results showed that normal lung tissues did not express FAK, while high FAK expression can be seen even in stage I cases. This finding is important and provides rationale for investigating FAK expression in premalignant lesions as a biomarker for disease progression into invasive lung cancer. In breast cancer, FAK expression can be detected in 66% of pre-invasive ductal carcinoma in-situ (DCIS) lesions and 21% of atypical ductal hyperplasia (ADH) tissues.15 Whether FAK expression will also be expressed in premalignant lesions in the respiratory tract remains to be investigated.
One of the earliest investigations suggesting a prognostic role for FAK expression in lung cancer was led by Nishimura et al who correlated the presence of phosphorylated 100–130kDa proteins with regional nodal involvement as well as survival independent of nodal involvement in Japanese patients who underwent surgery for NSCLC.12 Another study demonstrated correlation between FAK overexpression and more advanced stages of disease using 153 NSCLC frozen tissues in Chinese patients.10 The authors reported that FAK overexpression was associated with lymph node metastasis and more advanced stages of NSCLC. FAK overexpression was independently associated with worse overall survival, suggesting it as a significant prognostic factor in addition to pathologic stage. Another group evaluating FAK expression in NSCLC resected from Chinese patients also reported that FAK expression was associated with worse prognosis, higher incidence of tumor recurrence and distant metastasis.16 A different retrospective analysis in Chinese patients also revealed that FAK expression confers worse survival in surgically resected lung adenocarcinoma.17 In comparison, FAK expression was not a prognostic factor in North American patients with SCLC, as its expression did not correlate with disease stage, recurrence/progression-free survival or overall survival.18
Our study is the first publication to analyze FAK expression specifically in a large cohort of stage I NSCLC patients in North America. In contrast to the aforementioned studies in NSCLC, we did not detect a correlation between FAK expression and survival in our patient population. The survival curves that counterintuitively showed a trend of better survival in tumors with higher FAK scores (either ≥ 3 or ≥5) may be attributed to either random chance or true effect which warrants further investigation in this population. Indeed, a recent North American study reported in abstract form, analyzing FAK protein expression by IHC in archival tumors from 216 stage I-III NSCLC patients, revealed that FAK IHC score was higher in early stage (stage I versus stage II or stage III) tumors, particularly in adenocarcinomas and in never smokers.19 In the adenocarcinoma cases, FAK protein overexpression correlated with better overall survival even after adjusting for stage and adjuvant therapy and was significantly higher in tumors with epidermal growth factor receptor mutations.
Our study has some limitations. Due to the TMA approach, there could be heterogeneity in the pattern of FAK expression which accounted for these results as well. We also did not evaluate normal bronchial epithelium FAK expression and activation state which can be increased by exposure to cigarette smoke and thus hypothetically may potentially have prognostic impact on risk of local recurrences.20 Another limitation is that because phosphorylated FAK cannot be measured reliably due to its labile nature and inherent variability in FFPE specimen processing, we measured total FAK in this retrospective study which may not necessarily indicate an activated pathway. There may also be confounding variables found specifically in Asian patients that account for the lack of negative prognostic impact of FAK in our predominantly Caucasian population. The genomic changes found in NSCLC from Asian patients have a different profile from those found in patients from Western countries, even after controlling for histologic subtype.21 These differences need to be studied in future investigations of the impact of FAK expression and lung cancer prognosis.
Despite the lack of prognostic value of FAK in stage I NSCLC, our report provides additional data for other investigators evaluating the role of FAK in lung carcinogenesis. Novel therapeutics to block FAK signaling are in development. Further understanding of the impact of modulating FAK in various stages of NSCLC will influence the clinical development of these agents and the corresponding trial design in the future.
Acknowledgments
Sources of support:
We would like to thank members of the Pathology core for providing TMA samples, immunohistochemical staining, scoring and Aperio imaging. Biospecimens or research pathology services for this study were provided by the Pathology Resource Network, which is funded by the National Cancer Institute and is a Roswell Park Cancer Institute Cancer Center Support Grant shared resource. Clinical Data Delivery and Honest Broker services for this study were provided by the Clinical Data Network, which is funded by the National Cancer Institute and is a Roswell Park Cancer Institute Cancer Center Support Grant shared resource. The work was supported by RO-1 grant CA65910 and Roswell Park Cancer Institute and National Cancer Institute (NCI) grant.
REFERENCES
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 4.Chen HY, Yu SL, Chen CH, et al. A five-gene signature and clinical outcome in nonsmall- cell lung cancer. N Engl J Med. 2007;356:11–20. doi: 10.1056/NEJMoa060096. [DOI] [PubMed] [Google Scholar]
- 5.Schaller MD, Borgman CA, Cobb BS. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci U S A. 1992;89:5192–5196. doi: 10.1073/pnas.89.11.5192. Vet al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lark AL, Livasy CA, Calvo B, et al. Overexpression of focal adhesion kinase in primary colorectal carcinomas and colorectal liver metastases: immunohistochemistry and real-time PCR analyses. Clin Cancer Res. 2003;9:215–222. [PubMed] [Google Scholar]
- 7.Golubovskaya V, Beviglia L, Xu LH, et al. Dual inhibition of focal adhesion kinase and epidermal growth factor receptor pathways cooperatively induces death receptor-mediated apoptosis in human breast cancer cells. J Biol Chem. 2002;277:38978–3987. doi: 10.1074/jbc.M205002200. [DOI] [PubMed] [Google Scholar]
- 8.Park HB, Golubovskaya V, Xu L, et al. Activated Src increases adhesion, survival and alpha2-integrin expression in human breast cancer cells. Biochem J. 2004;378:559–567. doi: 10.1042/BJ20031392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golubovskaya VM, Finch R, Kweh F, et al. p53 regulates FAK expression in human tumor cells. Mol Carcinog. 2008;47:373–382. doi: 10.1002/mc.20395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ji HF, Pang D, Fu SB, et al. Overexpression of focal adhesion kinase correlates with increased lymph node metastasis and poor prognosis in non-small-cell lung cancer. J Cancer Res Clin Oncol. 2013;139:429–435. doi: 10.1007/s00432-012-1342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carelli S, Zadra G, Vaira V, et al. Up-regulation of focal adhesion kinase in non-small cell lung cancer. Lung Cancer. 2006;53:263–271. doi: 10.1016/j.lungcan.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Nishimura M, Machida K, Imaizumi M, et al. Tyrosine phosphorylation of 100-130 kDa proteins in lung cancer correlates with poor prognosis. Br J Cancer. 1996;74:780–787. doi: 10.1038/bjc.1996.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carretero J, Shimamura T, Rikova K, et al. Integrative genomic and proteomic analyses identify targets for Lkb1-deficient metastatic lung tumors. Cancer Cell. 2010;17:547–559. doi: 10.1016/j.ccr.2010.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carrasco JL, Jover L. Estimating the generalized concordance correlation coefficient through variance components. Biometrics. 2003;59:849–858. doi: 10.1111/j.0006-341x.2003.00099.x. [DOI] [PubMed] [Google Scholar]
- 15.Lightfoot HM, Jr, Lark A, Livasy CA, et al. Upregulation of focal adhesion kinase (FAK) expression in ductal carcinoma in situ (DCIS) is an early event in breast tumorigenesis. Breast Cancer Res Treat. 2004;88:109–116. doi: 10.1007/s10549-004-1022-8. [DOI] [PubMed] [Google Scholar]
- 16.Hsu NY, Chen CY, Hsu CP, et al. Prognostic significance of expression of nm23-H1 and focal adhesion kinase in non-small cell lung cancer. Oncol Rep. 2007;18:81–85. [PubMed] [Google Scholar]
- 17.Wang C, Yang R, Yue D, et al. Expression of FAK and PTEN in bronchioloalveolar carcinoma and lung adenocarcinoma. Lung. 2009;187:104–109. doi: 10.1007/s00408-008-9130-6. [DOI] [PubMed] [Google Scholar]
- 18.Ocak S, Chen H, Callison C, et al. Expression of focal adhesion kinase in small-cell lung carcinoma. Cancer. 2012;118:1293–1301. doi: 10.1002/cncr.26382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang X, Cai Y, Behrens C, et al. Focal adhesion kinsase (FAK) protein overexpression and gene copy number gain correlate with better outcome in patients with surgically resected NSCLC tumors. Presented on April 7, 2014 in the 105th Annual Meeting of the American Association for Cancer Research; San Diego, California. Abstract 2877. [Google Scholar]
- 20.Carter CA. Multiplexed high content screening reveals that cigarette smoke condensatealtered cell signaling pathways are accentuated through FAK inhibition in human bronchial cells. Int J Toxicol. 2012;31:257–266. doi: 10.1177/1091581812440890. [DOI] [PubMed] [Google Scholar]
- 21.Dearden S, Stevens J, Wu YL, et al. Mutation incidence and coincidence in non small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap) Ann Oncol. 2013;24:2371–2376. doi: 10.1093/annonc/mdt205. [DOI] [PMC free article] [PubMed] [Google Scholar]