Abstract
Neutralizing antibodies (NAbs) can confer immunity to primate lentiviruses by blocking infection in macaque models of AIDS1–4. However, earlier studies of anti-HIV 1 NAbs administered to infected individuals or humanized mice, reported poor control of virus replication and the rapid emergence of resistant variants 5–7. A new generation of anti-HIV 1 monoclonal antibodies (mAbs), possessing extraordinary potency and breadth of neutralizing activity, has recently been isolated from infected individuals 8. These NAbs target different regions of the HIV 1 envelope glycoprotein including the CD4 binding site (bs), glycans located in the V1/V2, V3, and V4 regions, and the membrane proximal external region of gp419–14. We have examined two of the new antibodies, directed to the CD4 bs and the V3 region (3BNC117 and 10-1074 respectively) for their ability to block infection and suppress viremia in macaques infected with the R5 tropic SHIVAD8 virus, which emulates many of the pathogenic and immunogenic properties of HIV 1 during infections of rhesus macaques15,16. Either antibody alone can potently block virus acquisition. When administered individually to recently infected monkeys, the 10-1074 antibody caused a rapid decline in virus loads to undetectable levels for 4 to 7 days, followed by virus rebound during which neutralization resistant variants became detectable. When administered together, a single treatment rapidly suppressed plasma viremia for 3 to 5 weeks in some long-term chronically SHIV infected animals with low CD4+ T cell levels. A second cycle of anti-HIV 1 mAb therapy, administered to two previously treated animals, successfully controlled virus rebound. These results suggest that immunotherapy or a combination of immunotherapy plus conventional antiretroviral drugs might be useful as a treatment for chronically HIV-1 infected individuals experiencing immune dysfunction.
SHIVAD8 was selected as challenge virus for this study because several clinical features observed during infections of macaques were similar to those reported in HIV 1 infected individuals. SHIVAD8 consistently establishes sustained set point viremia in monkeys inoculated by the intravenous or intrarectal routes and causes unrelenting depletion of CD4+ T lymphocytes15,16. During the acute infection, SHIVAD8 targets memory CD4+ T cells in blood and at effector sites in tissues. Their gradual depletion is subsequently followed by the loss of the naïve CD4+ T lymphocyte subset. The latter heralds the onset of symptomatic immunodeficiency in macaques characterized by the development of opportunistic infections (Mycobacterium, Pneumocystis, Cryptosporidium species), lymphomas, marked weight loss, and death within 2 to 4 years of virus inoculation. In addition, SHIVAD8 infected monkeys generate cross reactive antibodies, capable of neutralizing Tier 1 and Tier 2 HIV 1 isolates, including one “elite neutralizer” macaque producing potent cross-clade neutralizing activity 8,17,18. As is frequently the case for HIV 1 elite neutralizers, resistant variants emerged in this SHIVAD8 infected animal, which succumbed to AIDS at week 117 post infection 19.
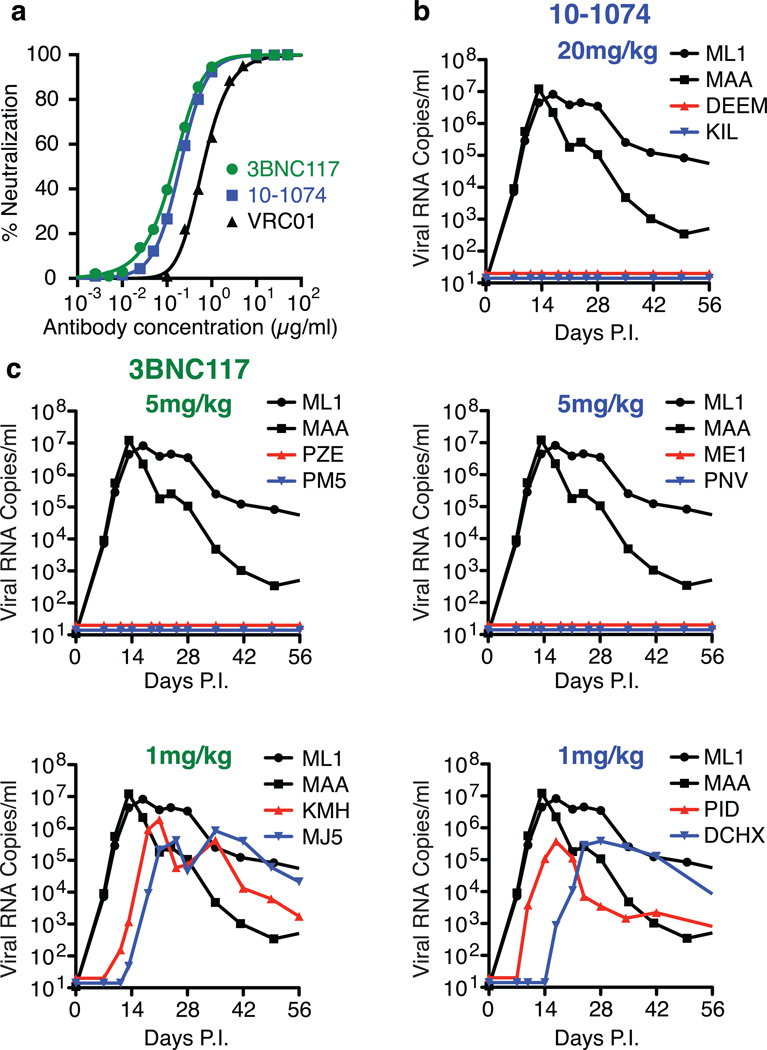
The neutralization sensitivities of SHIVAD8EO17, a molecularly cloned derivative of SHIVAD816, to the 10-107420, 3BNC11721, and VRC0122 mAbs, were measured in the TZM-bl cell assay (Fig. 1a). The IC50 values determined for 10-1074, 3BNC117 and VRC01 against SHIVAD8EO were 0.20 µg/ml, 0.14 µg/ml, and 0.63 µg/ml, respectively, indicating that the 10-1074 and 3BNC117 mAbs had similar activities and either one was more potent than VRC01 against SHIVAD8EO in vitro.
Figure 1.
HIV monoclonal antibodies block SHIV acquisition. a. Neutralization of SHIVAD8EO by three anti-HIV 1 mAbs assayed in TZM-bl cells. b. Pre-exposure passive transfer of 10-1074 mAb to macaques followed by SHIVAD8EO IR challenge. c. Pre-exposure passive transfer of 3BNC117 mAb to macaques followed by SHIVAD8EO IR challenge. Macaques ML1 and MAA received 20 mg/kg of control anti-dengue virus NS1 IgG1 mAb.
A more critical test of neutralization efficacy is the prevention of virus acquisition in vivo following passive transfer of NAbs and subsequent virus challenge. This was examined by administering the 10-1074 or 3BNC117 mAbs 24h prior to an intrarectal challenge of Indian origin rhesus monkeys with 1000 TCID50 (approximately 3 animal infectious doses50 [AID50] 15), an inoculum size we have previously determined is sufficient to establish SHIVAD8EO infections in vivo following a single inoculation by this route in 10/10 macaques. As shown in Fig. 1b, the transfer of the 10-1074 mAb at a dose of 20 mg/kg or 5 mg/kg to monkeys prevented virus acquisition in 2/2 and 2/2 macaques, respectively. The administration of 1 mg/kg of 10-1074, however, failed to protect either of two animals. For the 3BNC117 passive transfer, the mAb in vivo titration was initiated at a dose of 5 mg/kg, which blocked virus acquisition in 2/2 monkeys (Fig. 1c). In contrast, 2/2 macaques became infected when the dose of 3BNC117 was reduced to 1 mg/kg. The plasma concentrations at the time of challenge for both mAbs were comparable (approximately 100 µg/ml) in the four monkeys treated with a dose of 5 mg/kg.
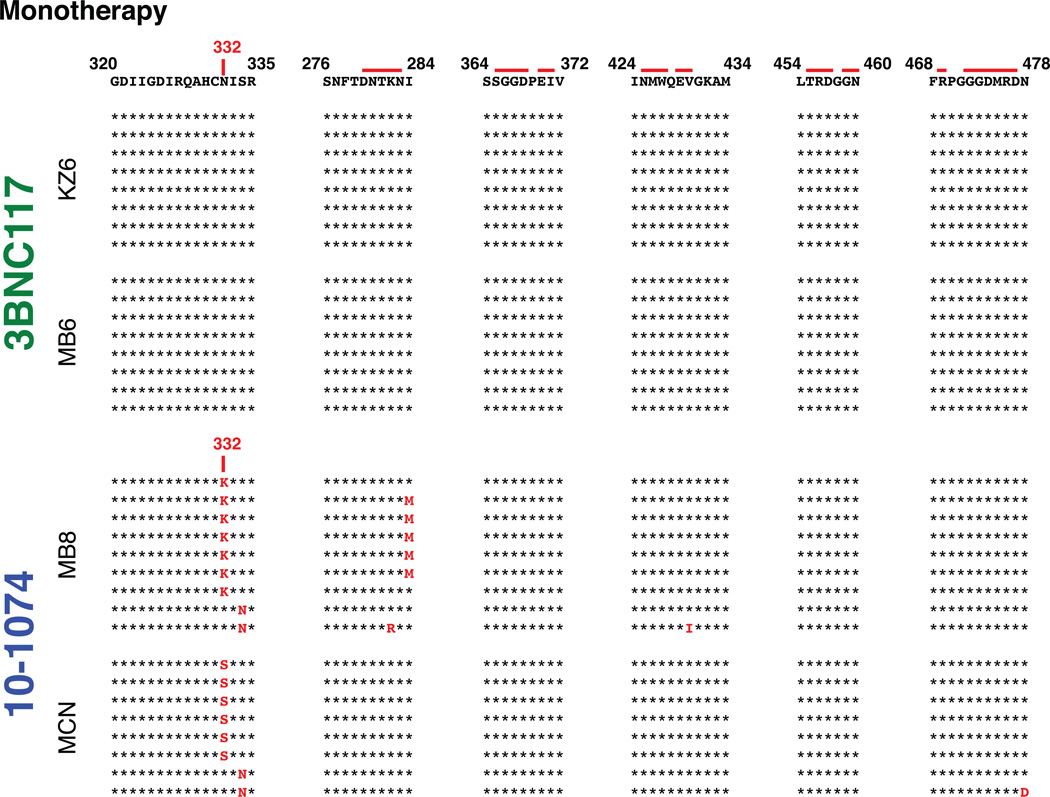
Recent studies have reported that combinations of three or more of the newly cloned broad and potent antibodies effectively suppressed HIV 1 viremia in humanized mice10,23,24. However, humanized mice carry a lower viral load, and do not have an intact adaptive or innate immune system. We have also previously reported that in the presence of neutralizing antibody, the clearance of circulating HIV-1 in vivo is accelerated from approximately 20 minutes to 3 or 4 minutes25. The potential therapeutic benefit of anti-HIV 1 NAbs controlling virus replication in infected monkeys was evaluated by administering either 10-1074 or 3BN117 alone (monotherapy) to two animals, 12 weeks following virus inoculation, when post-peak set point viremia levels had been established. Based on the pre-exposure prevention experiments described above, infected macaques were treated with either antibody at a dose of 10 mg/kg (Table 1). Both recipients of the 3BNC117 mAb experienced rapid declines of plasma viremia to background levels at day 10 following the start of treatment (Fig. 2a). Virus rebound became detectable on day 20 in these two macaques. The 10-1074 treated animal MCN sustained an unexpectedly rapid and a greater than a 103 reduction of plasma viral loads to undetectable levels by day 6 of treatment initiation. However, the effects of 10-1074 mAb administration were more variable and of short duration (4 days) (Fig. 2a and S1). Furthermore, single genome amplification (SGA) analysis of virus in the four monotherapy recipients revealed that the rebound virus present in both of the 10-1074 treated monkeys had sustained changes that eliminated the gp120 N332 glycan, rendering the virus resistant to this antibody (Fig. S2)10.
Table 1.
SHIVAD8 infected macaques treated with anti-HIV mAbs
| Animal | Pre-Infection | Treatment Initiation | Pre mAb Treatment | Clinical Status | mAb Administered | ||
|---|---|---|---|---|---|---|---|
| CD4+ T Cells (Cells/µl) |
Weeks Post Infection | CD4+ T Cells (Cells/µl) |
Viral Load (RNA Copies/ml) |
3BNC117 (mg/kg) |
10-1074 (mg/kg) |
||
| KZ6 | 881 | 12 | 1447 | 5.22E+03 | Post-Acute Set Point | 10 | 0 |
| MB6 | 545 | 12 | 1598 | 7.43E+03 | Post-Acute Set Point | 10 | 0 |
| MB8 | 1512 | 12 | 2625 | 1.14E+04 | Post-Acute Set Point | 0 | 10 |
| MCN | 210 | 12 | 599 | 2.14E+04 | Post-Acute Set Point | 0 | 10 |
| DBZ3 | 650 | 159 | 118 | 1.08E-04 | Asymptomatic | 10 | 10 |
| DC99A | 623 | 159 | 165 | 7.60E+03 | Asymptomatic | 10 | 10 |
| DBXE | 1585 | 163 | 158 | 1.96E+05 | Intermittent diarrhea | 10 | 10 |
| DCF1 | 1203 | 157 | 105 | 1.44E+05 | Intermittent diarrhea | 10 | 10 |
| DCM8 | 608 | 163 | 43 | 1.59E+0. | Intermittent diarrhea | 10 | 10 |
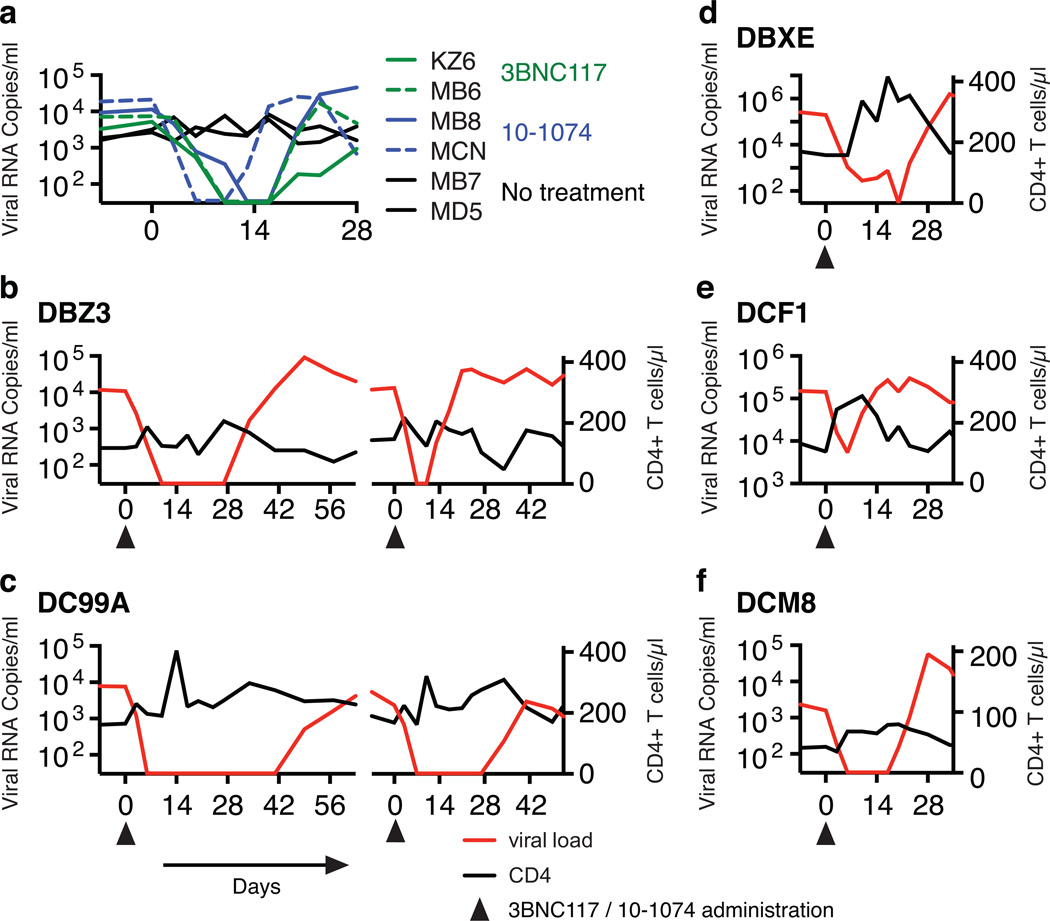
Figure 2.
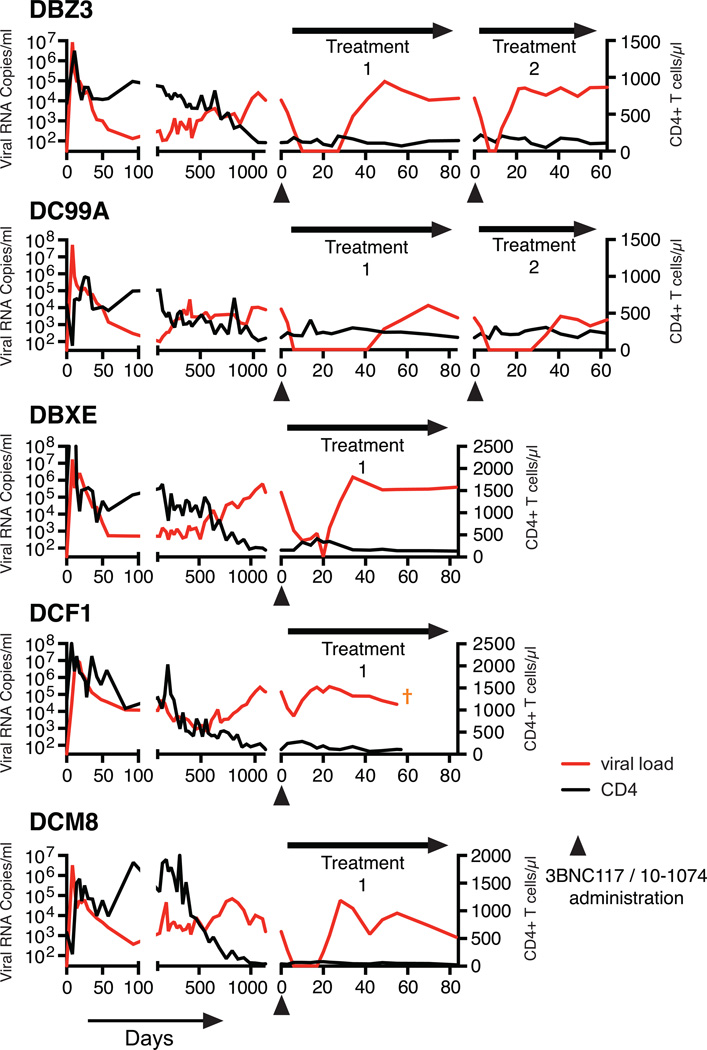
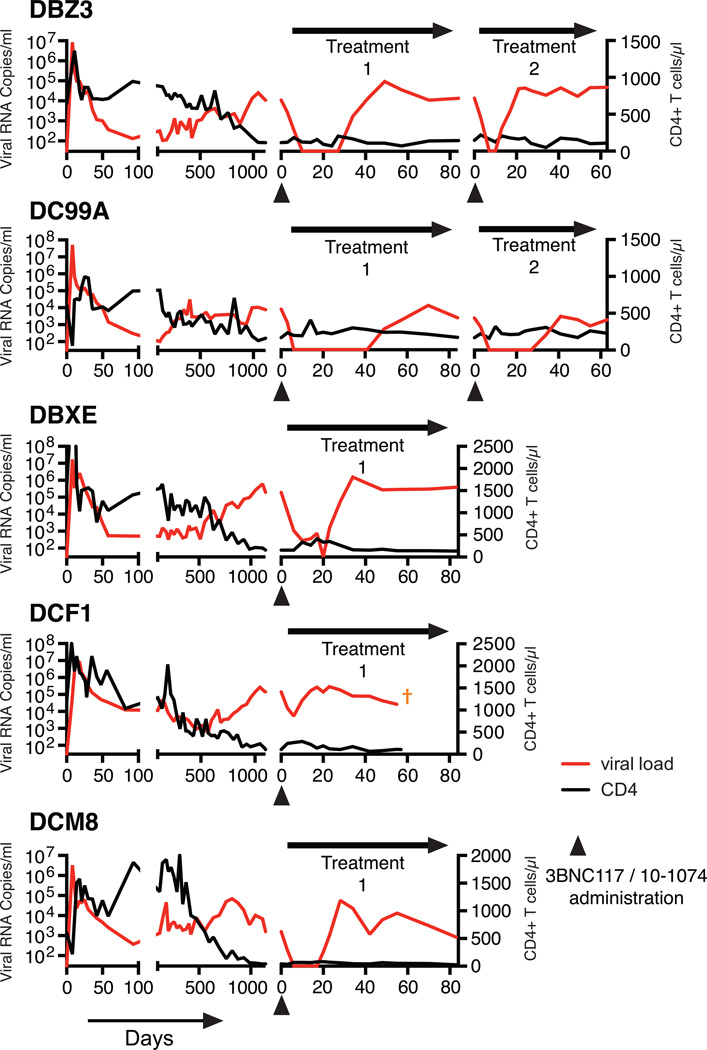
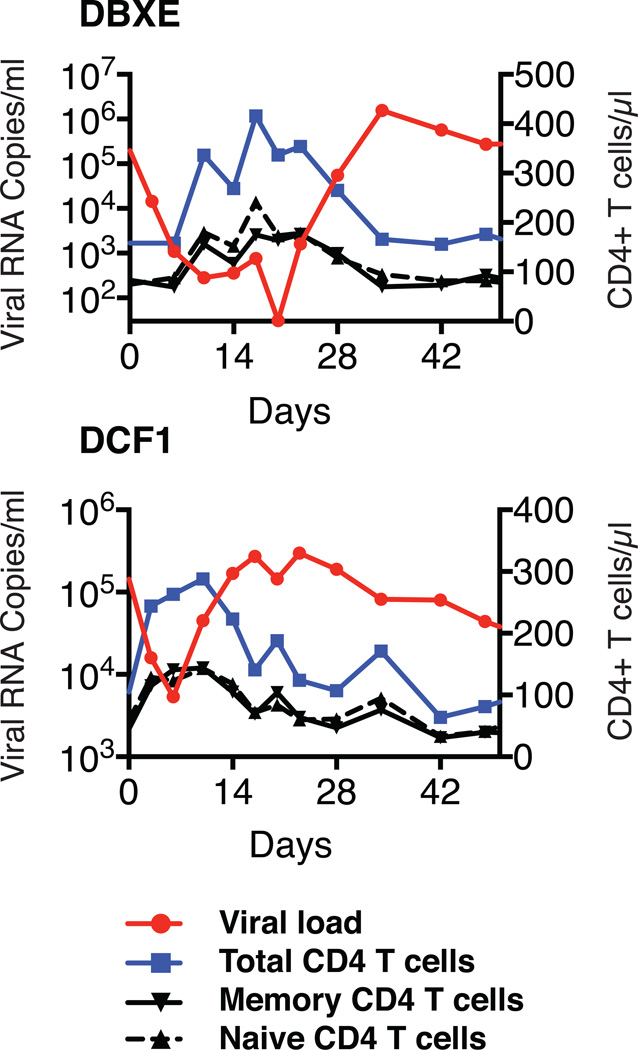
Suppression of plasma viremia following mono- or combination anti-HIV 1 neutralizing antibody treatment. (a) Plasma viral loads in post-acute set-point SHIV infected rhesus macaques with/without single mAb treatment. Plasma viral loads and total CD4+ T cell numbers in chronically SHIV infected rhesus macaques [DBZ3 (b), DC99A (c), DBXE (d), DCF1 (e) and DCM8 (f)] following combination mAb treatment.
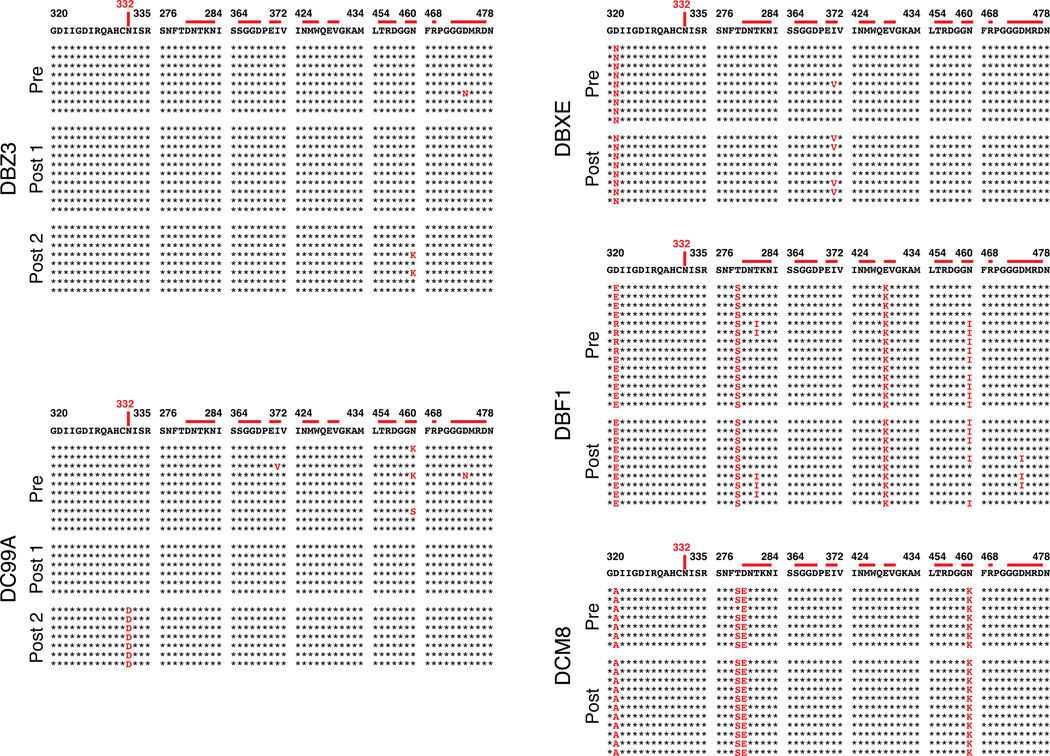
The known failure of antiretroviral drug monotherapy to durably control HIV 1 and SIV replication in vivo, prompted us to co-administer 3BNC117 and 10-1074 mAbs, each at a dose of 10mg/kg, to chronically SHIVAD8EO infected monkeys. In addition, the capacity of the molecularly cloned SHIVAD8EO to cause unrelenting CD4+ T cell depletion and symptomatic immunodeficiency provided the opportunity to assess the potential therapeutic effects of combination anti-HIV-1 neutralizing mAbs in two groups of infected macaques experiencing the pathogenic effects of SHIVAD8EO infection. The first group consisted of two clinically asymptomatic animals (DBZ3 and DC99A) that had been infected for 159 weeks and had sustained similar and significant declines of circulating and bronchoalveolar lavage (BAL) CD4+ T cells (Table 1, Figs. S3 and S4). At the time of mAb administration, the plasma viral loads in macaques DBZ3 and DC99A were 1.08×104 and 7.6×103 RNA copies/ml, respectively. Both monkeys responded to combination anti-HIV 1 mAb treatment with immediate and rapid reductions of plasma viremia to undetectable levels within 7 to 10 days (Figs. 2b and 2c). Suppression of measurable SHIVAD8EO in the plasma of macaques DBZ3 and DC99A, following a single administration of the two mAbs, lasted 18 and 36 days, respectively, exceeding the 4 to 7 day window of virus suppression observed during monotherapy. In each case, plasma viremia rebounded to pretreatment levels. A second cycle of combination immunotherapy was administered to monkeys DBZ3 and DC99A to determine whether the re-emergence of detectable plasma viremia simply reflected insufficient levels of circulating antibodies in these animals. As shown in the right panels of Figs 2b and 2c, the viral loads in each animal became undetectable by day 7 of the second treatment cycle. Viremia was suppressed for 4 days in macaque DBZ3 and 28 days in monkey DC99A. SGA analysis demonstrated that the rebound virus that emerged in monkey DC99A, following the second round of immunotherapy, carried a gp120 N332D mutation, indicating that resistance to the 10-1074 component of the antibody combination had occurred (Fig S5b).
Combination mAb therapy was also evaluated in a second group of three chronically infected animals (DBX3, DCF1, and DCM8), which had also been infected for more than 3 years but were clinically symptomatic, experiencing intermittent diarrhea and or anorexia (Table 1). At the time of mAb administration, the level of circulating CD4+ T cells in one of these macaques (DCM8) was only 43 cells/µl and was somewhat higher in animals DCF1 (105 cells/µl) and DBXE (158 cells/µl). Plasma viral loads exceeded 105 RNA copies/ml in animals DBXE and DCF1 and were significantly lower (1.59×103 RNA copies/ml) in monkey DCM8. As shown in Figs. 2d to f and Fig. S3, the administration of the two mAbs to monkey DBXE resulted in reduction of viremia from 2.0×105 RNA copies at day 0 to undetectable levels in plasma at day 20. This was followed, within a few days, by a resurgence of high levels of circulating virus in DBXE. Macaque DCM8, with more modest plasma virus loads and very low numbers of circulating CD4+ T cells, experienced a rapid decline of viremia to undetectable levels between days 6 and 17 following the initiation of mAb treatment. Finally, animal DCF1, previously reported to have generated broadly reacting anti HIV 1 NAbs17, exhibited a transient and a comparatively modest 27-fold reduction of plasma viremia by day 6 in response to combination mAb therapy, before the viral loads returned to high pretreatment levels.
SGA analysis of emerging virus populations in the 5 long-term chronically infected macaques revealed no changes affecting sensitivity to either 10-1074 or 3BNC117 except for the rebounding virus following the second treatment cycle of monkey DC99A noted above (Fig. S5).
Because some of the recipients of combination antibody treatment had prolonged control of detectable plasma viremia, PBMC associated viral RNA and DNA levels were evaluated prior to and following mAb administration. As shown in Table S1, mAb treatment resulted in reduced levels of cell associated viral RNA in each animal contemporaneous with the suppression of its plasma viremia. No consistent change in cell associated viral DNA levels occurred as a result of antibody treatment.
Co-administration of neutralizing mAbs to chronically SHIVAD8EO infected monkeys resulted in a transient elevation of circulating CD4+ T cell levels, particularly in late-stage animals with very high virus loads. The CD4+ T cell numbers in macaques DBXE and DCF1 increased 2 to 3 fold during the period of mAb mediated virus suppression, but gradually declined to pretreatment levels as viremia again became detectable (Fig S6). The corresponding elevations and declines of both the naïve and memory CD4+ T cell subsets in animals DBXE and DCF1 seen would be consistent with an antibody-mediated tissue redistribution mechanism, given that the R5 tropic SHIVAD8EO exclusively targets memory, not naïve CD4+ T lymphocytes. The levels of memory CD4+ T cells at an effector site (from BAL) did not change appreciably during immunotherapy (Fig. S4).
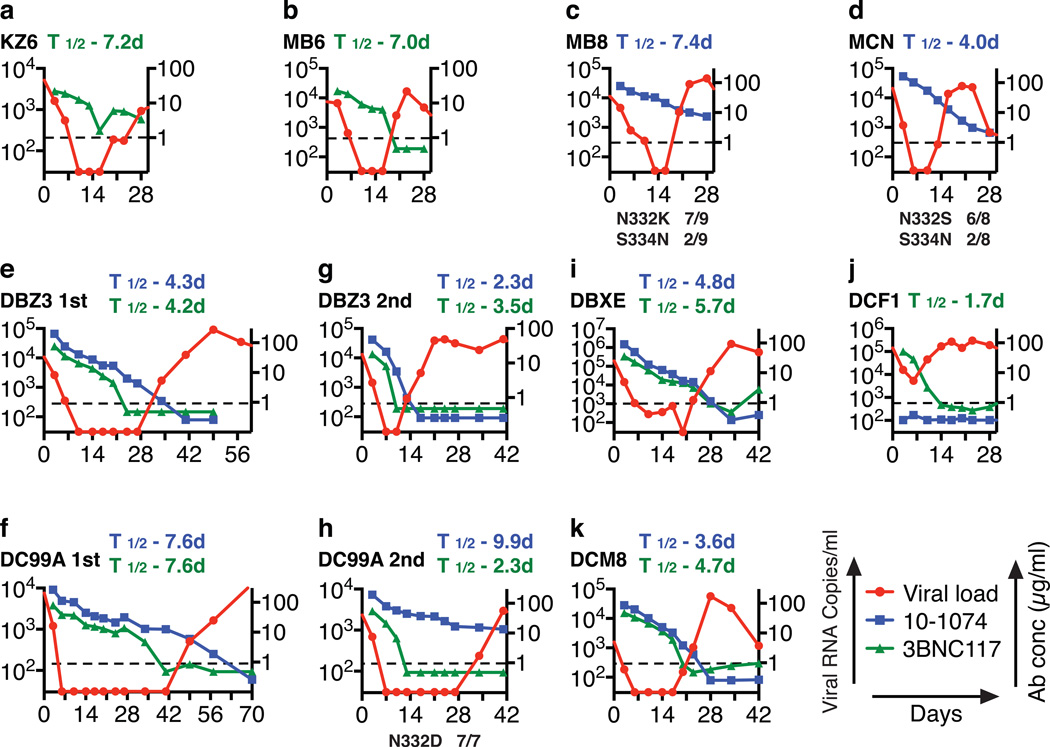
Plasma concentrations of each mAb were determined by measuring the neutralizing activity in plasma against selected HIV-1 pseudovirus strains sensitive to one or the other, but not to both antibodies (Figs. S7). In treated animals, suppression of SHIVAD8EO viremia was maintained until a threshold plasma mAb concentration of approximately 5 µg/ml was reached, except when escape variants emerged in animals MB8, MCN and the second cycle of treatment in macaque DC99A (Figs. 3 and S8). This was even the case for macaque DCF1, for which only a modest and transient reduction of plasma viral RNA levels was observed following combination therapy. Interestingly, the mAbs administered to this clinically symptomatic macaque had a shortened half-life or was undetectable (Fig. 3j). In this regard, macaque DCF1 had to be euthanized on day 56 post treatment initiation and a necropsy revealed severe enteropathy, characterized by disseminated gastrointestinal cryptosporidiosis, pancreatitis, and cholangitis, perhaps explaining the rapid loss of Nabs in this animal.
Figure 3.
Plasma viremia rebounds in SHIV infected monkeys when neutralizing antibody levels decline. Viral RNA levels and concentrations of 10-1074 or 3BNC117 mAbs in plasma at various times following initiation of single or combination antibody treatment. In panels c, d, and h, the gp120 changes in present in the rebound virus populations and their frequencies are indicated at the bottom.
The decay rate constants and the corresponding half-lives of plasma viral RNA following mono- or combination NAb treatments were calculated from the initial slopes of the HIV-1 plasma RNA decline (Table S2). Furthermore, unlike pharmacologic ART in which two phases of viral decay lasting several weeks is commonly seen, treatment with NAbs induced a single phase of exponential decline (T1/2 = 1.13 days). For the monkeys (DC99A and DBZ3) that received two cycles of treatment, the decay rate constant for the second treatment was higher than that for the first treatment for one of the animals and lower for the other (Table S2).
Our findings demonstrate that combination anti-HIV neutralizing mAb treatment is superior to monotherapy and can rapidly and potently suppress plasma virema in chronically SHIV infected macaques with low CD4+ T cell levels and symptomatic disease. Animals administered a single neutralizing mAb experienced a relatively short period of plasma viral RNA suppression and neutralization resistant progeny virus emerged in some monkeys, attesting to the robustness of SHIVAD8EO in vivo. Our results further suggest that administration of mAbs, alone or in combination with currently available anti-retroviral drug regimens 24, could be useful therapeutically, particularly for chronically infected individuals with compromised immune systems.
FULL METHODS
Virus
The origin and preparation of the tissue culture-derived SHIVAD8EO stock have been previously described17.
Animal Experiments
Rhesus macaques (Macaca mulatta) were housed in a biosafety level 2 NIAID facility and cared for in accordance with standards of the American Association for Accreditation of Laboratory Animal Care (AAALAC) in AAALAC-accredited facilities. All animal procedures were performed according to NIAID animal protocol LMM32, approved by the Institutional Animal Care and Use Committees of NIAID/NIH. Phlebotomies, euthanasia and sample collection were performed as previously described27. All animals were negative for the MHC class I Mamu-A*01, Mamu-B*08, and Mamu-B*17 allele.
Antibodies
Two monoclonal antibodies 3BNC11721 and 10-107420 were isolated and produced as described elsewhere (ref, ref). DEN3, a dengue virus NS1-specific human IgG1 antibody2, was used as the negative control antibodies in this study. All antibodies were administered intravenously.
Quantitation of Viral Nucleic Acids
Viral RNA levels in plasma were determined by real-time reverse transcription-PCR (ABI Prism 7900HT sequence detection system; Applied Biosystems) as previously reported27. Ultrasensitive measurement of cell associated SIV RNA and DNA were determined by a nested, hybrid real-time/digital PCR assay28.
Neutralization Assays
The in vitro potency of each mAb was assessed by TZM-bl entry assay with pseudotyped SHIVAD8EO as previously reported15,17.
Lymphocyte Immunophenotyping
EDTA-treated blood samples were stained for flow cytometric analysis as previously described16.
Antibody concentrations and t1/2 estimations in plasma
Plasma concentrations of 10-1074 and 3BNC117 neutralization activity were separately determined against HIV-1 virus strains that are sensitive to one but not the other mAb as well as not, or very weakly sensitive, to autologous antibodies in macaque plasma. All samples were heat-inactivated for 1h at 56°C for 1h and neutralizing activity was measured by using a TZM-bl assay as previously described29. ID50 titers were determined by serial plasma dilutions starting at 1:20; antibody concentrations were calculated by multiplying each ID50-value. T1/2 of plasma mAbs was estimated by ln(2)/k.
Single genomic analysis (SGA)
Single-genome amplification (SGA) of full length HIV 1 env genes was performed as reported26.
Determination of plasma viral RNA decay
The kinetics of plasma HIV-1 RNA after the start of therapy was analyzed as previously described30 by a simple one-exponential model: Ln(V)=c-kt, where V is the HIV-1 RNA concentration, k-virus decay rate constant (note that here k corresponds to k130 and c = a constant close to the ln of the baseline virus concentration. The half-life T1/2 was calculated as 0.693/k. The data were fitted using the program Excel and the goodness of fit was estimated by the correlation coefficients, which were in the range from 0.91 to 1.0. All time points in the exponential phase of decline were used for calculation.
Extended Data
Extended Data Figure 1.
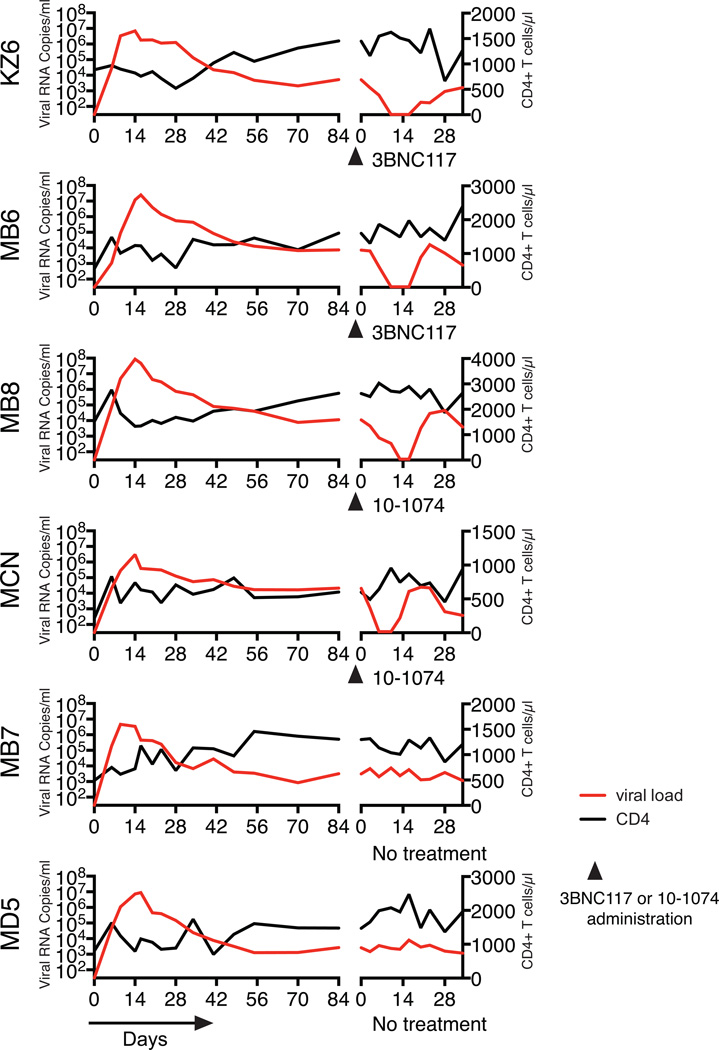
Treatment of SHIV infected macaques with single anti-HIV 1 neutralizing mAbs. Plasma viral loads and total CD4+ T cell numbers prior to (the initial 84 days of the SHIVAD8EO infection) and during single mAb treatment are shown. MZ6 and MB6 received the 3BNC117 mAb and MB8 and MCN were administered the 10-1074 mAb. Macaques MB7 and MD5 were not treated.
Extended Data Figure 2.
SGA analysis of selected SHIVAD8EO gp120 sequences, present in rebound virus following single mAb immunotherapy, and known to confer resistance to 10-1074 or 3BNC117 mAb. SGA was used to amplify plasma viral RNA following mAb treatment from the plasma of animals KZ6 [day 28], MB6 [day 23], MB8 [day 23], and MCN [day 23]. The gp120 sequences at the top are present in the SHIVAD8EO molecular clone inoculum. Mutations conferring resistance are highlighted in red.
Extended Data Figure 3.
Circulating CD4+ T cells in five chronically SHIV infected macaques treated with two anti-HIV 1 neutralizing mAbs. Plasma viral loads and total CD4+ T cell numbers prior to (first 1100 to 1140 days) and during the first or second cycle of combination mAb treatment are shown.
Extended Data Figure 4.
BAL CD4+ T cells in five chronically SHIV infected macaques treated with two anti-HIV 1 neutralizing mAbs. Plasma viral loads and the % CD4+ T cells in CD3+ gated BAL specimens, prior to (first 1100 to 1140 days) and during the first or second cycle of combination mAb treatment, are shown.
Extended Data Figure 5.
SGA analysis of selected SHIVAD8EO gp120 sequences known to confer resistance to 10-1074 or 3BNC117 mAb, prior to and following combination immunotherapy. Plasma from animals (a) DBZ3 [day 49], (b) DC99A [day 57], (c) DBXE [day 28], (d) DCF1 [day 28], and (e) DCM8 [day 28] were evaluated. The gp120 sequences at the top are present in the SHIVAD8EO molecular clone inoculum. Mutations conferring resistance are highlighted in red.
Extended Data Figure 6.
CD4+ T cell numbers increase during combination mAb treatment of SHIVAD8EO infected macaques. Levels of viral RNA and total CD4+T cell/CD4+ T cell subsets in symptomatic chronically infected macaques (a) DBX3 and (b) DCF1.
Extended Data Figure 7.
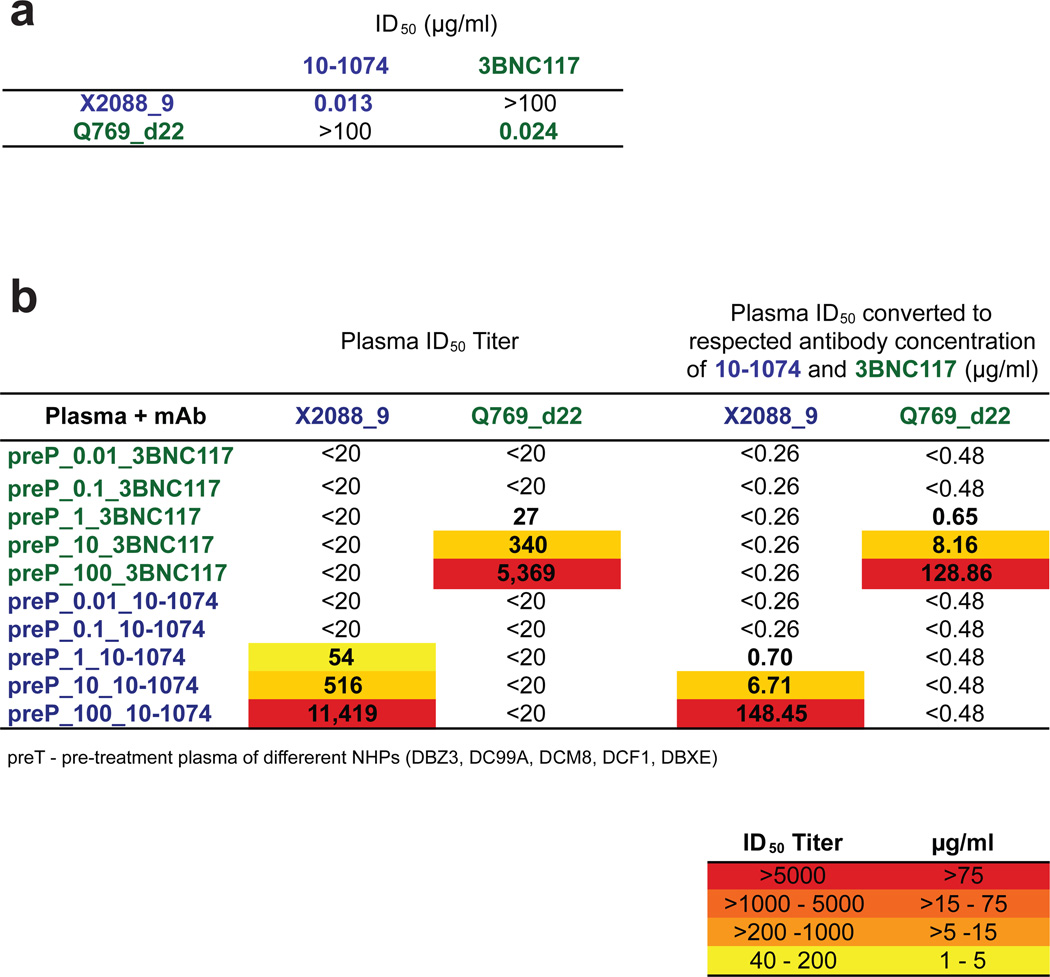
Assays to identify 10-1074- or 3BNC117-specific neutralizing activities in the plasma of mAb treated macaques. (a) ID50-values measured in the TZM-bl neutralization assay of 10-1074 and 3BNC117 against HIV-1 strains that are sensitive to one but not the other bNAb (i.e. HIV-1 strain X2088_9 (10-1074 sensitive); HIV-1 strain Q769_d22 (3BNC117 sensitive). (b) Neutralizing activities in plasma prior to antibody administration (preP), but spiked with 0.01, 0.1, 1, 10, and 100 µg/ml of antibodies 10-1074 (blue) or 3BNC117 (green). Neutralizing activities are reported as plasma ID50 titers (left columns) and converted to antibody concentrations (right columns) based on measured ID50-values in A.
Extended Data Figure 8.
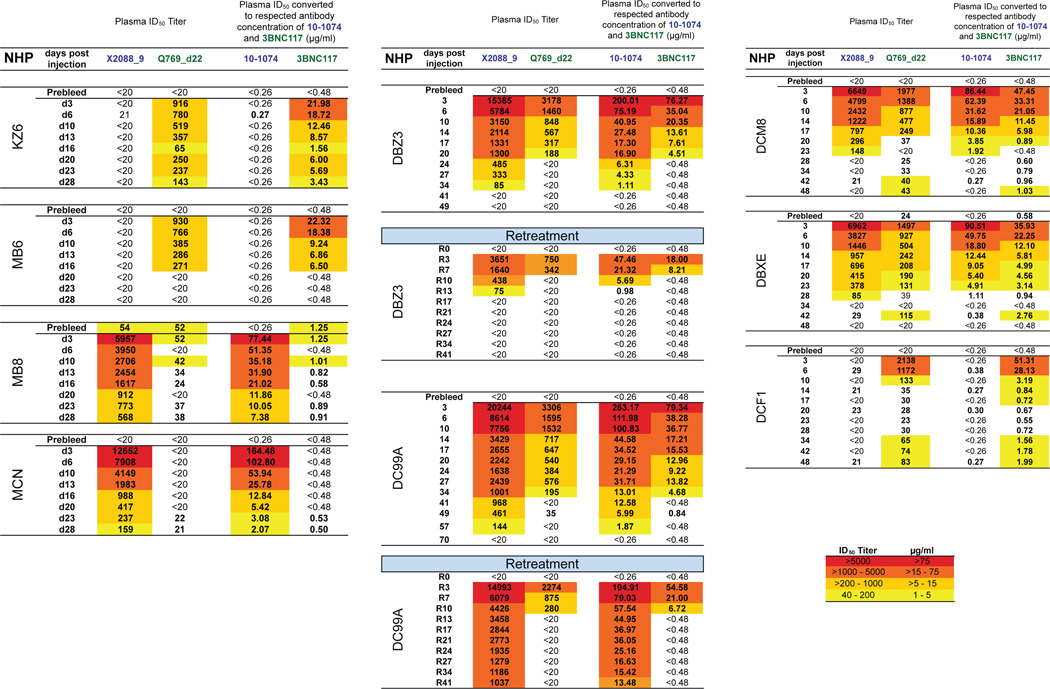
Monoclonal antibody levels in the plasmas of mono and combination mAb macaque recipients (a) Macaques treated with one neutralizing mAb; (b) Macaques receiving two cycles of combination mAb treatment; (c) Macaques receiving a single cycle of combination mAb treatment. ID50 titers (left columns) and mAb concentrations (right columns) were measured in the indicated macaque plasma samples prior to (Prebleed) and following (Day) mAb administration.
Extended Data Table 1.
Cell associated viral RNA/DNA in rhesus macaques receiving combination mAb therapy.
| Animal | Treatment Time (Days) |
Plasma Viral RNA (copies/ml) |
SIV Gag RNA Copies per 108 Cell Eq |
SIV Gag DNA Copies per 108 Cell Eq |
|---|---|---|---|---|
| DBZ3 1st | 0 | 1.08E+04 | 9,000 | 6,700 |
| 10 | < 100 | 360 | 7,500 | |
| 20 | < 100 | 2,600 | 14,000 | |
| 24 | < 100 | 1,600 | 6,400 | |
| 27 | < 100 | 670 | 5,700 | |
| DBZ3 2nd | 0 | 1.32E+04 | 52,000 | 15,000 |
| 10 | < 100 | 380 | 4,700 | |
| 13 | 4.09E+02 | 1,000 | 11,000 | |
| DC99A 1st | 0 | 7.60E+03 | 31,000 | 1,400 |
| 14 | < 100 | 18,000 | 5,600 | |
| 20 | < 100 | 8,100 | 2,700 | |
| 27 | < 100 | 400 | 790 | |
| 34 | < 100 | 550 | 1,100 | |
| 41 | < 100 | 7,200 | 780 | |
| DC99A 2nd | 0 | 2.35E+03 | 23,000 | 2,100 |
| 21 | < 100 | 570 | 1,100 | |
| 27 | < 100 | 1,100 | 2,100 | |
| DBXE | 0 | 1.96E+05 | 470,000 | 71,000 |
| 14 | 3.59E+02 | 17,000 | 33,000 | |
| 17 | 7.55E+02 | 11,000 | 22,000 | |
| 20 | < 100 | 11,000 | 33,000 | |
| 23 | 1.60E+03 | 17,000 | 27,000 | |
| DCM8 | 0 | 1.59E+03 | 110,000 | 8,600 |
| 14 | < 100 | 1,700 | 1,600 | |
| 17 | < 100 | 880 | 5,000 | |
| 20 | 1.51E+02 | 22,000 | 6,600 | |
| DCF1 | 0 | 1.44E+05 | 240,000 | 15,000 |
| 6 | 5.38E+03 | 34,000 | 5,500 | |
| 14 | 1.69E+05 | 190,000 | 11,000 | |
| 20 | 1.45E+05 | 1,100,000 | 14,000 |
Extended Data Table 2.
Decay rate constants of SHIVADS RNA in plasma following mAb treatment.
| Animal | k (days−1) | T1/2 (days) |
|---|---|---|
| DC99A 1st | 0.72 | 1.0 |
| DC99A 2nd | 0.45 | 1.5 |
| DBZ3 1st | 0.47 | 1.4 |
| DBZ3 2nd | 0.70 | 1.0 |
| DBXE | 0.66 | 1.0 |
| DCF1 | 0.55 | 1.3 |
| DCM8 | 0.46 | 1.5 |
| KZ6 | 0.40 | 1.8 |
| MB6 | 0.43 | 1.5 |
| MB8 | 0.36 | 1.9 |
| MCN | 0.89 | 0.8 |
Rate constants of initial viral RNA decline in plasma (decay rate constants k) and corresponding half-lives (T1/2) were determined as previously described (26). The decay rate constants k were calculated by fitting the data with an exponential function. The fit was very good with correlation coefficients in the range from 0.91 to 1.0.
ACKNOWLEDGEMENTS
We thank Keiko Tomioka and Robin Kruthers for determining plasma viral RNA loads and Boris Skopets, William Magnanelli and Rahel Petros for diligently assisting in the maintenance of animals and assisting with procedures. We are indebted to Dennis R. Burton, The Scripps Institute, for providing anti-dengue virus neutralizing mAb (DEN-3). This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH) and, in part, with federal funds from the National Cancer Institute, NIH, under contract HHSN261200800001E.
Footnotes
AUTHOR CONTRIBUTIONS
M.S., Y.N., M.C.N., and M.A.M. designed the experiments; M.S., Y.N., F.K., H.M., O.K.D., R.P., A. B-W., and M.P. Jr. performed the experiments; M.S., Y.N., F.K., M.P.Jr., J.D.L., D.D., M.C.N. and M.A.M. analyzed the data and M.S., Y.N., M.C.N. and M.A.M wrote the manuscript.
REFERENCES
- 1.Mascola JR, et al. Protection of Macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. Journal of virology. 1999;73:4009–4018. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moldt B, et al. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:18921–18925. doi: 10.1073/pnas.1214785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishimura Y, et al. Determination of a statistically valid neutralization titer in plasma that confers protection against simian-human immunodeficiency virus challenge following passive transfer of high-titered neutralizing antibodies. Journal of virology. 2002;76:2123–2130. doi: 10.1128/jvi.76.5.2123-2130.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parren PW, et al. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. Journal of virology. 2001;75:8340–8347. doi: 10.1128/JVI.75.17.8340-8347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehandru S, et al. Adjunctive passive immunotherapy in human immunodeficiency virus type 1-infected individuals treated with antiviral therapy during acute and early infection. Journal of virology. 2007;81:11016–11031. doi: 10.1128/JVI.01340-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poignard P, et al. Neutralizing antibodies have limited effects on the control of established HIV–1 infection in vivo. Immunity. 1999;10:431–438. doi: 10.1016/s1074-7613(00)80043-6. [DOI] [PubMed] [Google Scholar]
- 7.Trkola A, et al. Delay of HIV–1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nature medicine. 2005;11:615–622. doi: 10.1038/nm1244. [DOI] [PubMed] [Google Scholar]
- 8.Burton DR, et al. A Blueprint for HIV Vaccine Discovery. Cell host & microbe. 2012;12:396–407. doi: 10.1016/j.chom.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang J, et al. Broad and potent neutralization of HIV–1 by a gp41-specific human antibody. Nature. 2012;491:406–412. doi: 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klein F, et al. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature. 2012;492:118–122. doi: 10.1038/nature11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong L, et al. Supersite of immune vulnerability on the glycosylated face of HIV–1 envelope glycoprotein gp120. Nat Struct Mol Biol. 2013;20:796–803. doi: 10.1038/nsmb.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker LM, et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker LM, et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV–1 vaccine target. Science. 2009;326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu X, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV–1. Science. 2010;329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gautam R, et al. Pathogenicity and mucosal transmissibility of the R5-tropic simian/human immunodeficiency virus SHIV(AD8) in rhesus macaques: implications for use in vaccine studies. Journal of virology. 2012;86:8516–8526. doi: 10.1128/JVI.00644-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishimura Y, et al. Generation of the pathogenic R5-tropic simian/human immunodeficiency virus SHIVAD8 by serial passaging in rhesus macaques. Journal of virology. 2010;84:4769–4781. doi: 10.1128/JVI.02279-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shingai M, et al. Most rhesus macaques infected with the CCR5-tropic SHIV(AD8) generate cross-reactive antibodies that neutralize multiple HIV–1 strains. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:19769–19774. doi: 10.1073/pnas.1217443109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker LM, et al. Rapid development of glycan-specific, broad, and potent anti-HIV–1 gp120 neutralizing antibodies in an R5 SIV/HIV chimeric virus infected macaque. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:20125–20129. doi: 10.1073/pnas.1117531108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sadjadpour R, et al. Emergence of gp120 V3 Variants Confers Neutralization Resistance in an R5 Simian-Human Immunodeficiency Virus-Infected Macaque Elite Neutralizer That Targets the N332 Glycan of the Human Immunodeficiency Virus Type 1 Envelope Glycoprotein. Journal of virology. 2013;87:8798–8804. doi: 10.1128/JVI.00878-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mouquet H, et al. Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E3268–E3277. doi: 10.1073/pnas.1217207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scheid JF, et al. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. 2011;333:1633–1637. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou T, et al. Structural basis for broad and potent neutralization of HIV–1 by antibody VRC01. Science. 2010;329:811–817. doi: 10.1126/science.1192819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diskin R, et al. Restricting HIV–1 pathways for escape using rationally designed anti-HIV–1 antibodies. The Journal of experimental medicine. 2013;210:1235–1249. doi: 10.1084/jem.20130221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horwitz JA, et al. HIV–1 suppression and durable control by combining single broadly neutralizing antibodies and antiretroviral drugs in humanized mice. Proceedings of the National Academy of Sciences of the United States of America. 2013 doi: 10.1073/pnas.1315295110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Igarashi T, et al. Human immunodeficiency virus type 1 neutralizing antibodies accelerate clearance of cell-free virions from blood plasma. Nature medicine. 1999;5:211–216. doi: 10.1038/5576. [DOI] [PubMed] [Google Scholar]
- 26.Keele BF, et al. Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV–1. The Journal of experimental medicine. 2009;206:1117–1134. doi: 10.1084/jem.20082831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Endo Y, et al. Short- and long-term clinical outcomes in rhesus monkeys inoculated with a highly pathogenic chimeric simian/human immunodeficiency virus. Journal of virology. 2000;74:6935–6945. doi: 10.1128/jvi.74.15.6935-6945.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansen SG, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montefiori DC. Evaluating neutralizing antibodies against HIV, SIV, and SHIV in luciferase reporter gene assays. In: Coligan John E, et al., editors. Current protocols in immunology. 2005. Chapter 12, Unit 12 11. [DOI] [PubMed] [Google Scholar]
- 30.Polis MA, et al. Correlation between reduction in plasma HIV–1 RNA concentration 1 week after start of antiretroviral treatment and longer-term efficacy. Lancet. 2001;358:1760–1765. doi: 10.1016/s0140-6736(01)06802-7. [DOI] [PubMed] [Google Scholar]