Abstract
Olfactory receptors (ORs) detect volatile chemicals that lead to the initial perception of smell in the brain. The olfactory receptor (OR) is the first protein that recognizes odorants in the olfactory signal pathway and it is present in over 1,000 genes in mice. It is also the largest member of the G protein-coupled receptors (GPCRs). Most ORs are extensively expressed in the nasal olfactory epithelium where they perform the appropriate physiological functions that fit their location. However, recent whole-genome sequencing shows that ORs have been found outside of the olfactory system, suggesting that ORs may play an important role in the ectopic expression of non-chemosensory tissues. The ectopic expressions of ORs and their physiological functions have attracted more attention recently since MOR23 and testicular hOR17-4 have been found to be involved in skeletal muscle development, regeneration, and human sperm chemotaxis, respectively. When identifying additional expression profiles and functions of ORs in non-olfactory tissues, there are limitations posed by the small number of antibodies available for similar OR genes. This review presents the results of a research series that identifies ectopic expressions and functions of ORs in non-chemosensory tissues to provide insight into future research directions. [BMB Reports 2012; 45(11): 612-622]
Keywords: Ectopic expression, Non-chemosensory tissues, Odorant, Olfactory receptor, Smell
INTRODUCTION
The olfactory system in the nose acts as a window, monitoring external environmental chemical changes from the brain. The olfactory system is made up of four subsystems: the main olfactory epithelium (MOE), the vomeronasal organ (VNO), the septal organ (SO) of Masera and the Grueneberg ganglion (GG) (1). Odorants and pheromones are recognized by olfactory receptors (ORs) expressed in the MOE and pheromone receptor located in the VNO (2). The GG has recently been isolated and shown to detect alarm pheromones that result in freezing behavior (3-5).
ORs are localized in the cilia of olfactory sensory neurons (OSNs) in the olfactory epithelium (OE) and are activated by chemical cues, typically odorants at the molecular level, which lead to the perception of smell in the brain (6). Tremendous research was conducted since Buck and Axel isolated ORs as an OE-specific expression in 1991 (7). OR genes, the largest family among the G protein-coupled receptors (GPCRs) (8), constitute more than 1,000 genes on the mouse chromosome (9,10) and more than 450 genes in the human genome (11,12).
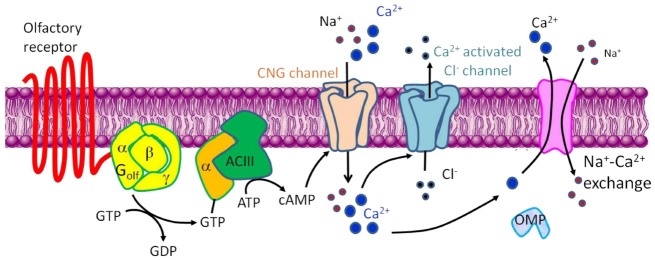
Odorant activation shows a distinct signal transduction pathway for odorant perception. Odorant signal transduction is initiated when odorants interact with specific ORs. ORs linked to Golf proteins are activated (13) and induce an increase of intracellular cAMP caused by the membrane form of adenylate cyclase III (ACIII) (14,15). Increased intracellular cAMP causes an external Ca2+ influx by activating a cation-selective cyclic nucleotide-gated (CNG) channel (16,17). Then, rapid plasma membrane depolarization is triggered by the Ca2+-activated Clchannel (18). The elevated intracellular Ca2+ concentration is reduced by expelling Ca2+ through the plasma membrane by a Na+/ Ca2+ exchanger (NCX), a potassium-dependent Na+/Ca2+ exchanger (NCKX4), and plasma membrane Ca2+-ATPase (PMCA) (19-22). Olfactory marker protein (OMP) facilitates NCX activity and allows rapid Ca2+ extrusion (19) (Fig. 1).
Fig. 1. A schematic diagram of olfactory signal transduction. Olfactory signal transduction begins with the activation of an olfactory receptor (OR) in the ciliary membrane; this leads to an increase in cyclic AMP (cAMP) synthesis through the activation of adenylate cyclase type III (ACIII) via a G protein (Golf)-coupled cascade. The increase in cAMP concentration causes cyclic nucleotide-gated (CNG) channels to open, leading to an increase in intracellular Ca2+ concentration and depolarization of the cell membrane by the Ca2+-activated Clchannel. Among several molecules of the olfactory signal transduction, OR, olfactory marker protein (OMP), Golf protein α-subunit (Gαolf), and ACIII have known to be olfactory specific molecules.
Many ORs were extensively identified in non-olfactory tissues since OR genes have been found in the testis and sperm (Table 1). Physiological functions of ectopic OR expressions are unlikely since most of the ectopic expressions of ORs from non-olfactory tissues are isolated by PCR-based amplification and cloning methods caused by low expression levels. However, novel testicular ORs and its ligands were isolated by Hatt's group, who presented human sperm chemotaxis to specific odorants through ORs by utilizing sperm swimming bioassay (23).
Table 1.
Overview of identified olfactory receptor in non-chemosensory tissues
| Tissue or cell used | Receptor identified | Species | Human- /rat- /mouse-ortholog by biogps database (81) | Analysis method used | Ref |
|---|---|---|---|---|---|
|
| |||||
| Testis | OR7A5 | Human | HTPCR2/Olr1571, Or7a17, ratchr11-86418029-86418739_ORF/Olfr19, M12, MOR140-1, MTPCR15 | RT-PCR, Q-PCR, CI, MA | (41) |
| OR4D1 | OR17-23, OR4D3, OR4D4P, TPCR16/Olr1523, tpcr04/Olfr464, MOR240-2 | ||||
| OR1D2 | OLFR1, OR17-4/Olr1519/Olfr412, MOR127-5P | ||||
| hOR17-4 | Refer to testis OR1D2 | RT-PCR, CI, MA | (23) | ||
| hOR17-2 | OR1E1, HGM071, OR13-66, OR17-2, OR17-32, OR1E5, OR1E6, OR1E8P, OR1E9P, OST547/Olr1468/Olfr23, MOR135-27, MTPCR50 | RT-PCR | |||
| MTPCR05 | Mouse | - /Olr737, tpcr09/Olfr32, MOR227-7P, MOR227-9_p | RT-PCR, RPA | (38) | |
| MTPCR07 | - /Olr1413, Olfr30, TPCR05/Olfr30, MOR2811 | ||||
| MTPCR18 | - /Olr1228/Olfr25, MOR170-4 | ||||
| MTPCR51 | OR1M1, OR19-5, OR19-6/Olr1121/Olfr24, MOR132-1 | ||||
| MTPCR53 | OR2T1, OR1-25/Olr1606, tpcr38/Olfr31, MOR274-1 | ||||
| MTPCR55 | OR1E2, OR17-135, OR17-93, OR1E4, OR1E7, OST529/Olr1466/Olfr20, MOR135-11, MTPCR06, Olfr21 | ||||
| MOR23 | OR10J5, OR1-28/Olr1576/Olfr16, MOR267-13 | RT-PCR, NB, RPA, ISH, 5’-RACE | (31) | ||
| MOR23 | RT-PCR, ISH, CI, TG mice, MA | (34) | |||
| MOR244-3 (MOR83) | OR4E2, OR14-42/Olr1643/Olfr1509, Or83 | RT-PCR, ISH | (33) | ||
| MOR139-3 | OR7A17/Olr1075/Olfr57, IF12 | ||||
| MOR248-11 | - /Olr750/Olfr1277 | ||||
| MOR267-13 (MOR23) | Refer to testis MOR23 | ||||
| MOR283-1 | - /Olr210/Olfr701, 4932441H21Rik, 4933433E02Rik | RT-PCR | |||
| MOR8-1 | - / - /Olfr575 | ||||
| MOR31-2 | - /Olr201/Olfr690, Ors18 | RT-PCR, ISH | |||
| HT2, HTPCR92, -16, -25, -86/RTPCR04, -19, -38 | Human/rat | RT-PCR, RPA | (38) | ||
| pSCR D, pSCR G | Rat | RPA, ISH, 5’-RACE | (39) | ||
| OD1, OD2 | RT-PCR, WB, IHC | (30) | |||
| MOR171-31 | Mouse | Olfr1037 | RT-PCR, ISH | (33) | |
| MOR264-10 | RT-PCR | ||||
| DTPCR64 | Dog | RT-PCR, RPA | (38) | ||
| HGMP07/HGMP0 7I, DTMT | NB | (24) | |||
| DTMT, DTPCRH02, -09 | RPA, WB, IHC | (29) | |||
| RPA | |||||
The importance of the ectopic expressions of ORs is raised since the physiological function of the sperm OR was characterized, but still unknown in many cases. Therefore, this paper presents a summary of the ectopic expressions of ORs and the physiological functions of ORs that are already known and identified from a variety of non-chemosensory tissues, suggests possible problems of this area, and shows important directions for future research.
ECTOPIC OLFACTORY RECEPTOR EXPRESSION
Ectopic expression of ORs has been widely found in non-chemosensory tissues since the OR was isolated from the OE twenty years ago (7,24). However, this phenomenon still requires further intensive investigation because we cannot exclude the possibility that ORs are expressed in non-olfactory tissues without any functional significance. There are two controversial inconsistencies. One inconsistency is that genetic mutations of promoter regions have resulted in leaky transcription and induced low levels of ectopic expression in non-chemosensory tissues (25). The other inconsistency is that we cannot understand the deep and hidden functions of ectopic expressions due to the limitations of current techniques and lack of scientific knowledge (25). However, ectopic expression of some ORs is conserved through the tissue of interspecies (26,27), while some ORs are not related to each other (28). Therefore, we will present the ectopic expression of ORs that have been isolated from each tissue through analytic methods, orthologs, and synonyms (Table 1).
Testis
The large multigene family that encodes ORs to detect odorants was originally discovered in the nose (7). This finding resulted in the exponential expansion of olfactory research. Parmentier's group initially isolated the olfactory-like proteins in the testis, which was the first case of ORs identified in non-chemosensory tissues (24). Antibodies against the ORs, abundantly expressed in the testis of dogs (29) and rats (30), were generated and used to characterize the location of ORs in mature sperm during spermatogenesis. In particular, the localization was characterized in the midpiece of mature sperm through the use of immunohistochemistry technique. Localization was also shown in the late round and elongated spermatids in the cytoplamic droplet during spermatogenesis.
MOR23, a mouse OR transcript, was isolated by PCR based cloning using degenerate primers in the testis and OE. This gene demonstrated different transcriptional initiation regions in the testis compared with those of OE, indicating that different 5'-untranslated regions (5'-UTR) of ORs were related to the context of tissue-specific expression of the OR genes (31).
A lot of effort has been made to show the presence of ORs' expression in the testis and mature sperm. Recent efforts include RNase protection assay, northern blot, cloning depending on RT-PCR, western blot, and in situ hybridization (ISH) (23,24,29-39). Also, many testicular ORs were recently identified by DNA array and bioinformatics (28,40). Ten years after the first isolation of OR genes in testis, Hatt's group has isolated synthetic agonists and antagonists of the newly identified human testicular OR for the investigation of physiological function. They demonstrated that sperms show chemotaxis to synthetic chemicals. The sperms’ swimming behavior suggests that sperm cells detect chemical cues that cause the change of intracellular Ca2+ level through ORs (23).
Transgenic (TG) mouse that highly expresses the testicular ORs in the testis and sperm was generated and this results demonstrated that the TG sperms show chemotaxis through the OR, similar to the human sperm, by sperm swimming behavior analysis (34). Recent studies have extended the functional characterization of two isolated novel ORs of human sperm. These results demonstrated that the activation of individual receptors induces a specific Ca2+ signaling pattern (41). These distinct Ca2+ dynamics function as sperm-egg chemical communication devices that cause a stereotyped stimulus-specific behavioral response.
Table 1.
Continued 1
| Tissue or cell used | Receptor identified | Species | Human- /rat- /mouse-ortholog by biogps database (81) | Analysis method used | Ref | |
|---|---|---|---|---|---|---|
|
| ||||||
| Tongue | Fetal | TPCR85/JCG8 | Human | OR8B8/Olr1201/Olfr145, K21, MOR161-6 | RT-PCR | (43) |
| JCG1 | OR5P3/Olr1871/Olfr508, MOR204-6 | |||||
| JCG2 | OR8D2/ - /Olfr926, MOR171-8 | |||||
| JCG6 | OR10A5, OR10A1, OR11-403/Olr227, ratchr1-164389755-164390708_ORF/Olfr713, MOR263-1, P3 | |||||
| JCG9 | OR8D1, OR8D3, OST004, PDJ9J14/ - /Olfr26, MOR171-9, MTPCR09 | |||||
| Adult | OR6Q1 | OR11-226/ - / - | RT-PCR | (42) | ||
| OR10A4/JCG5 | OR10A4P/Olr231, ratchr1-164484422-164485369_ORF/Olfr17, MOR263-5, P2 | |||||
| OR7A5/HTPCR2 | Refer to testis OR7A5 | |||||
| HTPCR06/OR2K2 | HSHTPCRH06, HTPCRH06, OR2AN1P, OR2AR1P/Olr854/Olfr267, MOR262-1 | |||||
| JCG3/OR5P2 | JCG4/Olr279/Olfr502, MOR204-8 | |||||
| JCG6/OR10A5 | Refer to tongue JCG6 | |||||
| mTPCR06 | Mouse | Refer to testis MTPCR55 | RT-PCR | (45) | ||
| Both | HGMP07I/OR1E1 | Human | Refer to testis hOR17-2 | RT-PCR | (43) | |
| HTPCR06 | Refer to tongue HTPCR06/OR2K2 | |||||
| JCG3/JCG4 | Refer to tongue JCG3/OR5P2 | |||||
| JCG5 | Refer to tongue OR10A4/JCG5 | |||||
| Heart | OL1 | Rat | OR2B2, OR2B2Q, OR2B9, OR6-1, dJ193B12.4, hs6M1-10/Olr1654/Olfr1359 , MOR256-35, MOR256-60 | RT-PCR, ISH | (46) | |
| PSGR | Rat, mouse | OR51E2, OR51E3P, OR52A2/Olr59, Olfr78, Or51e2, RA1c/Olfr78, 4633402A21Rik, MOL2.3, MOR18-2, PSGR, RA1c | NB | (61) | ||
| OR10G4 | Human | OR11-278/Olr1335/Olfr980, MOR223-2 | RT-PCR | (55) | ||
| MOR2.3 | Mouse | Refer to heart PSGR | MOR2.3-IGITL TG | (47) | ||
| mice, ISH, X-gal staining | ||||||
| Embryo | COR7b | Chicken | ISH | (48) | ||
| Spleen | PSGR | Human | Refer to heart PSGR | NB | (61) | |
| pSCR D, pSCR G | Rat | RPA | (39) | |||
| OL-2 | Mouse | RT-PCR | (50) | |||
| Pancreas | OL-2 | Mouse | RT-PCR | |||
| Blood | HPFH1OR | Mouse | OR52A1/- / - | RT-PCR, RPA | (51) | |
| Prostate | PSGR | Human | Refer to heart PSGR | NB | (61) | |
| PSGR | Human | Refer to heart PSGR | RT-PCR, WB, CI | (59) | ||
| PGC & oocytes | HT2 | Human | HPGMP07 | RT-PCR | (52) | |
| Brain | M71 | Mouse | OR8A1, OR11-318, OST025/Olr1194/Olfr151, MOR171-2 | RT-PCR, ISH, M71-IRES-tauLacZ mice | (56) | |
| C6 | RT-PCR, ISH | |||||
| OR3 | OR2C1, OLFmf3, OR2C2P/Olr1356, Or2c1/olfr15, MOR256-17 | RT-PCR | ||||
| MOR2.3 | Refer to heart MOR2.3 | MOR2.3-IGITL TG | (47) | |||
| mice, ISH, X-gal staining | ||||||
| PSGR | Rat, mouse | Refer to heart PSGR | NB | (61) | ||
| RA1c | Rat | Refer to heart MOR2.3 | RT-PCR, ISH | (82) | ||
Table 1.
Continued 2
| Tissue or cell used | Receptor identified | Species | Human- /rat- /mouse-ortholog by biogps database (81) | Analysis method used | Ref |
|---|---|---|---|---|---|
|
| |||||
| Muscle | MOR23 | Mouse | Refer to testis MOR23 | Real-time RT-PCR, WB, CMA, immunostaining | (64) |
| Liver | PSGR | Human/ rat/ mouse | Refer to heart PSGR | NB | (61) |
| OR10G4 | Human | Refer to heart OR10G4 | RT-PCR | (55) | |
| Lung | MOR2.3 | Mouse | Refer to heart MOR2.3 | MOR2.3-IGITL TG | (47) |
| mice, X-gal staining | |||||
| Kidney | OR6N2 | Human | OR1-23/Olr1588/Olfr430, MOR105-5P | RT-PCR | (55) |
| OR2T1 | Refer to testis MTPCR53 | ||||
| Olfr90 | Mouse | OR2H2, FAT11, OLFR2, OLFR42B, OR2H3, dJ271M21.2, hs6M1-12/Olr1750, MOR256-21, Olfr90, Or1/MOR256-21, bM573K1.2 | RT-PCR | (54) | |
| GI tract | PSGR | Mouse | Refer to heart PSGR | NB | (61) |
| OR73, hOR17-7/11 | Human | - / - /MOR174-9 | RT-PCR, CI, AR | (57) | |
| OR1A1, OR17-7/Olr1513/Olfr43, IA7, MOR125-1, MOR125-5_p, Olfr403 | |||||
| OR1G1 | OR17-130, OR17-209, OR1G2/Olr1406/- | ||||
| hOR17-210 | OR1E3, OR17-210, OR1E3P/ - / - | RT-PCR | |||
| Placenta | MOR125-1 | Human | Refer to GI tract hOR17-7/11 | RT-PCR | (53) |
| MOR126-1 | - / - /olfr54, F3 | ||||
| MOR140-1 | Refer to testis OR7A5 | ||||
| MOR145-5 | - / - /Olfr866 | ||||
| MOR216-1 | - /Olr1767, ratchrX-136588662-136587739_ORF/olfr1323 | ||||
| MOR263-9 | - /Olr1687/olfr129 | ||||
| HeLa cell | OR1A2 | Human | OR17-6/ - / - | RNAi | (63) |
| OR2A4 | OR2A10/Olr818/olfr13, K7, MOR261-6 | RNAi, WB, immunostaining | |||
GI tract: gastrointestinal tract, PGC: primordial germ cell, CI: Ca2+ imaging, MA: microcapillary assay, RPA: RNase protection assay, NB: Northern blot, ISH: in situ hybridization, WB: western blot, IHC: immunohistochemistry, AR: amperomeric recording, IGITL: IRES-GFP-IRES-Tau-LacZ, CMA: cell migration and adhesion assay 5’-RACE: rapid amplification of cDNA end.
Tongue
Several kinds of olfactory-like receptors were isolated in human tongue tissue by RT-PCR analysis. Some of them were also confirmed in the human fetal cDNA library (42-44). The mouse orthologs of the human tongue ORs were identified and the expression of these orthologs was studied in three types of mouse papillae (circumvallate, fungiform, foliate) utilizing RT-PCR. Only MOR262-1, ortholog of HTPCR06/OR2K2 was expressed in mouse tongue tissues enriched in circumvallate, foliate, fungiform of tongue muscle as well as in the olfactory epithelium (45).
Heart
Rat OL1, a putative OR transcript, was identified by RT-PCR and ISH analyses to not only expressed in the OE, but also in the heart. In particular, this unexpected cardiac expression was developmentally regulated, being maximal at early postnatal stages but hardly detectable at adult stages. This transient cardiac expression suggests that ORs are involved in odor coding as well as cardiac morphogenesis processes and cardiac cell growth (46). The other study of the OR in the heart was performed using a MOR2.3-IGITL (IRES-GFP-IRES-Tau-LacZ) TG mouse and X-gal staining analysis. This demonstrated that MOR2.3 is expressed in a small segment of the aorta of the thoracic region (47). These results show that the ORs are expressed in the heart providing evidence that ORs have a physiological function in the heart.
Embryo
COR7b is transiently expressed in the developing chick notochord in addition to the olfactory system. The COR7b transcript was found to be expressed in the notochord from E2 to E6. COR7b may also play a role in the notochord that is essential for the proper positioning of the neural tube and of the somitic mesoderm (48). It may play an important role in cell recognition during embryogenesis (49).
Spleen and pancreas
Two spermatid chemoreceptor (SCR) D and -G families, expressed in olfactory cilia, sperm, and spleen, were confirmed by RNase protection assay (39). Moreover, RT-PCR analysis demonstrated that rat OR-like protein (OL2) was expressed in the rat spleen and mouse insulin-secreting cell line (MIN6) (50). Although the ligands of these receptors have not been investigated, the ligands might control the modulation of insulin secretion in the pancreas. There is also the possibility that the ligands control the maturation and migration of white blood cells in the spleen.
Blood
HPFH1OR gene and its murine ortholog are expressed in primary tissues containing erythroid cells and in the erythroid tissue culture cell line. Also, these OR genes were expressed in both the mouse and human olfactory epithelium (51).
Primordial germ cell and oocyte
ORs (HT2) were identified from human primordial germ cells (PGC) by differential displays. ORs were not expressed in the preimplantation embryos, EC cells and somatic cells, but they were expressed in the female and male PGC and oocyte. This study suggests a role in the migration of PGC to the developing gonad and as a special function in germ-line development (52).
Placenta
Several different OR genes were identified from the rat placenta using the degenerative primers designed to correspond to highly conserved regions of ORs (53). This study suggests that ORs may recognize some small molecules in the placenta as environment chemicals. These cues from the fetus and/or mother help to regulate the synthesis of hormones and growth factors.
Lung
One OR (S25/mJCG1) was isolated from the human adult tongue and its mouse ortholog expressed in the lung was confirmed by RT-PCR (45). MOL2.3 was also observed on a distinct population of lung alveoli by analyzing the TG mouse line MOL2.3-IGITL (47).
Kidney
6 different ORs were identified and confirmed in the kidney. One of these ORs was detected in a macula densa (MD) cell line. The key olfactory signaling components are expressed in the renal distal nephron. They may have a sensory role in the MD to control both renin secretion and glomerular filtration rate (54). Two more ORs were identified by RT-PCR (55).
Brain
Three receptors, M71, C6, and OR3 were detected in the cortex of the brain. M71 and C6 transcripts are expressed in the layer II cortical pyramidal neurons located in the occipital pole during the postnatal development of the mouse. The X-gal staining analysis shows M71 expression at P3, and a peak at P8 that continues to adult using M71-IRES-tauLacZ mouse, in which M71 expression was genetically marked with tauLacZ (56). Additionally, the odorant receptor, MOL2.3, was shown to be expressed in the ganglia of the autonomic nervous system (47).
FUNCTIONS OF ECTOPIC OLFACTORY RECEPTORS IN THE NON-CHEMOSENSORY TISSUES
After the report of OR’s ectopic expression, various OR genes were isolated by PCR-dependent cloning at the transcription level. Furthermore, the generation of testicular ORs’ antibodies provided an opportunity to identify the cellular localization of ORs at a protein level (29,30). Ten years later, the physiological function of ORs expressed in the testis was first reported (23). A new human testicular OR, hOR17-4, was isolated, and agonist (Bourgeonal) and antagonist (Undecanal) were screened from an odorant mixture library utilizing heterogeneously expressed hOR17-4 within a HEK293 cell. The activation of ORs expressed in HEK293 by the isolated ligands was confirmed by Ca2+ dynamics. The ligand also increased the intracellular Ca2+ level in the human sperm. The subsequent behavioral bioassays showed that the chemotaxis of human sperm’s swimming behavior depended on ligand concentration (23). The chemotaxis function of testicular ORs was verified again through the quantity of testicular ORs (MOR23)-overexpressed in the TG mouse (34).
Though many studies of ectopic OR expression was focused on testis and sperm, the expression of 4 ORs (OR73, hOR17-7/11, OR1G1, hOR17-210) was recently identified in the gut enterochromaffin (EC) cell through the use of EC derived human cell lines. One of the known ligands for these ORs was tested for functional study in an EC cell. Serotonin was secreted by the odorant treatment through a L-type Ca2+ channel, phospholipase C (PLC), and IP3 receptor utilizing different combinations of various pharmacological agents (57). Collectively, this result suggests that several types of ORs are expressed in the gut EC cell. These expressions are activated by spicy odorants released during food ingestion. The odorant secrets serotonin used for regulating enterocinesia (57, 58).
The prostate-specific G-protein-coupled receptor (PSGR) was originally isolated as a prostate-specific tumor marker (59-62). The ligand of PSGR (olfr78)-OR51E2, androstenone derivative, was identified by heterologous expression systems. The steroid hormone (androstenone derivative) elicited a Ca2+ response in prostate cancer cell line (LNCaP) and primary human prostate epithelium cells. PSGR reduced the proliferation of prostate cancer cells by utilizing p38 phosphorylation and SAPK/JNK- MAPK cascade (59).
Another ectopic OR expression was elucidated during the study of GPCRs related to cytokinesis. OR1A2 and OR2A4 were isolated from a human cervical cancer cell line (HeLa) by RNAi knockdown screening. The knockdown of OR2A4 perturbed the actin cytoskeleton which increased binucleated and multinucleated cell formation 4-10 fold. OR2A4 localizes the spindle pole, midzone, and midbody ring and may be involved in cytokinesis by exerting a regulatory role on the actin cytoskeleton (63).
During myogenesis of primary cultured mouse muscle cells and muscle regeneration, changes of multiple ORs' expression pattern were observed. Typically, the expression of MOR23 increased during muscle regeneration. The MOR23 specific ligand, lyral concentration affected myocyte migration. MOR23 targeted siRNA treatment decreased the migration of myocytes which was recovered when MOR23 was rescued. Furthermore, the signaling mechanism of MOR23 is suggested to follow the canonical OR, Golf, and ACIII signaling pathway. Additionally, MOR23 may be involved in cell-cell adhesion which was shown by the same methods utilized in this experiment. These results indicate that MOR23 regulates muscle cell adhesion and migration (64). Previous studies show that MOR23 is related with mouse sperm migration and axonal growth cone migration (34). With the myocyte migration, this result suggests that MOR23 participates in migration of multiple cell types.
CHARACTERISTICS OF ECTOPIC EXPRESSIONS OF OLFACTORY RECEPTORS
One cell-one receptor
It is widely accepted that each OR selectively expresses only one of thousands of odorant receptor genes. Only one OR is expressed in each cell of the olfactory neuron in the OE (65,66). However, other tissues may violate the one cell-one receptor rule. It seems that more than one OR is expressed in one cell of testis and muscle (33,64). More than one OR mRNA signal is shown in one round of spermatid during spermatogenesis (33). This feature requires more accurate analysis. In the muscle system, ORs have multiple expressions (64), whereas subpopulations of muscle cells have a possibility of expressing one OR in each cell. Also, we cannot exclude the possibility that the "one cell-one receptor rule" applies to OR proteins since mRNAs were identified by ISH analysis.
5’-UTR structure of ectopic OR mRNAs
Tsuboi's group suggested that posttranscriptional regulation for ectopic expression of ORs was required by comparing the 5'-UTR structure of ectopic OR transcripts with that of the OE in 1996 (31). With the results of analysis of OR transcript with the same open reading frame (ORF) from testis and OE, transcription of testicular OR was initiated within the intron located before the exon containing ORF. Transcript of ORs in OE has a structure which contains exon with 5’-UTR regions. These results were demonstrated by the analysis of the transcript start site using 5’-RACE experiments in an isolated sample in each tissue. Also, the same result has been proved with different ORs in other tissues by other groups (33,39). These studies indicate that the localization of same OR's expression may be affected by posttranscriptional regulation depending on the heterologous transcript in other tissues (67).
However, there are other cases that same initiation site was demonstrated in transcripts of 5’-UTR of the M71 cDNA sequence from cerebral cortex and OE (56), and OR transcript in the spleen, OE, and testis (39). These evidences present the possibility that the 5’-UTR structure may be correlated with different tissues and different ORs.
Potential downstream pathway of ectopic OR signal transduction
For odorant perception, ORs have distinct signal pathways by the OR-Golf-ACIII-CNG channel in olfactory neurons. Increased intracellular Ca2+ induces action potential and Ca2+ efflux by NCX, NCKX4, and PMCA (19-22,68). Also, OMP is reported to be involved in this process (19). Although OR downstream signaling occurring in the ectopic expression is not clearly shown, some groups insist that the OR-Golf-ACIII pathway in olfactory sensory neurons is similar to that in sperm, kidney, and muscle (54,64,69).
On the other hand, another result suggests that intracellular Ca2+ levels are increased by OR activation through L-type Ca2+ channel, PLC, and IP3 receptors in EC cells in the gut (57). The activated OR-downstream by chemical cues may vary in the non-olfactory tissues or be the same in OE.
POSSIBLE PROBLEMS
There are several problems in studying ORs in non-chemosensory tissues. First, the expression level of OR transcript in the non-olfactory tissue is relatively lower than in the olfactory tissue. This is because the ectopic expression of ORs is mostly isolated by the amplification of the OR gene through PCR based cloning (24,29-31,38,39,41-43,46,53,61). Although the OR gene is detected by RT-PCR, the same ORs are not detected by ISH (45). However, testicular ORs are detected by ISH when using the tyramide signal amplification (TSA) system even though testicular ORs are not detected by normal ISH (33). These results show that the expression level of ORs in the OE is relatively high. Non-chemosensory tissues show low expression levels of transcript or rare numbers of specific cell-type expression of ORs. Second, it is very difficult to study ORs as proteins because there are not enough high quality specific OR antibodies, due to the similarity of the ORs. In a recently published paper in which ORs are involved in cytokinesis, the localization of the OR2A4 protein was revealed through IHC by utilizing high quality antibodies that provided the opportunity for a functional assay (63). In this way, the generation of useful OR antibodies met the level needed to study ORs as proteins. Lastly, there are other barriers to studying ORs because ligands have been isolated in less than 100 ORs (70-73). Identification of ligands, external cues to investigate the function of ORs, is necessary because ORs belong to the family of GPCRs that transmit external signals to the internal cellular signaling events. Several groups have tried to isolate specific OR-mediated chemicals on the basis of chemical libraries and synthesized specific derivatives, the synthetic agonist and antagonist, to activate OR using the isolated chemicals (23,41,59).
PERSPECTIVES
In previous parts, we described ectopic expressions of ORs by each tissue and highlighted the physiological functions identified thus far. Furthermore, we discussed the features of OR ectopic expressions and limitations for additional study in this area. In the following parts, future directions for the study of the ORs’ ectopic expressions will be suggested.
First, the future research of ectopic expression of ORs has to be diversified beyond the OR itself. As previously mentioned, if Golf and ACIII, located on the downstream of OR activations in the olfactory system, are to be targeted for screening, they will provide a useful method to figure out the ORs and OR-like pathways in non-chemosensory tissues.
In addition, strategies to use the olfactory specific proteins for the screening of ORs and OR-like pathways seem to be an alternative method. It is known that the receptor-transporting protein 1 short (RTP1S) is the olfactory specific protein (74) that facilitates the translocation and trafficking of ORs on the plasma membrane (75). Ric-8B, a putative guanine nucleotide exchange factor, is also mainly expressed in the olfactory epithelium and it enhances accumulation of Golf, promoting functional OR expression (76). Furthermore, according to the RTPCR results, Ric8B is also expressed in wholly within the eye, brain, skeletal muscle, heart, and kidney with a small amount of transcript (77). REEP1 is also an olfactory tissue-specific protein and it promotes the functional expression of a large number of ORs in HEK293T cells (78). Although OMP was known to be expressed in olfactory system for a long time, it is also expressed outside of the olfactory system in places such as the hypothalamus in the brain (79). It is suggested that an effective way to use olfactory specific proteins, olfactory receptor associated proteins, and olfactory signal transduction-specific proteins with OR is to find olfactory-like pathways in non-chemosensory tissues.
The generation of the antibody library against the specific OR peptides is the second avenue for the future study of ectopic expressions of OR. Since the homology of ORs is between 40-90% and the number of OR genes is greater than 1,000 in the mouse, the generation of a whole antibody library will be required for important resources. These libraries will be generated by analyzing the whole genome, followed by the selection of specific peptide regions of each OR. This work will be a significant contribution, not only for the research of OR ectopic expression, but also for research on the smell perception of ORs.
The establishment of a chemical library is another recommendable method for finding the agonist and antagonist of ORs. Some groups have succeeded in identifying ligands of specific ORs by establishing chemical libraries (70,71). If the odorant library is constructed and reciprocal access is possible, it will lead to a breakthrough in the research field of ORs in non-chemosensory tissues and the OE. In addition, the identification of endogenous ligands is a very significant rate-limiting step. Above all, an assay system for extracting active endogenous ligands in candidate sources such as blood and body fluid will be required for the identification of in vivo ligands. Also, pursuing the study of active compounds from fractionation by GC-Mass chromatography should be another accompanied study.
The establishment of an in vitro system for functional assay is the final suggestion. The effective heterologous expression system with several accessory proteins for ORs has already been set up by Matsunami's group (78). They isolated the ligand of specific ORs and characterized them by using this system. As the OR hardly expresses itself in the plasma membrane, co-expression of the accessory proteins such as RTP1S and REEP1 enhance expression of ORs (78). For instance, expression of bitter taste receptors (TAS2Rs) with their accessory proteins was promoted in vitro system (80). In spite of those accessory proteins, there is still intracellular accumulation of OR proteins during the process of OR expression on the plasma membrane in in vitro system. To overcome this problem, identification and purification of novel accessory proteins that enhance the rate of intracellular trafficking, produce stable locations on the plasma membrane, and cause proper OR expression will be investigated. Ultimately, these proteins enable effective expression of ORs in in vitro system and functional assay.
Lastly, if a conditional knockout mouse for specific ORs is generated in specific non-olfactory tissues using the Cre-loxP system, it will lead to the identification of physiological functions of specific ORs in specific non-chemosensory tissues or cells.
Acknowledgments
We thank the members of the Koo laboratory and Michael Koo for careful readings of the manuscript and helpful discussions. J.H.K. was supported by the DGIST MIREBrain and Convergence Science Center (12-BD-0403).
References
- 1.Munger S. D., Leinders-Zufall T., Zufall F. Subsystem organization of the mammalian sense of smell. Ann. Rev. Physiol. (2009);71:115–140. doi: 10.1146/annurev.physiol.70.113006.100608. [DOI] [PubMed] [Google Scholar]
- 2.Mombaerts P. Molecular biology of odorant receptors in vertebrates. Annu. Rev. Neurosci. (1999);22:487–509. doi: 10.1146/annurev.neuro.22.1.487. [DOI] [PubMed] [Google Scholar]
- 3.Brechbuhl J., Klaey M., Broillet M. C. Grueneberg ganglion cells mediate alarm pheromone detection in mice. Science. (2008);321:1092–1095. doi: 10.1126/science.1160770. [DOI] [PubMed] [Google Scholar]
- 4.Fuss S. H., Omura M., Mombaerts P. The Grueneberg ganglion of the mouse projects axons to glomeruli in the olfactory bulb. Eur. J. Neurosci. (2005);22:2649–2654. doi: 10.1111/j.1460-9568.2005.04468.x. [DOI] [PubMed] [Google Scholar]
- 5.Koos D. S., Fraser S. E. The Grueneberg ganglion projects to the olfactory bulb. Neuroreport. (2005);16:1929–1932. doi: 10.1097/01.wnr.0000186597.72081.10. [DOI] [PubMed] [Google Scholar]
- 6.Fleischer J., Breer H., Strotmann J. Mammalian olfactory receptors. Front. Cell. Neurosci. (2009);3:9. doi: 10.3389/neuro.03.009.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buck L., Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. (1991);65:175–187. doi: 10.1016/0092-8674(91)90418-X. [DOI] [PubMed] [Google Scholar]
- 8.Bjarnadottir T. K., Gloriam D. E., Hellstrand S. H., Kristiansson H., Fredriksson R., Schioth H. B. Comprehensive repertoire and phylogenetic analysis of the G protein-coupled receptors in human and mouse. Genomics. (2006);88:263–273. doi: 10.1016/j.ygeno.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Young J. M., Friedman C., Williams E. M., Ross J. A., Tonnes-Priddy L., Trask B. J. Different evolutionary processes shaped the mouse and human olfactory receptor gene families. Hum. Mol. Genet. (2002);11:535–546. doi: 10.1093/hmg/11.5.535. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X., Firestein S. The olfactory receptor gene superfamily of the mouse. Nat. Neurosci. (2002);5:124–133. doi: 10.1038/nn800. [DOI] [PubMed] [Google Scholar]
- 11.Olender T., Feldmesser E., Atarot T., Eisenstein M., Lancet D. The olfactory receptor universe--from whole genome analysis to structure and evolution. Genet. Mol. Res. (2004);3:545–553. [PubMed] [Google Scholar]
- 12.Glusman G., Bahar A., Sharon D., Pilpel Y., White J., Lancet D. The olfactory receptor gene superfamily: data mining, classification, and nomenclature. Mamm. Genome. (2000);11:1016–1023. doi: 10.1007/s003350010196. [DOI] [PubMed] [Google Scholar]
- 13.Jones D. T., Reed R. R. Golf: an olfactory neuron specific-G protein involved in odorant signal transduction. Science. (1989);244:790–795. doi: 10.1126/science.2499043. [DOI] [PubMed] [Google Scholar]
- 14.Lowe G., Nakamura T., Gold G. H. Adenylate cyclase mediates olfactory transduction for a wide variety of odorants. Proc. Natl. Acad. Sci. U.S.A. (1989);86:5641–5645. doi: 10.1073/pnas.86.14.5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong S. T., Trinh K., Hacker B., Chan G. C., Lowe G., Gaggar A., Xia Z., Gold G. H., Storm D. R. Disruption of the type III adenylyl cyclase gene leads to peripheral and behavioral anosmia in transgenic mice. Neuron. (2000);27:487–497. doi: 10.1016/S0896-6273(00)00060-X. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura T., Gold G. H. A cyclic nucleotide- gated conductance in olfactory receptor cilia. Nature. (1987);325:442–444. doi: 10.1038/325442a0. [DOI] [PubMed] [Google Scholar]
- 17.Brunet L. J., Gold G. H., Ngai J. General anosmia caused by a targeted disruption of the mouse olfactory cyclic nucleotide-gated cation channel. Neuron. (1996);17:681–693. doi: 10.1016/S0896-6273(00)80200-7. [DOI] [PubMed] [Google Scholar]
- 18.Billig G. M., Pal B., Fidzinski P., Jentsch T. J. Ca2+-activated Cl- currents are dispensable for olfaction. Nat. Neurosci. (2011);14:763–769. doi: 10.1038/nn.2821. [DOI] [PubMed] [Google Scholar]
- 19.Kwon H. J., Koo J. H., Zufall F., Leinders-Zufall T., Margolis F. L. Ca extrusion by NCX is compromised in olfactory sensory neurons of OMP mice. PLoS One. (2009);4:e4260. doi: 10.1371/journal.pone.0004260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stephan A. B., Tobochnik S., Dibattista M., Wall C. M., Reisert J., Zhao H. The Na(+)/Ca(2+) exchanger NCKX4 governs termination and adaptation of the mammalian olfactory response. Nat. Neurosci. (2012);15:131–137. doi: 10.1038/nn.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saidu S. P., Weeraratne S. D., Valentine M., Delay R., Van Houten J. L. Role of plasma membrane calcium ATPases in calcium clearance from olfactory sensory neurons. Chem. Senses. (2009);34:349–358. doi: 10.1093/chemse/bjp008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pyrski M., Koo J. H., Polumuri S. K., Ruknudin A. M., Margolis J. W., Schulze D. H., Margolis F. L. Sodium/calcium exchanger expression in the mouse and rat olfactory systems. J. Comp. Neurol. (2007);501:944–958. doi: 10.1002/cne.21290. [DOI] [PubMed] [Google Scholar]
- 23.Spehr M., Gisselmann G., Poplawski A., Riffell J. A., Wetzel C. H., Zimmer R. K., Hatt H. Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science. (2003);299:2054–2058. doi: 10.1126/science.1080376. [DOI] [PubMed] [Google Scholar]
- 24.Parmentier M., Libert F., Schurmans S., Schiffmann S., Lefort A., Eggerickx D., Ledent C., Mollereau C., Gerard C., Perret J., Grootegoed A., Vassart G. Expression of members of the putative olfactory receptor gene family in mammalian germ cells. Nature. (1992);355:453–455. doi: 10.1038/355453a0. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez-Trelles F., Tarrio R., Ayala F. J. Is ectopic expression caused by deregulatory mutations or due to gene-regulation leaks with evolutionary potential? BioEssays. (2005);27:592–601. doi: 10.1002/bies.20241. [DOI] [PubMed] [Google Scholar]
- 26.Gilad Y., Man O., Glusman G. A comparison of the human and chimpanzee olfactory receptor gene repertoires. Genome Res. (2005);15:224–230. doi: 10.1101/gr.2846405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De la Cruz O., Blekhman R., Zhang X., Nicolae D., Firestein S., Gilad Y. A signature of evolutionary constraint on a subset of ectopically expressed olfactory receptor genes. Mol. Biol. Evol. (2009);26:491–494. doi: 10.1093/molbev/msn294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feldmesser E., Olender T., Khen M., Yanai I., Ophir R., Lancet D. Widespread ectopic expression of olfactory receptor genes. BMC Genomics. (2006);7:121. doi: 10.1186/1471-2164-7-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vanderhaeghen P., Schurmans S., Vassart G., Parmentier M. Olfactory receptors are displayed on dog mature sperm cells. J. Cell Biol. (1993);123:1441–1452. doi: 10.1083/jcb.123.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walensky L. D., Roskams A. J., Lefkowitz R. J., Snyder S. H., Ronnett G. V. Odorant receptors and desensitization proteins colocalize in mammalian sperm. Mol. Med. (1995);1:130–141. [PMC free article] [PubMed] [Google Scholar]
- 31.Asai H., Kasai H., Matsuda Y., Yamazaki N., Nagawa F., Sakano H., Tsuboi A. Genomic structure and transcription of a murine odorant receptor gene: differential initiation of transcription in the olfactory and testicular cells. Biochem. Biophys. Res. Commun. (1996);221:240–247. doi: 10.1006/bbrc.1996.0580. [DOI] [PubMed] [Google Scholar]
- 32.Branscomb A., Seger J., White R. L. Evolution of odorant receptors expressed in mammalian testes. Genetics. (2000);156:785–797. doi: 10.1093/genetics/156.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukuda N., Touhara K. Developmental expression patterns of testicular olfactory receptor genes during mouse spermatogenesis. Genes Cells. (2006);11:71–81. doi: 10.1111/j.1365-2443.2005.00915.x. [DOI] [PubMed] [Google Scholar]
- 34.Fukuda N., Yomogida K., Okabe M., Touhara K. Functional characterization of a mouse testicular olfactory receptor and its role in chemosensing and in regulation of sperm motility. J. Cell Sci. (2004);117:5835–5845. doi: 10.1242/jcs.01507. [DOI] [PubMed] [Google Scholar]
- 35.Gaillard I., Rouquier S., Giorgi D. Olfactory receptors. Cell. Mol. Life Sci. (2004);61:456–469. doi: 10.1007/s00018-003-3273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spehr M., Hatt H. hOR17-4 as a potential therapeutic target. Drug News Perspect. (2004);17:165–171. doi: 10.1358/dnp.2004.17.3.829014. [DOI] [PubMed] [Google Scholar]
- 37.Spehr M., Schwane K., Riffell J. A., Zimmer R. K., Hatt H. Odorant receptors and olfactory-like signaling mechanisms in mammalian sperm. Mol. Cell. Endocrinol. (2006);250:128–136. doi: 10.1016/j.mce.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 38.Vanderhaeghen P., Schurmans S., Vassart G., Parmentier M. Specific repertoire of olfactory receptor genes in the male germ cells of several mammalian species. Genomics. (1997);39:239–246. doi: 10.1006/geno.1996.4490. [DOI] [PubMed] [Google Scholar]
- 39.Walensky L. D., Ruat M., Bakin R. E., Blackshaw S., Ronnett G. V., Snyder S. H. Two novel odorant receptor families expressed in spermatids undergo 5'-splicing. J. Biol. Chem. (1998);273:9378–9387. doi: 10.1074/jbc.273.16.9378. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X., Rogers M., Tian H., Zou D. J., Liu J., Ma M., Shepherd G. M., Firestein S. J. High-throughput microarray detection of olfactory receptor gene expression in the mouse. Proc. Natl. Acad. Sci. U.S.A. (2004);101:14168–14173. doi: 10.1073/pnas.0405350101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Veitinger T., Riffell J. R., Veitinger S., Nascimento J. M., Triller A., Chandsawangbhuwana C., Schwane K., Geerts A., Wunder F., Berns M. W., Neuhaus E. M., Zimmer R. K., Spehr M., Hatt H. Chemosensory Ca2+ dynamics correlate with diverse behavioral phenotypes in human sperm. J. Biol. Chem. (2011);286:17311–17325. doi: 10.1074/jbc.M110.211524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Durzynski L., Gaudin J. C., Myga M., Szydlowski J., Gozdzicka-Jozefiak A., Haertle T. Olfactorylike receptor cDNAs are present in human lingual cDNA libraries. Biochem. Biophys. Res. Commun. (2005);333:264–272. doi: 10.1016/j.bbrc.2005.05.085. [DOI] [PubMed] [Google Scholar]
- 43.Gaudin J. C., Breuils L., Haertle T. New GPCRs from a human lingual cDNA library. Chem. Senses. (2001);26:1157–1166. doi: 10.1093/chemse/26.9.1157. [DOI] [PubMed] [Google Scholar]
- 44.Thomas M. B., Haines S. L., Akeson R. A. Chemoreceptors expressed in taste, olfactory and male reproductive tissues. Gene. (1996);178:1–5. doi: 10.1016/0378-1119(96)00311-3. [DOI] [PubMed] [Google Scholar]
- 45.Gaudin J. C., Breuils L., Haertle T. Mouse orthologs of human olfactory-like receptors expressed in the tongue. Gene. (2006);381:42–48. doi: 10.1016/j.gene.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 46.Drutel G., Arrang J. M., Diaz J., Wisnewsky C., Schwartz K., Schwartz J. C. Cloning of OL1, a putative olfactory receptor and its expression in the developing rat heart. Receptors Channels. (1995);3:33–40. [PubMed] [Google Scholar]
- 47.Weber M., Pehl U., Breer H., Strotmann J. Olfactory receptor expressed in ganglia of the autonomic nervous system. J. Neurosci. Res. (2002);68:176–184. doi: 10.1002/jnr.10164. [DOI] [PubMed] [Google Scholar]
- 48.Nef S., Nef P. Olfaction: transient expression of a putative odorant receptor in the avian notochord. Proc. Natl. Acad. Sci. U.S.A. (1997);94:4766–4771. doi: 10.1073/pnas.94.9.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dreyer W. J. The area code hypothesis revisited: olfactory receptors and other related transmembrane receptors may function as the last digits in a cell surface code for assembling embryos. Proc. Natl. Acad. Sci. U.S.A. (1998);95:9072–9077. doi: 10.1073/pnas.95.16.9072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blache P., Gros L., Salazar G., Bataille D. Cloning and tissue distribution of a new rat olfactory receptor-like (OL2). Biochem. Biophys. Res. Commun. (1998);242:669–672. doi: 10.1006/bbrc.1997.8041. [DOI] [PubMed] [Google Scholar]
- 51.Feingold E. A., Penny L. A., Nienhuis A. W., Forget B. G. An olfactory receptor gene is located in the extended human beta-globin gene cluster and is expressed in erythroid cells. Genomics. (1999);61:15–23. doi: 10.1006/geno.1999.5935. [DOI] [PubMed] [Google Scholar]
- 52.Goto T., Salpekar A., Monk M. Expression of a testis-specific member of the olfactory receptor gene family in human primordial germ cells. Mol. Hum. Reprod. (2001);7:553–558. doi: 10.1093/molehr/7.6.553. [DOI] [PubMed] [Google Scholar]
- 53.Itakura S., Ohno K., Ueki T., Sato K., Kanayama N. Expression of Golf in the rat placenta: Possible implication in olfactory receptor transduction. Placenta. (2006);27:103–108. doi: 10.1016/j.placenta.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 54.Pluznick J. L., Zou D. J., Zhang X., Yan Q., Rodriguez-Gil D. J., Eisner C., Wells E., Greer C. A., Wang T., Firestein S., Schnermann J., Caplan M. J. Functional expression of the olfactory signaling system in the kidney. Proc. Natl. Acad. Sci. U.S.A. (2009);106:2059–2064. doi: 10.1073/pnas.0812859106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang X., De la Cruz O., Pinto J. M., Nicolae D., Firestein S., Gilad Y. Characterizing the expression of the human olfactory receptor gene family using a novel DNA microarray. Genome Biol. (2007);8:R86. doi: 10.1186/gb-2007-8-5-r86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Otaki J. M., Yamamoto H., Firestein S. Odorant receptor expression in the mouse cerebral cortex. J. Neurobiol. (2004);58:315–327. doi: 10.1002/neu.10272. [DOI] [PubMed] [Google Scholar]
- 57.Braun T., Voland P., Kunz L., Prinz C., Gratzl M. Enterochromaffin cells of the human gut: sensors for spices and odorants. Gastroenterology. (2007);132:1890–1901. doi: 10.1053/j.gastro.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 58.Breer H., Eberle J., Frick C., Haid D., Widmayer P. Gastrointestinal chemosensation: chemosensory cells in the alimentary tract. Histochem. Cell Biol. (2012);138:13–24. doi: 10.1007/s00418-012-0954-z. [DOI] [PubMed] [Google Scholar]
- 59.Neuhaus E. M., Zhang W., Gelis L., Deng Y., Noldus J., Hatt H. Activation of an olfactory receptor inhibits proliferation of prostate cancer cells. J. Biol. Chem. (2009);284:16218–16225. doi: 10.1074/jbc.M109.012096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu L. L., Stackhouse B. G., Florence K., Zhang W., Shanmugam N., Sesterhenn I. A., Zou Z., Srikantan V., Augustus M., Roschke V., Carter K., McLeod D. G., Moul J. W., Soppett D., Srivastava S. PSGR, a novel prostate-specific gene with homology to a G protein-coupled receptor, is overexpressed in prostate cancer. Cancer Res. (2000);60:6568–6572. [PubMed] [Google Scholar]
- 61.Yuan T. T., Toy P., McClary J. A., Lin R. J., Miyamoto N. G., Kretschmer P. J. Cloning and genetic characterization of an evolutionarily conserved human olfactory receptor that is differentially expressed across species. Gene. (2001);278:41–51. doi: 10.1016/S0378-1119(01)00709-0. [DOI] [PubMed] [Google Scholar]
- 62.Xia C., Ma W., Wang F., Hua S., Liu M. Identification of a prostate-specific G-protein coupled receptor in prostate cancer. Oncogene. (2001);20:5903–5907. doi: 10.1038/sj.onc.1204803. [DOI] [PubMed] [Google Scholar]
- 63.Zhang X., Bedigian A. V., Wang W., Eggert U. S. G protein-coupled receptors participate in cytokinesis. Cytoskeleton (Hoboken) (2012);69:810–818. doi: 10.1002/cm.21055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Griffin C. A., Kafadar K. A., Pavlath G. K. MOR23 promotes muscle regeneration and regulates cell adhesion and migration. Dev. Cell. (2009);17:649–661. doi: 10.1016/j.devcel.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chess A., Simon I., Cedar H., Axel R. Allelic inactivation regulates olfactory receptor gene expression. Cell. (1994);78:823–834. doi: 10.1016/S0092-8674(94)90562-2. [DOI] [PubMed] [Google Scholar]
- 66.Ressler K. J., Sullivan S. L., Buck L. B. Information coding in the olfactory system: evidence for a stereotyped and highly organized epitope map in the olfactory bulb. Cell. (1994);79:1245–1255. doi: 10.1016/0092-8674(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 67.Volz A., Ehlers A., Younger R., Forbes S., Trowsdale J., Schnorr D., Beck S., Ziegler A. Complex transcription and splicing of odorant receptor genes. J. Biol. Chem. (2003);278:19691–19701. doi: 10.1074/jbc.M212424200. [DOI] [PubMed] [Google Scholar]
- 68.Kleene S. J. Limits of calcium clearance by plasma membrane calcium ATPase in olfactory cilia. PLoS One. (2009);4:e5266. doi: 10.1371/journal.pone.0005266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spehr M., Schwane K., Riffell J. A., Barbour J., Zimmer R. K., Neuhaus E. M., Hatt H. Particulate adenylate cyclase plays a key role in human sperm olfactory receptor-mediated chemotaxis. J. Biol. Chem. (2004);279:40194–40203. doi: 10.1074/jbc.M403913200. [DOI] [PubMed] [Google Scholar]
- 70.Saito H., Chi Q., Zhuang H., Matsunami H., Mainland J. D. Odor coding by a Mammalian receptor repertoire. Sci. Signal. (2009);2:ra9. doi: 10.1126/scisignal.2000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nara K., Saraiva L. R., Ye X., Buck L. B. A large-scale analysis of odor coding in the olfactory epithelium. J. Neurosci. (2011);31:9179–9191. doi: 10.1523/JNEUROSCI.1282-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sanz G., Schlegel C., Pernollet J. C., Briand L. Comparison of odorant specificity of two human olfactory receptors from different phylogenetic classes and evidence for antagonism. Chem. Senses. (2005);30:69–80. doi: 10.1093/chemse/bji002. [DOI] [PubMed] [Google Scholar]
- 73.Matarazzo V., Clot-Faybesse O., Marcet B., Guiraudie-Capraz G., Atanasova B., Devauchelle G., Cerutti M., Etievant P., Ronin C. Functional characterization of two human olfactory receptors expressed in the baculovirus Sf9 insect cell system. Chem. Senses. (2005);30:195–207. doi: 10.1093/chemse/bji015. [DOI] [PubMed] [Google Scholar]
- 74.Saito H., Kubota M., Roberts R. W., Chi Q., Matsunami H. RTP family members induce functional expression of mammalian odorant receptors. Cell. (2004);119:679–691. doi: 10.1016/j.cell.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 75.Wu L., Pan Y., Chen G. Q., Matsunami H., Zhuang H. Receptor-transporting protein 1 short (RTP1S) mediates translocation and activation of odorant receptors by acting through multiple steps. J. Biol. Chem. (2012);287:22287–22294. doi: 10.1074/jbc.M112.345884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Von Dannecker L. E., Mercadante A. F., Malnic B. Ric-8B promotes functional expression of odorant receptors. Proc. Natl. Acad. Sci. U.S.A. (2006);103:9310–9314. doi: 10.1073/pnas.0600697103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Von Dannecker L. E., Mercadante A. F., Malnic B. Ric-8B, an olfactory putative GTP exchange factor, amplifies signal transduction through the olfactory-specific G-protein Galphaolf. J. Neurosci. (2005);25:3793–3800. doi: 10.1523/JNEUROSCI.4595-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhuang H., Matsunami H. Synergism of accessory factors in functional expression of mammalian odorant receptors. J. Biol. Chem. (2007);282:15284–15293. doi: 10.1074/jbc.M700386200. [DOI] [PubMed] [Google Scholar]
- 79.Koo J. H., Saraswati M., Margolis F. L. Immunolocalization of Bex protein in the mouse brain and olfactory system. J. Comp. Neurol. (2005);487:1–14. doi: 10.1002/cne.20486. [DOI] [PubMed] [Google Scholar]
- 80.Behrens M., Bartelt J., Reichling C., Winnig M., Kuhn C., Meyerhof W. Members of RTP and REEP gene families influence functional bitter taste receptor expression. J. Biol. Chem. (2006);281:20650–20659. doi: 10.1074/jbc.M513637200. [DOI] [PubMed] [Google Scholar]
- 81.Wu C., Orozco C., Boyer J., Leglise M., Goodale J., Batalov S., Hodge C. L., Haase J., Janes J., Huss J. W. 3rd, Su A. I. BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol. (2009);10:R130. doi: 10.1186/gb-2009-10-11-r130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Raming K., Konzelmann S., Breer H. Identification of a novel G-protein coupled receptor expressed in distinct brain regions and a defined olfactory zone. Receptors Channels. (1998);6:141–151. [PubMed] [Google Scholar]


