Abstract
Objective
This study tested the hypothesis that the antidepressant venlafaxine would be an effective treatment for cocaine abusers with concurrent depressive disorders.
Methods
This was a randomized, 12-week, double-blind, placebo-controlled trial of outpatients (N = 130) meeting DSM-IIIR criteria for cocaine dependence and major depression or dysthymia (by SCID interview). Participants were treated with venlafaxine, up to 300 mg/day versus placebo. All patients received weekly individual manual-guided relapse prevention therapy. Weekly outcome measures included Clinical Global Impression Scale (CGI), self-reported cocaine use, urine toxicology and the Hamilton Depression Scale (Ham-D).
Results
Mood response, defined as a 50% reduction in the Ham-D between randomization and end of study, was 41% (26/64) on venlafaxine, and 33% (22/66) on placebo (p = .39). Measures of depression (Ham-D and CGI) improved more rapidly on venlafaxine than placebo, but these differences disappeared by weeks 6–8. Cocaine outcomes did not differ between treatment groups, and the proportion of patients achieving three or more consecutive weeks of urine-confirmed abstinence was low (venlafaxine: 16%; placebo: 15%). Reduction in cocaine use was associated with mood response.
Conclusions
Overall, venlafaxine was not superior to placebo on either mood or cocaine use outcomes. Mood improvement was associated with improvement in cocaine use. However, placebo mood response was only moderate, and the proportion of patients achieving sustained abstinence was low. This suggests that the subgroup of cocaine-dependent patients with depressive disorders is relatively treatment resistant, and that further research is needed to improve outcomes for these patients.
INTRODUCTION
Depression is common among alcohol- and drug-dependent patients. In community-based surveys, a diagnosis of major depression or dysthymia increases the odds of alcohol or drug use disorders by a factor of 2–4.1,2 Among alcohol or drug-dependent patients seeking treatment, 20–50% meet criteria for a depressive disorder.3 Furthermore, a diagnosis of major depression has been associated with worsening progression of the concurrent alcohol or drug use disorders.3 This suggests that depression may be a causal factor promoting the onset or maintenance of substance use disorders.
Placebo controlled trials of antidepressant medications among patients with co-occurring substance and depressive disorders have been conducted. Meta-analyses suggest that among alcohol-dependent patients with major depression, antidepressant medication is effective in improving drinking outcome.4,5 However, there have been fewer such trials among drug-dependent patients. The results are uneven, with some positive,6 but more negative studies, most of which were done among opioid-dependent patients.7,8 A number of clinical trials have tested antidepressant medications among cocaine-dependent patients not selected for depression with similar patterns of results.9 Several of the negative trials suggested a benefit from antidepressants among depressed subgroups within their samples.9–11
To date, only four placebo-controlled trials of antidepressant medication have focused on cocaine-dependent patients with depressive disorders. A trial of the serotonin reuptake inhibitor fluoxetine was negative.8 Trials with nefazodone and mirtazapine were also negative.12,13 A trial of the norepinephrine reuptake inhibitor desipramine found a modest beneficial effect of desipramine on depression symptoms, no direct effect of the medication on cocaine use outcome, but mood improvement was associated with cocaine use improvement; poor tolerability limited adherence.14 An uncontrolled pilot trial of venlafaxine, a mixed serotonin-norepinephrine reuptake inhibitor, with a more benign side effect profile, showed promise for combined cocaine dependence and depression.15 We therefore conducted a placebo-controlled trial of venlafaxine among cocaine-dependent patients with major depression or dysthymia. We hypothesized that venlafaxine would be superior to placebo for mood and cocaine use outcomes, and that mood improvement would be associated with improvement in cocaine use.
METHODS
Participants
Adults aged 18–65 seeking treatment for cocaine dependence were screened by clinicians at a university-based research clinic using the Structured Clinical Interview for DSM-IIIR,16 modified for substance-dependent patients.17 Patients were deemed eligible only if they met DSM-IIIR criteria for both cocaine dependence and current major depressive disorder or dysthymia, with at least one of the following characteristics: (1) the depression was chronologically primary, antedating the onset of substance abuse during a lifetime history; (2) the depression was chronologically secondary, but persisted or emerged during a past instance of abstinence lasting at least 6 months; or (3) the depression was of at least 3 months duration in the current episode. The Institutional Review Board of the New York State Psychiatric Institute approved the study. All participants gave written informed consent.
Patients were excluded if they had a history of bipolar disorder, psychotic illness other than brief psychotic symptoms attributable to cocaine intoxication, were judged to be at risk of suicidal behavior, were medically unstable, or had a seizure disorder. Patients dependent on nicotine, alcohol, or cannabis were not excluded, as long as cocaine dependence was the predominant clinical problem.
Procedures
Eligible patients began with a 7-day single-blind placebo lead-in to remove placebo-responders (Clinical Global Impression Scale [CGI] rating = 1 or 2; Hamilton Depression Scale [Ham-D] > 50% reduction or ≤8). Those remaining were randomized to venlafaxine (target dose 300 mg) or placebo, stratified by baseline level of cocaine use: (a) periodic use (< 1 day/week); (b) low use (1–2 days/week); or (c) high use (=3 days/week). A research pharmacy, otherwise independent of the research team, conducted the randomizations, maintained the blind, and prepared medications. Extended release venlafaxine (75 mg) and placebo was packaged in unmarked gelatin capsules containing 25 mg of riboflavin to monitor compliance by urine fluorescence under ultraviolet light. Patients and all clinic staff were blind to medication assignment.
Patients were asked to come to the clinic twice a week for the 12 weeks of the trial. At these visits, they met with nursing and research staff to review possible side effects, and monitor medication compliance and vital signs. A breach in medication compliance prompted the research staff to reemphasize the importance of medication compliance with the patient. At one of these visits, patients met with a trained clinician for manual-guided relapse prevention therapy.18 A psychiatrist met patients at the second visit to administer clinical ratings of mood and cocaine use, assess side effects and clinical status, and adjust medication dosage as needed. Venlafaxine was titrated on a fixed-flexible schedule, beginning at 37.5 mg for 4 days, and then twice a day for the remaining 3 days, and then increased every week by 75 mg to reach 300 mg or the maximum tolerated dosage.
Measures
Mood outcome was evaluated with the Ham-D19 every 2 weeks, and the Clinical Global Impression Scale weekly.20 Cocaine and other drug use (opiates, marijuana, alcohol, nicotine, prescription drugs) were assessed by self-report for quantity and the monetary value of drugs used. Urine samples were collected at every visit. The sample was placed under ultraviolet light to assess compliance by riboflavin fluorescence, and analyzed by immunoassay for semi-quantitative levels of cocaine metabolite and other drugs. Of the 1,241 urine samples collected, 992 (70%) corresponded with self-reports. Of the 249 urine samples that did not correspond, 96 (8%) were positive for cocaine when the patient denied having used, while 153 (12%) were negative for cocaine when the patient reported using. Overall, this suggested good concordance between urine and self-report. Blood levels of venlafaxine were drawn at weeks 3, 6, and 12.
The primary outcomes were dichotomous measures of treatment response, intended to reflect clinically significant improvement. At week 12, or at the last week of the study participation, the treating psychiatrist rendered a global rating of depression response based on all available data; and of cocaine response, based on whether the patient had achieved at least a 75% reduction in cocaine use compared to baseline, based on self-report and urine toxicology. A dichotomous depression response measure typically employed in antidepressant trials was also computed, namely >50% decrease in Ham-D scores between randomization and end of study. Urine-confirmed abstinence (both urine and self-reports negative for cocaine) was determined weekly, and the proportion of patients achieving at least three consecutive weeks of abstinence during the trial was computed. This measure was found to be a sensitive index of treatment effects in previous trials.9,14
Concurrent Psychosocial Treatment
To promote retention and compliance with clinical trial procedures, and provide a foundation of treatment, all patients in the trial participated in weekly individual cognitive-behavioral relapse prevention therapy.18 Motivational interviewing techniques were also used at the outset of treatment.21 All therapists were supervised with weekly review of audio taped sessions and therapist adherence checklists.
Data Analysis
All analyses were conducted on the intent-to-treat sample and all tests were 2-tailed with the alpha significance level set at .05. Baseline demographic and clinical characteristics and summary outcome measures were compared between groups using chi-square test for categorical variables and t-test for continuous variables. Retention rates between the groups were compared using Kaplan-Meier survival curves and the log-rank test. Longitudinal analyses of depression and cocaine outcome measures were performed using generalized estimating equations for dichotomous outcomes (using SAS PROC GENMOD), and mixed effects models for continuous outcomes (using SAS PROC MIXED). For each outcome, the dependent variable was modeled as a function of time, treatment assignment, time by treatment interaction, and a baseline (pre-treatment) measure of the outcome variable. Backward elimination was used to remove interactions from the models not significant at the .05 level.
RESULTS
Sample Description
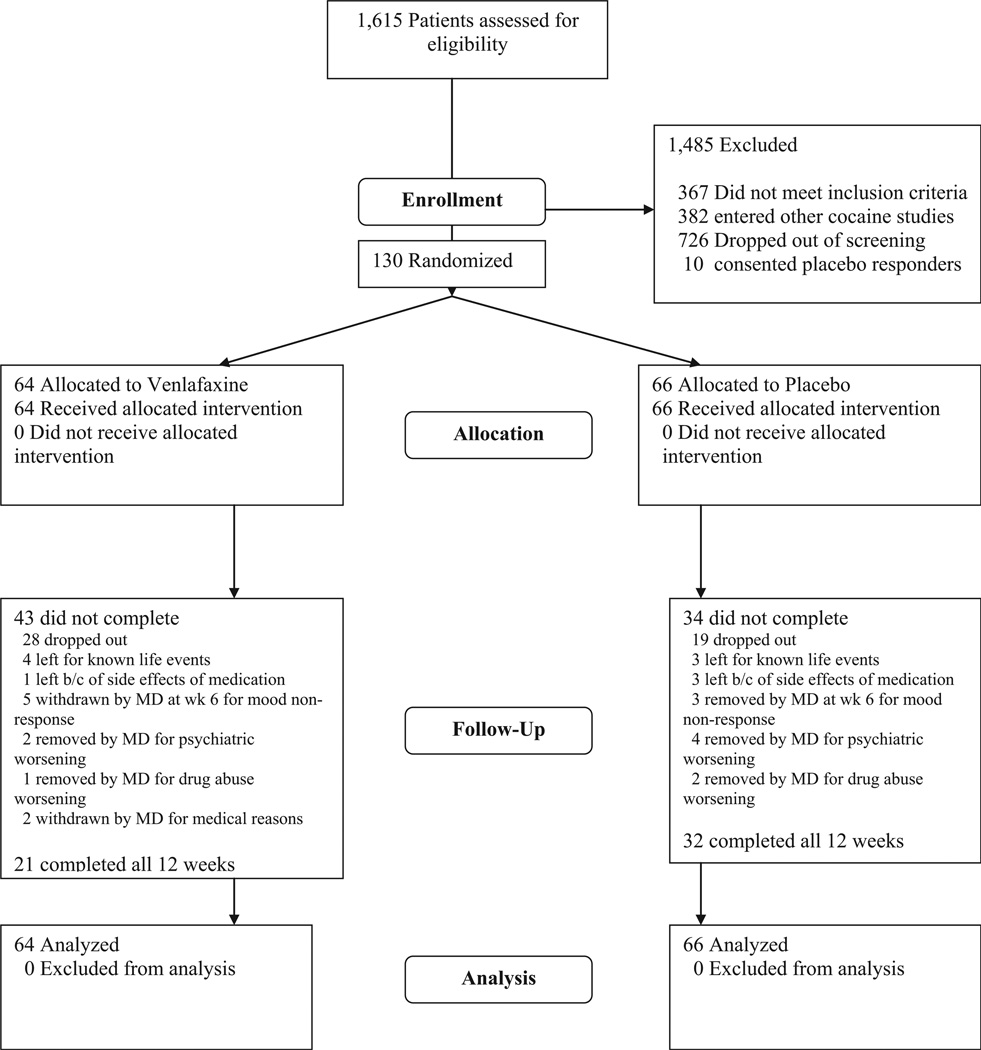
One hundred and forty patients were consented to participate (Fig. 1), 10 were removed during the placebo lead-in period and 130 were randomized to placebo (PBO; n = 66) or venlafaxine (VEN; n = 64). Demographic and baseline clinical characteristics of the sample are shown in Table 1. Ninety-four (72%) of the randomized sample were male, 50 (38%) Caucasian, 34 (26%) Hispanic, and 33 (25%) African American. The average age of the sample was 37 ± 8 (range 21–58 years). The only significant baseline difference between treatment groups was the CGI cocaine severity score, which was modestly greater in the PBO group (4.09 ± 1.21) compared with the venlafaxine group (3.53 ± 1.52; t = 2.30, p = .02).
FIGURE 1.
Consort E-flowchart.
TABLE 1.
Baseline demographics and clinical characteristics of depressed, cocaine-dependent patients (N = 130) randomized to venlafaxine or placebo in a double-blind, placebo-controlled clinical trial of venlafaxine
| Variable | PBO (n = 66) | Ven (n = 64) |
|---|---|---|
| Age (years) | 38 (8) | 37 (8) |
| Male | 73% (48/66) | 72% (46/64) |
| Race | ||
| African American | 29% (19/66) | 23% (14/62) |
| Hispanic | 29% (19/66) | 24% (15/62) |
| Caucasian | 33% (22/66) | 45% (28/62) |
| Other | 9% (6/66) | 8% (5/62) |
| Education (% post HS) | 48% (30/63) | 60% (37/62) |
| Marital status | ||
| Not married | 48% (31/64) | 53% (33/62) |
| Married | 31% (20/64) | 24% (15/62) |
| Divorced/separated | 20% (13/64) | 23% (14/62) |
| Employment | ||
| Full-time | 68% (41/60) | 79% (46/58) |
| Part-time | 8% (5/60) | 12% (7/58) |
| Unemployed | 23% (14/60) | 9% (5/58) |
| Income | ||
| <25 K | 44% (24/55) | 40% (21/52) |
| 25–50 K | 49% (27/55) | 39% (20/52) |
| >50 K | 7% (4/55) | 21% (11/52) |
| Ham-D 21: total score | 16.39 (4.99) | 15.70 (4.77) |
| CGI Dep: severity score | 4.49 (.82) | 4.42 (.90) |
| Type of depression | ||
| Primary | 42% (27/65) | 40% (25/63) |
| Secondary | 40% (26/65) | 38% (24/63) |
| Diagnosis of dysthymia | 18% (12/65) | 22% (14/63) |
| Diagnosis of dysthymia + major depression | 9% (6/65) | 10% (6/63) |
| CGI Coc: severity score | 4.09 (1.21)* | 3.53 (1.52)* |
| Days/week: using cocaine | 1.97 (1.98) | 1.57 (1.80) |
| Days/week: craving cocaine | 4.61 (2.27) | 3.98 (2.50) |
| Diagnosis of alcohol dependence | 21% (14/66) | 23% (15/64) |
| Diagnosis of cannabis dependence | 14% (9/66) | 11% (7/64) |
There were no significant differences between placebo and venlafaxine groups, except for CGI cocaine severity score (t = 2.3, p = .02).
Retention in Treatment
One hundred and four (80%) out of the 130 randomized participants completed at least 4 weeks of the trial (PBO = 53 [80%], Ven = 51 [70%]). Fifty-three (41%) completed the 12-week treatment phase (PBO = 32 [49%], Ven = 21 [33%]). Survival analysis on weeks to dropout did not reach significance (log-rank = 2.24, df = 1, p = .13), although inspection of the survival curves suggested greater dropout on venlafaxine over the later weeks of the trial. Reasons for dropout are summarized in Fig. 1. Non-compliance was the most common reason for dropout, with a non-significant trend toward more non-compliance on venlafaxine. The 77 participants who did not complete the 12 weeks of the trial did not differ in baseline demographic or clinical characteristics from those who completed the trial.
Mood Response
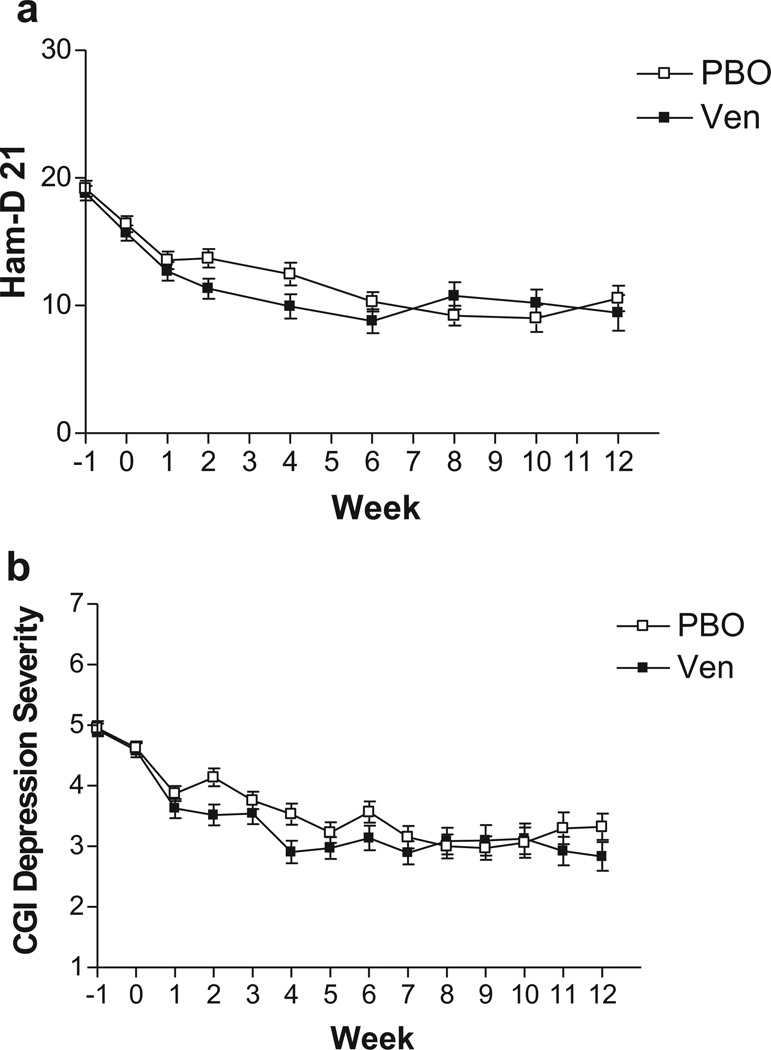
As can be seen in Table 2, the proportion of patients meeting the standard depression response criterion of 50% reduction in Ham-D score from randomization was 33% (22/66) on placebo, compared to 41% (26/64) on venlafaxine. The proportion meeting the clinician’s global response rating was 48% (32/66) on placebo, compared to 56% (36/64) on venlafaxine. Neither difference is significant, while the placebo response rates are in the moderate range. The course of depression severity scores over time in this trial is shown in Fig. 2a (Ham-D scores) and Fig. 2b (CGI depression severity scores). The mixed effect model for Ham-D yielded significant effects of baseline Ham-D score (high baseline scores associated with higher scores during treatment; t = 6.75, p < .0001), and time (scores decreasing overtime; t = –4.40, p < .0001), while the main effect of treatment was not significant (t = .68, p = .50). For the CGI depression severity score, the model yielded a main effect of baseline CGI score (t = 4.59, p < .0001), but not time (t = .88, p = .38), while there was a significant effect of treatment (t = 2.06, p = .042). Interactions were not significant. Inspection of the Fig. 2a and 2b suggests depression improves more rapidly on venlafaxine over the first 6 weeks of the trial, and several of the pairwise differences between groups are significant between weeks 2 and 6 by post hoc t-tests. However, after week 6, placebo appears to catch up with similar mean scores on venlafaxine and placebo between weeks 7 and 12.
TABLE 2.
Summary measures of mood and cocaine use outcome at end-of-study among depressed, cocaine-dependent patients during a 12-week randomized, double-blind, placebo-controlled trial of venlafaxine, first by assignment to venlafaxine or placebo, second by mood response as defined by a 50% or greater decrease in 21-item Hamilton Depression Scale score between randomization and end of study
| Effect of medication treatment |
|||
|---|---|---|---|
| Mood outcome | Placebo (n = 66) | Venlafaxine (n = 64) | X or t, p-value |
| Mood responder by clinician’s global rating | 48% (32/66) | 56% (36/64) | .67, .42 |
| 50% drop in Hamilton Depression Scale | 33% (22/66) | 41% (26/64) | .74, .39 |
| Cocaine use outcome | |||
| Cocaine responder by clinician’s global rating | 42% (28/66) | 51% (33/64) | 1.09, .30 |
| CGI cocaine severity | 3.05 (1.56) | 2.91 (1.59) | .51, .61 |
| Days per week using cocaine | 1.64 (1.57) | 1.49 (1.46) | .60, .55 |
| Proportion of urines positive for cocaine | .64 (.36) | .62 (35) | .34, .738 |
| ≥3 consecutive weeks of urine confirmed abstinence | 15% (10/66) | 16% (10/64) | .001, .94 |
| Effect of mood improvement* |
|||
| Cocaine use outcome | Mood non-responder (n = 82) |
Mood responder (n = 48) |
X or t, p-value |
| Cocaine responder by clinician’s global rating | 37% (30/82) | 65% (31/48) | 9.53, .002 |
| CGI cocaine severity | 3.31 (1.64) | 2.42 (1.26) | 3.23, .002 |
| Days per week using cocaine | 1.76 (1.56) | 1.23 (1.37) | 1.96, .053 |
| Proportion of urines positive for cocaine | .67 (.34) | .51 (.34) | 2.57, .01 |
| ≥3 consecutive weeks of urine confirmed abstinence | 11% (9/82) | 23% (11/48) | 3.32, .07 |
Mood response, defined as at least 50% reduction in Hamilton Depression Scale score at end of study, compared to randomization.
FIGURE 2.
(a) Average Ham-D severity scores with standard deviation bars by week, from consent (week −1; baseline), randomization (week 0) to week 12 of a randomized, double-blind, placebo-controlled study of venlafaxine (up to 300 mg) versus placebo. A single-blind placebo lead-in occurs between week −1 and 0. In the mixed effect model, there was a significant effect of time, but no main or interactive effects of treatment, while post hoc t-tests indicated venlafaxine separated from placebo at week 2 (t = 2.26, p= .02) and week 4 (t= 1.96, p= .05). (b) Average CGI Depression Severity score with standard deviation bars by week, from consent (week −1; baseline), randomization (week 0), to week 12 of a randomized, double-blind, placebo-controlled study of venlafaxine (up to 300 mg) versus placebo. A single-blind placebo lead-in occurs between week −1 and 0. In the mixed effect model, there was a significant effect of treatment, with post hoc t-tests indicating venlafaxine separated from placebo at week 2 (t= 2.38, p= .01) and week 4 (t= 2.57, p= .01).
Cocaine Response
As can be seen in Table 2, around half of the patients were rated as cocaine responders (placebo: 42% [28/66]; venlafaxine: 51% [33/64]), reflecting clinician’s judgment of clinically significant reduction in cocaine use. However, over half of urine toxicologies were positive in both treatment groups, and few patients achieved three or more consecutive weeks of urine-confirmed abstinence (placebo: 15% [10/66]; venlafaxine: 16% [10/64]). None of these global measures of cocaine outcome differed significantly between treatment groups. Mood response (50% drop in Ham-D score from randomization) was associated with better outcome on the clinicians’ global rating of cocaine severity (CGI), and trends toward fewer days per week using cocaine, and more mood responders achieving three or more weeks of urine-confirmed abstinence. With the longitudinal model of weekly urine toxicology (dichotomous: positive/negative), there was a main effect of baseline (Z = −5.42, p < .0001) indicating that negative urine toxicology at baseline strongly predicts negative urine toxicologies during the study, but no effect of medication treatment (Z = −.16, p = .87) or time (Z = −1.40, p = .16). Interactions were not significant. When mood response (50% reduction in Ham-D from randomization) was added as a factor into the model, the interaction between mood response and baseline was significant (Z = 2.71, p = .0068), indicating that when urine toxicology at baseline is negative, mood responders have improved cocaine outcome compared with mood non-responders, regardless of medication assignment. In the longitudinal model predicting weekly self-reported days using cocaine there were no main, or interactive effects of medication.
Medication Compliance
Blood samples were obtained from 44/64 participants assigned to venlafaxine. Measurable levels of venlafaxine were found in 37/64 patients (58%). Clinical response to venlafaxine in clinical trials for depression has been related to plasma levels of venlafaxine (V) and of its primary metabolite O-desmethylvenlafaxine (ODV), as both V and ODV are reported to exhibit antidepressant properties.22 Plasma medication levels are thus expressed as the sum of V + ODV concentrations in ng/ml (23). Among 37 patients with detectable blood levels, the mean plasma concentration (V + ODV) was 477.82 ± 215.5 ng/ml. Forty-one percent of all collected urine samples failed to display riboflavin fluorescence under ultraviolet light. The difference in venlafaxine + ODV levels between mood responders and non-responders was not significant. Taken together, the presence of undetectable blood levels of medication among those randomized to venlafaxine, the wide variation in measured blood levels that do not relate to mood response, and the frequency of riboflavin-negative urine samples, suggest poor medication compliance by many patients. Moreover, in a previous trial of venlafaxine among depressed patients without addiction, mood improvement was associated with a mean plasma concentration of 639.5 ± 120.1 ng/ml (V + ODV). Only 18 patients in the venlafaxine group in the present trial (28%) displayed V + ODV levels within one standard deviation of this mean value (519.1–759.6).
Side Effects and Adverse Events
Side effects that occurred at a frequency greater than 1% while on venlafaxine or placebo include insomnia, headache, sexual dysfunction, nausea, lethargy, agitation, sedation, dizziness, chest pain, night sweat, diarrhea, shortness of breath, sweating, and decreased appetite. Those encountered exclusively in the venlafaxine group include diarrhea, shortness of breath, sweating, decreased appetite, weight loss, flatulence, vivid dreams, increased blood pressure, flushing, tremor and difficulty urinating. Overall, side effects did not differ significantly between groups. There were six serious adverse events, all involving patients in the venlafaxine arm. Three patients were suicidal; one patient was involved in a car accident while intoxicated; another suffered a motorcycle accident while abstinent; one patient was found to have an abdominal mass. There were no serious adverse events in the placebo group.
DISCUSSION
A placebo-controlled trial of the antidepressant venlafaxine was undertaken among cocaine-dependent patients with depressive disorders. It was hypothesized that venlafaxine would improve mood, which would in turn reduce cocaine use. Disappointingly, venlafaxine produced no clear antidepressant effect. Depression severity scores were lower on venlafaxine compared to placebo between weeks 2 and 6 after randomization, but after week 6 these differences disappeared. Dichotomous measures of depression response did not differ significantly between venlafaxine and placebo. Improvement in mood was associated with improvement in measures of cocaine use outcome. However, there was no direct effect of venlafaxine on cocaine use outcome, which was poor overall in that only 15% of patients achieved sustained abstinence. In addition, a high drop-out rate, more adverse events on venlafaxine, and poor medication compliance hampered the detection of a medication effect. Prior trials had suggested some promise for the tricyclic antidepressants imipramine and desipramine among depressed cocaine-dependent patients. It was hoped that venlafaxine with its similar pharmacological profile (norepinephrine and serotonin re-uptake inhibition), but better tolerability and safety, would produce more robust effects. Instead, the results are reminiscent of other small, open pilot studies not replicating in larger controlled trials, and of negative trials of serotonin reuptake inhibitors, nefazodone, or mirtazapine among alcohol,24,25 or drug-dependent patients. 7,8,12,13,25,26
These disappointing results may be understood in the context of prior studies that have tested antidepressant medications among alcohol- or drug-dependent patients with depressive disorders. In meta-analyses of this literature, beneficial effects of medication (compared to placebo) for improving both mood and substance abuse were more likely in studies of alcohol dependent, as opposed to drug-dependent patients, 4,5 and in studies with the following features: (1) low placebo response rates of less than 25%; (2) diagnosis of depression and enrollment in the trial after at least a 1-week period of abstinence, enforced through hospitalization in several studies; and (3) noradrenergic antidepressants—most positive trials involved tricyclic antidepressants, as opposed to serotonin uptake inhibitors, where most trials were negative.4 Further, provision of a manual-guided intervention was associated with high placebo response and lack of medication effect.4 Thus, the present study shares features associated with other negative studies, as it had moderate placebo mood response in the 30–40% range, enrolled outpatients actively using cocaine, provided manual-guided cognitive-behavioral relapse prevention (CBT-RP) 27 to all participants, and tested a medication, venlafaxine, which, while a mixed serotonin-norepinephrine reuptake inhibitor, has predominantly serotonin effects at lower doses and blood levels.28
These considerations might suggest future studies that select more severely ill patients (eg, inpatients, or those with more severe depression), or patients who fail to improve after an initial trial of CBT, in order to reduce placebo response and increase the likelihood of observing a beneficial effect of antidepressant medication. That said, the placebo mood response rate observed here, in the 30–40% range depending on the measure, still represents over half of patients not achieving substantial improvement in mood, while the rate of abstinence on placebo or medication was low. Thus, it can be argued that this trial selected patients with relatively treatment-resistant cocaine dependence, despite provision of an evidence-based behavioral treatment (CBT-RP). This focuses scrutiny on the failure of the medication, either because of its low efficacy or poor tolerability.
Poor treatment adherence has been a problem for medication development trials among drug-dependent patients. Riboflavin fluorescence was a poor marker of compliance given low or undetectable blood levels. Procedures that might improve medication compliance in future studies include provision of voucher incentives for protocol adherence (visit attendance, returning pill bottles), and a concurrent behavioral intervention that emphasizes medication compliance.29
Poor compliance may also be a referendum on the acceptability and effectiveness of the medication. As noted above, serotonin reuptake inhibitors have not generally been successful in trials for treatment of alcohol and cocaine dependence. Some trials have even suggested that SRIs may worsen alcohol use outcome among some alcoholics.30,31 Another possibility is that antidepressants exert a weak beneficial effect, which by itself is insufficient to produce much impact on substance use, but which might synergize with another treatment to produce larger and more clinically significant effects. Examples of such combinations that have shown efficacy include sertraline plus naltrexone for depressed alcoholics,32 and contingency management with voucher incentives combined with antidepressant medications for cocaine dependence.33,34
The observed association between mood improvement and cocaine use improvement was predicted and does encourage further research on treating depression among cocaine-dependent patients. It also raises methodological points related to statistical power and the nature of the relationship between depression and cocaine use. The average effect size for antidepressant medications in trials with routine depressed outpatients is only in the small to medium range,35 corresponding to a number needed to treat of around 5. This would leave a trial like the present one just marginally powered, despite a sample size (N = 130) fairly typical for single-site efficacy trials. Further, if the effect of an antidepressant medication on cocaine use is mediated by its effect on depression, then the problem of low power is compounded, since the effect on cocaine outcome would depend on the fraction of the sample (1/5, perhaps) with a medication-specific mood response. Rather than clinical trials testing single antidepressant medications, it may make more sense to test adaptive treatment algorithms that change or augment antidepressant treatments until a mood response is achieved. By achieving a higher overall mood response rate, this could in turn increase the likelihood of seeing an impact on substance use.
In summary, this placebo controlled trial failed to find a beneficial effect of venlafaxine for cocaine-dependent patients with depression, when delivered in combination with cognitive behavioral relapse prevention therapy. The modest overall rate of depression improvement, low rate of abstinence achieved, and association between improvements in depression and cocaine use, suggest depressed cocaine-dependent patients should continue to be the focus of treatment development efforts. Future trials should place more emphasis on medication compliance and consider designs that might boost effect size, such as testing synergistic combinations of antidepressant medications with other pharmacological or behavioral treatments, or testing adaptive treatment algorithms for depression.
Acknowledgments
The authors wish to thank NIDA for support of this study through NIDA P50 DA09236 to Dr. Herbert D. Kleber, Professor of Psychiatry, Columbia University and a K23 (DA277044-01) to Dr Raby.
The authors wish to thank all the personnel of the Division on Substance Abuse, and the STARS clinic for their assistance in conducting this clinical trial.
Footnotes
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
REFERENCES
- 1.Kessler RC, Crum RM, Warner LA, et al. Lifetime co-occurrence of DSM-IIIR alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Arch Gen Psychiatry. 1997;54:313–321. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- 2.Jane-Llopis E, Matytsina I. Mental health and alcohol, drugs and tobacco: A review of the comorbidity between mental disorders and the use of alcohol, tobacco, and illicit drugs. Drug Alcohol Depend. 2006;25:515–536. doi: 10.1080/09595230600944461. [DOI] [PubMed] [Google Scholar]
- 3.Hasin DS, Nunes EV. Comorbidity of alcohol, drug, and psychiatric disorders: Epidemiology. In: Kranzler HR, Rounsaville BJ, editors. Dual Diagnosis and Treatment: Substance Abuse and Psychiatric Disorders. New York, NY: Marcel Decker, Inc.; 1988. pp. 1–32. [Google Scholar]
- 4.Nunes EV, Levin FR. Treatment of depression in patients with alcohol or other drug dependence: A meta-analysis. JAMA. 2004;291:1887–1896. doi: 10.1001/jama.291.15.1887. [DOI] [PubMed] [Google Scholar]
- 5.Torrens M, Fonseca F, Mateu G, et al. Efficacy of antidepressants in substance use disorders with and without comorbid depression. A systematic review and meta-analysis. Drug Alcohol Depend. 2005;78:1–22. doi: 10.1016/j.drugalcdep.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Nunes EV, Quitkin FM, Donovan SJ, et al. Imipramine treatment of opiate-dependent patients with depressive disorders. A placebo-controlled trial. Arch Gen Psychiatry. 1998;55:153–160. doi: 10.1001/archpsyc.55.2.153. [DOI] [PubMed] [Google Scholar]
- 7.Petrakis I, Carroll KM, Nich C, et al. Fluoxetine treatment of depressive disorders in methadone-maintained opioid addicts. Drug Alcohol Depend. 1998;50:221–226. doi: 10.1016/s0376-8716(98)00032-5. [DOI] [PubMed] [Google Scholar]
- 8.Schmitz JM, Averill P, Stotts AL, et al. Fluoxetine treatment of cocaine-dependent patients with major depressive disorder. Drug Alcohol Depend. 2001;63:207–214. doi: 10.1016/s0376-8716(00)00208-8. [DOI] [PubMed] [Google Scholar]
- 9.Nunes EV, McGrath PJ, Quitkin FM, et al. Imipramine treatment of cocaine abuse: Possible boundaries of efficacy. Drug Alcohol Depend. 1995;39:185–195. doi: 10.1016/0376-8716(95)01161-6. [DOI] [PubMed] [Google Scholar]
- 10.Margolin A, Kosten TR, Avants SK, et al. A multicenter trial of bupropion for cocaine dependence in methadone-maintained patients. Drug Alcohol Depend. 1995;40:125–131. doi: 10.1016/0376-8716(95)01198-6. [DOI] [PubMed] [Google Scholar]
- 11.Carroll KM, Nich C, Rounsaville BJ. Differential symptom reduction in depressed cocaine abusers treated with psychotherapy and pharmacotherapy. J Nerv Ment Dis. 1995;183:251–259. doi: 10.1097/00005053-199504000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Ciraulo DA, Knapp C, Rotrosen J, et al. Nefazodone treatment of cocaine dependence with comorbid depressive symptoms. Addiction. 2005;100(Suppl. 1):23–31. doi: 10.1111/j.1360-0443.2005.00984.x. [DOI] [PubMed] [Google Scholar]
- 13.Afshar M, Knapp CM, Sarid-Segal O, et al. The efficacy of mirtazapine in the treatment of cocaine dependence with comorbid depression. Am J Drug Alcohol Abuse. 2012;38:181–186. doi: 10.3109/00952990.2011.644002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDowell DM, Nunes EV, Seracini AM, et al. Desipramine treatment of cocaine-dependent patients with depression: A placebo-controlled trial. Drug Alcohol Depend. 2005;80:209–221. doi: 10.1016/j.drugalcdep.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 15.McDowell DM, Levin FR, Seracini AM, et al. Venlafaxine treatment in cocaine abusers with depressive disorders. Am J Drug Alcohol Abuse. 2000;26:25–31. doi: 10.1081/ada-100100588. [DOI] [PubMed] [Google Scholar]
- 16.Spitzer RL, Williams JDW, Gibbon M, et al. The structured clinical interview for DSM-III-R (SCID). I: History, rational, and description. Arch Gen Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 17.Nunez EV, Goehl L, Seracini A, et al. A modification of the structured clinical interview for DSM-III-R to evaluate methadone patients: Test-retest reliability. Am J Addictions. 1996;5:241–248. [Google Scholar]
- 18.Carrol KM, Rounsaville BJ, Gordon LT, et al. Psychotherapy and pharmacotherapy for ambulatory cocaine abusers. Arch Gen Psychiatry. 1994;51:177–187. doi: 10.1001/archpsyc.1994.03950030013002. [DOI] [PubMed] [Google Scholar]
- 19.Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry. 1988;45:742–747. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- 20.Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: U.S. Department of Health, Education, and Welfare Publication ADM 76-338, National Institute of Mental Health; 1976. pp. 217–222. [Google Scholar]
- 21.Miller WR, Rollnick S. Motivational Interviewing. New York: The Guilford Press; 2002. [Google Scholar]
- 22.Lobello KW, Preskorn SH, Guico-Pabia CJ, et al. Cytochrome P450 phenotype predicts antidepressant efficacy of venlafaxine: A secondary analysis of 4 studies in major depressive disorder. J Clin Psychiatry. 2010;71:1482–1487. doi: 10.4088/JCP.08m04773blu. [DOI] [PubMed] [Google Scholar]
- 23.Gex-Fabry M, Balant-Gorgia AE, Balant LP, et al. Time course of clinical response to venlafaxine: Relevance of plasma levels and chirality. Eur J Clin Pharmacol. 2004;59:883–891. doi: 10.1007/s00228-003-0710-3. [DOI] [PubMed] [Google Scholar]
- 24.Pettinati HM, Volpicelli JR, Luck G, et al. Double-blind clinical trial of sertraline treatment for alcohol dependence. J Clin Psychopharmacol. 2001;21:143–153. doi: 10.1097/00004714-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Kranzler HR, Mueller T, Cornelius J, et al. Sertraline treatment of co-occurring alcohol dependence and major depression. J Clin Psychopharmacol. 2006;26:13–20. doi: 10.1097/01.jcp.0000194620.61868.35. [DOI] [PubMed] [Google Scholar]
- 26.Carpenter KM, Brooks AC, Vosburg SK, et al. The effect of sertraline and environmental context on treating depression and illicit substance use among methadone maintained opiate dependent patients: A controlled clinical trial. Drug Alcohol Depend. 2004;74:123–134. doi: 10.1016/j.drugalcdep.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 27.Carroll KM, Rounsaville BJ, Nich C, et al. One-year follow-up of psychotherapy and pharmacotherapy for cocaine dependence. Delayed emergence of psychotherapy effects. Arch Gen Psychiatry. 1994;51:989–997. doi: 10.1001/archpsyc.1994.03950120061010. [DOI] [PubMed] [Google Scholar]
- 28.Roseboom PH, Kalin NH. Neuropharmacology of venlafaxine. Depress Anxiety. 2000;12(Suppl. 1):20–29. doi: 10.1002/1520-6394(2000)12:1+<20::AID-DA3>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 29.Anton RF, O’Malley SS, Ciraulo DA, et al. Combine Study Group. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 30.Kranzler HR, Burleson JA, Brown J, et al. Fluoxetine treatment seems to reduce the beneficial effects of cognitive-behavioral therapy in type B alcoholic. Alcohol Clin Exp Res. 1996;20:1534–1541. doi: 10.1111/j.1530-0277.1996.tb01696.x. [DOI] [PubMed] [Google Scholar]
- 31.Kranzler HR, Armeli S, Tennen H, et al. A double-blind, randomized trial of sertraline for alcohol dependence: Moderation by age of onset [corrected] and 5-hydroxytryptamine transporter-linked promoter region genotype. J Clin Psychopharmacol. 2011;31:22–30. doi: 10.1097/JCP.0b013e31820465fa. Erratum in: J Clin Psychopharmacol 2011;31:576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pettinati HM, Oslin DW, Kampman KM, et al. A double-blind, placebo-controlled trial combining sertraline and naltrexone for treating co-occurring depression and alcohol dependence. Am J Psychiatry. 2010;167:668–675. doi: 10.1176/appi.ajp.2009.08060852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poling J, Oliveto A, Petry N, et al. Six-month trial of bupropion with contingency management for cocaine dependence in a methadone-maintained population. Arch Gen Psychiatry. 2006;63:219–228. doi: 10.1001/archpsyc.63.2.219. [DOI] [PubMed] [Google Scholar]
- 34.Moeller FG, Schmitz JM, Steinberg JL, et al. Citalopram combined with behavioral therapy reduces cocaine use: A double-blind, placebo-controlled trial. Am J Drug Alcohol Abuse. 2007;33:367–378. doi: 10.1080/00952990701313686. [DOI] [PubMed] [Google Scholar]
- 35.Walsh BT, Seidman SN, Sysko R, et al. Placebo response in studies of major depression; variable, substantial, and growing. JAMA. 2002;287:1840–1847. doi: 10.1001/jama.287.14.1840. [DOI] [PubMed] [Google Scholar]