Version Changes
Revised. Amendments from Version 1
We added the location of the study to title.
We revised the Abstract to include more specific statements of results.
We removed the hypothesis regarding epiphyte functional type differences because sufficient observations to detect trends were only available for 1-2 species per functional type.
We added new text describing the photographic analysis of cloud base to the Methods.
We added text to the Methods describing the identification and use of morpho-species in the analysis and now refer consistently to morpho-species throughout.
We added text to the Methods describing the focal taxa.
We fixed the misspelling of Scaphyglottis throughout.
We added text to the Discussion describing limitations to interpretation of the results imposed by the length of study.
We added text to the Discussion noting that both temperature and moisture are correlated with elevation.
We added text to the Discussion describing the possible interaction between rainfall compensation and drought tolerance.
We added a figure showing that rainfall during installation of the experiment in 2005 was well below average.
Abstract
The high diversity and abundance of vascular epiphytes in tropical montane cloud forest is associated with frequent cloud immersion, which is thought to protect plants from drought stress. Increasing temperature and rising cloud bases associated with climate change may increase epiphyte drought stress, leading to species and biomass loss. We tested the hypothesis that warmer and drier conditions associated with a lifting cloud base will lead to increased mortality and/or decreased recruitment of epiphyte ramets, altering species composition in epiphyte mats. By using a reciprocal transplant design, where epiphyte mats were transplanted across an altitudinal gradient of increasing cloud immersion, we differentiated between the effects of warmer and drier conditions from the more general prediction of niche theory that transplanting epiphytes in any direction away from their home elevation should result in reduced performance. Effects differed among species, but effects were generally stronger and more negative for epiphytes in mats transplanted down slope from the highest elevation, into warmer and drier conditions, than for epiphyte mats transplanted from other elevations. In contrast, epiphytes from lower elevations showed greater resistance to drought in all treatments. Epiphyte community composition changed with elevation, but over the timescale of the experiment there were no consistent changes in species composition. Our results suggest some epiphytes may show resistance to climate change depending on the environmental and evolutionary context. In particular, sites where high rainfall makes cloud immersion less important for epiphyte water-balance, or where occasional drought has previously selected for drought-resistant taxa, may be less adversely affected by predicted climate changes.
Introduction
Tropical montane forests, often referred to as cloud forests, harbor high species diversity, provide water and protect water quality for numerous people in tropical countries, and are under particular threat from climate change 1– 3. Most cloud forest regions of the world, including the tropical Andes, are considered hotspots of biological diversity 4, and plant species endemism often reaches high levels within cloud forest 5, 6. Epiphytes, non-parasitic plants that depend on other plants for support and are not in contact with terrestrial soil, are key components of cloud forest biodiversity and play critical roles in the hydrological and nutrient cycling of montane ecosystems. Not only can vascular epiphytes make up 30 percent or more of plant species diversity in tropical montane forests 7, they also provide keystone resources for birds, insects, and other animals 8– 11. Through cloud stripping, epiphytes increase total moisture captured by forest canopies 12– 14, and are important in nutrient cycling 15, 16. Cloud immersion is important for many epiphyte species to maintain a positive water balance and avoid desiccation 17; this makes them sensitive to changes in moisture regimes. Because of their sensitivity to moisture levels, epiphytes are considered indicator species in cloud forests for changing water balance conditions 18, particularly those in the wet tropics 19.
On a typical tropical mountain, where temperature decreases with altitude, there is a gradient of increasing cloud incidence with altitude. Cloud formation is dependent on the vapor content of air and air temperature, both of which are predicted to change with global warming 1. Atmospheric moisture levels are much less easily predicted than temperature in climate models, but a multi-model ensemble of climate simulations showed a trend towards drying in many tropical regions 20. Climate model projections in the Andes include warmer temperatures and lower precipitation in the dry season 21– 23, which could place cloud forest plants under increased drought stress. Cloud base height is also predicted to rise, due to a combination of higher temperature and lower atmospheric moisture input by vegetation due to lowland deforestation and reduced transpiration because of increased atmospheric CO 2 24– 26. In Costa Rica, an increase in the elevation of cloud base has been demonstrated and has already lead to the extinction of cloud forest species 27, although this may have been associated with a severe El Niño event in 1986–87 rather than a long term drying trend 28.
The sensitivity of vascular epiphytes to changes in cloud incidence was demonstrated experimentally in Monteverde, Costa Rica, where vascular epiphytes transplanted below the cloud base had shorter lifespans and higher leaf mortality 29. It is not clear, however, whether this result can be generalized to continental cloud forests such as the eastern Andes. Cloud forests vary worldwide, with differences in cloud base height and the proportion of moisture received by the vegetation via cloud stripping versus rainfall 30, 31. Cloud forests near coastlines are heavily influenced by ocean conditions 32, while cloud formation on continental mountain ranges is dependent on moisture flux across continents driven by synoptic weather patterns. Deforested areas in Costa Rica have fewer clouds than adjacent forested areas 33, but simulations suggest that sea-surface temperature has a greater impact on lifting condensation level than deforestation 34. Cloud forests in continental mountains like the Andes are expected to be more sensitive to conditions of the adjoining lowland ecosystems, particularly deforestation 25, 32. In addition, complex topography in the eastern Andes and its interaction with prevailing winds leads to wet and dry areas within regions broadly considered cloud forest 35. Given the diversity of cloudiness and precipitation regimes that epiphytes as a group are exposed to, it is reasonable to expect that epiphytes may be adapted to local moisture regimes. For instance, epiphytes in lowland dry or seasonal forest have high desiccation tolerance 36, 37. Epiphytes in continental cloud forests like the Andes, which experience variable cloudiness regimes, may have more resistance to drought than those in locations with more stable cloud bases. Likewise, epiphytes growing at lower elevations, below the cloud base, may have greater drought tolerance than epiphytes growing above the cloud base.
Beginning in the 2005 austral winter (June and July), we conducted a year-long reciprocal transplant experiment across an elevational gradient in cloud formation in the eastern Andes of southern Peru to test the effect that cloud immersion has on the performance of vascular epiphytes. The reciprocal transplant design also allowed us to distinguish between the effects of moving mats away from their home elevation versus moving plants into lower moisture conditions. This is a key control, not often made climate change transplant studies [e.g. 29, 38], for if epiphytes are locally adapted, reduced performance is expected if moved in any direction from their bioclimatic optimum. The specific questions we addressed were: (1) Does demographic performance decline when epiphytes are moved farther from their home elevation? (2) Is this effect greater when transplanted down-slope into drier and warmer conditions as predicted by results from Costa Rica? 29
We focused on three aspects of demographic performance, ramet survival, recruitment, and change in population size. Ramet survival allows us to examine treatment effects on existing individuals, while ramet recruitment and population change gives insight into treatment effects on epiphyte populations. We expected that ramet survival and recruitment would decrease and population change become negative as mats were moved farther from their home elevation. We also hypothesized that the decrease in ramet survival and recruitment would be greater for mats moved down-slope than for mats moved upslope if moisture level is the dominant factor in determining epiphyte species distributions.
Materials and methods
Study area
The Kosñipata Valley (13°03′S, 71°33′W) lies along the eastern slope of the Andes in southern Peru. Elevations range from about 800 m to over 4000 m, and vegetation changes from pre-montane rainforest at the lowest elevations to tropical subalpine forest and puna (alpine grassland) at the highest elevations 39. The experiment was installed along a single forested ridge, with three transplant sites at 1500, 1650, and 1800 m. We chose these elevations because the large increase in vascular epiphyte and bryophyte biomass 40, and step changes in soil properties 41 and biomass carbon stocks 42 between 1500 and 2000 m elevation in the Kosñipata Valley are likely associated with higher cloud incidence and lower temperatures, as seen on other tropical mountains 43, 44. The bedrock underlying the ridge is Permian granite, and soils are classified as umbric Gleysols 45.
The Kosñipata Valley has a perhumid climate described in detail in Rapp and Silman 46. Temperature decreases linearly with altitude and annual rainfall is high across the gradient, with a distinct, but weak dry season (monthly rainfall > monthly potential evapotranspiration except during drought). Vapor pressure deficit (VPD) typically decreases with altitude across the east Andean slope above 1500 m for all months except June 46. While differences in VPD between 1500 m (lowest experimental elevation) and 1800 m (highest experimental elevation) are typically small, excursions to higher VPD (greater desiccation) are more severe at 1500 m 46. The dry season (May–August) is when cloud immersion is expected to be most important for cloud forest plants. In July, the driest month, high relative humidity associated with cloud immersion (>95%) is more common at 1840 m than at 1500 m at climate stations approximately 1 km from the study site, while vapor pressure deficits greater than 1.0 kPa are more common at the higher elevation ( Table 1). Vapor pressure deficits greater than 1.0 kPa are associated with moisture stress in cloud forest plants 47, 48. We took daily photographs at 16:00 local time from a fixed location at 1400 m facing up-valley towards the ridge were the experiment was installed during June and July 2005 and July 2006. We categorized cloud base height using these photographs as being less than 1500 m (below experimental elevations), 1500–1800 m (within experimental elevations), or greater than 1800 m (above the experimental elevations). This analysis confirmed that cloud base height did not differ between years (Χ 2 = 3.32, p = 0.19), and that cloud frequency increased with elevation (cloud base <1500 m in 9% of observations; 1500–1800 m in 30% of observations; >1800 in 61% of observations).
Table 1. July climate for weather stations within 1 kilometer of the transplant sites.
Values represent the mean for July in 2007, 2008, and 2009, except for precipitation, which does not include data for 2007. RH95 is the proportion of time relative humidity was greater than 95%. VPD excursions is the number of days per month in which vapor pressure deficit (VPD) was 1.0 kPa or greater.
| Elevation
(m) |
Temperature
(°C) |
Precipitation
(mm/day) |
Relative humidity
(%) |
RH95
(%) |
VPD excursions
(days/month) |
|---|---|---|---|---|---|
| 1500 | 17.2 | 7.5 | 88.3 | 0.27 | 1.7 |
| 1840 | 16.1 | 4.6 | 88.4 | 0.32 | 0 |
Data collection
In the 2005 austral winter (dry season: June and July), we selected five Alzatea verticillata Ruiz & Pav. trees at each of three elevations, 1500 meters, 1650 meters, and 1800 meters elevation. Alzatea was an appropriate choice for a host tree because: (1) it was common at all three elevations; (2) it attains large size and has strong wood suitable for supporting climbers working in the trees; and (3) its unique architecture resulted in many large horizontal branches that supported sizeable epiphyte mats. We accessed trees using roped arborist techniques 49. In each tree, we chose four sections of epiphyte mat that were at least 25 cm wide and 30–40 cm long. Within each of the 60 mats, we marked an area of 25 × 25 cm with wire, and marked all ramets of vascular epiphytes within it, identified them to morpho-species, and recorded the length of shoots and number of leaves of each ramet.
Most taxa, and all of the focal taxa (see below), were non-reproductive when surveyed. This, combined with the fact that the epiphytic flora of the Andes is relatively poorly known, made it impossible to identify all taxa to species. We therefore used morpho-species designations in our analysis. Taxonomic uncertainty therefore, could affect our results if individuals of multiple cryptic species were combined in the analysis. Nonvascular epiphytes were present, but not considered in this experiment.
Most mat dwelling epiphytes are clonal, with individual ramets connected by subsurface stems, but capable of surviving without connection to other ramets. It was difficult to determine individual genets without excavating the plants, so we identified and measured individual ramets rather than genetically distinct plants. For strap-leafed ferns in the genus Elaphoglossum, each ramet was a single leaf, while ramets for other taxa consisted of one or more stems with multiple leaves.
On each tree, one of the mats was left in place to serve as an undisturbed control. We cut each of the other three mats from the tree, lowered them to the ground, and then transplanted one each to a random tree at each of the three elevations. After all transplants were complete, each tree had one undisturbed mat, one mat that had been removed and then replaced at the same elevation, and one mat from each of the other two elevations. We tied each mat in place using wire, and then watered it with one liter of water to minimize any desiccation effect that handling may have had. Supplementary Figure 1– Supplementary Figure 4 illustrate the process of transplanting the epiphyte mats.
We left the mats undisturbed for one year, and resurveyed them the following year in June and July 2006 ( Dataset 1). We searched for all marked ramets and counted the new ramets of each morpho-species. We assumed ramets obviously more than a year old (28 out of 1400+ original ramets) had lost their tag if previously marked ramets of the same morpho-species were not found in the same mat. Eight ‘old’ ramets were still not accounted for; we assumed these were missed during the first census. Any other missing ramets were assumed to be dead.
Full data set including all epiphyte ramets surveyed in 2005, before transplanting epiphyte mats, and in 2006, after transplantation. Columns are as follows: Mat.num, a unique number for each transplanted mat; From.elev, the elevation of the mat before transplantation; To.elev, the elevation of the mat after transplanting; From.tree, the tree the mat was in before transplantation; To.tree, the tree the mat was in after transplanting; Ramet.num, unique number for each ramet in a mat; mSpecies, morpho-species of the ramet; Guild, life-form of the ramet (fern, orchid, or shrub); Ramet.condition, condition of the ramet in 2006 (a: alive, d: dead, f: fresh, m: mature, n: new, s: senescent); treatment, undisturbed control or transplanted mat. Study location: Kosñipata Valley, Peru (13°03′S, 71°33′W). Data was collected between June-July in 2005 and 2006.
With these data, we defined three measures of population performance: 1) survival, 2) recruitment, and 3) population change. Survival was defined as:
Survival = (N 2005 – D 2006)/N 2005,
where N 2005 was the number of ramets surveyed in a mat in each year, and D 2006 was the number of ramets surveyed in 2005 that had died by 2006. Recruitment was defined as:
Recruitment = n 2006/N 2005,
where n 2006 was the number of new ramets surveyed in 2006, which were not present in 2005. Population change was defined as:
Population change = (N 2006 – N 2005)/N 2005.
Focal species
We conducted analyses at the community level and for the most common morpho-species individually. The common morpho-species occurred in at least half (10) of all epiphyte mats transplanted from at least one elevation. These included four common morpho-species identified to genus, by which we will refer to them: a strap-leaf fern ( Elaphoglossum Schott ex J. Sm.), two orchid morpho-species ( Maxillaria Ruiz & Pav.; Scaphyglottis Poepp. & Endl), and an ericaceous shrub ( Cavendishia Lindl.). Collectively, these morpho-species accounted for 78% (1127/1452) of the ramets surveyed in the initial 2005 survey ( Table 2). Elaphoglossum was abundant across the gradient, Maxillaria and Scaphyglottis were most abundant at upper elevations, and Cavendishia was most common at the central elevation ( Table 2).
Table 2. Number of ramets in surveyed mats before transplanting.
Numbers in parentheses indicate number of mats the ramets were found in. Bold indicates ramets used in single species analyses.
| Species | ||||||
|---|---|---|---|---|---|---|
| Elevation (m) | Elaphoglossum | Maxillaria | Cavendishia | Schaphyglottis | All other species | Total |
| 1500 | 346 (19) | 16 (3) | 11 (4) | 0 (0) | 181 (12) | 554 (20) |
| 1650 | 163 (19) | 118 (17) | 46 (11) | 33 (8) | 55 (10) | 415 (20) |
| 1800 | 197 (19) | 96 (14) | 20 (8) | 81 (10) | 89 (17) | 483 (20) |
| Total | 706 (57) | 230 (34) | 77 (23) | 114 (18) | 325 (39) | 1452 (60) |
Statistical analysis
All analyses were performed using the mat as the experimental unit to account for within-mat correlations between ramets, i.e. to avoid pseudo replication. We fitted models to data that included all ramets irrespective of morpho-species to explore the overall community patterns of ramet survival, recruitment, and population change, and then modeled common morpho-species separately to look at individual morpho-species responses. We tested whether ramet recruitment was different than mortality in transplanted mats using a two-sided t-test. We then analyzed ramet survival, recruitment, and population change with respect to experimental manipulations using generalized linear mixed-effects models (GLMMs). Survival was modeled as a binomial distribution with a logit link function to account for the binary nature of the response (alive, dead). Recruitment (new ramets in 2006) and population change (total ramets in 2006) were modeled as a rate relative to the initial ramets per mat by using a Poisson distribution with a log link, and adding an offset of the log of the number of initial ramets in 2005. To account for the natural blocking by tree in our experimental design, models that included multiple source elevations and transplant elevations also included random effects for source tree and transplant tree. Models including only one source elevation included a random effect for source tree only. Likelihood ratio tests were used to assess the fixed effects, while Wald z-tests were used to evaluate differences between levels of fixed effects. We did not evaluate the significance of random effects because they were a required part of our experimental design. Finally, we confirmed that the residuals of the final model were not overdispersed 50 using code from Bolker et al. 51. All analyses were done in R [Version 2.15.2; 52]. In all analyses we considered an effect significant if the P-value was less than 0.05.
First, we tested for an effect of manipulating mats using data for undisturbed control mats and mats transplanted within elevation. Source elevation and treatment (transplant versus undisturbed) were modeled as fixed effects in this analysis. Then, we tested for effects of source and transplant elevation on the response variable, using data from just the transplanted mats. We took this two-tiered approach because a full model including all mats was unbalanced (e.g. there could not be a control mat that moved between elevations) and statistical models accounting for this would not converge computationally. For analysis of individual morpho-species, we used only source elevations for which the morpho-species was present in at least half (10) of the source mats from that elevation (see Table 2).
To investigate patterns in mat species composition we used Detrended Correspondance Analysis (DCA) because our compositional data collected across a directional gradient matched the assumptions of DCA. First, we investigated the change in composition versus elevation using the pre-transplantation composition of all mats. We then investigated compositional change due to experimental treatments by ordinating the composition of all mats during 2005 before transplantation, with the composition of mats in 2006, one year after transplantation. Permutation Multivariate Analysis of Variance using distance matrices [function adonis in the vegan R package; 53] was used to test for compositional changes with altitude and among years due to the transplantation.
Results
Transplant effect: transplants within elevation
First, we tested for an effect of transplantation independent of elevational distance moved by asking whether epiphytes in mats transplanted to the same elevation had different ramet survival, recruitment, and population change or turn-over than those in intact mats. Across all morpho-species, there was no significant effect of transplant, elevation, or their interaction on survival, recruitment, or population change of epiphyte ramets ( Table 3, Figure 1). However, individual morpho-species were affected by transplantation. For Elaphoglossum, survival was lower in mats transplanted to another site at the same elevation than in undisturbed controls, but there was no effect of elevation on survival or any interaction between elevation and transplantation ( Table 4, Figure 1). There was an interaction between elevation and transplantation for recruitment and population change in Elaphoglossum, however ( Table 4); both were lower for transplanted mats at 1500 m and 1650 m, but higher for mats transplanted at 1800 m ( Figure 1). For Maxillaria, recruitment was lower in transplanted mats, but not affected by elevation, and neither survival nor population change was affected by either transplanting or elevation ( Table 4, Figure 1). For Cavendishia, recruitment and population change were lower for transplanted mats ( Figure 1), but only significantly so for recruitment; survival was unaffected by transplantation ( Table 4). Transplanting did not affect survival, recruitment, or population change in Scaphyglottis ( Table 4, Figure 1).
Table 3. Results of generalized linear mixed-effects models (GLMMs) for all species in mats transplanted within elevations.
SE: standard error.
| Estimate | SE | Statistic | P | |
|---|---|---|---|---|
| (a) Survival | ||||
| Intercept | 0.16 | 0.08 | ||
| Treatment | Χ 2 (1) = 2.15 | 0.142 | ||
| Transplant vs. Control | -0.23 | 0.15 | z = -1.47 | 0.143 |
| Elevation | Χ 2 (2) = 1.20 | 0.548 | ||
| 1650 m vs. 1500 m | -0.11 | 0.18 | z = -0.63 | 0.530 |
| 1800 m vs. 1500 m | -0.20 | 0.19 | z = -1.07 | 0.287 |
| 1800 m vs. 1650 m | -0.09 | 0.21 | z = -0.42 | 0.675 |
| Treatment × elevation | Χ 2 (2) = 2.86 | 0.239 | ||
| (levels not shown) | ||||
| Source tree [R] | 0.00 | |||
| Transplant tree [R] | 0.00 | |||
| (b) Recruitment | ||||
| Intercept | -1.20 | 0.20 | ||
| Treatment | Χ 2 (1) = 0.00 | 0.983 | ||
| Transplant vs. Control | 0.00 | 0.14 | z = -0.02 | 0.982 |
| Elevation | Χ 2 (2) = 0.13 | 0.938 | ||
| 1650 m vs. 1500 m | -0.17 | 0.49 | z = -0.35 | 0.729 |
| 1800 m vs. 1500 m | -0.13 | 0.50 | z = -0.25 | 0.801 |
| 1800 m vs. 1650 m | 0.05 | 0.50 | z = 0.09 | 0.927 |
| Treatment × elevation | Χ 2 (2) = 4.53 | 0.104 | ||
| (levels not shown) | ||||
| Source tree [R] | 0.47 | |||
| Transplant tree [R] | 0.56 | |||
| (c) Population change | ||||
| Intercept | -0.08 | 0.06 | ||
| Treatment | Χ 2 (1) = 2.18 | 0.140 | ||
| Transplant vs. Control | -0.12 | 0.08 | z = -1.48 | 0.138 |
| Elevation | Χ 2 (2) = 0.32 | 0.852 | ||
| 1650 m vs. 1500 m | -0.03 | 0.14 | z = -0.20 | 0.842 |
| 1800 m vs. 1500 m | 0.05 | 0.14 | z = 0.37 | 0.710 |
| 1800 m vs. 1650 m | 0.08 | 0.14 | z = 0.56 | 0.575 |
| Treatment × elevation | Χ 2 (2) = 4.45 | 0.108 | ||
| (levels not shown) | ||||
| Source tree [R] | 0.13 | |||
| Transplant tree [R] | 0.10 | |||
[R] indicates random effect
Figure 1. Survival, recruitment, and population change of ramets of all epiphyte species pooled and four abundant epiphyte species from a reciprocal transplant experiment within three elevations.
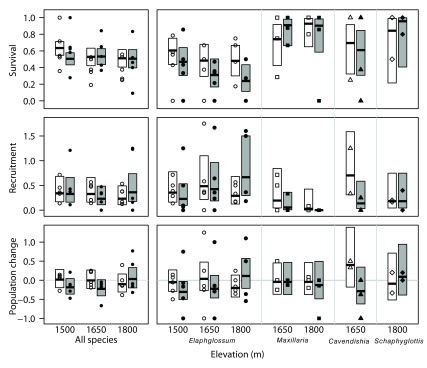
Points show survival (top), recruitment (middle), and population change (bottom) of individual mats in 2006, each expressed as a proportion relative to the number of ramets present in 2005. Thick horizontal lines and boxes depict the modeled mean and 95% confidence intervals, respectively. White shading depicts controls, dark shading transplant.
Table 4. Results of generalized linear mixed-effects models (GLMMs) for ramet survival, recruitment, and population change of the four most abundant species in control mats and mats transplanted within elevations.
SE: standard error.
| Survival | Recruitment | Population change | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | Statistic | P | Estimate | SE | Statistic | P | Estimate | SE | Statistic | P | |
| (a) Elaphoglossum | ||||||||||||
| Intercept | 0.1 | 0.23 | -1.04 | 0.39 | -0.05 | 0.15 | ||||||
| Treatment | Χ 2 (1) = 9.03 | 0.003 | Χ 2 (1) = 0.14 | 0.713 | Χ 2 (1) = 0.86 | 0.353 | ||||||
| Transplant vs. Control | -0.77 | 0.24 | z = -3.17 | 0.0015 | -0.44 | 0.34 | z = -1.32 | 0.187 | -0.31 | 0.18 | z = -1.70 | 0.089 |
| Elevation | Χ 2 (2) = 2.48 | 0.29 | Χ 2 (2) = 0.61 | 0.738 | Χ 2 (2) = 0.24 | 0.886 | ||||||
| 1650 m vs. 1500 m | -0.57 | 0.45 | z = -1.28 | 0.202 | 0.31 | 0.57 | z = 0.55 | 0.581 | 0.09 | 0.23 | z = 0.39 | 0.699 |
| 1800 m vs. 1500 m | -0.73 | 0.44 | z = -1.67 | 0.094 | -0.2 | 0.58 | z = -0.35 | 0.727 | -0.17 | 0.23 | z = -0.76 | 0.447 |
| 1800 m vs. 1650 m | -0.16 | 0.47 | z = -0.34 | 0.732 | -0.51 | 0.59 | z = -0.87 | 0.384 | -0.26 | 0.25 | z = -1.05 | 0.293 |
| Treatment × elevation | Χ 2 (2) = 0.74 | 0.689 | Χ 2 (2) = 7.96 | 0.019 | Χ 2 (2) = 5.98 | 0.05 | ||||||
| (levels not shown) | ||||||||||||
| Source tree [R] | 0.32 | 0.52 | 0.14 | |||||||||
| Transplant tree [R] | 0.52 | 0.52 | 0.13 | |||||||||
| (b) Maxillaria | ||||||||||||
| Intercept | 2.35 | 0.75 | -2.72 | 0.87 | -0.06 | 0.11 | ||||||
| Treatment | Χ 2 (1) = 1.18 | 0.277 | Χ 2 (1) = 3.48 | 0.062 | Χ 2 (1) = 0.03 | 0.86 | ||||||
| Transplant vs. Control | 0.8 | 0.71 | z = 1.13 | 0.26 | -1.32 | 0.84 | z = -1.58 | 0.115 | -0.04 | 0.22 | z = -0.16 | 0.87 |
| Elevation | Χ 2 (1) = 0.25 | 0.617 | Χ 2 (1) = 1.91 | 0.167 | Χ 2 (1) = 0.03 | 0.86 | ||||||
| 1800 m vs. 1650 m | 0.63 | 1.36 | z = 0.46 | 0.642 | -2.18 | 1.55 | z = -1.41 | 0.16 | -0.04 | 0.22 | z = -0.16 | 0.87 |
| Treatment × elevation | Χ 2 (1) = 1.17 | 0.279 | Χ 2 (1) = 0.33 | 0.565 | Χ 2 (1) = 0.04 | 0.848 | ||||||
| (levels not shown) | ||||||||||||
| Source tree [R] | 0.65 | 1.71 | 0 | |||||||||
| Transplant tree [R] | 1.57 | 0 | 0 | |||||||||
| (c) Cavendishia | ||||||||||||
| Intercept | 0.65 | 0.56 | -0.35 | 0.41 | 0.34 | 0.27 | ||||||
| Treatment | Χ 2 (1) = 0.10 | 0.75 | Χ 2 (1) = 4.86 | 0.028 | Χ 2 (1) = 2.69 | 0.101 | ||||||
| Transplant vs. Control | -0.38 | 0.97 | z = -0.39 | 0.699 | -1.63 | 0.83 | z = -1.98 | 0.048 | -0.67 | 0.41 | z = -1.63 | 0.104 |
| Source tree [R] | 0.68 | 0.2 | 0 | |||||||||
| (d) Scaphyglottis | ||||||||||||
| Intercept | 2.23 | 1.41 | -1.71 | 0.5 | 0 | 0.21 | ||||||
| Treatment | Χ 2 (1) = 1.17 | 0.279 | Χ 2 (1) = 0 | 1 | Χ 2 (1) = 0.18 | 0.67 | ||||||
| Transplant vs. Control | 1.4 | 1.37 | z = 1.03 | 0.304 | 0 | 1 | z = 0 | 1 | 0.18 | 0.43 | z = 0.43 | 0.67 |
| Source tree [R] | 1.66 | 0 | 0 | |||||||||
[R] indicates random effect
Transplants across elevation
Across all morpho-species, there were no significant effects on survival of any of the treatments for mats transplanted across elevations ( Table 5 and Figure 2). For recruitment and population change, there was a significant interaction between source and transplant elevation ( Table 5), with both positively associated with elevation for mats transplanted from 1500 and 1800 m, but negatively associated with altitude for mats from 1650 m ( Figure 2). Overall for transplanted mats, more ramets died than were recruited (mean change number of ramets per mat between years = -1.38; two-sided t-test, P = 0.01).
Table 5. Results of generalized linear mixed-effects models (GLMMs) for all species in mats transplanted between elevations.
SE: standard error.
| Estimate | SE | Statistic | P | |
|---|---|---|---|---|
| (a) Survival | ||||
| Intercept | 0.13 | 0.06 | ||
| Source elevation | Χ 2 (2) = 0.31 | 0.858 | ||
| 1650 m vs. 1500 m | -0.07 | 0.15 | z = -0.50 | 0.619 |
| 1800 m vs. 1500 m | -0.06 | 0.14 | z = -0.41 | 0.683 |
| 1800 m vs. 1650 m | 0.02 | 0.16 | z = 0.11 | 0.916 |
| Transplant elevation | Χ 2 (2) = 1.64 | 0.440 | ||
| 1650 m vs. 1500 m | 0.16 | 0.14 | z = 1.17 | 0.241 |
| 1800 m vs. 1500 m | 0.01 | 0.14 | z = 0.07 | 0.947 |
| 1800 m vs. 1650 m | -0.15 | 0.15 | z = -1.03 | 0.301 |
| Source elevation × transplant elevation | Χ 2 (4) = 1.09 | 0.895 | ||
| (levels not shown) | ||||
| Source tree [R] | 0.00 | |||
| Transplant tree [R] | 0.00 | |||
| (b) Recruitment | ||||
| Intercept | -1.33 | 0.42 | ||
| Source elevation | Χ 2 (2) = 0.41 | 0.816 | ||
| 1650 m vs. 1500 m | 0.27 | 0.48 | z = 0.57 | 0.570 |
| 1800 m vs. 1500 m | -0.48 | 0.51 | z = -0.94 | 0.345 |
| 1800 m vs. 1650 m | -0.46 | 0.51 | z = -0.89 | 0.373 |
| Transplant elevation | Χ 2 (2) = 2.37 | 0.305 | ||
| 1650 m vs. 1500 m | -0.29 | 0.42 | z = -0.69 | 0.492 |
| 1800 m vs. 1500 m | 0.47 | 0.40 | z = 1.16 | 0.244 |
| 1800 m vs. 1650 m | -0.09 | 0.46 | z = -0.20 | 0.845 |
| Source elevation × transplant elevation | Χ 2 (4) = 13.31 | 0.010 | ||
| (levels not shown) | ||||
| Source tree [R] | 0.66 | |||
| Transplant tree [R] | 0.53 | |||
| (c) Population change | ||||
| Intercept | -0.27 | 0.16 | ||
| Source elevation | Χ 2 (2) = 0.30 | 0.860 | ||
| 1650 m vs. 1500 m | 0.24 | 0.18 | z = 1.36 | 0.174 |
| 1800 m vs. 1500 m | -0.26 | 0.19 | z = -1.41 | 0.157 |
| 1800 m vs. 1650 m | 0.03 | 0.18 | z = 0.15 | 0.885 |
| Transplant elevation | Χ 2 (2) = 1.03 | 0.597 | ||
| 1650 m vs. 1500 m | 0.06 | 0.19 | z = 0.33 | 0.742 |
| 1800 m vs. 1500 m | 0.13 | 0.19 | z = 0.67 | 0.506 |
| 1800 m vs. 1650 m | 0.03 | 0.21 | z = 0.13 | 0.898 |
| Source elevation × transplant elevation | Χ 2 (4) = 13.57 | 0.009 | ||
| (levels not shown) | ||||
| Source tree [R] | 0.17 | |||
| Transplant tree [R] | 0.21 | |||
[R] indicates random effect
Figure 2. Survival, recruitment, and population change of epiphyte ramets from a reciprocal transplant experiment across three elevations.
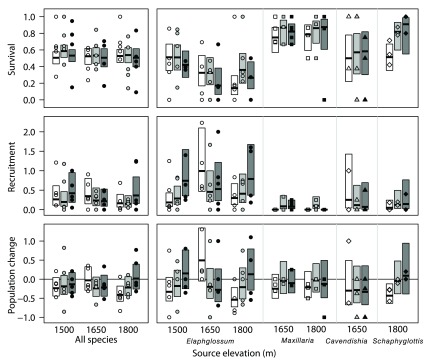
Points show survival (top), recruitment (middle), and population change (bottom) of individual mats in 2006, each expressed as a proportion relative to the number of ramets present in 2005. Thick horizontal lines and boxes depict the modeled mean and 95% confidence intervals, respectively. White shading depicts 1500 m elevation, light grey 1650 m elevation and dark grey 1800 m elevation.
Elaphoglossum ramets in mats originating at 1500 m had consistently and significantly higher survival than those originating at 1650 m or 1800 m, but there was no effect of transplant elevation on survival ( Table 6, Figure 2). For both recruitment and population change, however, there was a significant interaction between source elevation and transplant elevation, with both recruitment and population change declining in mats transplanted at lower elevations for mats originating at 1500 m and 1800 m, but for mats originating at 1650 m recruitment was greater and population change more positive for mats transplanted to 1500 m than for mats transplanted to higher elevation ( Table 6, Figure 2).
Table 6. Results of generalized linear mixed-effects models (GLMMs) for ramet survival of the four most abundant species in mats transplanted between elevations.
SE: standard error.
| Survival | Recruitment | Population change | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | Statistic | P | Estimate | SE | Statistic | P | Estimate | SE | Statistic | P | |
| (a) Elaphoglossum | ||||||||||||
| Intercept | -0.97 | 0.31 | -1.68 | 0.42 | -0.4 | 0.22 | ||||||
| Source elevation | Χ 2 (2) = 8.28 | 0.02 | Χ 2 (2) = 1.80 | 0.407 | Χ 2 (2) = 1.21 | 0.547 | ||||||
| 1650 m vs. 1500 m | -0.92 | 0.36 | z = -2.57 | 0.01 | 1.68 | 0.43 | z = 3.94 | <0.001 | 0.8 | 0.24 | z = 3.32 | 0.001 |
| 1800 m vs. 1500 m | -1.07 | 0.35 | z = -3.02 | 0 | 0.47 | 0.45 | z = 1.05 | 0.292 | -0.37 | 0.28 | z = -1.34 | 0.181 |
| 1800 m vs. 1650 m | -0.15 | 0.39 | z = -0.38 | 0.7 | -0.11 | 0.43 | z = -0.26 | 0.795 | -0.01 | 0.28 | z = -0.03 | 0.974 |
| Transplant elevation | Χ 2 (2) = 1.34 | 0.51 | Χ 2 (2) = 2.74 | 0.254 | Χ 2 (2) = 1.87 | 0.393 | ||||||
| 1650 m vs. 1500 m | 0.31 | 0.37 | z = 0.85 | 0.39 | 0.43 | 0.5 | z = 0.86 | 0.389 | 0.21 | 0.28 | z = 0.73 | 0.465 |
| 1800 m vs. 1500 m | -0.13 | 0.38 | z = -0.34 | 0.74 | 1.38 | 0.49 | z = 2.83 | 0.005 | 0.54 | 0.28 | z = 1.92 | 0.055 |
| 1800 m vs. 1650 m | -0.44 | 0.38 | z = -1.17 | 0.24 | 0.15 | 0.52 | z = 0.29 | 0.774 | -0.1 | 0.35 | z = -0.29 | 0.772 |
| Source elevation × transplant elevation | Χ 2 (4) = 5.86 | 0.21 | Χ 2 (4) = 24.42 | <0.001 | Χ 2 (4) = 24.79 | <0.001 | ||||||
| (levels not shown) | ||||||||||||
| Source tree [R] | 0.37 | 0.4 | 0.19 | |||||||||
| Transplant tree [R] | 0.49 | 0.57 | 0.32 | |||||||||
| (b) Maxillaria | ||||||||||||
| Intercept | 1.52 | 0.2 | -21.33 | 5515.88 | -0.14 | 0.08 | ||||||
| Source elevation | Χ 2 (1) = 0.20 | 0.658 | Χ 2 (1) = 0.23 | 0.632 | Χ 2 (1) = 0.00 | 0.946 | ||||||
| 1800 m vs. 1650 m | 0.19 | 0.41 | z = 0.45 | 0.651 | -0.38 | 0.83 | z = -0.46 | 0.645 | 0.02 | 0.17 | z = 0.10 | 0.918 |
| Transplant elevation | Χ 2 (2) = 1.95 | 0.377 | Χ 2 (2) = 5.97 | 0.051 | Χ 2 (2) = 1.27 | 0.529 | ||||||
| 1650 m vs. 1500 m | 0.67 | 0.51 | z = 1.32 | 0.187 | 18.86 | 5515.88 | z = 0.00 | 0.997 | 0.23 | 0.2 | z = 1.11 | 0.268 |
| 1800 m vs. 1500 m | 0.44 | 0.48 | z = 0.93 | 0.353 | 18.39 | 5515.88 | z = 0.00 | 0.99 | 0.15 | 0.21 | z = 0.73 | 0.464 |
| 1800 m vs. 1650 m | -0.23 | 0.55 | z = -0.42 | 0.674 | -0.47 | 0.83 | z = -0.56 | 0.576 | -0.08 | 0.2 | z = -0.38 | 0.704 |
| Source elevation × transplant elevation | Χ 2 (2) = 0.17 | 0.917 | Χ 2 (2) = 1.78 | 0.412 | Χ 2 (2) = 0.03 | 0.986 | ||||||
| (levels not shown) | ||||||||||||
| Source tree [R] | 0 | 0 | 0 | |||||||||
| Transplant tree [R] | 0 | 0.49 | 0 | |||||||||
| (c) Cavendishia | ||||||||||||
| Intercept | 0.22 | 0.34 | -1.97 | 0.45 | -0.36 | 0.2 | ||||||
| Transplant elevation | Χ 2 (2) = 0.18 | 0.916 | Χ 2 (2) = 0.77 | 0.681 | Χ 2 (2) = 0.02 | 0.989 | ||||||
| 1650 m vs. 1500 m | 0.29 | 0.83 | z = 0.35 | 0.729 | -0.77 | 1.14 | z = -0.67 | 0.502 | 0.02 | 0.49 | z = 0.04 | 0.967 |
| 1800 m vs. 1500 m | 0.34 | 0.86 | z = 0.39 | 0.696 | -1.26 | 1.4 | z = -0.90 | 0.368 | -0.05 | 0.52 | z = -0.09 | 0.925 |
| 1800 m vs. 1650 m | 0.05 | 0.8 | z = 0.06 | 0.951 | -0.49 | 1.29 | z = -0.38 | 0.702 | -0.07 | 0.47 | z = -0.15 | 0.884 |
| Source tree [R] | 0 | 0 | 0 | |||||||||
| (d) Scaphyglottis | ||||||||||||
| Intercept | 0.05 | 0.33 | -2.44 | 0.54 | -0.26 | 0.14 | ||||||
| Transplant elevation | Χ 2 (2) = 9.81 | 0.007 | Χ 2 (2) = 1.64 | 0.44 | Χ 2 (2) = 4.37 | 0.112 | ||||||
| 1650 m vs. 1500 m | 1.45 | 0.64 | z = 2.25 | 0.024 | 0.95 | 0.95 | z = 1.01 | 0.314 | 0.52 | 0.31 | z = 1.69 | 0.092 |
| 1800 m vs. 1500 m | 2.25 | 1.1 | z = 2.05 | 0.041 | 1.1 | 1.03 | z = 1.07 | 0.285 | 0.65 | 0.36 | z = 1.81 | 0.071 |
| 1800 m vs. 1650 m | 0.8 | 1.19 | z = 0.67 | 0.501 | 0.15 | 0.96 | z = 0.15 | 0.877 | 0.13 | 0.36 | z = 0.37 | 0.712 |
| Source tree [R] | 0 | 0.63 | 0 | |||||||||
[R] indicates random effect
For Maxillaria, the only significant effect for transplanted mats was that for transplant elevation on recruitment ( Table 6); recruitment was low in all transplanted mats, but there was zero recruitment in mats transplanted to 1500 m ( Figure 2). There were no significant effects of source elevation or transplant elevation on survival or population change ( Table 6), but survival was lower and population change more negative for ramets transplanted to 1500 m ( Figure 2).
All three measures of performance were unaffected by transplant elevation in Cavendishia ( Table 6, Figure 2). For Scaphyglottis, survival, recruitment, and population change were all progressively lower in mats transplanted to lower elevations ( Figure 2), but the difference was significant only for survival ( Table 6).
Community composition
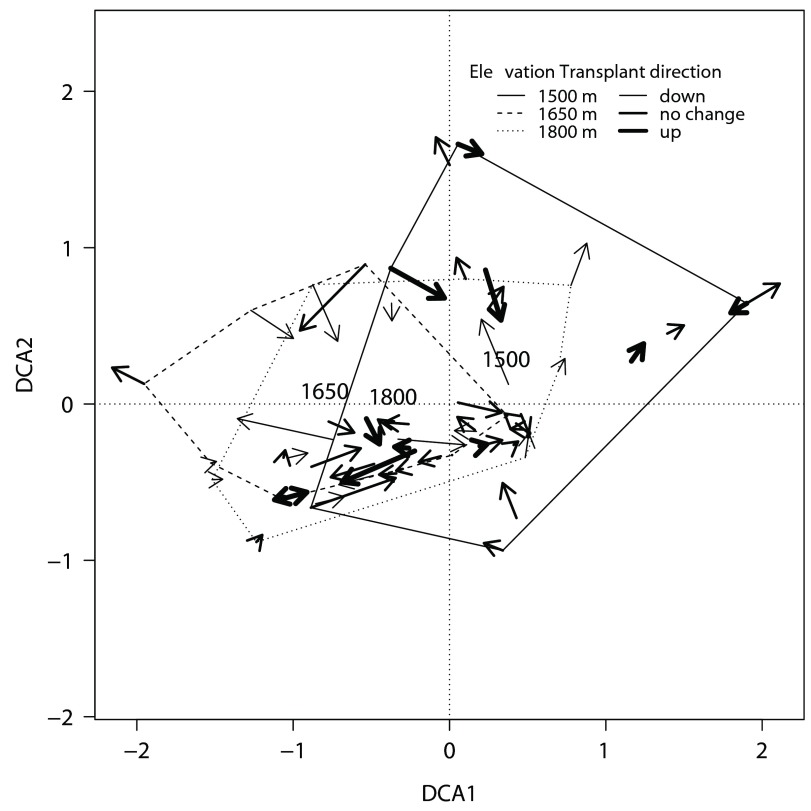
Prior to transplanting mats, the epiphyte community composition showed significant differences across the elevational gradient, although relatively little of the variation could be explained by elevation ( Table 7); most of the compositional separation was between mats at 1500 m and the other two elevations ( Figure 3). Morpho-species richness increased with elevation (Poisson regression, Z = 2.446, P = 0.0144), while the number of ramets per mat declined (Poisson regression, Z = -2.281, P = 0.0225; Table 8). Comparison of pre- and post-treatment species compositions in mats revealed no directional shift in community composition due to transplantation ( Table 7). A few mats did show large changes ( Figure 3), likely because of large changes in abundance in Elaphoglossum, either through high ramet mortality or recruitment ( Figure 2).
Table 7. ANOVA table from permutational multivariate Analysis of Variance to test differences in composition between mats at different elevations across years and treatments.
| Source of variation | df | SS | MS | F | R 2 |
|---|---|---|---|---|---|
| Elevation | 1 | 0.75 | 0.75 | 3.38 | 0.0283** |
| Treatment | 1 | 0.25 | 0.25 | 1.14 | 0.0095 |
| Year | 1 | 0.071 | 0.07 | 0.32 | 0.0027 |
| Elevation × Treatment | 1 | 0.17 | 0.17 | 0.74 | 0.0062 |
| Elevation × Year | 1 | 0.31 | 0.31 | 1.40 | 0.0117 |
| Treatment × Year | 1 | 0.04 | 0.04 | 0.20 | 0.0016 |
| Elevation × Treatment
× Year |
1 | 0.10 | 0.10 | 0.44 | 0.0037 |
| Residuals | 112 | 24.92 | 0.22 | 0.9363 | |
| Total | 119 | 26.61 | 1 | ||
Significance levels: **p < 0.01
Figure 3. First two axis of a Detrended Correspondance Analysis (DCA) on the species composition of epiphyte mats both before and after mats were transplanted.
Arrows connect the compositions of individual mats before and a year after transplantation. Line width depicts direction of transplanting. Hulls are drawn around the 2005 composition of mats that originated at the same elevation, and labels are placed at the hull centroid.
Table 8. Species richness or morpho-species and ramet density of mats surveyed in 2005.
Per mat values are means with standard error in parentheses.
| Elevation (m) | Total
species |
Species per mat | Ramets per mat |
|---|---|---|---|
| 1500 | 13 | 2.35 (1.04) | 27.70 (11.20) |
| 1650 | 14 | 3.65 (1.14) | 20.75 (6.82) |
| 1800 | 16 | 3.75 (1.16) | 24.15 (11.81) |
Discussion
Vascular epiphytes transplanted down slope from our highest elevation had lower ramet recruitment and the number of ramets declined ( Table 5, Figure 2) when transplanted to the lowest elevation, suggesting warmer temperatures and lower cloud immersion will cause community-level changes for species currently above the cloud base. This result corroborates previous work in another tropical montane site, which found fewer leaves and shorter life-spans for vascular epiphytes moved down slope 29. However, reciprocal transplants between all elevations revealed unexpected dynamics, with demographic rates differing in their response and morpho-species responding individualistically to the treatments ( Figure 2). In general, survival was less sensitive than recruitment; for all ramets combined there was a significant interaction between source and transplant elevation for ramet recruitment and population change, but not for survival ( Table 5). Morpho-species also differed in the strength of their response to transplantation across elevation. Cavendishia, a small woody shrub showed the least response, while Elaphoglossum, a strap-leafed fern in which individual leaves were the measurement unit, was most responsive to treatments; there were significant effects for both ramet recruitment and population change ( Table 6). The two orchid morpho-species were intermediate, with Scaphyglottis responding more strongly (significant effect for survival, Table 6) than Maxillaria which has stouter stems.
Given the relatively short 1-year duration of the experiment, the relatively modest effects observed should perhaps be expected. Stronger effects would be expected for an experiment carried out over multiple years, since plants often react to stressful conditions through physiological responses such as closing stomata, which lowers carbon acquisition 54– 57. While this could eventually lead to mortality, plants are likely to first lower investment in growth and reproduction 57. In this context, it is not surprising that recruitment was more responsive that survival. It is also possible that functional differences in ramet construction may account for the differences in response among morpho-species to the elevational transplants, although our experiment was not set up to test this hypothesis directly. More species in each functional type would be needed to rigorously test this, as well as physiological measurements to demonstrate functional differences among species.
In general, it appears that epiphytes responded to water stress and/or higher temperatures but we also found evidence for local adaptation. The response to transplanting was strongest in those transplanted from the highest elevation, which is coolest and has the highest degree of cloud immersion. Epiphytes from lower elevations only benefitted slightly from increased water availability and cooler temperatures, possibly indicating they are better adapted to withstand heat and drought stress. Epiphytes from the middle elevations, where temperatures and cloud immersion are intermediate, responded in more idiosyncratic ways to transplantation. Finally, while composition changed across the elevational gradient, there was no significant directional shift in composition due to any of the transplant treatments ( Figure 3, Table 7). The relative resistance of epiphytes to expected transplant-induced moisture stress found in this study could be due to two competing factors, described below in more detail: (1) rainfall compensation in this pluvial system, where high rainfall is sufficient to maintain epiphyte water-balance below cloud base; and/or (2) higher epiphyte drought tolerance from a history of variable rainfall and occasional drought in these continental mountains. While these factors act in opposite directions, both are plausible mechanisms for epiphyte resilience to decreased cloud immersion. It is even possible that they work in concert, with rainfall compensation maintaining epiphyte water-balance in most years, while occasional drought provides a selective pressure for drought tolerance. We describe each of these mechanisms in detail below.
Rainfall compensation
While our results for epiphytes transplanted from the highest elevation are consistent with the hypothesized altitudinal gradient in moisture stress, this gradient had less of an effect on epiphyte performance than the one in Monteverde, Costa Rica 29. The relative importance of cloud immersion for the distribution of epiphytes in this system may account for the difference. A consistent cloud base is a significant feature of many tropical montane forests 2, 58, and regular low cloud is assumed to maintain the diversity and abundance of cloud forest epiphytes, and control many of the unique structural and functional features of cloud forests 43, 44. Indeed, we chose the elevations for this experiment because of a suite of changes in ecosystem structure and function that occur at these elevations, including a step-change in bryophyte and vascular epiphyte biomass 40, tree height, above ground biomass, and forest productivity declining 42, and soil organic matter increasing 41 above 1500 m. Tree diversity also begins to decline above 1500 m in the study region 59, 60 mimicking the general pattern in the Andes 61, 62. These clear changes in forest structure, diversity and productivity contrast with smoother changes in climate. Mean temperature, precipitation, and VPD, a measure of moisture stress on plants, all decrease linearly with elevation above 1000 m 46.
High rainfall in this part of the Andes may mean that epiphytes here are less dependent on cloud immersion to maintain their water balance than their counterparts in other cloud forests. Even in 2005 under drought conditions, total precipitation for the year was 3273 mm. In this pluvial system, cloud base may be less important in determining epiphyte distributions than in other systems. It is noteworthy that the Nadkarni and Solano 29 experiment was carried out on the leeward Pacific slope of Monteverde, which is drier than the Caribbean slope 63, 64. Mean annual precipitation on the Pacific slope is 2155 mm at 1480 m in the cloud forest 65, and declines at lower elevations 64, and there is a 5–6 month dry season where much of the hydrologic balance is maintained by cloud immersion 65. This steep moisture gradient between cloud forest and lower elevations probably leads to a greater dependence of epiphytes on cloud immersion. If this previous study had been carried out on the Caribbean slope, where precipitation is higher at lower elevation 64, the results may have been similar to our study. On leeward slopes rainfall compensation may occur, in which epiphyte survival is enhanced by high rainfall even when there is less frequent cloud immersion.
Drought tolerance
Even though high rainfall may maintain epiphytes under normal conditions in the eastern Andes, droughts do occur, and epiphytes may be adapted to infrequent drought, especially at the lower fringe of the cloud forest. Drought in the Amazon basin during 2005 66, 67 resulted in lower precipitation in the cloud forest. Although microclimate data were not available at the experimental elevations during the study, rainfall at the Peruvian SENAMHI meterological station at Rocotal (13°06′41″S, 71°34′14″, approximately 7 km from the transplant site at 2010 m elevation) for May–August in 2005 was the lowest for any year measured (mean May–August precipitation for 2000–2008: 601 mm; 2005: 175 mm; Figure 4). There was no recorded rainfall in July 2005, the only month during the nine-year measurement period with no recorded precipitation (mean July precipitation: 112 mm). In addition, actual cloud water interception based on fog collectors in place during the experiment did not show a gradient of increasing moisture with elevations during the 2005 dry season (four week total weight of water collected: 1500 m, 1109 g m -2; 1750 m, 35 g m -2; 1900 m, 72 g m -2). All elevations were very dry, and desiccation was evident in bryophytes and non-succulent vascular epiphytes in the study area. However, ramet mortality in undisturbed control mats was not significantly greater than recruitment at any elevation ( Figure 1). In addition, mat species composition did not change directionally between years ( Figure 3). Thus, undisturbed epiphytes between 1500 and 1800 m in this Andean cloud forest appeared resistant to drought over the one-year time scale of our experiment.
Figure 4. Monthly precipitation (mm) recorded at the Rocotal meteorological station at 2010 m maintained by SENAMHI in the Kosñipata Valley.
Monthly means with 95% confidence intervals are shown for 2000–2008 exclusive of 2005, and compared with 2005 monthly totals.
This resistance to drought may be related to the normally variable and seasonal rainfall at the study site. Annual rainfall totals ranged between 3 and 6 m per year in a five year period not including the 2005 Amazonian drought. Precipitation was lowest in June and July 46, when temporary drought is possible, though for no month did potential evapotranspiration exceed precipitation at these elevations in most years. However, prolonged (days-to-weeks) periods of direct sun can induce drought stress, and epiphyte species that live in this part of the Andes may possess adaptations for surviving drought, similar to those in lowland dry or seasonal forests 36, 37, 68. Epiphyte drought tolerance is higher in areas where drought occurs more frequently 19, 69, and many epiphyte species have adaptations for surviving drought – crassulacean acid metabolism, desiccation tolerance, pseudobulbs, succulent leaves and other water-storing organs. Consistent with this idea, Elaphoglossum ramets transplanted from 1500 m had higher survival than those from higher elevations, regardless of the transplant elevation ( Figure 2). The stronger response of epiphyte mats transplanted from normally cloud immersed elevations (i.e. 1800 m) compared to those transplanted from lower elevations suggests that lower elevation populations may be better adapted to drought stress due to less frequent cloud immersion. Another example of locally adapted epiphytes was observed in subtropical China, where bryophytes transplanted downslope lost biomass, while in situ measurements showed no change in biomass across the gradient 38.
Conclusion
Greater epiphyte resistance to drought in this part of the Andes compared to previous studies may indicate that even seemingly benign dry seasons or dry periods can be important for structuring epiphyte communities, with potential implications for larger scale patterns of diversity. More generally, while epiphyte response to global climate change on tropical mountains is discussed in the literature 18, 19, 29, 38, tropical mountains and their climates are highly heterogeneous, and predictions may defy all but the broadest generalizations. Fundamental differences in the climate and biogeographical contexts may lead to differences in species response to climate change. Long-term experimental studies in tropical montane systems are needed to understand the drivers of patterns of epiphyte abundance, in particular why there is a change in biomass and abundance at putative ‘cloud base’ (which is correlated with changes throughout the ecosystem), and how these diverse communities will respond to climate change. While our experiment suggests that epiphytes in our study system show some resistance to climate change, climate models predict more severe droughts in parts of the Andes 20, 21. Pervasive changes in the tree canopy of the western Amazon following the 2005 Amazon drought persisted until an even stronger drought in 2010 70; it is unknown whether similarly long-lasting effects were present in Andean cloud forest. Given the keystone position of epiphytes in cloud forests, drought-induced changes in epiphyte communities could have cascading effects throughout the ecosystem.
Acknowledgements
This paper is a product of the Andes Biodiversity and Ecosystem Research Group (ABERG). We would like to thank Norma Salinas for help with permits, the Cock-of-the-Rock Lodge for letting us set up the experiment on their land, Luis Imunda for clearing a trail, Richard Amick for helping with canopy access, and William Farfan for identifying morpho-species to genus.
Funding Statement
Funding was generously provided by a Wake Forest University Biology Department Vecellio Fund grant to J.M. Rapp, and grants from the Gordon and Betty Moore Foundation Andes to Amazon Program, NSF EAR 0711414, NSF DEB-0237684, and an REU supplement to DEB-0237684 to M.R. Silman.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
v2; ref status: indexed
Supplementary materials
Figure S1. Study branch.
Experimental mats in a tree at 1800 m were all on the same large horizontal branch. From lower left to upper right: two mats transplanted from trees at 1500 and 1650 m, an open space awaiting a transplanted mat from 1800 m, and a surveyed undisturbed control mat.
Figure S2. Mat removed.
A transplanted mat was removed from this branch at 1650 m.
Figure S3. Transplanted mat.
A mat from another tree was transplanted into the same location.
Figure S4. Control mat.
A control epiphyte mat after being surveyed in 2005. This mat at 1800 m elevation included primarily Elaphoglossum and Schaphyglottis.
References
- 1.Foster P: The potential negative impacts of global climate change on tropical montane cloud forests. Earth Sci Rev. 2001;55(1–2):73–106 10.1016/S0012-8252(01)00056-3 [DOI] [Google Scholar]
- 2.Loope LL, Giambelluca TW: Vulnerability of island tropical montane cloud forests to climate change, with special reference to East Maui, Hawaii. Clim Change. 1998;39(2–3):503–517 10.1023/A:1005372118420 [DOI] [Google Scholar]
- 3.Herzog S, Martinez R, Jorgensen P, et al. : Climate change and biodiversity in the tropical Andes. Inter-American Institute for Global Change Research (IAI) and Scientific Committee on Problems of the Environment (SCOPE).2011. Reference Source [Google Scholar]
- 4.Myers N, Mittermeier RA, Mittermeier CG, et al. : Biodiversity hotspots for conservation priorities. Nature. 2000;403(6772):853–858 10.1038/35002501 [DOI] [PubMed] [Google Scholar]
- 5.Gentry AH: Tropical Forest Biodiversity - Distributional Patterns and Their Conservational Significance. Oikos. 1992;63(1):19–28 Reference Source [Google Scholar]
- 6.Knapp S: Assessing patterns of plant endemism in neotropical uplands. Bot Rev. 2002;68(1):22–37 Reference Source [Google Scholar]
- 7.Kuper W, Kreft H, Nieder J, et al. : Large-scale diversity patterns of vascular epiphytes in Neotropical montane rain forests. J Biogeogr. 2004;31(9):1477–1487 10.1111/j.1365-2699.2004.01093.x [DOI] [Google Scholar]
- 8.Cruz-Angon A, Baena ML, Greenberg R: The contribution of epiphytes to the abundance and species richness of canopy insects in a Mexican coffee plantation. J Trop Ecol. 2009;25(5):453–463 10.1017/S0266467409990125 [DOI] [Google Scholar]
- 9.Cruz-Angon A, Greenberg R: Are epiphytes important for birds in coffee plantations? An experimental assessment. J Appl Ecol. 2005;42(1):150–159 10.1111/j.1365-2664.2004.00983.x [DOI] [Google Scholar]
- 10.Nadkarni NM, Matelson TJ: Bird Use of Epiphyte Resources in Neotropical Trees. Condor. 1989;91(4):891–907 10.2307/1368074 [DOI] [Google Scholar]
- 11.Yanoviak SP, Nadkarni NM, Solano R: Arthropod assemblages in epiphyte mats of Costa Rican cloud forests. Biotropica. 2007;39(2):202–210 10.1111/j.1744-7429.2006.00261.x [DOI] [Google Scholar]
- 12.Gomez-Peralta D, Oberbauer SF, McClain ME, et al. : Rainfall and cloud-water interception in tropical montane forests in the eastern Andes of Central Peru. For Ecol Manage. 2008;255(3–4):1315–1325 10.1016/j.foreco.2007.10.058 [DOI] [Google Scholar]
- 13.Holscher D, Kohler L, van Dijk A, et al. : The importance of epiphytes to total rainfall interception by a tropical montane rain forest in Costa Rica. J Hydrol. 2004;292(1–4):308–322 10.1016/j.jhydrol.2004.01.015 [DOI] [Google Scholar]
- 14.Munoz-Villers LE, Holwerda F, Gomez-Cardenas M, et al. : Water balances of old-growth and regenerating montane cloud forests in central Veracruz, Mexico. J Hydrol. 2012;462–463:53–66 10.1016/j.jhydrol.2011.01.062 [DOI] [Google Scholar]
- 15.Nadkarni NM, Schaefer D, Matelson TJ, et al. : Biomass and nutrient pools of canopy and terrestrial components in a primary and a secondary montane cloud forest, Costa Rica. For Ecol Manage. 2004;198(1–3):223–236 10.1016/j.foreco.2004.04.011 [DOI] [Google Scholar]
- 16.Umana NHN, Wanek W: Large canopy exchange fluxes of inorganic and organic nitrogen and preferential retention of nitrogen by epiphytes in a tropical lowland rainforest. Ecosystems. 2010;13(3):367–381 10.1007/s10021-010-9324-7 [DOI] [Google Scholar]
- 17.Weathers KC: The importance of cloud and fog in the maintenance of ecosystems. Trends Ecol Evol. 1999;14(6):214–215 10.1016/S0169-5347(99)01635-3 [DOI] [PubMed] [Google Scholar]
- 18.Benzing DH: Vulnerabilities of tropical forests to climate change: The significance of resident epiphytes. Clim Change. 1998;39(2–3):519–540 10.1023/A:1005312307709 [DOI] [Google Scholar]
- 19.Zotz G, Bader MY: Epiphytic Plants in a Changing World-Global: Change Effects on Vascular and Non-vascular Epiphytes. In Progress in Botany, U. Lüttge, et al., Editors. Springer-Verlag: Berlin Heidelberg.2009; 70:147–170 10.1007/978-3-540-68421-3_7 [DOI] [Google Scholar]
- 20.Neelin JD, Munnich M, Su H, et al. : Tropical drying trends in global warming models and observations. Proc Nat Acd Sci U S A. 2006;103(16):6110–6115 10.1073/pnas.0601798103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urrutia R, Vuille M: Climate change projections for the tropical Andes using a regional climate model: Temperature and precipitation simulations for the end of the 21st century. J Geophys Res Atmos. 2009;114(D2). 10.1029/2008JD011021 [DOI] [Google Scholar]
- 22.Vuille M, Francou B, Wagnon P, et al. : Climate change and tropical Andean glaciers: Past, present and future. Earth Sci Rev. 2008;89(3–4):79–96 10.1016/j.earscirev.2008.04.002 [DOI] [Google Scholar]
- 23.Martinez R, Ruiz D, Andrade M, et al. : Synthesis of the climate of the tropical Andes, in Climate change and biodiversity in the tropical Andes. SK Herzog, et al., Editors. Inter-American Institute for Global Change Research (IAI) and Scientific Committee on Problems of the Environment (SCOPE).2011. Reference Source [Google Scholar]
- 24.Cowling SA, Shin Y, Pinto E, et al. : Water recycling by Amazonian vegetation: coupled versus uncoupled vegetation-climate interactions. Philos Trans R Soc Lond B Biol Sci. 2008;363(1498):1865–1871 10.1098/rstb.2007.0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinto E, Shin Y, Cowling SA, et al. : Past, present and future vegetation-cloud feedbacks in the Amazon Basin. Clim Dynamics. 2009;32(6):741–751 10.1007/s00382-009-0536-5 [DOI] [Google Scholar]
- 26.Still CJ, Foster PN, Schneider SH: Simulating the effects of climate change on tropical montane cloud forests. Nature. 1999;398(6728):608–610 10.1038/19293 [DOI] [Google Scholar]
- 27.Pounds JA, Fogden MPL, Campbell JH: Biological response to climate change on a tropical mountain. Nature. 1999;398(6728):611–615 10.1038/19297 [DOI] [Google Scholar]
- 28.Anchukaitis KJ, Evans MN: Tropical cloud forest climate variability and the demise of the Monteverde golden toad. Proc Natl Acad Sci U S A. 2010;107(11):5036–5040 10.1073/pnas.0908572107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nadkarni NM, Solano R: Potential effects of climate change on canopy communities in a tropical cloud forest: an experimental approach. Oecologia. 2002;131(4):580–586 10.1007/s00442-002-0899-3 [DOI] [PubMed] [Google Scholar]
- 30.Hamilton LS, Juvik JO, Scatena FN: The Puerto Rico Tropical Cloud Forest Symposium: Introduction and Workshop Synthesis, in Tropical Montane Cloud Forests. LS Hamilton, JO Juvik, and FN Scatena, Editors. Springer-Verlag: New York.1994. Reference Source [Google Scholar]
- 31.Stadtmuller T: Cloud Forests in the Humid Tropics: A Bibliographic Review. Centro Agronómico Tropical de Investigación y Enseñanza: Turrialba, Costa Rica: United Nations University Press: Tokyo.1987;82 Reference Source [Google Scholar]
- 32.Bruijnzeel LA: Hydrological functions of tropical forests: not seeing the soil for the trees? Agric Ecosystems Environ. 2004;104(1):185–228 10.1016/j.agee.2004.01.015 [DOI] [Google Scholar]
- 33.Lawton RO, Nair US, Pielke RA, Sr, et al. : Climatic impact of tropical lowland deforestation on nearby montane cloud forests. Science. 2001;294(5542):584–587 10.1126/science.1062459 [DOI] [PubMed] [Google Scholar]
- 34.Pounds JA, Bustamante MR, Coloma LA, et al. : Widespread amphibian extinctions from epidemic disease driven by global warming. Nature. 2006;439(7073):161–167 10.1038/nature04246 [DOI] [PubMed] [Google Scholar]
- 35.Killeen TJ, Douglas M, Consiglio T, et al. : Dry spots and wet spots in the Andean hotspot. J Biogeogr. 2007;34(8):1357–1373 10.1111/j.1365-2699.2006.01682.x [DOI] [Google Scholar]
- 36.Andrade JL: Dew deposition on epiphytic bromeliad leaves: an important event in a Mexican tropical dry deciduous forest. J Trop Ecol. 2003;19(5):479–488 10.1017/S0266467403003535 [DOI] [Google Scholar]
- 37.Bader MY, Menke G, Zotz G: Pronounced drought tolerance characterizes the early life stages of the epiphytic bromeliad Tillandsia flexuosa. Funct Ecol. 2009;23(3):472–479 10.1111/j.1365-2435.2009.01547.x [DOI] [Google Scholar]
- 38.Song L, Liu WY, Nadkarni NM: Response of non-vascular epiphytes to simulated climate change in a montane moist evergreen broad-leaved forest in southwest China. Biol Conserv. 2012;152:127–135 10.1016/j.biocon.2012.04.002 [DOI] [Google Scholar]
- 39.Malhi Y, Silman M, Salinas N, et al. : Introduction: Elevation gradients in the tropics: laboratories for ecosystem ecology and global change research. Glob Chang Biol. 2010;16(12):3171–3175 10.1111/j.1365-2486.2010.02323.x [DOI] [Google Scholar]
- 40.Horwath AB: Epiphytic Bryophytes as Cloud Forest Indicators: Stable Isotopes, Biomass and Diversity along an Altitudinal Gradient in Peru. In Jesus College, University of Cambridge: Cambridge, U.K.2011;284 [Google Scholar]
- 41.Zimmermann M, Meir P, Bird MI, et al. : Climate dependence of heterotrophic soil respiration from a soil-translocation experiment along a 3000 m tropical forest altitudinal gradient. Eur J Soil Sci. 2009;60(6):895–906 10.1111/j.1365-2389.2009.01175.x [DOI] [Google Scholar]
- 42.Girardin CAJ, Malhi Y, Aragao LEOC, et al. : Net primary productivity allocation and cycling of carbon along a tropical forest elevational transect in the Peruvian Andes. Glob Chang Biol. 2010;16(12):3176–3192 10.1111/j.1365-2486.2010.02235.x [DOI] [Google Scholar]
- 43.Frahm JP, Gradstein SR: An Altitudinal Zonation of Tropical Rain-Forests Using Byrophytes. J Biogeogr. 1991;18(6):669–678 10.2307/2845548 [DOI] [Google Scholar]
- 44.Grubb PJ: Factors controlling the distribution of forest types on tropical mountains: new facts and a new perspective, in Altitudinal zonationin Malesia, JR Flenley, Editor, University of Hull, Department of Geography: Hull, England.1974;13–46 [Google Scholar]
- 45.Zimmermann M, Meir P, Bird MI, et al. : Temporal variation and climate dependence of soil respiration and its components along a 3000 m altitudinal tropical forest gradient. Global Biogeochem Cycles. 2010; 24(4). 10.1029/2010GB003787 [DOI] [Google Scholar]
- 46.Rapp JM, Silman MR: Diurnal, seasonal, and altitudinal trends in microclimate across a tropical montane cloud forest. Clim Res. 2012;55(1):17–32 10.3354/cr01127 [DOI] [Google Scholar]
- 47.Motzer T, Munz N, Kuppers M, et al. : Stomatal conductance, transpiration and sap flow of tropical montane rain forest trees in the southern Ecuadorian Andes. Tree Physiol. 2005;25(10):1283–1293 10.1093/treephys/25.10.1283 [DOI] [PubMed] [Google Scholar]
- 48.Cunningham SC: Stomatal sensitivity to vapour pressure deficit of temperate and tropical evergreen rainforest trees of Australia. Trees. 2004;18(4):399–407 10.1007/s00468-004-0318-y [DOI] [PubMed] [Google Scholar]
- 49.Dial R, Tobin SC: Description of arborist methods for forest canopy access and movement. Selbyana. 1994;15(2):24–37 Reference Source [Google Scholar]
- 50.Venables WN, Ripley BD: Modern Applied Statistics with S. 4th ed New York: Springer.2002;497 Reference Source [Google Scholar]
- 51.Bolker BM, Brooks ME, Clark CJ, et al. : Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol. 2009;24(3):127–135 10.1016/j.tree.2008.10.008 [DOI] [PubMed] [Google Scholar]
- 52.R Development Core Team, R: A language and environment for statistical computing. R Foundation for Statistical Computing: Vienna, Austria;2012. Reference Source [Google Scholar]
- 53.Oksanen J, Blanchet FG, Kindt R, et al. : vegan: Community Ecology Package.2010. Reference Source [Google Scholar]
- 54.McDowell NG: Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiol. 2011;155(3):1051–1059 10.1104/pp.110.170704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farquhar GD, Sharkey TD: Stomatal Conductance and Photosynthesis. Ann Rev Plant Physiol Plant Mol Biol. 1982;33:317–345 10.1146/annurev.pp.33.060182.001533 [DOI] [Google Scholar]
- 56.Hsiao TC: Plant Responses to Water Stress. Ann Rev Plant Physiol Plant Mol Biol. 1973;24:519–570 10.1146/annurev.pp.24.060173.002511 [DOI] [Google Scholar]
- 57.Chaves MM, Pereira JS, Maroco J, et al. : How plants cope with water stress in the field. Photosynthesis and growth. Ann Bot. 2002;89:907–916 10.1093/aob/mcf105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ritter A, Regalado CM, Aschan G: Fog reduces transpiration in tree species of the Canarian relict heath-laurel cloud forest (Garajonay National Park, Spain). Tree Physiol. 2009;29(4):517–528 10.1093/treephys/tpn043 [DOI] [PubMed] [Google Scholar]
- 59.Fierer N, McCain CM, Meir P, et al. : Microbes do not follow the elevational diversity patterns of plants and animals. Ecology. 2011;92(4):797–804 10.1890/10-1170.1 [DOI] [PubMed] [Google Scholar]
- 60.Jankowski JE, Merkord CL, Rios WF, et al. : The relationship of tropical bird communities to tree species composition and vegetation structure along an Andean elevational gradient. J Biogeogr. 2013;40(5):950–962 10.1111/jbi.12041 [DOI] [Google Scholar]
- 61.Gentry AH: Patterns of Diversity and Floristic Composition in Neotropical Montane Forests, in Biodiversity and Conservation of Neotropical Montane Forests. SP Churchill, et al., Editors. The New York Botanical Garden: New York.1995;p.103–126 Reference Source [Google Scholar]
- 62.Gentry AH: Changes in plant community diversity and floristic composition on environmental and geographical gradients. Ann Mo Bot Gard. 1988;75(1):1–34 Reference Source [Google Scholar]
- 63.Guswa AJ, Rhodes AL, Newell SE: Importance of orographic precipitation to the water resources of Monteverde, Costa Rica. Adv Water Resour. 2007;30(10):2098–2112 10.1016/j.advwatres.2006.07.008 [DOI] [Google Scholar]
- 64.Häger A, Dohrenbusch A: Hydrometeorology and structure of tropical montane cloud forests under contrasting biophysical conditions in north-western Costa Rica. Hydrol Process. 2011;25(3):392–401 10.1002/hyp.7726 [DOI] [Google Scholar]
- 65.Clark KL, Lawton RO, Butler PR: The physical environment, in Monteverde: Ecology and conservation of a tropical cloud forest. NM Nadkarni and NT Wheelwright, Editors. Oxford University Press: New York.2000;15–38 Reference Source [Google Scholar]
- 66.Marengo JA, Nobre CA, Tomasella J, et al. : The drought of Amazonia in 2005. J Climate. 2008;21(3):495–516 10.1175/2007JCLI1600.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zeng N, Yoon JH, Marengo JA, et al. : Causes and impacts of the 2005 Amazon drought. Environ Res Lett. 2008;3(1):1–9 10.1088/1748-9326/3/1/014002 [DOI] [Google Scholar]
- 68.Stancato GC, Mazzafera P, Buckeridge MS: Effect of a drought period on the mobilisation of non-structural carbohydrates, photosynthetic efficiency and water status in an epiphytic orchid. Plant Physiol Biochem. 2001;39(11):1009–1016 10.1016/S0981-9428(01)01321-3 [DOI] [Google Scholar]
- 69.Higuera D, Wolf JH: Vascular epiphytes in dry oak forests show resilience to anthropogenic disturbance, Cordillera Oriental, Colombia. Caldasia. 2010;32(1):161–174 Reference Source [Google Scholar]
- 70.Saatchi S, Asefi-Najafabady S, Malhi Y, et al. : Persistent effects of a severe drought on Amazonian forest canopy. Proc Natl Acad Sci U S A. 2013;110(2):565–570 10.1073/pnas.1204651110 [DOI] [PMC free article] [PubMed] [Google Scholar]