Abstract
This paper reviews and synthesizes functional imaging research that over the past decade has begun to offer new insights into the brain mechanisms underlying emotion regulation. Towards that end, the first section of the paper outlines a model of the processes and neural systems involved in emotion generation and regulation. The second section surveys recent research supporting and elaborating the model, focusing primarily on studies of the most commonly investigated strategy, which is known as reappraisal. At its core, the model specifies how prefrontal and cingulate control systems modulate activity in perceptual, semantic and affect systems as a function of one's regulatory goals, tactics, and the nature of the stimuli and emotions being regulated. This section also shows how the model can be generalized to understand the brain mechanisms underlying other emotion regulation strategies as well as a range of other allied phenomena. The third and last section considers directions for future research, including how basic models of emotion regulation can be translated to understand changes in emotion across the lifespan and in clinical disorders.
Keywords: emotion, emotion regulation, cognitive control, amygdala, prefrontal cortex
…Thy fate is the common fate of all,
Into each life some rain must fall…
- Henry Wadsworth Longfellow
The Rainy Day (1842)
…'Every cloud', says the proverb, 'has a silver lining.'
- P. T. Barnum
Struggles and Triumphs (1869)
It might be said that emotions are the weather of our lives. Some days, we experience the blue skies of happiness and the sunshine of joy. Other days, we are drenched by the rain clouds of sadness or buffeted by the hot winds of anger. How we respond adaptively to our emotional weather patterns - finding the silver lining in every dark cloud - has important consequences for our physical and mental well-being1-7.
Although we can't control the weather outside, we are capable of using myriad emotion regulation strategies to take control of our internal climates8. Such strategies allow us to wholly or partially alter the nature, magnitude, and duration of our emotional responses, including initiating new ones. In recent years great strides have been taken in using neuroscience techniques to understand the mechanisms underlying emotion regulation. In humans, this research has primarily used functional imaging to examine our ability to control affective responses using cognitive strategies. The overarching goals of this paper are to review the progress made by such research, synthesize from it conclusions that suggest expansion on and elaborations of a model of the cognitive control of emotion, and show how the model can make sense of a wide range of emotion regulatory abilities and allied phenomena.
Towards these ends, the remainder of the paper is divided into three parts. In the first, we outline a basic model of the cognitive control of emotion whose core elements have been described previously9, 10. In the second section we review current imaging research suggesting ways in which the model can evolve to integrate new findings on the brain bases of emotion regulation as well as be applied to account for other related phenomena, such as affective learning, affect-based decision-making, and affective expectancies. Throughout these first two sections we focus primarily on one strategy in particular – known as reappraisal – because it has received the bulk of empirical attention. In the third and last section we summarize and consider directions for future basic and translational research.
A Model of The Cognitive Control of Emotion
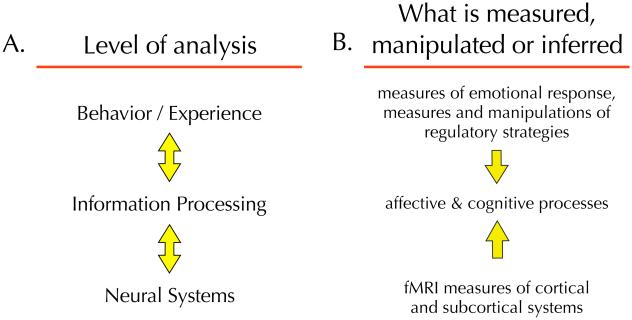
Any model of emotion regulation (or any other phenomenon) is predicated on assumptions about how different levels of analysis fit together. Our assumptions follow those now commonplace in cognitive, affective and social neuroscience where researchers seek to describe phenomena in terms of the relationships among three levels of analysis: behavior/experience, process and neural systems (11-13; Figure 1A). Neuroimaging research on emotion and its regulation can observe phenomena at the behavioral level (e.g. measures of emotional response and the specific regulatory strategies one might employ) and the neural level (e.g. fMRI measures of brain activity) and use these observations to infer the nature of the intervening cognitive and/or affective processes (Figure 1B).
Figure 1.
A multi-level approach to building model of emotion regulation. A. In cognitive, affective and social neuroscience research we seek to describe phenomena in terms of relationships between three levels of analysis: experience and behavior, psychological processes and neural systems. The bidirectional arrows between levels indicate that the relationships among them are bidirectional. B. Through measurement and/or experimental manipulation, neuroimaging research on emotion regulation can observe phenomena at the behavioral level and the neural level and use these observations to infer the nature of the intervening cognitive and/or affective processes. The direction of the arrows from the behavioral and neural levels towards the process level indicates the direction of causal inference (i.e. we can't observe the operation of these processes directly, but infer their operation based on behavioral and neural observations).
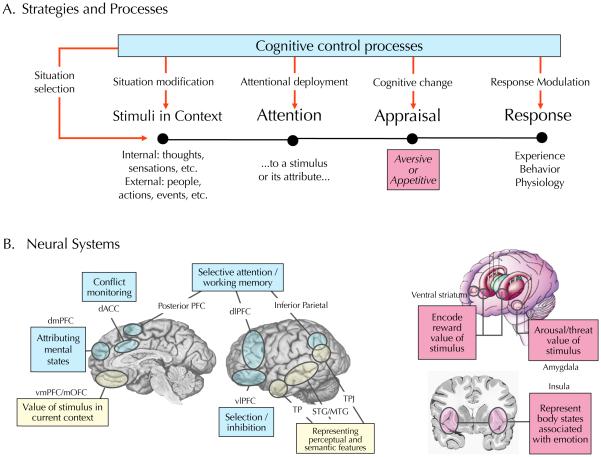
With this in mind, our review of current research will sometimes be organized in terms of phenomena described at the level of behavior, including regulatory goals, tactics and target stimuli. In other cases it will be organized in terms of issues concerning the neural-level pathways on which the field has begun to make progress. Taken together, the data reviewed in each section constrains and influences our model of the cognitive control of emotion (MCCE, see Figure 2).
Figure 2.
A model of the cognitive control of emotion (MCCE). A. Diagram of the processing steps involved in generating an emotion and the ways in which cognitive control processes (blue box) might be used to regulate them. As described in the text, the effects of different emotion regulation strategies (the red arrows descending from the cognitive control processes box) can be understood in terms of the stages of of the emotion generation sequence that they impact. The pink box seen at the appraisal stage is meant to indicate that neural systems involved in generating emotion support this process. B. Neural systems involve in using cognitive strategies, such as reappraisal, to regulate emotion (left, blue boxes),systems involved in generating those responses (left, pink boxes), and systems with an undefined or intermediary role in reappraisal (left, yellow boxes).
To understand how emotion regulation works, we must first have an idea of how emotions are generated. As such, our model has two main parts – descriptions of the mechanisms supporting emotion generation on the one hand and the mechanisms supporting emotion regulation on the other. For the sake of simplicity, we present the psychological and neural systems involved in the generation and regulation of emotion as being distinct yet it should be noted that there is evidence to suggest that the underlying psychological 14 and neural mechanisms 15, 16 are at least partially overlapping. Indeed, elsewhere we have noted that the distinction between emotion generation and regulation is blurry at best (e.g. 16), and which term one uses may reflect their usefulness for addressing a particular question more than hard and fast differences in their mechanisms. Here we treat them separately in order to make points about the ways in which putative control and affect-triggering systems interact.
Mechanisms of emotion generation
Our account of how emotions are generated is multi-leveled12 in its description of both the processes and the neural systems that give rise to emotional responses.
Processes involved in generating emotion
The black time line at the bottom of Figure 2A shows a simple model of four steps involved in generating emotional responses17. In the first step, a stimulus is perceived in its current situational context. The stimulus could be an internal thought, feeling, or sensation or any number of external cues, ranging from a facial expression or gesture to an action or event. At the second stage, one attends to some of these stimuli or their attributes. Whatever is in the focus of attention is passed along to subsequent emotion generative stages, whereas ignored or unattended stimuli may be either excluded from these stages or receive diminished subsequent processing. The third stage involves appraising the significance of stimuli in terms of their relevance to one's current goals, wants or needs. This is the stage focused on by appraisal theories of emotion, which describe the structure of different appraisals that lead to positive vs. negative reactions in general and to specific types of emotional responses in particular18. Because the current neuroscience literature suggests that there may not be specific neural systems for different discrete emotions19, 20, for present purposes, we simply distinguish between basic positive/appetitive vs. negative/aversive appraisals that have been reliably associated with specific neural systems that are described below. Finally, the fourth stage involves translating these appraisals into changes in experience, emotion-expressive behavior, and autonomic physiology. Although these three indicators of emotional response do not always correlate with one another for reasons that are not perfectly understood21, as noted below, emotion regulation strategies can effect changes in some or all of them, depending on the strategy.
Neural systems involved in generating emotion
Reviews and meta-analyses of functional imaging studies19, 20 indicate that a number of cortical and subcortical brain systems may play key roles in the appraisal and/or response stages of emotion generation. For present purposes we focus on the four that have been most frequently discussed in studies of reappraisal in particular, and emotion regulation strategies more generally (see Figure 2B; for examples of other emotion systems that may be modulated by emotion regulation see9, 22)
The first is the amygdala, which is involved in the perception and encoding of stimuli relevant to current or chronic affective goals23, 24, ranging from rewards or punishments to facial expressions of emotion to aversive or pleasant images and films25-27. While the amygdala generally is sensitive to detecting and triggering responses to arousing stimuli28, it exhibits a bias towards detecting cues signaling potential threats, like expressions of fear29-31.
The second is the ventral striatum, which is involved in learning which cues (ranging from social signals, like smiling faces, to actions to abstract objects) predict rewarding or reinforcing outcomes32-34.
The third is the ventromedial prefrontal cortex (vmPFC), which integrates affective valuations of specific stimuli made by the amygdala and ventral striatum with inputs from other regions, including medial temporal lobe systems that provide historical information about prior encounters with the stimuli as well as inputs from brainstem motivational and prefrontal control centers that provide information about current behavioral goals35-43. As such, vmPFC tracks the positive or negative valuation of stimuli in a context and goal-dependent manner41, 44-46. Examples of this include the finding that vmPFC activity to an image of a healthy but not tasty food depends upon whether one has the goal to eat healthily47, and the findings that vmPFC lesions lead to context-inappropriate affective responses in both humans and animals39, 48, 49.
The fourth brain system is the insula, which is thought to represent a viscerotopic map of ascending viscerosensory inputs from the body50 and has been implicated in negative affective experience in general51, 52. There appears to be posterior-anterior functional gradient in the insula with posterior regions associated with primary representations of sensations from the body and anterior regions associated interoceptive awareness of the body and in motivational and affective states, like disgust, that have a strong visceral component51, 53-57.
Mechanisms of Emotion Regulation
With an understanding of how emotions are generated in the first place we can turn to an account of the processes and neural systems involved in regulating them.
Processes involved in emotion regulation
While many behaviors can change our emotions, often these effects are unintended or incidental (e.g. your mood improves because you happen to have lunch with a friend) and as such as not considered to be examples of emotion regulation, per se. Instead, emotion regulation entails the modification of ongoing - or the initiation of new - emotional responses through the active engagement of regulatory processes. That said, we can further distinguish between cases where emotion regulation is guided by regulatory goals that are implicit or outside awareness (e.g.58) as compared to explicit and accessible to awareness. Although both are interesting and important, no neuroscience research has addressed the former case and a great deal has addressed the latter case. Therefore, we focus here on the deliberate deployment of an emotion regulation strategy in the service of explicit goals to change one's emotions. To understand how such explicit emotion regulation strategies work, it is useful to distinguish among five classes of strategies whose effects on emotion can be understood in terms of the stage of the emotion generation sequence that they impact59.
It is important to note that the distinctions made below originally were based on behavioral analyses of the aspects of emotional responses targeted by different strategies59. As such, this analysis was agnostic to the specific nature of the regulatory processes supporting each strategy, but tacitly assumed that all strategies drew upon some combination of cognitive control processes (designated by the blue box in Figure 2A). In this regard, functional imaging has made a substantial contribution to our understanding of how emotion regulation works because it provides insight into the nature of the control processes supporting emotion regulation that is not obtainable frombehavioral data alone9.
As illustrated by the top portion of Figure 2A, the first two strategies involve changing the nature of the stimulus inputs to the emotion generation cycle. In situation selection, you keep yourself away from stimuli that elicit unwanted emotions and put yourself in the presence of stimuli that elicit desired emotions. An example is staying away from a party where an old flame will be present if you don't want to feel pangs of sadness for having been dumped by her. Situation modification is when you find yourself in the presence of a stimulus that elicits an unwanted emotion and change something about the situation to alter its impact on you. In the old flame example, you might leave a party at which she is unexpectedly present or leave the room in which she is having a conversation. While these two strategies are undoubtedly effective (e.g.60), they can be difficult to study neurally and have received little attention in imaging or using other neuroscience techniques (see below).
The remaining three strategies are all amenable to, and have been studied, using imaging, albeit to varying degrees. Attentional deployment controls what stimuli are gated into, or out of, the emotion generation process. The two most commonly studied exemplars10 are selective attention, which involves moving the focus of attention towards or away from stimuli or their attributes, and distraction, which involves limiting attention to an external stimulus by focusing internally on information maintained in working memory. These types of strategies differ from situation selection in that they do not involve physically altering one's proximity or relationship to an emotional stimulus but rather they manipulate attention so as to alter one’s emotional response. Cognitive change involves changing the way one appraises the meaning of a stimulus. It is one of the most cognitively complex strategies insofar as it draws on any of a number of different higher cognitive processes to support changes in stimulus meaning, including language and memory, as well processes that also support other strategies, such as attention and response selection. The most commonly studied exemplar is reappraisal, which involves reinterpreting the meaning of a stimulus, including one's personal connection to it, in order to change one's emotional response. Finally, response modulation strategies target the systems for emotion-expressive behavior. The most commonly studied exemplar is expressive suppression61, which entails keeping the face still so that observers would not know what emotion you are experiencing.
A great deal of behavioral and psychophysiological research has been devoted to comparing and contrasting the behavioral consequences of deploying each of these strategies. For example, it's known that attentional deployment and reappraisal can have downstream effects on various components of an emotional response because they target the early stages of the emotion generation sequence61-66. By contrast, expressive suppression impacts only the behaviors it targets at the final response stage of emotion generation; when keeping your face still, emotional experience may subtly diminish67, 68, if at all, and your physiological arousal will increase from the effort61. There also is evidence that strategies differ in their long-term effects. For example, reappraisal but not distraction has been shown to have long-lasting effects on one's tendency to have an emotional response to a stimulus66, presumably because only reappraisal involves an active change in how one represents the affective meaning of that stimulus.
Neural systems involved in emotion regulation
As foreshadowed above, the use of functional imaging has provided insight into the nature of the control systems that support regulatory strategies as well as the affect systems that strategies modulate in order to change your emotional response. This section discusses core conclusions that can be drawn from reappraisal studies and a model of emotion regulation that can be derived from it.
Reappraisal as a paradigm case
Reappraisal is an appropriate starting point for developing a model of the cognitive control of emotion for three reasons. First, because reappraisal is among the most cognitively complex strategies, a model of emotion regulation derived from reappraisal work may be generally applicable to other strategies and phenomena that typically will be cognitively simpler. Second, the majority of studies to date have focused on reappraisal because a) it can be studied easily in an imaging environment and b) because it is the strategy referenced by countless aphorisms that advise us, "[to] look on the bright side…", "[to] turn a sow's ear into a silk purse…", "When life gives you lemons, make lemonade," and, "[that] every dark cloud has a silver lining." Third, in contrast to other areas of emotion regulation research (reviewed below) reappraisal studies tend to be more methodologically and conceptually similar to one another and therefore provide a stronger base for mechanistic inferences. With these considerations in mind, we now describe five key insights into the brain mechanisms supporting emotion regulation that have been derived from studies of reappraisal9.
Basic control system-affect system relationships
When the first fMRI studies of reappraisal were published approximately ten years ago there were no imaging studies of any form of emotion regulation. To develop hypotheses about how reappraisal might work, an analogy was drawn between the use of cognition to control emotion and the use of cognition to control memory, attention, and other thought processes43. The simple idea was that prefrontal and cingulate systems would support control processes that modulate activity in posterior and subcortical systems that generate emotional responses10. A decade and over 50 imaging studies later, this initial hypothesis has been strongly supported.
Figure 2B schematically illustrates the brain systems shown by current research to be involved in the cognitive control of emotion via reappraisal. As such, Figure 2B diagrams the core elements of the MCCE. Three types of neural systems are primarily involved in generating and applying reappraisals10. First, dorsolateral and posterior prefrontal cortex, along with inferior parietal regions generally implicated in selective attention and working memory, may be used to direct attention to reappraisal-relevant stimulus features and hold in mind reappraisal goals as well as the content of one's reappraisal69-71. Second, dorsal regions of the anterior cingulate cortex implicated in performance monitoring may help track the extent to which one's current reappraisals are changing emotional responses in the intended way72. Third, regions of ventrolateral prefrontal cortex implicated in selecting goal-appropriate (and inhibiting goal-inappropriate) responses and information from semantic memory may be used to deliberately select a new stimulus-appropriate reappraisal in favor of one's initial pre-potent appraisal of that stimulus73, 74. Finally, to the extent that one's reappraisal involves focusing on and interpreting or reinterpreting one's own emotional states-or those of others, dorsomedial prefrontal regions implicated in attributing mental states also may be active 75, 76.
With respect to the emotion-related regions that are modulated by reappraisal, the four regions described earlier in the section on neural systems for emotion generation all have been implicated - albeit to differing extents. Far and away, the most commonly modulated region is the amygdala, followed by the ventral striatum. The insula and the vmPFC are the least commonly modulated regions9, 10 (although see section on pathways below for a potential role of vmPFC in reappraisal as a modulator).
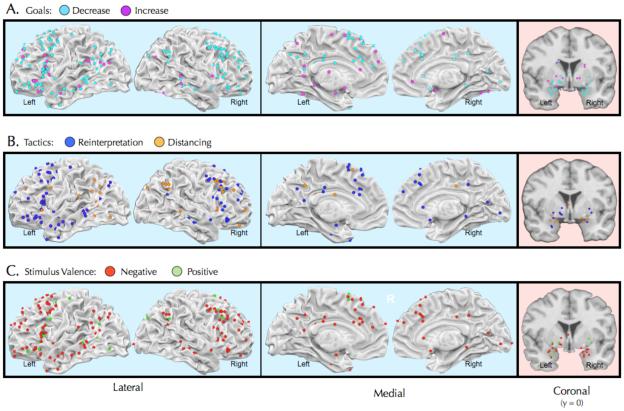
While we will discuss the significance of the differential modulation of these regions in more detail later (see later sections on valence-specificity and pathways), for now we can highlight the consistency with which they have been observed. Figure 3 plots peak activation foci for 43 studies (see Table 1) of reappraisal in healthy individuals as a function of reappraisal goals (panel A), reappraisal tactics (panel B), and the valence of the emotion being regulated (panel C). Ignoring these distinctions for a moment, one can see that the control system-affect system relationships shown in Figure 2B and described above have been observed reliably across numerous studies.
Figure 3.
Plots of activation foci from the 43 studies of reappraisal described in the text and Table. A. Plots of foci as a function of the goals to descrease or increase emotion. B. Plots of foci as a function of the specific reappraisal tactics used – either reinterpeting the meaning of events depicted in stimuli or actively changing one’s psychological distance from them. C. Plots of foci as a function of the valence of the stimuli eliciting the emotions that participants attempted to regulate. Blue boxes illustrate regions that are purported to support reappraisal (increase>look and decrease>look contrasts). Pink boxes illustrate regions that are purported to be modulated by reappraisal (look>decrease and increase>look contrasts; for clarity only foci falling within the boundaries of the amygdala and striatum are shown).
Table.
Studies are ordered first by year and second by alphabetical order. Only studies that reported contrasts (i.e., not only functional connectivity or correlational analyses) for psychologically healthy individuals are included here. If a study included a patient sample but still reported results for its healthy adult controls separately, it was included. Column labels: Study=study listed in references; For Participants, HYA= healthy young adult participants typically 18-30 yrs old, HOA=healthy older adults participants typically aged 60 years or older; HC=healthy adult control participants matched to patients; For Design, Goal=Goal pursued by participants to increase or decrease emotional responses, Valence=Positive or negatively valenced emotional stimuli, Tactic=Type of reappraisal used - distancing or reinterpreting, Stim Type=stimulus type, Timing of reapp cue=timing of instruction cue to reappraise relative to onset of stimulus, where early is just prior to simulus onset and late is a few seconds after stimulus onset; Amygdala?=whether modulation of amygdala was reported.
| Design |
|||||||
|---|---|---|---|---|---|---|---|
| Study | Participants | Goal | Valence | Tactic | Stimulus Type | Timing of reapp cue | Amygdala? |
| Beauregard et al., 2001205 | HYA | Dec | Pos | Dist | Videos | Early* | No |
| Domes et al., 201082 | HYA | Both | Neg | Both | Photos | Late | Yes |
| Eippert et al., 200785 | HYA | Both | Neg | Both | Photos | Late | Yes |
| Erk et al., 2010a169 | HC | Dec | Neg | Dist | Photos | Early | Yes |
| Goldin et al., 200867 | HYA | Dec | Neg | Reint | Videos | Early | Yes |
| Harenski et al., 2006206 | HYA | Dec | Neg | Both | Photos | Early | Yes |
| Hayes et al., 2010140 | HYA | Dec | Neg | Reint | Photos | Late | Yes |
| Herwig et al., 2007207 | HYA | Dec | Both | Reint | Anticipate Photos |
Early | Yes |
| Hollmann et al., 2011208 | HYA | Dec | Pos (food) | Reint | Photos | Early | No |
| Ichikawa et al., 201183 | HYA | Both | Neg (errors) |
Reint | Task Errors | Early* | No |
| Kanske et al., 2010128 | HYA | Dec | Both | Both | Photos | Late | Yes |
| Kim et al., 200796 | HYA | Dec | Both | Reint | Photos | Early | Inc pos only |
| Kober et al., 2010119 | HYA smokers & non-smokers |
Dec | Pos (food/ cigs) |
Reint | Photos | Early | Yes |
| Koenigsburg et al., 2010200 | HYA | Dec | Neg | Dist | Photos | Early | Yes |
| Krendl et al., 2011209 | HYA | Dec | Neg | Unclear | Photos | Early | Yes |
| Kross et al., 2009113 | HYA | Dec | Neg | Reint | Memories | Late | No |
| Lang et al., 201184 | HC | Both | Neg | Dist | Scripts | Early | Inc only |
| Levesque et al., 2003210 | HYA | Dec | Neg | Dist | Videos | Early* | No |
| Mak et al., 2009211 | HYA | Dec | Both | Unclear | Photos | Early* | No |
| McRae et al., 2008212 | HYA | Dec | Neg | Reint | Photos | Early | Yes |
| McRae et al., 201062 | HYA | Dec | Neg | Reint | Photos | Early | Yes |
| McRae et al., 2012181 | Healthy aged 10-22 |
Dec | Neg | Reint | Photos | Early | No |
| McRae et al., 201215 | HYA | Dec | Neg | Reint | Photos of faces | Early | Yes |
| Modinos et al., 2010213 | HYA | Dec | Neg | Reint | Photos | Late | Yes |
| New et al., 200986 | HC | Both | Neg | Reint | Photos | Late | Yes |
| Ochsner et al., 200243 | HYA | Dec | Neg | Reint | Photos | Late | Yes |
| Ochsner et al., 200477 | HYA | Both | Neg | Both | Photos | Early | Yes |
| Ochnser et al., 200916 | HYA | Inc | Neg | Both | Photos | Early | Yes |
| Ohira et al., 2006116 | HYA | Dec | Both | Unclear | Photos | Early* | Yes |
| Opitz et al., 201197 | HYA & HOA | Both | Neg | Reint | Photos | Late | No |
| Phan et al., 2005115 | HYA | Dec | Neg | Reint | Photos | Early* | Yes |
| Pitskel et al., 201187 | Healthy aged 7-17 |
Both | Neg | Reint | Photos | Early | Yes |
| Schardt et al., in press214 | HYA | Dec | Neg | Dist | Photos | Early | Yes |
| Schulze et al., 201088 | HC | Both | Neg | Both | Photos | Late | No |
| Staudinger et al., 2009215 | HYA | Dec | Pos | Dist | Reward | Early* | No |
| Staudinger et al., 2011216 | HYA | Dec | Pos | Dist | Anticipate reward |
Early* | No |
| Urry et al., 200695 | HOA | Both | Neg | Reint | Photos | Late | Inc only |
| Urry et al., 2009217 | HOA | Both | Neg | Reint | Photos | Late | Yes |
| van Reekum et al., 200789 | HOA | Both | Neg | Reint | Photos | Late | Dec only |
| Vrticka et al., 2011218 | HYA | Dec | Both | Reint | Photos | Early* | Yes |
| Wager et al., 2008118 | HYA | Dec | Neg | Reint | Photos | Early | Yes |
| Walter et al., 2009219 | HYA | Dec | Neg | Dist | Photos | Early | Yes |
| Winecoff et al., 2010196 | HYA & HOA | Dec | Both | Dist | Photos | Late | Yes |
Notes: All studies used event-related designs (different types of trials are presented in a randomized fashion so as to estimate responses on a trial-by-trial basis) except the nine studies designated by * in the ‘Timing of reapp cue’ column, which indicates that they used a block design (trials are ‘blocked’ by type, such that many of one type appear consecutively). Also, for the stimulus type column, photo stimuli were drawn from the international affective picture system112 unless otherwise specified.
Abbreviations: Goal: Dec=Decrease, Inc=Increase, Both=Both increase and decrease conditions were used; Valence: Neg=Negative, Pos=Positive, Both=Both positive and negative stimuli were used; Strategy: Both: Both distancing and reinterpreting were used (this only applies to Ochsner, 2004) or participants were given choice of distancing or reappraising; Dist=Become more or less psychologically distant; Reint=Cognitively reinterpret; Unclear=Unclear as to what tactic was instructed.
Moving Beyond the Basic Model
With the consistency of the core control-affect system relationship as a foundation, we are now in a position to consider how this basic model – first proposed in 200243 and elaborated in 200510 – has evolved. Below we discuss first new conclusions that can be drawn about the model from recent studies of reappraisal. In this section, we pay special attention to two emerging features of the model: 1) the potential intermediary role of semantic/perceptual systems in reappraisal, and 2) pathways linking control and affect systems. Next, we discuss the way in which the model can be applied to understanding regulatory strategies other than reappraisal as well as various allied phenomena involving control-affect system interactions.
Integrating new research on reappraisal
Recent research provides new insight into the distribution of emotion regulation-related activation foci as a function of reappraisal goals (i.e., what outcome one hopes to achieve by regulating, e.g. increasing or decreasing an emotional experience), tactics (i.e., the specific subtype of reappraisal one implements), and the emotional valence of stimuli (i.e., whether the stimulus evokes a positive or negative emotional response). Here we consider the implications of this work for the evolving MCCE.
Goal-specificity
Arguably, the most common goal when using reappraisal is to decrease negative emotion, as when we attempt to make ourselves feel better about a disappointing paper rejection, an argument, and the like. Given this, it is not surprising that this goal has been the focus of the majority of reappraisal studies (see Table 1). This is not the only goal that guides reappraisal, however. In some cases, as when we worry, ruminate, or make ourselves more anxious or fearful by elaborating on the meaning of unpleasant events, we are using reappraisal in service of the goal to increase emotion. A small, but growing, number of studies have examined this reappraisal goal as well.
Figure 3A plots peak activation foci for reappraisal studies of healthy individuals as a function of decrease vs. increase goals. Perusal of this figure highlights three findings. First, whereas both increase and decrease goals recruit left prefrontal regions, decrease goals recruit right prefrontal regions to a much greater extent than do increase goals. There are two interpretations of this finding. First, it may be attributable to the fact that decreasing an emotional response is more difficult than increasing one, and therefore may require additional cognitive control resources 77. Second, decreasing - but not increasing - an emotional response requires inhibiting or limiting the expression of a prepotent appraisal of a stimulus (e.g. as negative) in favor of selecting an alternative reappraisal (e.g. as neutral or even positive) Research shows that right dorsal - and especially ventrolateral - prefrontal cortex is involved in the selection and/or inhibition of various kinds of responses78-81.
Second, there is some evidence that increase goals differentially involve anterior portions of dorsomedial prefrontal cortex (dmPFC). Of the 12 studies directly comparing increasing emotion to a control condition where participants respond naturally, six show increases in anterior dmPFC 16, 77, 82-84]. Of the six not, most showed activation in neighboring areas (such as anterior cingulate cortex) 63, 85-89. Given the role of dmPFC in making judgments about mental states76, 90, 91 and that the majority of reappraisal studies use photographs of people as stimuli (see Table 1), it is likely that these regions support attention to and elaboration of emotional states, intentions, and outcomes of the individuals depicted in these photos.
Third, whereas increase and decrease goals both seem to modulate the striatum (including both the caudate and putamen), they may differ in the way they modulate the amygdala. On the one hand, decrease goals reliably modulate the amygdala’s ventral (corresponding to the basal and lateral amygdala nuclei) and dorsal portions (corresponding to the central nucleus) as well as the sublenticular extended amygdala (SLEA30, 92, 93) that lies between the amygdala and the striatum. On the other hand, increase goals may modulate only the dorsal amygdala/SLEA. One speculative interpretation of these data is that decrease goals influence perceptual and semantic inputs to the amygdala, which come through the basolateral complex, whereas increase goals influence the outputs of the amygdala, which flow from the central nucleus43, 77. This hypothesis would fit with anatomical data showing that the basolateral complex has reciprocal connections with ventrolateral PFC as well as temporal and parietal regions implicated in visuospatial and semantic representation whereas the central nucleus recives inputs from medial prefrontal regions and sends outputs to autonomic centers that implement various components of an emotional response94.
The major caveat for all of these conclusions, however, is that very few studies have examined increase goals, and as a consequence, conclusions about the goal-specificity of reappraisal-related activations must be considered tentative. That being said, the first study to directly compare increase and decrease goals within subjects obtained exactly the results described above77 – both increasing and decreasing negative affect recruited left vlPFC and dlPFC and modulated the dorsal amygdala/SLEA (increasing affect increased amygdala activity while decreasing negative affect decreased activity), yet it was also revealed that increasing negative affect recruited the dmPFC to a greater degree than did decreasing negative affect and decreasing negative affect recruited right vlPFC and modulated ventral amygdala to a greater extent than did increasing negative affect. At least two thirds of subsequent studies comparing these goals have obtained results that are generally consistent with them77, 85, 88, 95-97 (other findings also have been reported, including increase vs. decrease differences only in the amygdala82, striatal modulation77, 89, 95 and greater right PFC activation for increasing than decreasing85).
Tactic-specificity
In the military, a distinction is commonly made between strategy and tactics. Strategy is the overall means by which a goal (e.g., win the war) is to be achieved (e.g., divide and conquer). Tactics are the specific ways in which strategies are implemented in a given circumstance (e.g. a quick infantry advance, an airstrike, etc.). In the same way, one can distinguish between reappraisal as a strategy that involves changing the meaning of a stimulus and the tactics used to implement that strategy98.
Two different reappraisal tactics have been studied with imaging9, 77. The first can be called reinterpretation, which involves changing one's interpretation of the elements of the situation or stimulus that elicits emotion. For example, if one is presented with a photo of a sick man in the hospital that elicits feelings of sadness, one might reinterpret this image in a way that decreases emotion by thinking about the man's hearty constitution and that he will be healthy and well in the future. To increase emotion, one might instead think about how the man is in a great deal of pain and may, in fact, get worse and even perish. The second can be called distancing, which involves changing one's personal connection to, or psychological distance from, the stimulus that elicits emotion. In the example of the photo of the sick man, one might decrease emotion by viewing the image from the detached perspective of an objective, third person observer and/or imagining that the pictured event took place a long time ago or in a faraway location. One might increase emotion by instead imagining that one is experiencing pictured events in the present moment, from a first-person perspective, which enables you to smell, hear, and directly observe what is taking place.
As Table 1 shows, about twice as many studies have examined reinterpretation as have examined distancing, with a few allowing participants to engage in either tactic, and only a single study directly comparing them77. Figure 3B plots peak activation foci for reappraisal studies of healthy individuals as a function of reinterpretation vs. distancing tactics.
This Figure illustrates three conclusions that can be drawn about reappraisal tactics. First, reinterpretation seems to differentially call upon ventral lateral prefrontal regions implicated in response selection and inhibition74, 99, 100. Presumably, this reflects the fact that reinterpretation requires that one must look up and select alternative meanings for stimuli from semantic memory to a greater extent than does distancing. Second, distancing seems to recruit parietal regions implicated in spatial attention and representation to a greater extent, including perspective taking and the sense of agency101-104. This may reflect the fact that distancing involves changing the conceptual and spatiotemporal perspective from which stimuli are experienced. Third, in general the regions involved in reinterpretation appear to be more strongly left lateralized in prefrontal and temporal cortices whereas regions involved in distancing appear to be more strongly right lateralized in prefrontal cortex. These patterns may reflect the differential dependence of reinterpretation and distancing on linguistic and semantic processes as opposed to spatial and attentional processes, which generally show a left vs. right hemisphere pattern of relative specialization77, 105.
Here again, however, because comparatively fewer studies have examined distancing firm conclusions concerning the tactic-specificity of reappraisal-related activations await further research that directly test the conclusions drawn above.
Valence-specificity
On average, the impact of negative emotional experiences seems to be greater than the impact of positive emotional experiences, both in the short and long term106. Indeed, problems with regulating negative emotion are more often a hallmark of clinical disorders than are problems with regulating positive emotion107. As such, it is not surprising that Table 1 shows that the number of reappraisal studies examining negative emotion outnumber those examining positive emotion more than three to one.
That said, two conclusions can be drawn from examining Figure 3C, which plots peak activation foci for reappraisal studies of healthy individuals as a function of the negative vs. positive valence of stimuli (and the emotions they presumably elicit). First, whereas reappraisal of both negative and positive stimuli depends upon left-hemisphere regions, reappraising negative stimuli depends on right hemisphere regions as well. These findings might reflect the fact that, to date, the majority of studies of negative emotion involve decrease goals. As noted earlier, decrease goals may require more cognitive resources than increase goals, including placing greater demands on selection/inhibitory functions associated with right vlPFC108-110. An alternative explanation is that positive and negative emotion generally involve approach vs. avoidance motivations, which have been associated with the left vs. right prefrontal cortex. This interpretation seems less likely, however, given that this motivation-related prefrontal asymmetry is commonly observed in EEG111 but not in fMRI studies19.
Second, it's apparent that reappraising negative stimuli typically modulates activity in the amygdala and less commonly activity in the striatum. By contrast, the handful of studies examining reappraisal of positive stimuli more commonly show modulation of the striatum, including the ventral portions associated with reward and reinforcement learning33, 34.
These conclusions are again tentative, however, because so few studies have examined reappraisal of positive stimuli and in general, studies of reappraisal have focused overwhelmingly on decrease rather than increase goals. As a consequence, it is not yet clear whether the patterns noted above are attributable to the pursuit of decrease vs. increase goals, the use of negative vs. positive stimuli, or both.
Stimulus-specificity
To date, 33 out of the 43 reappraisal studies shown in Table 1 have used photographic stimuli pulled from the International Affective Picture System (IAPS). These stimuli have been shown to reliably elicit experiential, physiological and facial expressive components of an emotional response in a valence-specific manner112. As such, they provide a straightforward means of eliciting affective reactions in the scanner environment.
That said, the emotions elicited by such stimuli may or may not generalize to other contexts. For example, IAPS photos are selected so as to be normatively positive or negative112. While this is suitable for many experimental agendas, other stimuli may be appropriate if one wants to examine the ability to reappraise specific emotions, the emotions elicited by idiosyncratically self-relevant autobiographical experiences113, 114, and so on.
With these considerations in mind, small numbers of studies have examined the ability to reappraise the specific emotions elicited by sad, sexual or disgusting videos, scripts that elicit particular emotions, the recollection of autobiographical memories, anticipation of reward or shock, or the commission of an error (see Table 1). Because so few studies have used each of these stimuli it is not useful at present to plot activation foci for them or to attempt to draw conclusions about how they might differ as a function of stimulus type. It remains for future research to directly address the question of how the nature of the stimulus per se, as opposed to the kind of emotion elicited, influences the neural systems involved in reappraisal.
Pathways linking control and affect systems
Studies of reappraisal - and more generally studies of any form of emotion regulation - implicitly or explicitly assume that prefrontal regions modulate emotional responses via their impact on affect systems like the amygdala and ventral striatum. Given the prevalence of this assumption it is somewhat surprising that it has seldom been put to a direct test. To be sure, a number of studies have shown correlations between prefrontal and amygdala activity77, 96, 115 or correlations between some measure of emotional response (typically self-report) and either prefrontal77, 115, 116 or amygdala activity77, 95, 117. Only four studies, however, have directly tested the mediation model implied by the hypothesis that control systems impact emotional response by influencing activity in affect systems (see Figure 4).
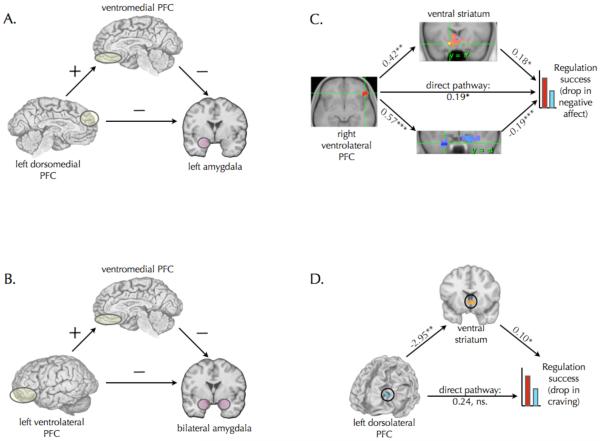
Figure 4.
Two kind of mediation pathways involved in reappraisal. A. and B. show pathways identified in two studies of the down regulation of negative emotion whereby dorsomedial or ventrolateral prefrontal regions diminish amygdala responses via their impact on ventromedial prefrontal cortex. These studies did not report weights for the mediation paths between regions or test for full vs. partial mediation. C. and D. show pathways identified in two studies of the down regulation of negative or positive emotion whereby ventrolateral or dorsolateral prefrontal regions diminish self-reports of negative affect or craving via their impact on the amygdala or ventral striatum, respectively. Path weights in the mediation model are shown. * = p < .05, ** = p < .01, *** = p < .001.
The first two studies to use mediation examined the use of reappraisal to diminish responses to negative photos95, 117. While both studies used amygdala reactivity as their measure of emotional response, neither reported a main effect of reappraisal on diminishing amygdala activity. Motivated by known connections between the amygdala and vmPFC, both studies looked for and found that individual differences in amygdala response were correlated inversely with responses of vmPFC. Mediation analyses showed that vmPFC mediated a relationship between either left dmPFC95 or left vlPFC117 and the amygdala (Figure 4A-B) such that activity in these prefrontal regions was positively related with vmPFC activity, which in turn was negatively related to amygdala responses. There are a couple qualifiers in interpreting these results, however. First, the study95 identifying the left dmPFC region did so in a increase > attend (i.e., a no regulation baseline) > decrease contrast, meaning that it generally is less active when decreasing negative emotion than when responding naturally in a baseline “attend” condition. This suggests that to the extent one shows less deactivation when decreasing (relative to baseline), one will show greater activity in vmPFC, and in turn lesser amygdala response. It is not immediately clear how to interpret lesser degrees of dmPFC deactivation in this context. Second, the study117 identifying the vlPFC->vmPFC->amygdala pathway collapsed across activity in both amygdalae that was extracted from structural ROIs. As such, its not clear whether the prefrontal effects were more or less strong for one amygdala or the other. That said, when taken together these two studies suggest that effective reappraisal involves PFC->vmPFC->amygdala pathways.
The second two studies used similar analytic approaches to study either the use of reappraisal to diminish responses to negative photos118 or for smokers, the use of reappraisal to diminish craving elicited by photographs of appetitive foods or cigarettes119. The study of negative emotion118 showed that right vlPFC activity predicted drops in self-reported negative emotion, and that this relationship was independently mediated by separate pathways through the amygdala and the ventral striatum (Figure 4C). These two pathways were taken to reflect the use of reappraisal to minimize negative appraisals and enhance positive reappraisals, respectively (see also Table S1 from that paper, which shows left vlPFC involvement as well). The study of craving119 showed that left dlPFC activity predicted drops in self-reported craving via modulation of activity in the ventral striatum (Figure 4D). Together, these two studies suggest that effective reappraisal involves a pathway linking PFC -> subcortex-> emotion change, with the specific elements of the pathway depending on the nature of the stimulus and emotion involved.
Why the differences between the results of these pairs of studies? On one hand, because different dependent measures of emotional response were used (amygdala response vs. self-reported emotion) it's possible that different reappraisal pathways will emerge depending on the type of response. On the other hand, it's also possible that differences in methodology may lead participants to reappraise differently, and in turn recruit different pathways for effective emotion regulation. Here, two differences between the pairs of studies may be relevant.
The first is that the first pair of studies that identifed the vmPFC mediated pathway both had participants that were up to 40 years older than the average participants in studies of reappraisal in young adults – aged 62-64 in one case95 and 19-53 (avg. 33) years in the other117. Participants in the second pair of studies were younger, as is the norm, averaging 22.3118 and 26.8119 years, respectively. Given findings that older adults may be impaired at some kinds of reappraisal, that lateral PFC thins while vmPFC thickens with age120, and that even when not told to regulate, older adults can show greater connectivity between vmPFC and amygdala121, its possible that vmPFC will play a bigger role in reappraisal for older as compared to younger adults.
Second, the first pair of studies cued participants to reappraise ~4 seconds into an ~10 second presentation of an aversive photo (a late cue), whereas the second pair presented the cue to reappraise just prior to onset of aversive photos (an early cue). The early cue method is intended to provide participants with an opportunity to first have a naturalistic emotional response to an aversive photo before they begin to regulate and has been used in 14 studies (see Table). The late cue method models real-world situations where the goal to reappraise comes online just as one encounters an emotionally evocative stimulus and has been used in 29 studies (see Table).
While the early cue method is analogous to real-world situations where the goal to regulate comes online only after one already is having an emotional response, there is a potential problem with trying to model this in the lab. During the initial free viewing of an aversive photo, participants may try out a few reappraisals just in case they are asked to subsequently reappraise on that trial. If this were the case, then we might expect one or both of two kinds of results.
One possibility is the ability to detect an effect of reappraisal on amygdala responses would be diminished for late cue studies either because the amygdala responded early and then habituated, or because when finally participants are asked to reappraise, the amygdala’s response could already have decreased a bit because they already had time to begin generating/practicing potential reappraisals before the explicit instruction cue to do so appeared. While neither of the mediation studies in question showed whole-brain amygdala effects, and only one showed effects using ROIs, weak effects of reappraisal on the amygdala are probably related to other factors (like age – see above) given that roughly the same ratio (roughly 2/3 to 3/4) of studies using the late and early cue methods show reappraisal-related amygdala modulation, especially for studies using photos (see Table).
A second possibility is that the late vs. early timing of reappraisal cues changes the nature of one’s reappraisals, even if, on average, they have similar effects on amygdala responding. For example, in late cues studies, if participants have had a chance to view stimuli for a few seconds and think about potential reappraisals before being explicitly told to go ahead and reappraise, then vmPFC recruitment could reflect decision processes about which of a set of prepared reappraisals they prefer and can best use for the stimulus at hand (see also section on decision-making below).
To date no imaging studies have compared late and early cues. But one behavioral/psychophyiological study has compared them and found that the effects of increase goals on some physiological measures are greater for late than early cues but that the effects of decrease goals were similar for each cue type. Future work could fruitfully illuminate these issues.
All this said, it is of course possible that both kinds of pathways are important and that a multi-step vlPFC->vmPFC->amydala/striatum->emotion response pathway may be observed in future studies. To date, however, no published studies have expressly tested for the existence of this complex pathway underlying reappraisal success.
The role of perceptual and semantic systems
A related issue is whether and how reappraisal involves modulation not just of systems involved in affective appraisal and response, but of systems involved in representing the perceptual and semantic properties of stimuli as well. As shown in Figure 3, activation of a number of these systems is often seen during reappraisal, including: regions along the middle and superior temporal sulci involved in representing the visual properties of stimuli, including nonverbal social cues to emotion like movements of lips and eyes122-124; temporal polar regions implicated in representing episodic and semantic emotion knowledge125; and regions near the temporal-parietal junction involved in representations of beliefs, including “false” beliefs of the sort one generates when considering alternative reappraisals of stimuli126, 127.
These data raise at least three questions. First, there is the question of when activation of these regions will be seen. Certainly, cognitive change strategies like reappraisal may involve these regions, given that it involves an active reworking of the meaning of a stimulus. Other strategies that do not focus on meaning may not involve these regions, however. Consistent with this, two studies directly comparing reappraisal and distraction found that reappraisal differentially recruited all three of the temporal regions listed above62, 128. Along these lines, it also is likely that these regions will be more involved in regulating responses to visual stimuli given the role of the temporal lobe in the ‘ventral visual stream’ for representing information about object identity129-131 (although this remains to be tested directly). As noted above, there is not yet enough work using different kinds of stimuli to say whether reappraisal of stimuli in non-visual modalities (e.g. somatosensory or auditory) may involve modulation of corresponding modality-specific regions (e.g. somatosensory of auditory cortices).
Second, if these regions are more active during reappraisal, there is the question as to why this is the case? Does greater activity here reflect increased attention to perceptual and semantic aspects of stimuli? Access to/retrieval of alternative ‘views’ of reappraised stimuli? The process of actively restructuring one’s (visual) mental image of a stimulus? All three interpretations are possible and could be tested in future work.
Third, there is the question of whether these temporal regions play a part in the regulation pathways described earlier – playing an intermediary role, for example, between prefrontal control systems and affective appraisal systems. This possibility was raised in early reappraisal studies (e.g. 43) where it was suggested that even though dorsolateral PFC regions do not have direct connections to subcortical regions like the amygdala, they may nevertheless modulate them via their impact on perceptual/semantic systems. On this view, PFC could change one’s mental representation of a stimulus’s meaning from the top down and that representation of the reappraised stimulus would feed forward to the amygdala (and other structures that trigger affective responses). Because the amygdala now, ‘sees,’ the reappraised stimulus, its response changes. While plausible, this hypothesis has yet to be directly tested.
Summary
Extant data from functional imaging studies of reappraisal strongly support the model of the cognitive control of emotion depicted in Figure 2B. Although many questions remain to be addressed about how specific control systems modulate specific affect systems as a function of reappraisal goals and tactics or various aspects of stimuli and emotions they elicit, a core control-affect system dynamic is now well established.
Generalizability to other forms of regulation
Given the robustness of the MCCE (Figure 2) in accounting for reappraisal, the question naturally arises as to whether this model can be generalized to account for other types of emotion regulation strategies.
As noted above, the majority of functional imaging studies of emotion regulation have focused on reappraisal. That said, the other four main classes of emotion regulation strategies diagrammed in Figure 2A have been targeted by imaging studies to varying degrees. Here, we consider each in turn.
Situation selection and modification
The two situation-focused strategies, situation selection and situation modification, have received little attention thus far in human imaging research. As noted earlier, this is at least partially attributable to the difficulty of devising appropriate lab paradigms for studying them. The lone human imaging study of situation selection builds on the rodent literature on avoidance conditioning. In a typical task, a rat learns to perform an action that allows it to avoid presentation of an aversive stimulus (e.g. 132, 133). In a human analogue of this procedure, Delgado et al. found that avoidance conditioning activates vlPFC and dlPFC control systems and modulates the amygdala134. These findings provide an initial suggestion that situation selection may call systems that maintain regulatory goals and select context-appropriate avoidance responses.
Attentional Deployment
By contrast, studies of attentional deployment have been relatively common, second in number only to studies of reappraisal. One set of these studies have examined the use of selective attention to shift visual spatial attention away from an affectively valenced stimulus or stimulus attribute and towards a neutral one. Another set of these studies has examined the use of distraction to shift the focus of attention inwards onto some internally maintained mental representation (e.g. a relevant working memory load, self generated stimulus-irrelevant thoughts, a pleasant mental image, and so on). As has been reviewed in detail elsewhere10, 135, interpreting the findings of both of these kinds of studies is clouded by three issues. First, almost all of the studies of selective attention, and many studies of distraction, use stimuli that do not elicit strong emotional responses, such as facial expressions of emotion. As such, these studies are concerned with the regulation of evaluative judgment or perception rather than affective responding, per se. Second, when highly arousing and affect-inducing stimuli are employed, they most often are stimuli that cause physical pain. While responses to painful stimuli have a strong negatively-valenced affective component, this component may itself have a distinct neural signature due to its recruitment of dedicated pain-specific neural pathways 136, 137. As such, it is an empirical question whether the regulation of pain is similar to or different from the regulation of negative affective responses more generally. Third, these studies are highly heterogenous, often employing very different stimuli and methods of controlling the focus and level of attention, without a clear metric for assessing the extent to which attention has or has not been paid to a given affective stimulus. Given these limitations, we refer the reader to other reviews of this literature 138, 139 while noting that it is generally consistent with the model depicted in Figure 2B in so far as activation of prefrontal systems and modulation of affect systems (like the amygdala) often (but not always) is reported.
Response Modulation
Finally, both behavioral and imaging studies of response modulation have focused on expressive suppression, which is the ability to hide behavioral manifestations of emotion61. The two imaging studies of expressive suppression asked participants to suppress facial expressions of disgust elicited by a film clip67, 140. Both found that expressive suppression not only activated dorsolateral and ventrolateral PFC regions associated with maintaining goals, response selection and inhibition73, 74, 78, it also increased activation of the insula, which is involved in triggering affective responses. Amygdala findings were more mixed, however, with one study reporting increases67 and one decreases140 in activity during suppression. Increases in insula and amygdala fit with psychophysiological studies demonstrating that expressive suppression boosts the autonomic component of emotional responding61.
In total, the available literature on emotion regulation strategies other than reappraisal is in some cases limited and in other cases somewhat confusing, but in general supports the idea that all emotion regulation strategies involve interactions between cognitive control and affect regions. Future neuromaging research must apply the same rigorous and thorough approach to these other strategies that has already been applied to reappraisal.
Generalizability to other related phenomena
Given the robustness of the MCCE (Figure 2B) in accounting for multiple forms of regulation, a next natural question is whether it can be generally applied to other allied phenomena, such as affective/emotional learning, decision making, and expectancies. These phenomena are typically considered in separate literatures, but seem to involve related cognitive-affective dynamics. Although space limitations prohibit an in-depth discussion, here we briefly examine the broad applicability of the model in each of these three cases. .
Affective/Emotional Learning
At the outset of this paper we made a distinction between goal-directed forms of emotion regulation, which are the focus of this review, and other behaviors that may have regulatory effects on emotion despite lacking a specific goal to do so. There are a number of forms of affective or emotional learning that fit the latter description. One of the most common examples is extinction of a conditioned fear response. In the traditional fear conditioning paradigm141, an animal learns that an ostensibly neutral stimulus, such as a light (known as the conditioned stimulus or CS), predicts the occurrence of an intrinsically aversive stimulus, such as electric shock (known as the unconditioned stimulus or UCS). Over time the repeated pairing of the light and shock lead the animal to respond to the light itself with an anticipatory fear response. Elegant animal studies have shown that fear conditioning depends upon communication between input and output nuclei of the amygdala141, 142. Fear extinction, involves the repeated presentation of the CS in the absence of the UCS143. Over time, the organism learns that the CS no longer predict shock, ceases to have its anticipatory fear response, and fear is said to be extinguished. Importantly, extinction is known to involve the laying down of a new context-dependent memory144. In the current temporal context, the CS does not predict shock, whereas in the past temporal context it did. Rodent lesion studies have shown that whereas the initial acquisition of extinction requires only the amygdala, the ability to retain and express memory for extinction depends upon vmPFC143. In keeping with this finding, studies in humans have shown that both the magnitude of vmPFC activation and vmPFC thickness predict the speed of extinction145-147.
In the present model, phenomena like extinction (or stimulus-reward reversal learning, which also depends upon vmPFC148, 149), are somewhat hybrid phenomena. On the one hand, they can be viewed as an example of emotion generation, in so far as one is learning to express a new emotional response to a given stimulus. On the other hand, they can be viewed as an implicit form of emotion regulation where one does not have an explicit goal to regulate, but the behaviors in which one engages directly alter the nature of one's emotional response.
Beyond this, there are a number of ways in which prefrontal control systems may have a regulatory impact on affective learning. For example, as noted earlier, in some cases reappraisal may involve interactions between PFC, vmPFC, and the amygdala, when reappraisal paradigms give participants a chance to respond emotionally and potentially plan reappraisals prior to deciding whether to implement them. Interactions of this sort also have been observed in studies that use distraction to regulate a conditioned response150, 151. In these studies, one is initially conditioned to expect either a painful shock or reward UCS following a visual CS (e.g. a yellow triangle). Later, one regulates the conditioned response to the CS by thinking about a calm and neutral scene unrelated to either the CS or the UCS. In both cases effective regulation involves activation of left dlPFC and modulation of both the amygdala and/or ventral striatum and the vmPFC.
Affective Decision Making
Affective decision making involves choosing among several stimuli that one may purchase, consume, or own. In some cases, these choices are a simple matter of selecting the option that has the greatest value. Imaging research suggests that activation in systems thought to represent affect and value like the ventral striatum, insula, and vmPFC is sufficient to support and even predict such choices152, 153. But in other cases, the choice options may be of similar value, or the reasons for valuing them may conflict with one another. In the model, such cases may draw on the control systems shown in Figure 2B to modulate the values associated with choice options, essentially guiding a top down re-valuation of them in order to facilitate choice.
Perhaps the simplest example of this is where the act of choice itself arouses conflict as one decides which features of choice options they can't live without and which features of choice options they must forgo. Classically, this decision conflict is thought to arouse cognitive dissonance, which the act of choosing reduces by placing a higher value on chosen and a lesser value on unchosen stimuli154. Imaging studies show that these choice-induced changes in value involve control systems like the anterior cingulate cortex, which may signal the presence of choice conflict and motivate value change, and systems like the ventral striatum, which may represent the revalued stimuli154-157.
Another type of choice that commonly requires the use of control occurs when an individual must decide between options that fit short-term vs. long-term goals. This is the dilemma faced by a dieter who must decide whether to eat a cupcake or an apple. Consuming the cupcake satisfies the short-term goal of hedonic pleasure whereas eating the apple satisfies the long-term goal of living a healthy lifestyle. A recent imaging study47 of this choice dilemma showed that food choices reflecting a greater valuation of long-term health over short-term tastiness involve the modulation of vmPFC by dlPFC. This is consistent with the idea that the cognitive control of choice involves interactions between systems for maintaining choice goals (e.g. dlPFC) and systems representing the value of choices with respect to those goals (e.g. vmPFC).
This same logic applies to studies of intertemporal choice and delay of gratification158 where imaging159, 160 and TMS161 studies suggest that lateral PFC control systems can be used to effortfully represent the value of a larger delayed reward and guide selection of it over a smaller but immediately available reward.
More generally, the model can be applied to other choices where control is needed to modulate the affective valuations placed on choice options, ranging from risky decision making162 to interpersonal contexts where one must decide whether to be fair towards or punish others163, 164.
Affective Expectancies
In parallel to the growth and development of imaging research on emotion regulation there has been a tremendous surge of interest in the brain mechanisms underlying the influence of expectancies on behavior165. In imaging, expectancies have been studied either by cueing participants that an upcoming stimulus will have particular properties (e.g. that it will or will not be painful, will be a neutral or aversive image, and so on) or by inducing beliefs about the effects of a placebo drug on their experience (e.g. that an analgesic cream will reduce pain).
In the model, these phenomena all involve the use of prefrontal control systems to set and maintain an expectation, which in turn influences the responses of affect generating systems. For example, imaging studies show that expectancies and placebo beliefs about pain activate lateral prefrontal/parietal control systems and/or medial prefrontal systems165-168 that may maintain expectations about upcoming events. In turn, these systems may influence the way one attends to and appraises the meaning of expected stimuli, thereby increasing or decreasing activity in affect systems to be consistent with the nature of one's expectations.
Summary and Future Directions for Basic and Translational Research
The overarching goal of this paper has been to review and synthesize current functional imaging research on emotion regulation. Towards that end, we outlined a basic model of the processes and neural systems involved in emotion generation and emotion regulation and surveyed various domains of research that support it. At its core, the MCCE specifies how prefrontal and cingulate control systems modulate activity in affect systems as a function of one's regulatory goal, tactics, and the nature of the stimuli and emotions being regulated. While the model was built primarily from studies of one type of cognitive change strategy known as reappraisal, it is generally applicable to understanding the brain mechanisms underlying the other emotion regulation strategies depicted in Figure 2A as well as a range of other allied phenomena.
That said, there is much work yet to be done. At various points during the review we've highlighted the limitations of current knowledge and the shortcomings of current methodologies. Future work is needed to clarify the mechanisms underlying all of the emotion regulation strategies discussed here as well as the roles the brain systems supporting emotion regulation (Figure 2B) play in affective learning, affective decision making, and affective expectancies. Essential will be progress made not just in refining our understanding of the distinctions made here, but also addressing new questions about how emotion regulation mechanisms operate. For example, while it is certainly important that regulation strategies have immediate effects on emotional responses, it is also important that their effects be long-lasting. Indeed, whether regulatory effects last is critical both in everyday and clinical contexts where one could repeatedly reencounter an emotionally evocative stimulus (e.g. the risk of running into a girlfriend who dumped you because you work for the same company). To date, this issue has been addressed only twice – once in an fMRI study169 reporting that the effects of reappraisal on diminishing amygdala responses may endure for up to 40 minutes in healthy adults – but not those with major depression – and once in an ERP study170 showing that the effects of reappraisal on arousal-related responses endure for up to 30 minutes. Clearly, more work is needed here.
In so doing, it will be important for this work to increasingly make use of techniques other than functional imaging (e.g. ERP170-178, TMS161, 179, and lesion methodologies180), as well as to integrate insights gained from human studies with the large body of literature on affective and regulatory phenomena in nonhuman primates and rodents39, 143, 145. Progress on all of these fronts is absolutely critical if we are to develop a model of interactions between control and affect systems that can make sense not just of emotion regulatory phenomena, but of all the other types of phenomena that recruit these systems as well.
Another important direction for future research is the translation of basic research on emotion regulation to understanding the full range of normal to abnormal differences in emotional responding and regulatory ability. This is critical both for understanding the mechanisms underlying this variability and for testing the boundaries of basic models of emotion regulatory mechanisms.
One domain in which this will prove important is understanding how and why our emotional lives evolve as we grow from childhood through adolescence into adulthood and old age. On one hand, there is growing evidence that childhood and adolescence are critical times for the development of the emotion regulatory abilities needed to adaptively regulate affective impulses and the deleterious health behaviors (e.g. obesity, substance use) they can promote. A small but growing number of studies have begun to address this issue by asking how the neural mechanisms of reappraisal and emotional reactivity develop from adolescence into young adulthood. Some early results suggest that reappraisal ability increases linearly with age whereas emotional reactivity remains relatively constant181, 182 (but see183). On the other hand, while physical health and cognitive abilities tend to decline with age184-186, older adults report more emotional stability and a greater ratio of positive to negative experiences in their daily life, with the extent of positive emotion predicting longevity187, 188,189. While many have hypothesized that this, "rosy glow," of old age is due in part to more effective emotion regulation, to date there is little evidence directly testing this idea188, 190, 191. One conundrum to resolve here will be the apparent dependence of emotion regulation on the same kinds of prefrontal control systems that decline with age. This raises the question of how regulatory abilities improve as the underlying neural machinery declines192, 193. Early results suggest that it may depend on the strategies older adults deploy, with spared or greater regulatory ability shown for strategies and tactics that fit with long-term goals and have become habitual9, 97, 191, 194-197.
A second important goal for translational research will be to understand how potential dysfunction in the mechanisms of emotion generation and regulation may underlie various forms of psychiatric and substance use disorders (for a more in depth discussion, please see Denny, Silvers & Ochsner, 2009). This translational direction is being pursued in studies of reappraisal across various disorders, ranging from depression117, 169, 199 to borderline personality disorder84, 88, 200, social anxiety disorder201, 202, phobia203, posttraumatic stress disorder84, 86, cocaine users204 and smokers119. These studies can be useful in two ways. First they may show disorder-specific patterns of altered function in control and affect systems. For example, current data suggests depressed individuals may show impaired recruitment of vlPFC during reappraisal117, suggestive of an impairment of top-down control, whereas borderline individuals may show heightened amygdala responses coupled with diminished cingulate responses, suggestive of a failure to monitor paradoxical increases in affective responding when attempting to decrease emotion200. Second, imaging methods for studying emotion regulation may be used before and after treatment regimes as predictors of and markers of improvement. While such studies are only beginning to emerge, they hold great promise for understanding why some individuals improve, and whether different treatments (e.g. drug vs. cognitive behavioral therapy) have different mechanisms of action.
In the long run, the hope is that integrating basic and translational perspectives will help specify which individuals are at greatest risk for maladaptive health behaviors and emotional outcomes, at what ages this risk is greatest, and which regulatory mechanisms could be targeted in future interventions during particular points in the life course. While realization of this dream is still a long ways away, current research provides a strong foundation for getting there.
Acknowledgements
Preparation of this article was supported by NIH grants AG039279, MH076137 and DA022541 awarded to Kevin N. Ochsner as well as fellowship F31MH094056 awarded to Jennifer A. Silvers.
Footnotes
IN PRESS, Annals of the New York Academy of Sciences, Special Volume: The Year in Cognitive Neuroscience
References
- 1.Eisenberg N. Emotion, regulation, and moral development. Annu Rev Psychol. 2000;51:665–97. doi: 10.1146/annurev.psych.51.1.665. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez E, Turk DC. The utility of cognitive coping strategies for altering pain perception: a meta-analysis. Pain. 1989;38:123–35. doi: 10.1016/0304-3959(89)90230-3. [DOI] [PubMed] [Google Scholar]
- 3.Diener E. Subjective well-being. Psychological Bulletin. 1984;95:542–575. [PubMed] [Google Scholar]
- 4.Wilson BJ, Gottman JM. In: Stress, coping, and resiliency in children and families. Hetherington EM, Blechman EA, editors. Lawrence Erlbaum; Hillsdale, NJ: 1996. pp. 189–228. [Google Scholar]
- 5.Ayduk O, et al. Regulating the interpersonal self: strategic self-regulation for coping with rejection sensitivity. J Pers Soc Psychol. 2000;79:776–92. doi: 10.1037//0022-3514.79.5.776. [DOI] [PubMed] [Google Scholar]
- 6.Gross JJ, Munoz RF. Emotion regulation and mental health. Clinical Psychological: Science and Practice. 1995;2:151–164. [Google Scholar]
- 7.Mittal D, Torres R, Abashidze A, Jimerson N. Worsening of post-traumatic stress disorder symptoms with cognitive decline: case series. Journal of Geriatr Psychiatry Neurol. 2001;14:17–20. doi: 10.1177/089198870101400105. [DOI] [PubMed] [Google Scholar]
- 8.Gross JJ, Thompson RA. Handbook of emotion regulation. Guilford Press; US, New York, NY: 2007. [Google Scholar]
- 9.Ochsner KN, Gross JJ. Cognitive emotion regulation: Insights from social cognitive and affective neuroscience. Currents Directions in Psychological Science. 2008;17:153–158. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Marr D. Vision: a computational investigation into the human representation and processing of visual information. W.H. Freeman; San Francisco: 1982. [Google Scholar]
- 12.Ochsner K. In: Social Psychology: A Handbook of Basic Principles. Kruglanksi A, Higgins ET, editors. Guilford Press; New York: 2007. pp. 39–66. [Google Scholar]
- 13.Ochsner KN, Kosslyn SM. In: The Handbook of Cognitive Neuroscience. Ochsner KN, Kosslyn SM, editors. Oxford University Press; New York: in press. [Google Scholar]
- 14.Gross JJ, Barrett LF. Emotion Generation and Emotion Regulation: One or Two Depends on Your Point of View. Emot Rev. 2011;3:8–16. doi: 10.1177/1754073910380974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McRae K, Misra S, Prasad AK, Pereira SC, Gross JJ. Bottom-up and top-down emotion generation: implications for emotion regulation. Soc Cogn Affect Neurosci. 2012;7:253–62. doi: 10.1093/scan/nsq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ochsner KN, et al. Bottom-Up and Top-Down Processes in Emotion Generation: Common and Distinct Neural Mechanisms. Psychological Science. 2009;20:1322–1331. doi: 10.1111/j.1467-9280.2009.02459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrett LF, Mesquita B, Ochsner KN, Gross JJ. The experience of emotion. Annual Review of Psychology. 2007;58:373–403. doi: 10.1146/annurev.psych.58.110405.085709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scherer KR, Schorr A, Johnstone T, editors. Appraisal processes in emotion: Theory, methods, research. Oxford University Press; New York, NY: 2001. [Google Scholar]
- 19.Wager TD, et al. In: The handbook of emotion. Lewis M, Haviland-Jones JM, Barrett LF, editors. Guilford; New York: 2008. pp. 249–271. [Google Scholar]
- 20.Kober H, et al. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mauss IB, Levenson RW, McCarter L, Wilhelm FH, Gross JJ. The Tie That Binds? Coherence Among Emotion Experience, Behavior, and Physiology. Emotion. 2005;5:175–190. doi: 10.1037/1528-3542.5.2.175. [DOI] [PubMed] [Google Scholar]
- 22.Kalisch R. The functional neuroanatomy of reappraisal: time matters. Neurosci Biobehav Rev. 2009;33:1215–26. doi: 10.1016/j.neubiorev.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Cunningham WA, Van Bavel JJ, Johnsen IR. Affective flexibility: evaluative processing goals shape amygdala activity. Psychol Sci. 2008;19:152–60. doi: 10.1111/j.1467-9280.2008.02061.x. [DOI] [PubMed] [Google Scholar]
- 24.Cunningham WA, Arbuckle NL, Jahn A, Mowrer SM, Abduljalil AM. Aspects of neuroticism and the amygdala: chronic tuning from motivational styles. Neuropsychologia. 2011;48:3399–404. doi: 10.1016/j.neuropsychologia.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 25.Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- 26.Hariri AR, Whalen PJ. The amygdala: inside and out. F1000 Biol Rep. 2011;3:2. doi: 10.3410/B3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 28.Anderson AK, et al. Dissociated neural representations of intensity and valence in human olfaction. Nature Neuroscience. 2003;6:196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- 29.Neta M, Whalen PJ. The primacy of negative interpretations when resolving the valence of ambiguous facial expressions. Psychol Sci. 2011;21:901–7. doi: 10.1177/0956797610373934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whalen PJ, et al. Human amygdala responsivity to masked fearful eye whites. Science. 2004;306:2061. doi: 10.1126/science.1103617. [DOI] [PubMed] [Google Scholar]
- 31.Vuilleumier P, Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia. 2007;45:174–94. doi: 10.1016/j.neuropsychologia.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Curr Opin Neurol. 2005;18:411–7. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- 33.O'Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol. 2004;14:769–76. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 34.Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci. 2007;30:259–88. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- 35.Cunningham WA, Johnsen IR, Waggoner AS. Orbitofrontal cortex provides cross-modal valuation of self-generated stimuli. Soc Cogn Affect Neurosci. 2011;6:286–93. doi: 10.1093/scan/nsq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ongur D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. Journal of Comp Neurol. 2003;460:425–49. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- 37.Price JL. Prefrontal cortical networks related to visceral function and mood. Annals Of the New York Academy Science. 1999;877:383–96. doi: 10.1111/j.1749-6632.1999.tb09278.x. [DOI] [PubMed] [Google Scholar]
- 38.Davachi L. Item, context and relational episodic encoding in humans. Curr Opin Neurobiol. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 39.Murray EA, O'Doherty JP, Schoenbaum G. What we know and do not know about the functions of the orbitofrontal cortex after 20 years of cross-species studies. J Neurosci. 2007;27:8166–9. doi: 10.1523/JNEUROSCI.1556-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fellows LK. Orbitofrontal contributions to value-based decision making: evidence from humans with frontal lobe damage. Ann N Y Acad Sci. 2011;1239:51–8. doi: 10.1111/j.1749-6632.2011.06229.x. [DOI] [PubMed] [Google Scholar]
- 41.Schoenbaum G, Takahashi Y, Liu TL, McDannald MA. Does the orbitofrontal cortex signal value? Ann N Y Acad Sci. 2011;1239:87–99. doi: 10.1111/j.1749-6632.2011.06210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rudebeck PH, Murray EA. Balkanizing the primate orbitofrontal cortex: distinct subregions for comparing and contrasting values. Ann N Y Acad Sci. 2011;1239:1–13. doi: 10.1111/j.1749-6632.2011.06267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14:1215–29. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- 44.Schoenbaum G, Saddoris MP, Stalnaker TA. Reconciling the roles of orbitofrontal cortex in reversal learning and the encoding of outcome expectancies. Ann N Y Acad Sci. 2007;1121:320–35. doi: 10.1196/annals.1401.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci. 2012;16:147–56. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oya H, et al. Electrophysiological correlates of reward prediction error recorded in the human prefrontal cortex. Proc Natl Acad Sci U S A. 2005;102:8351–6. doi: 10.1073/pnas.0500899102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–8. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- 48.Beer JS, Heerey EA, Keltner D, Scabini D, Knight RT. The regulatory function of self-conscious emotion: insights from patients with orbitofrontal damage. Journal of Personality and Social Psychology. 2003;85:594–604. doi: 10.1037/0022-3514.85.4.594. [DOI] [PubMed] [Google Scholar]
- 49.Damasio A. Descartes' error : emotion, reason, and the human brain. G.P. Putnam; New York: 1994. [Google Scholar]
- 50.Mufson EJ, Mesulam MM. Insula of the old world monkey. II: Afferent cortical input and comments on the claustrum. J Comp Neurol. 1982;212:23–37. doi: 10.1002/cne.902120103. [DOI] [PubMed] [Google Scholar]
- 51.Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 52.Wager TD, Feldman Barrett L. PsycExtra. 2004 [Google Scholar]
- 53.Calder AJ, Lawrence AD, Young AW. Neuropsychology of fear and loathing. Nature Review Neuroscience. 2001;2:352–63. doi: 10.1038/35072584. [DOI] [PubMed] [Google Scholar]
- 54.Wager TD, Barrett LF. 2004.
- 55.Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev. 1996;22:229–44. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- 56.Nitschke JB, Sarinopoulos I, Mackiewicz KL, Schaefer HS, Davidson RJ. Functional neuroanatomy of aversion and its anticipation. Neuroimage. 2006;29:106–16. doi: 10.1016/j.neuroimage.2005.06.068. [DOI] [PubMed] [Google Scholar]
- 57.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 58.Williams LE, Bargh JA, Nocera CC, Gray JR. The unconscious regulation of emotion: nonconscious reappraisal goals modulate emotional reactivity. Emotion. 2009;9:847–54. doi: 10.1037/a0017745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gross JJ. The emerging field of emotion regulation: An integrative review. Review of General Psychological. 1998;2:271–299. [Google Scholar]
- 60.Davis JI, Gross JJ, Ochsner KN. Psychological distance and emotional experience: What you see is what you get. Emotion. 2009;11 doi: 10.1037/a0021783. [DOI] [PubMed] [Google Scholar]
- 61.Gross JJ. Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology. 1998;74:224–37. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- 62.McRae K, et al. The neural bases of distraction and reappraisal. J Cogn Neurosci. 2010;22:248–62. doi: 10.1162/jocn.2009.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Urry HL. Using reappraisal to regulate unpleasant emotional episodes: goals and timing matter. Emotion. 2009;9:782–97. doi: 10.1037/a0017109. [DOI] [PubMed] [Google Scholar]
- 64.Jackson DC, Malmstadt JR, Larson CL, Davidson RJ. Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology. 2000;37:515–22. [PubMed] [Google Scholar]
- 65.Ayduk O, Kross E. Enhancing the pace of recovery: Self-distanced analysis of negative experiences reduces blood pressure reactivity. Psychological Science. 2008;19 doi: 10.1111/j.1467-9280.2008.02073.x. [DOI] [PubMed] [Google Scholar]
- 66.Kross E, Ayduk O. Facilitating Adaptive Emotional Analysis: Distinguishing Distanced-Analysis of Depressive Experiences From Immersed-Analysis and Distraction. Personality and Social Psychology Bulletin. 2008;34:924–938. doi: 10.1177/0146167208315938. [DOI] [PubMed] [Google Scholar]
- 67.Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry. 2008;63:577–86. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Davis JI, Senghas A, Ochsner KN. How does facial feedback modulate emotional experience? Journal of Research in Personality. 2009;43 doi: 10.1016/j.jrp.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miller EK. The prefrontal cortex and cognitive control. Nat Rev Neurosci. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- 70.Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: a meta-analysis. Neuroimage. 2004;22:1679–93. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- 71.Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:255–74. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- 72.Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–46. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 73.Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 74.Thompson-Schill SL, Bedny M, Goldberg RF. The frontal lobes and the regulation of mental activity. Curr Opin Neurobiol. 2005;15:219–24. doi: 10.1016/j.conb.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 75.Olsson A, Ochsner KN. The role of social cognition in emotion. Trends in Cognitive Sciences. 2008;12:65–71. doi: 10.1016/j.tics.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 76.Mitchell JP. Inferences about mental states. Philos Trans R Soc Lond B Biol Sci. 2009;364:1309–16. doi: 10.1098/rstb.2008.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ochsner KN, et al. For Better or for Worse: Neural Systems Supporting the Cognitive Down- and Up-regulation of Negative Emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 78.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–7. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 79.Konishi S, et al. Common inhibitory mechanism in human inferior prefrontal cortex revealed by event-related functional MRI. Brain. 1999;122:981–91. doi: 10.1093/brain/122.5.981. [DOI] [PubMed] [Google Scholar]
- 80.Lieberman MD, et al. Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychol Sci. 2007;18:421–8. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- 81.Ochsner KN. In: Social Neuroscience: People thinking about people. Cacioppo JT, editor. MIT Press; Cambridge, MA: 2005. pp. 245–268. [Google Scholar]
- 82.Domes G, et al. The neural correlates of sex differences in emotional reactivity and emotion regulation. Hum Brain Mapp. 2010;31:758–69. doi: 10.1002/hbm.20903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ichikawa N, et al. Feeling bad about screwing up: emotion regulation and action monitoring in the anterior cingulate cortex. Cogn Affect Behav Neurosci. 2011;11:354–71. doi: 10.3758/s13415-011-0028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lang S, et al. Cognitive reappraisal in trauma-exposed women with borderline personality disorder. Neuroimage. 2011;59:1727–34. doi: 10.1016/j.neuroimage.2011.08.061. [DOI] [PubMed] [Google Scholar]
- 85.Eippert F, et al. Regulation of emotional responses elicited by threat-related stimuli. Hum Brain Mapp. 2007;28:409–23. doi: 10.1002/hbm.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.New AS, et al. A Functional Magnetic Resonance Imaging Study of Deliberate Emotion Regulation in Resilience and Posttraumatic Stress Disorder. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 87.Pitskel NB, Bolling DZ, Kaiser MD, Crowley MJ, Pelphrey KA. How grossed out are you? The neural bases of emotion regulation from childhood to adolescence. Dev Cogn Neurosci. 2011;1:324–337. doi: 10.1016/j.dcn.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schulze L, et al. Neuronal correlates of cognitive reappraisal in borderline patients with affective instability. Biol Psychiatry. 2010;69:564–73. doi: 10.1016/j.biopsych.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 89.van Reekum CM, et al. Gaze fixations predict brain activation during the voluntary regulation of picture-induced negative affect. Neuroimage. 2007;36:1041–55. doi: 10.1016/j.neuroimage.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 90.Zaki J, Ochsner KN. Reintegrating the Study of Accuracy Into Social Cognition Research (Target article) Psychological Inquiry. 2011;22:159–182. [Google Scholar]
- 91.Zaki J, Ochsner KN. The neuroscience of empathy: Progress, pitfalls and promise. Nature Neuroscience. doi: 10.1038/nn.3085. in press. [DOI] [PubMed] [Google Scholar]
- 92.Davis M, Shi C. he extended amygdala: are the central nucleus of the amygdala and the bed nucleus of the stria terminalis differentially involved in fear versus anxiety? Ann N Y Acad Sci. 1999;877:281–91. doi: 10.1111/j.1749-6632.1999.tb09273.x. [DOI] [PubMed] [Google Scholar]
- 93.Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: a critical review. Prog Brain Res. 2008;167:151–69. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- 94.Aggleton JP, Saunders RC. In: The Amygdala: A functional Analysis. Aggleton JP, editor. Oxford University Press; New York, NY: 2000. pp. 1–30. [Google Scholar]
- 95.Urry HL, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26:4415–25. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim SH, Hamann S. Neural Correlates of Positive and Negative Emotion Regulation. Journal of Cognitive Neuroscience. 2007;19:1–23. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- 97.Opitz PC, Rauch LC, Terry DP, Urry HL. Prefrontal mediation of age differences in cognitive reappraisal. Neurobiol Aging. 2011;33:645–55. doi: 10.1016/j.neurobiolaging.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 98.McRae K, Ciesielski B, Gross JJ. Unpacking cognitive reappraisal: Goals, tactics, and outcomes. Emotion. doi: 10.1037/a0026351. in press. [DOI] [PubMed] [Google Scholar]
- 99.Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci. 2007;27:3743–52. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Badre D, Poldrack RA, Pare-Blagoev EJ, Insler RZ, Wagner AD. issociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47:907–18. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 101.Chun MM, Golomb JD, Turk-Browne NB. A taxonomy of external and internal attention. Annual Review of Psychology. 2011;62:73–101. doi: 10.1146/annurev.psych.093008.100427. [DOI] [PubMed] [Google Scholar]
- 102.Gobbini MI, Koralek AC, Bryan RE, Montgomery KJ, Haxby JV. Two takes on the social brain: a comparison of theory of mind tasks. J Cogn Neurosci. 2007;19:1803–14. doi: 10.1162/jocn.2007.19.11.1803. [DOI] [PubMed] [Google Scholar]
- 103.Powell LJ, Macrae CN, Cloutier J, Metcalfe J, Mitchell JP. Dissociable neural substrates for agentic versus conceptual representations of self. J Cogn Neurosci. 2011;22:2186–97. doi: 10.1162/jocn.2009.21368. [DOI] [PubMed] [Google Scholar]
- 104.Vogeley K, Fink GR. Neural correlates of the first-person-perspective. Trends Cognitive Science. 2003;7:38–42. doi: 10.1016/s1364-6613(02)00003-7. [DOI] [PubMed] [Google Scholar]
- 105.Gazzaniga M, Ivry RB, Mangun GR. Cognitive Neuroscience: The Biology of the Mind. W. W. Norton & Company; New York, NY: 2008. [Google Scholar]
- 106.Baumeister RF, Bratslavsky E, Finkenauer C, Vohs KD. Bad is stronger than good. Review of General Psychology. 2001;5 [Google Scholar]
- 107.Diagnostic and statistical manual of mental disorders (4th ed.): Primary care version. 4. American Psychiatric Publishing, Inc ; US, Diagnostic and statistical manual of mental disorders; Arlington, VA, US: 1995. p. 223. Primary care version xv. 1995. [Google Scholar]
- 108.Tabibnia G, et al. Different forms of self-control share a neurocognitive substrate. J Neurosci. 2011;31:4805–10. doi: 10.1523/JNEUROSCI.2859-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Aron AR, et al. Converging evidence for a fronto-basal-ganglia network for inhibitory control of action and cognition. J Neurosci. 2007;27:11860–4. doi: 10.1523/JNEUROSCI.3644-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Aron AR. The neural basis of inhibition in cognitive control. Neuroscientist. 2007;13:214–28. doi: 10.1177/1073858407299288. [DOI] [PubMed] [Google Scholar]
- 111.Davidson RJ. What does the prefrontal cortex "do" in affect: perspectives on frontal EEG asymmetry research. Biol Psychol. 2004;67:219–33. doi: 10.1016/j.biopsycho.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 112.Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–73. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- 113.Kross E, Davidson M, Weber J, Ochsner K. Coping with emotions past: the neural bases of regulating affect associated with negative autobiographical memories. Biol Psychiatry. 2009;65:361–6. doi: 10.1016/j.biopsych.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kross E, Ayduk O, Mischel W. When Asking "Why" Does Not Hurt: Distinguishing Rumination From Reflective Processing of Negative Emotions. Psychological Science. 2005;16:709–715. doi: 10.1111/j.1467-9280.2005.01600.x. [DOI] [PubMed] [Google Scholar]
- 115.Phan KL, et al. Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:210–9. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 116.Ohira H, et al. Association of neural and physiological responses during voluntary emotion suppression. Neuroimage. 2006;29:721–33. doi: 10.1016/j.neuroimage.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 117.Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27:8877–84. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–50. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kober H, et al. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci U S A. 2010;107:14811–6. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fjell AM, et al. High consistency of regional cortical thinning in aging across multiple samples. Cereb Cortex. 2009;19:2001–12. doi: 10.1093/cercor/bhn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ritchey M, Bessette-Symons B, Hayes SM, Cabeza R. Emotion processing in the aging brain is modulated by semantic elaboration. Neuropsychologia. 2011;49:640–50. doi: 10.1016/j.neuropsychologia.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the STS region. Trends in Cognitive Sciences. 2000;4:267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- 123.Brefczynski-Lewis JA, Berrebi ME, McNeely ME, Prostko AL, Puce A. In the blink of an eye: neural responses elicited to viewing the eye blinks of another individual. Front Hum Neurosci. 2011;5:68. doi: 10.3389/fnhum.2011.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wheaton KJ, Thompson JC, Syngeniotis A, Abbott DF, Puce A. Viewing the motion of human body parts activates different regions of premotor, temporal, and parietal cortex. Neuroimage. 2004;22:277–88. doi: 10.1016/j.neuroimage.2003.12.043. [DOI] [PubMed] [Google Scholar]
- 125.Olson IR, Plotzker A, Ezzyat Y. The Enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130:1718–31. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- 126.Young L, Camprodon JA, Hauser M, Pascual-Leone A, Saxe R. Disruption of the right temporoparietal junction with transcranial magnetic stimulation reduces the role of beliefs in moral judgments. Proc Natl Acad Sci U S A. 2010;107:6753–8. doi: 10.1073/pnas.0914826107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Saxe R. Uniquely human social cognition. Curr Opin Neurobiol. 2006;16:235–9. doi: 10.1016/j.conb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 128.Kanske P, Heissler J, Schonfelder S, Bongers A, Wessa M. How to regulate emotion? Neural networks for reappraisal and distraction. Cereb Cortex. 2011;21:1379–88. doi: 10.1093/cercor/bhq216. [DOI] [PubMed] [Google Scholar]
- 129.Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:20–5. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- 130.Mishkin M, Ungerleider LG. Contribution of striate inputs to the visuospatial functions of parieto-preoccipital cortex in monkeys. Behav Brain Res. 1982;6:57–77. doi: 10.1016/0166-4328(82)90081-x. [DOI] [PubMed] [Google Scholar]
- 131.Ettlinger G. "Object vision" and "spatial vision": the neuropsychological evidence for the distinction. Cortex. 1990;26:319–41. doi: 10.1016/s0010-9452(13)80084-6. [DOI] [PubMed] [Google Scholar]
- 132.Everitt BJ, et al. Associative processes in addiction and reward. The role of amygdala-ventral striatal subsystems. Ann N Y Acad Sci. 1999;877:412–38. doi: 10.1111/j.1749-6632.1999.tb09280.x. [DOI] [PubMed] [Google Scholar]
- 133.LeDoux JE, Gorman JM. A call to action: overcoming anxiety through active coping. Am J Psychiatry. 2001;158:1953–5. doi: 10.1176/appi.ajp.158.12.1953. [DOI] [PubMed] [Google Scholar]
- 134.Delgado MR, Jou RL, Ledoux JE, Phelps EA. Avoiding negative outcomes: tracking the mechanisms of avoidance learning in humans during fear conditioning. Front Behav Neurosci. 2009;3:33. doi: 10.3389/neuro.08.033.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Buhle J, Wager T, Smith E. In: Self Control in Society, Mind, and Brain. Hassin R, Ochsner KN, Trope Y, editors. Oxford University Press; New York: 2010. pp. 93–113. [Google Scholar]
- 136.Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55:377–91. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 137.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–84. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 138.Villemure C, Bushnell MC. Cognitive modulation of pain: how do attention and emotion influence pain processing? Pain. 2002;95:195–9. doi: 10.1016/S0304-3959(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 139.Dolcos F, Iordan AD, Dolcos S. Neural correlates of emotion-cognition interactions: A review of evidence from brain imaging investigations. J Cogn Psychol (Hove) 2011;23:669–694. doi: 10.1080/20445911.2011.594433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hayes JP, et al. Staying cool when things get hot: emotion regulation modulates neural mechanisms of memory encoding. Front Hum Neurosci. 2010;4:230. doi: 10.3389/fnhum.2010.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.LeDoux JE. The emotional brain: The mysterious underpinnings of emotional life. Simon Schuster; New York N. Y., U. S.: 1996. [Google Scholar]
- 142.LeDoux JE. Emotion circuits in the brain. Annu Review Neuroscience. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 143.Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr Opin Neurobiol. 2006;16:723–7. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 144.Bouton ME. In: Emotions: Conscious and Unconscious. Feldman Barrett L, Winkielman P, editors. Guilford; New York: 2004. P.N. [Google Scholar]
- 145.Delgado MR, Olsson A, Phelps EA. Extending animal models of fear conditioning to humans. Biol Psychol. 2006;73:39–48. doi: 10.1016/j.biopsycho.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 146.Milad MR, et al. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc Natl Acad Sci U S A. 2005;102:10706–11. doi: 10.1073/pnas.0502441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 148.Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain. 2003;126:1830–7. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- 149.Fellows LK, Farah MJ. Different underlying impairments in decision-making following ventromedial and dorsolateral frontal lobe damage in humans. Cereb Cortex. 2005;15:58–63. doi: 10.1093/cercor/bhh108. [DOI] [PubMed] [Google Scholar]
- 150.Delgado MR, Nearing KI, Ledoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59:829–38. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Delgado MR, Gillis MM, Phelps EA. Regulating the expectation of reward via cognitive strategies. Nat Neurosci. 2008;11:880–1. doi: 10.1038/nn.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Knutson B, Rick S, Wimmer GE, Prelec D, Loewenstein G. Neural predictors of purchases. Neuron. 2007;53:147–56. doi: 10.1016/j.neuron.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nat Rev Neurosci. 2008;9:545–56. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lieberman MD, Ochsner KN, Gilbert DT, Schacter DL. Do amnesics exhibit cognitive dissonance reduction? The role of explicit memory and attention in attitude change. Psychol Science. 2001;12:135–40. doi: 10.1111/1467-9280.00323. [DOI] [PubMed] [Google Scholar]
- 155.van Veen V, Krug MK, Schooler JW, Carter CS. Neural activity predicts attitude change in cognitive dissonance. Nat Neurosci. 2009;12:1469–74. doi: 10.1038/nn.2413. [DOI] [PubMed] [Google Scholar]
- 156.Sharot T, Shiner T, Dolan RJ. Experience and choice shape expected aversive outcomes. J Neurosci. 2010;30:9209–15. doi: 10.1523/JNEUROSCI.4770-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sharot T, De Martino B, Dolan RJ. How choice reveals and shapes expected hedonic outcome. J Neurosci. 2009;29:3760–5. doi: 10.1523/JNEUROSCI.4972-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mischel W, Shoda Y, Rodriguez MI. Delay of gratification in children. Science. 1989;244:933–8. doi: 10.1126/science.2658056. [DOI] [PubMed] [Google Scholar]
- 159.McClure SM, Ericson KM, Laibson DI, Loewenstein G, Cohen JD. Time discounting for primary rewards. J Neurosci. 2007;27:5796–804. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–7. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- 161.Figner B, et al. Lateral prefrontal cortex and self-control in intertemporal choice. Nat Neurosci. 2011;13:538–9. doi: 10.1038/nn.2516. [DOI] [PubMed] [Google Scholar]
- 162.Gianotti LR, et al. Tonic activity level in the right prefrontal cortex predicts individuals' risk taking. Psychol Sci. 2009;20:33–8. doi: 10.1111/j.1467-9280.2008.02260.x. [DOI] [PubMed] [Google Scholar]
- 163.Knoch D, Pascual-Leone A, Meyer K, Treyer V, Fehr E. Diminishing reciprocal fairness by disrupting the right prefrontal cortex. Science. 2006;314:829–32. doi: 10.1126/science.1129156. [DOI] [PubMed] [Google Scholar]
- 164.Knoch D, et al. Studying the neurobiology of social interaction with transcranial direct current stimulation--the example of punishing unfairness. Cereb Cortex. 2008;18:1987–90. doi: 10.1093/cercor/bhm237. [DOI] [PubMed] [Google Scholar]
- 165.Wager TD. The Neural Bases of Placebo Effects in Pain. Current Directions in Psychological Science. 2005;14:175–179. [Google Scholar]
- 166.Atlas LY, Bolger N, Lindquist MA, Wager TD. Brain mediators of predictive cue effects on perceived pain. J Neurosci. 2010;30:12964–77. doi: 10.1523/JNEUROSCI.0057-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lieberman MD, et al. The neural correlates of placebo effects: a disruption account. Neuroimage. 2004;22:447–55. doi: 10.1016/j.neuroimage.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 168.Wager TD, Atlas LY, Leotti LA, Rilling JK. Predicting individual differences in placebo analgesia: contributions of brain activity during anticipation and pain experience. J Neurosci. 2011;31:439–52. doi: 10.1523/JNEUROSCI.3420-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Erk S, et al. Acute and sustained effects of cognitive emotion regulation in major depression. J Neurosci. 2010;30:15726–34. doi: 10.1523/JNEUROSCI.1856-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Macnamara A, Ochsner KN, Hajcak G. Previously reappraised: the lasting effect of description type on picture-elicited electrocortical activity. Soc Cogn Affect Neurosci. 2011;6:348–58. doi: 10.1093/scan/nsq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hajcak G, Nieuwenhuis S. Reappraisal modulates the electrocortical response to unpleasant pictures. Cogn Affect Behav Neurosci. 2006;6:291–7. doi: 10.3758/cabn.6.4.291. [DOI] [PubMed] [Google Scholar]
- 172.MacNamara A, Hajcak G. Anxiety and spatial attention moderate the electrocortical response to aversive pictures. Neuropsychologia. 2009;47:2975–80. doi: 10.1016/j.neuropsychologia.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 173.Foti D, Hajcak G. Deconstructing reappraisal: descriptions preceding arousing pictures modulate the subsequent neural response. J Cogn Neurosci. 2008;20:977–88. doi: 10.1162/jocn.2008.20066. [DOI] [PubMed] [Google Scholar]
- 174.Moser JS, Hajcak G, Bukay E, Simons RF. Intentional modulation of emotional responding to unpleasant pictures: an ERP study. Psychophysiology. 2006;43:292–6. doi: 10.1111/j.1469-8986.2006.00402.x. [DOI] [PubMed] [Google Scholar]
- 175.Krompinger JW, Moser JS, Simons RF. Modulations of the electrophysiological response to pleasant stimuli by cognitive reappraisal. Emotion. 2008;8:132–7. doi: 10.1037/1528-3542.8.1.132. [DOI] [PubMed] [Google Scholar]
- 176.Deveney CM, Pizzagalli DA. The cognitive consequences of emotion regulation: an ERP investigation. Psychophysiology. 2008;45:435–44. doi: 10.1111/j.1469-8986.2007.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Decicco JM, Solomon B, Dennis TA. Neural Correlates of Cognitive Reappraisal in Children: An ERP Study. Dev Cogn Neurosci. 2012;2:79–80. doi: 10.1016/j.dcn.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Moser JS, Most SB, Simons RF. Increasing negative emotions by reappraisal enhances subsequent cognitive control: a combined behavioral and electrophysiological study. Cogn Affect Behav Neurosci. 2010;10:195–207. doi: 10.3758/CABN.10.2.195. [DOI] [PubMed] [Google Scholar]
- 179.Knoch D, Fehr E. Resisting the power of temptations: the right prefrontal cortex and self-control. Ann N Y Acad Sci. 2007;1104:123–34. doi: 10.1196/annals.1390.004. [DOI] [PubMed] [Google Scholar]
- 180.Beer JS, Lombardo MV. Gross JJ, Thompson DG, editors. The Handbook of Emotion Regulation. 2007 [Google Scholar]
- 181.McRae K, et al. The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. Soc Cogn Affect Neurosci. 2012;7:11–22. doi: 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Silvers JA, et al. Age-related differences in emotional reactivity, regulation and rejection sensitivity in adolescence. Emotion. doi: 10.1037/a0028297. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Carthy T, Horesh N, Apter A, Edge MD, Gross JJ. Emotional reactivity and cognitive regulation in anxious children. Behav Res Ther. 2010;48:384–93. doi: 10.1016/j.brat.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 184.Grady CL. Cognitive neuroscience of aging. Ann N Y Acad Sci. 2008;1124:127–44. doi: 10.1196/annals.1440.009. [DOI] [PubMed] [Google Scholar]
- 185.Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–96. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Rajah MN, D'Esposito M. Region-specific changes in prefrontal function with age: a review of PET and fMRI studies on working and episodic memory. Brain. 2005;128:1964–83. doi: 10.1093/brain/awh608. [DOI] [PubMed] [Google Scholar]
- 187.Carstensen LL, Mikels JA. At the Intersection of Emotion and Cognition: Aging and the Positivity Effect. Current Directions in Psychological Science. 2005;14:117–121. [Google Scholar]
- 188.Scheibe S, Carstensen LL. Emotional aging: recent findings and future trends. J Gerontol B Psychol Sci Soc Sci. 2010;65B:135–44. doi: 10.1093/geronb/gbp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Carstensen LL, et al. Emotional experience improves with age: evidence based on over 10 years of experience sampling. Psychol Aging. 2011;26:21–33. doi: 10.1037/a0021285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Nashiro K, Sakaki M, Mather M. Age Differences in Brain Activity during Emotion Processing: Reflections of Age-Related Decline or Increased Emotion Regulation? Gerontology. 2011 doi: 10.1159/000328465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Urry HL, Gross JJ. Emotion regulation in older age. Current Directions in Psychological Science. 2010;19 doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mather M. The emotion paradox in the aging brain. Ann N Y Acad Sci. doi: 10.1111/j.1749-6632.2012.06471.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Nashiro K, Sakaki M, Mather M. Age differences in brain activity during emotion processing: reflections of age-related decline or increased emotion regulation? Gerontology. 2012;58:156–63. doi: 10.1159/000328465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Shiota MN, Levenson RW. Effects of aging on experimentally instructed detached reappraisal, positive reappraisal, and emotional behavior suppression. Psychology and Aging. 2009;24 doi: 10.1037/a0017896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tucker AM, Feuerstein R, Mende-Siedlecki P, Ochsner KN, Stern Y. Double Dissociation: Circadian Off-Peak Times Increase Emotional Reactivity; Aging Impairs Emotion Regulation Via Reappraisal. Emotion. doi: 10.1037/a0028207. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Winecoff A, Labar KS, Madden DJ, Cabeza R, Huettel SA. Cognitive and neural contributors to emotion regulation in aging. Soc Cogn Affect Neurosci. 2010;6:165–76. doi: 10.1093/scan/nsq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Blanchard-Fields F. Everyday Problem Solving and Emotion: An Adult Developmental Perspective. Current Directions in Psychological Science. 2007;16:26–31. [Google Scholar]
- 198.Denny BT, Silvers JA, Ochsner KN. In: Emotion Regulation and Psychopathology: A Transdiagnostic Approach to Etiology and Treatment. Kring AM, Sloan DM, editors. Guilford; New York: 2009. pp. 59–87. [Google Scholar]
- 199.Heller AS, et al. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proc Natl Acad Sci U S A. 2009;106:22445–50. doi: 10.1073/pnas.0910651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Koenigsberg HW, et al. Neural Correlates of using distancing to regulate emotional responses to social cues. Neuropsychologia. 2010;48:1813–22. doi: 10.1016/j.neuropsychologia.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Goldin PR, Manber-Ball T, Werner K, Heimberg R, Gross JJ. Neural Mechanisms of Cognitive Reappraisal of Negative Self-Beliefs in Social Anxiety Disorder. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Goldin PR, Manber T, Hakimi S, Canli T, Gross JJ. Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Arch Gen Psychiatry. 2009;66:170–80. doi: 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hermann A, et al. Emotion regulation in spider phobia: role of the medial prefrontal cortex. Soc Cogn Affect Neurosci. 2009;4:257–67. doi: 10.1093/scan/nsp013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Volkow ND, et al. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. Neuroimage. 2010;49:2536–43. doi: 10.1016/j.neuroimage.2009.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. Journal of Neuroscience. 2001;21:RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Harenski CL, Hamann S. Neural correlates of regulating negative emotions related to moral violations. Neuroimage. 2006;30:313–24. doi: 10.1016/j.neuroimage.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 207.Herwig U, et al. Modulation of anticipatory emotion and perception processing by cognitive control. Neuroimage. 2007;37:652–62. doi: 10.1016/j.neuroimage.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 208.Hollmann M, et al. Neural correlates of the volitional regulation of the desire for food. Int J Obes (Lond) 2011 doi: 10.1038/ijo.2011.125. [DOI] [PubMed] [Google Scholar]
- 209.Krendl AC, Kensinger EA, Ambady N. How does the brain regulate negative bias to stigma? Soc Cogn Affect Neurosci. 2011 doi: 10.1093/scan/nsr046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Levesque J, et al. Neural circuitry underlying voluntary suppression of sadness. Biol Psychiatry. 2003;53:502–10. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- 211.Mak AK, Hu ZG, Zhang JX, Xiao ZW, Lee TM. Neural correlates of regulation of positive and negative emotions: an fmri study. Neurosci Lett. 2009;457:101–6. doi: 10.1016/j.neulet.2009.03.094. [DOI] [PubMed] [Google Scholar]
- 212.McRae K, Ochsner KN, Mauss IB, Gabrieli JDE, Gross JJ. Gender Differences in Emotion Regulation: An fMRI study of Cognitive Reappraisal. Group Processes and Intergroup Relations. 2008;11:145–162. doi: 10.1177/1368430207088035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Modinos G, Ormel J, Aleman A. Individual differences in dispositional mindfulness and brain activity involved in reappraisal of emotion. Soc Cogn Affect Neurosci. 2010;5:369–77. doi: 10.1093/scan/nsq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Schardt DM, et al. Volition diminishes genetically mediated amygdala hyperreactivity. Neuroimage. 2009 doi: 10.1016/j.neuroimage.2009.11.078. [DOI] [PubMed] [Google Scholar]
- 215.Staudinger MR, Erk S, Abler B, Walter H. Cognitive reappraisal modulates expected value and prediction error encoding in the ventral striatum. Neuroimage. 2009;47:713–21. doi: 10.1016/j.neuroimage.2009.04.095. [DOI] [PubMed] [Google Scholar]
- 216.Staudinger MR, Erk S, Walter H. Dorsolateral prefrontal cortex modulates striatal reward encoding during reappraisal of reward anticipation. Cereb Cortex. 2011;21:2578–88. doi: 10.1093/cercor/bhr041. [DOI] [PubMed] [Google Scholar]
- 217.Urry HL, van Reekum CM, Johnstone T, Davidson RJ. Individual differences in some (but not all) medial prefrontal regions reflect cognitive demand while regulating unpleasant emotion. Neuroimage. 2009;47:852–63. doi: 10.1016/j.neuroimage.2009.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Vrticka P, Sander D, Vuilleumier P. Effects of emotion regulation strategy on brain responses to the valence and social content of visual scenes. Neuropsychologia. 2011;49:1067–82. doi: 10.1016/j.neuropsychologia.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 219.Walter H, et al. The temporal dynamics of voluntary emotion regulation. PLoS One. 2009;4:e6726. doi: 10.1371/journal.pone.0006726. [DOI] [PMC free article] [PubMed] [Google Scholar]