Abstract
Human Ly-6/uPAR molecules are a superfamily composed of two subfamilies; one is the membrane bound proteins with a GPI-anchor and the other are secreted proteins without the GPI-anchor. Ly-6/uPAR molecules have remarkable amino acid homology through a distinctive 8-10 cysteine-rich domain that is associated predominantly with O-linked glycans. These molecules are encoded by multiple tightly linked genes located on Chr. 8q23, and have a conserved genomic organization. Ly-6/uPAR molecules have an interesting expression pattern during hematopoiesis and on specific tumors indicating that Ly-6/uPAR molecules are associated with development of the immune system and carcinogenesis. Thus, Ly-6/uPAR molecules are useful antigens for diagnostic and therapeutic targets. This review summarizes our understanding of human Ly-6/uPAR molecules with regard to molecular structure as well as what is known about their function in normal and malignant tissues and suggest Ly-6/uPAR molecules as target antigens for cancer immunotherapy. [BMB Reports 2012; 45(11): 595-603]
Keywords: Cancer metastasis, Cancer therapy, Gene family, Hematopoiesis, Immune system, Tumorigenesis
INTRODUCTION
Ly-6 molecules were initially identified in mice as lymphocyte differentiation antigens (1). Ly-6 molecules are encoded by multiple tightly linked genes located on mouse chromosome 15 and have conserved genomic organization (2). Murine Ly-6 molecules are a set of structurally related and distinct collection of low molecular weight GPI-anchored cell surface glycoproteins such as Ly6a (Sca-1), Ly6b, Ly6c1, Ly6d (Thb), Ly6e (Sca-2), Ly6f, Ly6g, Ly6h, Ly6i, Ly6k, and TSA-1 antigens and are characterized by leukocyte expression (3). Secreted Ly-6 molecules without a GPI-anchoring sequence have been identified in mouse seminal vesicles (4). Murine Ly-6 molecules are expressed on fully differentiated cells in specific compartments of peripheral lymphoid tissues including the spleen, lymph nodes, and peripheral blood but limited expression occurs on non-lymphoid tissues (5). The binding of an anti-Ly-6 monoclonal antibody triggers cellular activation associated with T-cell activation, lymphoblast transformation, and signal transduction through the TcR-CD3 complex (3, 5).
The CD59 human Ly-6/uPAR molecule was first identified in human lymphoid cells (6). Since 1989, several human Ly-6/uPAR family members exhibiting varying degrees of similarity to murine Ly-6 molecules have been identified. To date, a total 20 human Ly-6/uPAR proteins, ranging from 11 to 36 kDa, have been identified and classified into transmembrane protein and secretory protein subfamilies according to the GPIanchored signaling sequence. We review the structure and function of human Ly-6/uPAR molecules in detail as well as their clinical value as biomarkers and molecular targets for cancer therapy.
CHARACTERIZATION OF HUMAN Ly-6/uPAR MOLECULES
A cluster of genes has been mapped to human chromosome 8 for many members of the human Ly-6/uPAR superfamily, particularly 8q24.3, which is synthenic to the murine Ly6 locus, the E band on mouse chromosome 15 (2). Other genes encoding the human Ly-6/uPAR superfamily members are localized on human chromosome 19q13 (uPAR, LYPD5, SAMP14, and LYPD3), chromosome 2q21-23 (LYPD1, LYPD6, and LYPD7), chromosome 11 (CD59 and SP-10), and chromosome 7 (CD177).
The Ly-6/uPAR superfamily of molecules is a single chain that has one or more LU domains. Ly-6/uPAR molecules have two distinct regions including: (i) a -NH2 terminal polypeptide, which contains a consensus leader sequence, Leu(Val/Ala) LeuLeuLeu(Ala/Val) Leu(Ala/Val) within a signal peptide 11-30 amino acids long that are destined towards the secretory pathway; and (ii) a peptide that forms a conserved 8-10 cysteine residue, hydrophilic LU domain. Ly-6/uPAR can be classified into two subfamilies based on the theoretical absence or presence of the GPI-anchor domain: one represents transmembrane proteins and the other represents secretory proteins without a GPI anchor. The transmembrane proteins of the Ly-6/uPAR superfamily have a-COOH terminal polypeptide that contains a consensus signal sequence located within 8–10 amino acids of the −NH2 terminal of a hydrophobic domain, which is required for cleavage of the -COOH peptide during phosphatidyl inositol anchor biosynthesis.
Cell surface expression of CD59, GHPIHBP1, GML, LY-6D, LY-6E, LY-6H, LY-6K, LYPD1, LYPD2, LYPD5, and PSCA and the secretion of SLURP1, SLURP2, SAMP14, and SP-10 is characterized by a single LU domain containing cysteine residues that have remarkable conservation within the mature peptide (Fig. 1). Internal disulfide bonds form between the sulfur atoms of two cysteine residues within the LU domain. In contrast to other cell surface proteins, CD177 and LYPD3 and uPAR contain two and three contiguous copies of the LU domain, respectively. LYPD6 and LYPD7 (also known as LYPD6B) have a LU domain but it contains 12 cysteine residues within the mature peptide. Cysteine residues are numbered in the context of the amino acid sequence of the Ly-6/uPAR superfamily molecules; equal spacing is observed between Cys3 and Cys6, Cys25 and Cys37, and Cys96 and Cys102 in CD59, GML, LY-6D, LY-6E, LY-6H, LY-6K, LYPD2, LYPD5, PSCA, SLURP1, SLURP2, LYPD3.1, uPAR(1-3), LYPD6, and LYPD7 amino acid sequences, although the GHPIHBP1, LYPD1, LYPD3.2 and SAMP14 sequences have different cysteine spacing between Cys25 and Cys37. Heterogeneity in cysteine residue spacing, which is observed between Cys6 and Cys15, Cys15 and Cys25, Cys37 and Cys62, Cys62 and Cys68, Cys68 and Cys96 in Ly-6/uPAR superfamily molecules, may impart specific structural epitopes.
Fig. 1. Amino acid sequence analysis of human Ly-6/uPAR molecules. The amino acid sequences of human Ly-6/uPAR superfamilies, from Leu1 to Asn103, were aligned with other sequences for comparison. Conserved cysteine residues are represented * and the boxes show amino acid identity.
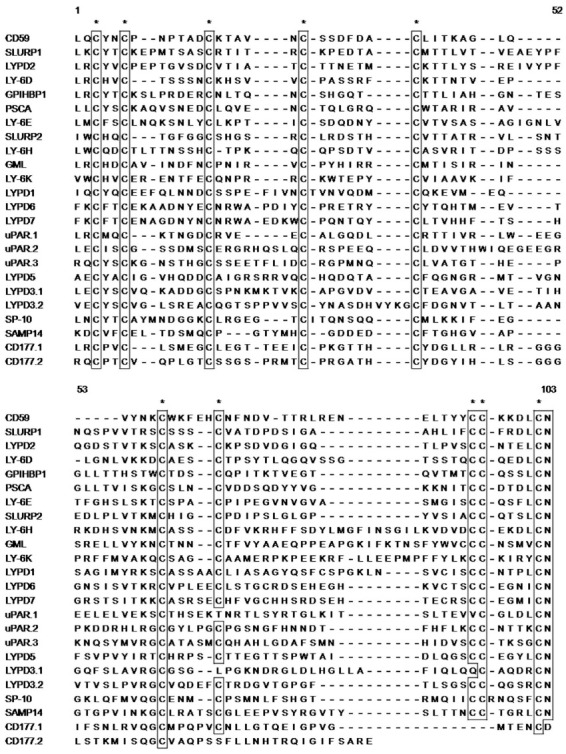
Other obvious amino acid identity and homology regions have been observed (Fig. 1). Many Ly-6 family genes have a highly conserved sequence located at the -NH2 terminus; a LeuXxxCysXxxXxxCys motif at the -COOH terminus; a CysCysXxxXxxXxxXxxCys Asn motif is found in almost Ly-6/uPAR related amino acid sequence except for SP-10, which has a CysCysXxxXxxXxxXxxXxxCysAsn motif, whereas CD177 does not.
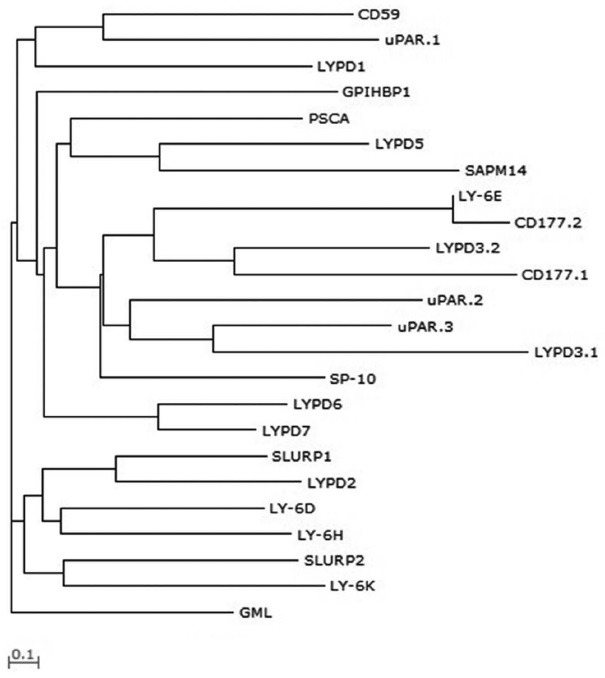
A three-dimensional crystal and solution structure of α-bungarotoxin was the first murine Ly-6/uPAR family structural model, and the amino acid sequence of the predicted β-sheet is shared between snake toxins and murine Ly-6 (7). The structural confirmation of α-bungarotoxin is maintained by internal disulfide bonds between the first and fifth, second and third, fourth and sixth, seventh and eighth, and ninth and tenth cysteine residues. Similarly, Ly-6/uPAR molecule structure can be predicted. The evolutionary relationship among the Ly-6/uPAR protein family was clarified by constructing a phylogenetic tree and Ly-6/uPAR superfamily amino acid sequences from Leu1 to Asn103 (Fig. 2). This analysis showed early divergence of the GML amino acid sequence, and a progenitor sequence of the other formed a common ancestral sequence.
Fig. 2. Phylogenetic tree of the human Ly-6/uPAR superfamily of molecules constructed based on the amino acid sequence distance method for Ly-6/uPAR superfamilies from Leu1 to Asn103, using the neighbor-joining algorithm (99) and the WAG alpha substitution model (100). Scale bar indicates branch length.
FUNCTIONAL ANALYSIS OF Ly-6/uPAR MOLECULES
CD59
The plasma membrane glycoprotein CD59 (protectin) was the first member of Ly-6/uPAR sueprfamily found in humans (6). Complement regulatory protein CD59 functions as a major protective element against C5b-9, thereby blocking assembly of the membrane attack complex for human complementmediated cell lysis (8,9). Second, CD59 induces T lymphocyte activation and participates in regulating the immune response (10). Third, CD59 acts as a CD2 complex ligand and activates T cells by secreting the cytokines interleukin (IL)-1 alpha, IL-6, and granulocyte-macrophage colony-stimulating factor (11). Fourth, CD 59 functions as a regulator of tumor cell growth and apoptosis; overexpressed CD59 induces proliferation and reduces anti-apoptotic Bcl-2 expression in MCF7 cells (12). These results suggest that CD59 may be a promising target for cancer immunotherapy.
CD177
CD177 (human neutrophil antigen-2a, NB1 glycoprotein, or polycythemia rubra vera 1) is a hematopoietic cell surface marker that is exclusively expressed on neutrophils (13). CD177 regulates neutrophil migration by binding with CD31 (platelet endothelial cell adhesion molecule-1) as a heterophilic binding partner of CD177 and induces the cytokine signaling cascade including thrombopoietin and IL-3-induced proliferation, and proteinase 3 expression, which is target antigen of anti-neutrophil cytoplasm auto-antibodies (14-16). These data indicate that regulating CD177 expression may be a novel strategy for cancer therapy by regulating the immune response.
GPIHBP1
GPIHBP1 is expressed exclusively in capillary endothelial cells along the capillary lumen and is highly expressed in heart and brown adipose tissue, which are crucial for processing of triglyceride-rich lipoproteins (17,18). These GPIHBP1 expression patterns suggest that GPIHBP1 serves as a lipoprotein lipase binding site within capillaries and may play a particularly important role in lipoprotein metabolism.
LY-6 molecules
LY-6D (E48 antigen) is expressed exclusively on normal squamous epithelia and transitional epithelium and their malignant counterparts (19). LY-6D is an effective target antigen for diagnosis and therapeutic exploitation in the management of patients with head and neck squamous cell carcinoma (HNSCC) and micrometastases to lymph nodes from HNSCC (20,21).
LY-6E (RIG-E, stem cell antigen 2, and thymic shared antigen) are expressed in squamous cells, peripheral blood, and bone marrow cells. LY-6E plays an important role in T-cell differentiation, activation of the T-cell receptor signaling pathway, and proliferation and differentiation of T2ECs (transforming growth factor [TGF]-α and TGF-β induced erythrocytic cells) that self-renew primary avian erythroid progenitors (22-24). Interestingly, LY-6E is highly expressed in various human cancers, such as colon cancer and malignant kidney cancer (25). Thus, LY-6E affects tumorigenesis by changing cell proliferation and differentiation.
LY-6H is highly expressed in the human brain, such as the cerebral cortex and also in acute lymphoblastic leukemia cells; thus, LY-6H might play an important role in both the central nervous (CNS) and immune systems (26).
LY-6K, as a cancer-testis antigen, was initially identified as a HNSCC diagnostic and therapeutic target antigen similar to LY-6D (27). Elevated LY-6K is a serologic diagnostic biomarker and therapeutic target for breast cancer, lung and esophageal carcinomas, bladder cancer, and esophageal squamous cell carcinoma (ESCC) (28-31). Induced LY-6K regulates cell growth, invasion, and migration of lung, esophageal, breast, and bladder cancer cell lines (29,30,32). Additionally, LY-6K is an immunotherapeutic target of cancer vaccine therapies because LY-6K stimulates cytotoxic T lymphocytes that have specific cytotoxic activity against ESCC cells that endogenously express LY-6K (33). Taken together, these results suggest that inhibiting LY-6K expression may be a promising target for control of these cancers and that targeting LY-6K may be a novel cancer therapy strategy.
LYPD molecules
LYPD1 (putative tumor suppressor) acts as a putative tumor suppressor in the Hela cell p53-independent pathway (34). The LYPD1 gene is highly expressed throughout the CNS, such as the cerebellum, cortex, pons, hippocampus, hypothalamus, striatum, amygdale, and septum based on quantitative reverse transcription-polymerase chain reaction and in situ hybridization analyses (35). Therefore, LYPD1 may play important roles in both tumorigenesis and the CNS.
LYPD3, also known as metastasis-associated membrane protein C4.4A, has been observed in placental tissue, skin, esophagus, and peripheral blood leukocytes (36). Human LYPD3, a uPAR structural homologue, has been detected in cancer cell lines, including melanoma, breast, bladder, and renal cell carcinoma, as well as in tumor tissue samples from malignant melanoma, metastatic breast cancer, tumor budding, and the epithelial-mesenchymal transition of colorectal cancer, non-small cell lung carcinoma with a poor prognosis, invasion and metastasis of esophageal squamous cell carcinoma, and urothelial tumors (37-42). Wound healing and metastasis promoted by LYPD3 is associated with α(6)β(4) integrin and MT1-MMP1 (43). These findings indicate that LYPD3 may also be a cancer therapy target.
LYPD6 is ubiquitously expressed in human tissues but highly expressed in brain and heart and localized in the cytoplasm where it suppresses activator protein-1 (AP-1)-mediated transcriptional activation (44).
LYPD7 is also known as LYPD6B and is highly expressed in testis, lung, stomach, and prostate, localized in the cytoplasm and activates AP-1 and phorbol myristate actetate-mediated transcriptional activation (45). Further studies are needed to gain a better understanding of the role of LYPD under normal conditions and diseases.
PSCA
Previous studies have reported that PSCA expression is upregulated in prostate cancer, pancreatic adenocarcinoma, transitional cell carcinoma, clear cell renal cell carcinoma, diffuse-type gastric cancer, endometrial adenocarcinoma, non small cell lung cancer, cervical squamous cell carcinoma, invasive micropapillary carcinoma, and glioma (46-55). In contrast, PSCA is downregulated in esophageal, gastric, stomach tumors, HNSCC, and gallbladder carcinogenesis (27,56,57). PSCA genetic variations affect susceptibility to diffuse-type gastric cancer, urinary bladder cancer, stomach cancer, and breast cancer (50,58-60). Additionally, PSCA regulates cell growth through the p53-related or immune signaling pathways, adhesion of epithelial cells, and tumor migration and metastasis (61-64). Therefore, PSCA expression may be a diagnostic marker and therapeutic target antigen for cancer therapy.
SAMP14
SAMP14 is localized near the uPAR shows testis-specific expression through the outer and inner acrosomal membranes as well as the acrosomal matrix. Antibodies against SAMP14 inhibit binding and fusion of human sperm to zona-free eggs (65). These results suggest that SAMP14 may regulate the sperm-egg interaction. However, further studies are needed to gain a better understanding of the role of SAMP14.
SLURP1 and SLURP-2
SLURP1 was the first secreted mammalian member of the Ly-6/uPAR protein superfamily identified without a GPI-anchoring signal sequence (66). SLURP1 and SLUPR2 have been identified in Mal de Meleda and hyperproliferative skin of patients with psoriasis vulgaris, respectively (67,68). SLURP1 regulates epidermal homeostasis, inflammation, keratinization, and programmed cell death as well as tumorigenesis of the colon, survival of fibroblasts, and T cell activation (69-72). SLURP2 plays a role not only in kerationcyte hyperproliferation and T cell differentiation/activation but is also competes with SLURP1 for the human nicotinic acetylcholine receptor (hAChR), thereby delaying keratinocyte differentiation and preventing apoptosis (68,73). Both SLURP1 and SLURP2 are efficient autocrine and paracrine ligands of keratinocyte nAChRs and activating the nAChR-mediated signaling pathways prevents malignant transformation of oral cells by regulating lymphocyte function and the immune system (74). Taken together, these results suggest that SLURP1 and SLURP2 may also be a target for cancer therapy by regulating the immune system and tumorigenesis.
SP-10
SP-10 is also known as acrosomal protein SP-10 or acrosomal vesicle protein 1 and has been identified as a potential contraceptive immunogen with germ cell specificity because a monoclonal antibody specific to SP-10 inhibits sperm penetration (75). SP-10, like the SAMP14 testis-specific acrosomal protein, is a spermatid differentiation marker localized to the developing acrosome of round and elongating spermatids and is associated with the inner and outer acrosomal membranes of most mature spermatids (76,77).
uPAR
uPAR was first identified in human blood monocytes and the histolytic lymphoma cell line U937 (78). uPAR plays an important role regulating degradation of extracellular matrix proteins and intravascular fibrinolysis on the cell surface (79). uPAR expression is overexpressed in various cancers, including colon cancer, invasive breast, melanocytic tumor progression, invasive brain tumor, squamous cell lung carcinoma, renal cell carcinoma, hepatocellular carcinoma, gastric cancer, ovarian cancer, HNSCC, oral cancer, aggressiveness lingual squamous cell carcinoma, and ductal pancreatic carcinoma (80-92). uPAR influences cellular behavior during proliferation, inflammation, wound healing repair, adhesion, angiogenesis, and metastasis by forming a complex with integrins and uPA, which activates the ERK signaling pathway (93). uPAR activation also leads to extravasation and migration of activated T lymphocytes and chemotaxis of polymophonuclear leukocytes (94,95). Interestingly, soluble uPAR is observed in ovarian cancer, multiple myeloma, breast cancer, burn-injuries, and severe sepsis and regulates uPA enzymatic activity and mobilization of hematopoietic stem cells (96-98).
CONCLUSION
The first human Ly-6/uPAR molecule was discovered relatively recently, and various Ly-6/uPAR family proteins have been identified. The studies described in this review have revealed many interesting properties of the human Ly-6/uPAR family of molecules in the context of tissue expression, molecular structure, and function. These molecules share remarkable amino acid homology in terms of conserved cysteine residues, which are crucial for the conformation of the Ly-6/uPAR domain. Some of the Ly-6/uPAR encoding tumor-specific antigens and their recognition by tumor specific cytotoxicity T lymphocytes have opened new avenues for developing effective anticancer immunotherapies. The Ly-6/uPAR members are considered promising candidates for cancer immunotherapy because they are strictly tumor specific and are shared by many kinds of tumors. The Ly-6/uPAR molecules are considered not only cancer vaccine targets but also target antigens for antibody-based immunotherapy. Other Ly-6/uPAR molecules such as SAMP14 and SP-10 are associated with testis-specific expression, and their function in tumorigenesis is unknown. Many aspects of the Ly-6/uPAR family are not fully understood, and future studies in the context of: (i) the relationship between the regulation of human Ly-6/uPAR expression in hematopoiesis and peripheral tissues to cell development and function in vivo and (ii) understanding the mechanisms of tumorigenesis or migration will allow insight into the functional roles of Ly-6/uPAR molecules in vivo.
Acknowledgments
This research was supported by the Sookmyung Women’s University Research Grants 2010.
References
- 1.McKenzie I. F., Gardiner J., Cherry M., Snell G. D. Lymphocyte antigens: Ly-4, Ly-6, and Ly-7. Transplant Proc. (1977);9:667–669. [PubMed] [Google Scholar]
- 2.LeClair K. P., Rabin M., Nesbitt M. N., Pravtcheva D., Ruddle F. H., Palfree R. G., Bothwell A. Murine Ly-6 multigene family is located on chromosome 15. Proc. Natl. Acad. Sci. U.S.A. (1987);84:1638–1642. doi: 10.1073/pnas.84.6.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gumley T. P., McKenzie I. F., Sandrin M. S. Tissue expression, structure and function of the murine Ly-6 family of molecules. Immunol. Cell. Biol. (1995);73:277–296. doi: 10.1038/icb.1995.45. [DOI] [PubMed] [Google Scholar]
- 4.Li S. H., Lee R. K., Lin M. H., Hwu Y. M., Lu C. H., Chen Y. J., Chen H. C., Chang W. H., Chang W. C. SSLP-1, a secreted Ly-6 protein purified from mouse seminal vesicle fluid. Reproduction. (2006);132:493–500. doi: 10.1530/rep.1.01183. [DOI] [PubMed] [Google Scholar]
- 5.Havran W. L., Lancki D. W., Moldwin R. L., Dialynas D. P., Fitch F. W. Characterization of an anti-Ly-6 monoclonal antibody which defines and activates cytolytic T lymphocytes. J. Immunol. (1988);140:1034–1042. [PubMed] [Google Scholar]
- 6.Davies A., Simmons D. L., Hale G., Harrison R. A., Tighe H., Lachmann P. J., Waldmann H. CD59, an LY-6-like protein expressed in human lymphoid cells, regulates the action of the complement membrane attack complex on homologous cells. J. Exp. Med. (1989);170:637–654. doi: 10.1084/jem.170.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleming T. J., O'HUigin C., Malek T. R. Characterization of two novel Ly-6 genes. Protein sequence and potential structural similarity to alpha-bungarotoxin and other neurotoxins. J. Immunol. (1993);150:5379–5390. [PubMed] [Google Scholar]
- 8.Meri S., Morgan B. P., Davies A., Daniels R. H., Olavesen M. G., Waldmann H., Lachmann P. J. Human protectin (CD59), an 18,000-20,000 MW complement lysis restricting factor, inhibits C5b-8 catalysed insertion of C9 into lipid bilayers. Immunology. (1990);71:1–9. [PMC free article] [PubMed] [Google Scholar]
- 9.Chang C. P., Husler T., Zhao J., Wiedmer T., Sims P. J. Identity of a peptide domain of human C9 that is bound by the cell-surface complement inhibitor, CD59. J. Biol. Chem. (1994);269:26424–26430. [PubMed] [Google Scholar]
- 10.Treon S. P., Shima Y., Grossbard M. L., Preffer F. I., Belch A. R., Pilarski L. M., Anderson K. C. Treatment of multiple myeloma by antibody mediated immunotherapy and induction of myeloma selective antigens. Ann. Oncol. (2000);11(Suppl 1):107–111. doi: 10.1093/annonc/11.suppl_1.S107. [DOI] [PubMed] [Google Scholar]
- 11.Naderi S., Hofmann P., Seiter S., Tilgen W., Abken H., Reinhold U. CD2-mediated CD59 stimulation in keratinocytes results in secretion of IL-1alpha, IL-6, and GM-CSF: implications for the interaction of keratinocytes with intraepidermal T lymphocytes. Int. J. Mol. Med. (1999);3:609–614. doi: 10.3892/ijmm.3.6.609. [DOI] [PubMed] [Google Scholar]
- 12.Li B., Chu X., Gao M., Xu Y. The effects of CD59 gene as a target gene on breast cancer cells. Cell Immunol. (2011);272:61–70. doi: 10.1016/j.cellimm.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Temerinac S., Klippel S., Strunck E., Roder S., Lubbert M., Lange W., Azemar M., Meinhardt G., Schaefer H. E., Pahl H. L. Cloning of PRV-1, a novel member of the uPAR receptor superfamily, which is overexpressed in polycythemia rubra vera. Blood. (2000);95:2569–2576. [PubMed] [Google Scholar]
- 14.Sachs U. J., Andrei-Selmer C. L., Maniar A., Weiss T., Paddock C., Orlova V. V., Choi E. Y., Newman P. J., Preissner K. T., Chavakis T., Santoso S. The neutrophil-specific antigen CD177 is a counter-receptor for platelet endothelial cell adhesion molecule-1 (CD31). J. Biol. Chem. (2007);282:23603–23612. doi: 10.1074/jbc.M701120200. [DOI] [PubMed] [Google Scholar]
- 15.Dillon M., Minear J., Johnson J., Lannutti B. J. Expression of the GPI-anchored receptor Prv-1 enhances thrombopoietin and IL-3-induced proliferation in hematopoietic cell lines. Leuk Res. (2008);32:811–819. doi: 10.1016/j.leukres.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 16.Korkmaz B., Kuhl A., Bayat B., Santoso S., Jenne D. E. A hydrophobic patch on proteinase 3, the target of autoantibodies in Wegener granulomatosis, mediates membrane binding via NB1 receptors. J. Biol. Chem. (2008);283:35976–35982. doi: 10.1074/jbc.M806754200. [DOI] [PubMed] [Google Scholar]
- 17.Beigneux A. P., Davies B. S., Gin P., Weinstein M. M., Farber E., Qiao X., Peale F., Bunting S., Walzem R. L., Wong J. S., Blaner W. S., Ding Z. M., Melford K., Wongsiriroj N., Shu X., de Sauvage F., Ryan R. O., Fong L. G., Bensadoun A., Young S. G. Glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 plays a critical role in the lipolytic processing of chylomicrons. Cell Metab. (2007);5:279–291. doi: 10.1016/j.cmet.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davies B. S., Beigneux A. P., Barnes R. H. 2nd, Tu Y., Gin P., Weinstein M. M., Nobumori C., Nyren R., Goldberg I., Olivecrona G., Bensadoun A., Young S. G., Fong L. G. GPIHBP1 is responsible for the entry of lipoprotein lipase into capillaries. Cell Metab. (2010);12:42–52. doi: 10.1016/j.cmet.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quak J. J., Balm A. J., van Dongen G. A., Brakkee J. G., Scheper R. J., Snow G. B., Meijer C. J. A 22-kd surface antigen detected by monoclonal antibody E 48 is exclusively expressed in stratified squamous and transitional epithelia. Am. J. Pathol. (1990);136:191–197. [PMC free article] [PubMed] [Google Scholar]
- 20.Bartelink H., Keus R. B. Progress in radiotherapy by treatment tailoring in head and neck cancer. Curr. Opin. Oncol. (1991);3:523–528. doi: 10.1097/00001622-199106000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Nieuwenhuis E. J., Leemans C. R., Kummer J. A., Denkers F., Snow G. B., Brakenhoff R. H. Assessment and clinical significance of micrometastases in lymph nodes of head and neck cancer patients detected by E48 (Ly-6D) quantitative reverse transcription-polymerase chain reaction. Lab. Invest. (2003);83:1233–1240. doi: 10.1097/01.LAB.0000083532.46536.56. [DOI] [PubMed] [Google Scholar]
- 22.Antica M., Wu L., Scollay R. Stem cell antigen 2 expression in adult and developing mice. Immunol. Lett. (1997);55:47–51. doi: 10.1016/S0165-2478(96)02682-X. [DOI] [PubMed] [Google Scholar]
- 23.Noda S., Kosugi A., Saitoh S., Narumiya S., Hamaoka T. Protection from anti-TCR/CD3-induced apoptosis in immature thymocytes by a signal through thymic shared antigen-1/stem cell antigen-2. J. Exp. Med. (1996);183:2355–2360. doi: 10.1084/jem.183.5.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saitoh S., Kosugi A., Noda S., Yamamoto N., Ogata M., Minami Y., Miyake K., Hamaoka T. Modulation of TCR-mediated signaling pathway by thymic shared antigen-1 (TSA-1)/stem cell antigen-2 (Sca-2). J. Immunol. (1995);155:5574–5581. [PubMed] [Google Scholar]
- 25.Bresson-Mazet C., Gandrillon O., Gonin-Giraud S. Stem cell antigen 2: a new gene involved in the self-renewal of erythroid progenitors. Cell Prolif. (2008);41:726–738. doi: 10.1111/j.1365-2184.2008.00554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horie M., Okutomi K., Taniguchi Y., Ohbuchi Y., Suzuki M., Takahashi E. Isolation and characterization of a new member of the human Ly6 gene family (LY6H). Genomics. (1998);53:365–368. doi: 10.1006/geno.1998.5462. [DOI] [PubMed] [Google Scholar]
- 27.de Nooij-van Dalen A. G., van Dongen G. A., Smeets S. J., Nieuwenhuis E. J., Stigter-van Walsum M., Snow G. B., Brakenhoff R. H. Characterization of the human Ly-6 antigens, the newly annotated member Ly-6K included, as molecular markers for head-and-neck squamous cell carcinoma. Int. J. Cancer. (2003);103:768–774. doi: 10.1002/ijc.10903. [DOI] [PubMed] [Google Scholar]
- 28.Lee J. W., Lee Y. S., Yoo K. H., Lee K. H., Park K., Ahn T., Ko C., Park J. H. LY-6K gene: a novel molecular marker for human breast cancer. Oncol. Rep. (2006);16:1211–1214. [PubMed] [Google Scholar]
- 29.Ishikawa N., Takano A., Yasui W., Inai K., Nishimura H., Ito H., Miyagi Y., Nakayama H., Fujita M., Hosokawa M., Tsuchiya E., Kohno N., Nakamura Y., Daigo Y. Cancer-testis antigen lymphocyte antigen 6 complex locus K is a serologic biomarker and a therapeutic target for lung and esophageal carcinomas. Cancer Res. (2007);67:11601–11611. doi: 10.1158/0008-5472.CAN-07-3243. [DOI] [PubMed] [Google Scholar]
- 30.Matsuda R., Enokida H., Chiyomaru T., Kikkawa N., Sugimoto T., Kawakami K., Tatarano S., Yoshino H., Toki K., Uchida Y., Kawahara K., Nishiyama K., Seki N., Nakagawa M. LY6K is a novel molecular target in bladder cancer on basis of integrate genome-wide profiling. Br. J. Cancer. (2011);104:376–386. doi: 10.1038/sj.bjc.6605990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang B., Zhang Z., Zhang X., Gao X., Kernstine K. H., Zhong L. Serological antibodies against LY6K as a diagnostic biomarker in esophageal squamous cell carcinoma. Biomarkers. (2012);17:372–378. doi: 10.3109/1354750X.2012.680609. [DOI] [PubMed] [Google Scholar]
- 32.Choi S. H., Kong H. K., Park S. Y., Park J. H. Metastatic effect of LY-6K gene in breast cancer cells. Int. J. Oncol. (2009);35:601–607. doi: 10.3892/ijo_00000320. [DOI] [PubMed] [Google Scholar]
- 33.Suda T., Tsunoda T., Daigo Y., Nakamura Y., Tahara H. Identification of human leukocyte antigen-A24-restricted epitope peptides derived from gene products upregulated in lung and esophageal cancers as novel targets for immunotherapy. Cancer Sci. (2007);98:1803–1808. doi: 10.1111/j.1349-7006.2007.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu D. H., Fan W., Liu G., Nguy V., Chatterton J. E., Long S., Ke N., Meyhack B., Bruengger A., Brachat A., Wong-Staal F., Li Q. X. PHTS, a novel putative tumor suppressor, is involved in the transformation reversion of HeLaHF cells independently of the p53 pathway. Exp. Cell Res. (2006);312:865–876. doi: 10.1016/j.yexcr.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Egerod K. L., Holst B., Petersen P. S., Hansen J. B., Mulder J., Hokfelt T., Schwartz T. W. GPR39 splice variants versus antisense gene LYPD1: expression and regulation in gastrointestinal tract, endocrine pancreas, liver, and white adipose tissue. Mol. Endocrinol. (2007);21:1685–1698. doi: 10.1210/me.2007-0055. [DOI] [PubMed] [Google Scholar]
- 36.Wurfel J., Seiter S., Stassar M., Claas A., Klas R., Rosel M., Marhaba R., Savelyeva L., Schwab M., Matzku S., Zoller M. Cloning of the human homologue of the metastasis-associated rat C4.4A. Gene. (2001);262:35–41. doi: 10.1016/S0378-1119(00)00515-1. [DOI] [PubMed] [Google Scholar]
- 37.Seiter S., Stassar M., Rappl G., Reinhold U., Tilgen W., Zoller M. Upregulation of C4.4A expression during progression of melanoma. J. Invest. Dermatol. (2001);116:344–347. doi: 10.1046/j.1523-1747.2001.01230.x. [DOI] [PubMed] [Google Scholar]
- 38.Fletcher G. C., Patel S., Tyson K., Adam P. J., Schenker M., Loader J. A., Daviet L., Legrain P., Parekh R., Harris A. L., Terrett J. A. hAG-2 and hAG-3, human homologues of genes involved in differentiation, are associated with oestrogen receptor-positive breast tumours and interact with metastasis gene C4.4a and dystroglycan. Br. J. Cancer. (2003);88:579–585. doi: 10.1038/sj.bjc.6600740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paret C., Hildebrand D., Weitz J., Kopp-Schneider A., Kuhn A., Beer A., Hautmann R., Zoller M. C4.4A as a candidate marker in the diagnosis of colorectal cancer. Br. J. Cancer. (2007);97:1146–1156. doi: 10.1038/sj.bjc.6604012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hansen L. V., Skov B. G., Ploug M., Pappot H. Tumour cell expression of C4.4A, a structural homologue of the urokinase receptor, correlates with poor prognosis in non-small cell lung cancer. Lung Cancer. (2007);58:260–266. doi: 10.1016/j.lungcan.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 41.Hansen L. V., Laerum O. D., Illemann M., Nielsen B. S., Ploug M. Altered expression of the urokinase receptor homologue, C4.4A, in invasive areas of human esophageal squamous cell carcinoma. Int. J. Cancer. (2008);122:734–741. doi: 10.1002/ijc.23082. [DOI] [PubMed] [Google Scholar]
- 42.Oshiro R., Yamamoto H., Takahashi H., Ohtsuka M., Wu X., Nishimura J., Takemasa I., Mizushima T., Ikeda M., Sekimoto M., Matsuura N., Doki Y., Mori M. C4.4A is associated with tumor budding and epithelial-mesenchymal transition of colorectal cancer. Cancer Sci. (2012);103:1155–1164. doi: 10.1111/j.1349-7006.2012.02263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ngora H., Galli U. M., Miyazaki K., Zoller M. Membrane-bound and exosomal metastasis-associated C4.4A promotes migration by associating with the alpha(6)beta(4) integrin and MT1-MMP. Neoplasia. (2012);14:95–107. doi: 10.1593/neo.111450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y., Lang Q., Li J., Xie F., Wan B., Yu L. Identification and characterization of human LYPD6, a new member of the Ly-6 superfamily. Mol. Biol. Rep. (2010);37:2055–2062. doi: 10.1007/s11033-009-9663-7. [DOI] [PubMed] [Google Scholar]
- 45.Ni J., Lang Q., Bai M., Zhong C., Chen X., Wan B., Yu L. Cloning and characterization of a human LYPD7, a new member of the Ly-6 superfamily. Mol. Biol. Rep. (2009);36:697–703. doi: 10.1007/s11033-008-9231-6. [DOI] [PubMed] [Google Scholar]
- 46.Reiter R. E., Gu Z., Watabe T., Thomas G., Szigeti K., Davis E., Wahl M., Nisitani S., Yamashiro J., Le Beau M. M., Loda M., Witte O. N. Prostate stem cell antigen: a cell surface marker overexpressed in prostate cancer. Proc. Natl. Acad. Sci. U.S.A. (1998);95:1735–1740. doi: 10.1073/pnas.95.4.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Argani P., Rosty C., Reiter R. E., Wilentz R. E., Murugesan S. R., Leach S. D., Ryu B., Skinner H. G., Goggins M., Jaffee E. M., Yeo C. J., Cameron J. L., Kern S. E., Hruban R. H. Discovery of new markers of cancer through serial analysis of gene expression: prostate stem cell antigen is overexpressed in pancreatic adenocarcinoma. Cancer Res. (2001);61:4320–4324. [PubMed] [Google Scholar]
- 48.Amara N., Palapattu G. S., Schrage M., Gu Z., Thomas G. V., Dorey F., Said J., Reiter R. E. Prostate stem cell antigen is overexpressed in human transitional cell carcinoma. Cancer Res. (2001);61:4660–4665. [PubMed] [Google Scholar]
- 49.Elsamman E. M., Fukumori T., Tanimoto S., Nakanishi R., Takahashi M., Toida K., Kanayama H. O. The expression of prostate stem cell antigen in human clear cell renal cell carcinoma: a quantitative reverse transcriptase-polymerase chain reaction analysis. BJU Int. (2006);98:668–673. doi: 10.1111/j.1464-410X.2006.06350.x. [DOI] [PubMed] [Google Scholar]
- 50.Sakamoto H., Yoshimura K., Saeki N., Katai H., Shimoda T., Matsuno Y., Saito D., Sugimura H., Tanioka F., Kato S., Matsukura N., Matsuda N., Nakamura T., Hyodo I., Nishina T., Yasui W., Hirose H., Hayashi M., Toshiro E., Ohnami S., Sekine A., Sato Y., Totsuka H., Ando M., Takemura R., Takahashi Y., Ohdaira M., Aoki K., Honmyo I., Chiku S., Aoyagi K., Sasaki H., Yanagihara K., Yoon K. A., Kook M. C., Lee Y. S., Park S. R., Kim C. G., Choi I. J., Yoshida T., Nakamura Y., Hirohashi S. Genetic variation in PSCA is associated with susceptibility to diffuse-type gastric cancer. Nat. Genet. (2008);40:730–740. doi: 10.1038/ng.152. [DOI] [PubMed] [Google Scholar]
- 51.Liu W. K., Jiang X. Y., Zhang Z. X. Expression of PSCA, PIWIL1, and TBX2 in endometrial adenocarcinoma. Onkologie. (2010);33:241–245. doi: 10.1159/000305098. [DOI] [PubMed] [Google Scholar]
- 52.Kawaguchi T., Sho M., Tojo T., Yamato I., Nomi T., Hotta K., Hamada K., Suzaki Y., Sugiura S., Kushibe K., Nakajima Y., Taniguchi S. Clinical significance of prostate stem cell antigen expression in non-small cell lung cancer. Jpn. J. Clin. Oncol. (2010);40:319–326. doi: 10.1093/jjco/hyp181. [DOI] [PubMed] [Google Scholar]
- 53.Liu W. K., Jiang X. Y., Zhang Z. X. Expression of PSCA, PIWIL1 and TBX2 and its correlation with HPV16 infection in formalin-fixed, paraffin-embedded cervical squamous cell carcinoma specimens. Arch. Virol. (2010);155:657–663. doi: 10.1007/s00705-010-0635-y. [DOI] [PubMed] [Google Scholar]
- 54.Hao J. Y., Yang Y. L., Li S., Qian X. L., Liu F. F., Fu L. PSCA expression in invasive micropapillary carcinoma of breast. Zhonghua Bing Li Xue Za Zhi. (2011);40:382–386. [PubMed] [Google Scholar]
- 55.Geiger K. D., Hendruschk S., Rieber E. P., Morgenroth A., Weigle B., Juratli T., Senner V., Schackert G., Temme A. The prostate stem cell antigen represents a novel glioma-associated antigen. Oncol Rep. (2011);26:13–21. doi: 10.3892/or.2011.1265. [DOI] [PubMed] [Google Scholar]
- 56.Bahrenberg G., Brauers A., Joost H. G., Jakse G. Reduced expression of PSCA, a member of the LY-6 family of cell surface antigens, in bladder, esophagus, and stomach tumors. Biochem. Biophys. Res. Commun. (2000);275:783–788. doi: 10.1006/bbrc.2000.3393. [DOI] [PubMed] [Google Scholar]
- 57.Ono H., Hiraoka N., Lee Y. S., Woo S. M., Lee W. J., Choi I. J., Saito A., Yanagihara K., Kanai Y., Ohnami S., Chiwaki F., Sasaki H., Sakamoto H., Yoshida T., Saeki N. Prostate stem cell antigen, a presumable organ-dependent tumor suppressor gene, is down-regulated in gallbladder carcinogenesis. Genes Chromosomes Cancer. (2012);51:30–41. doi: 10.1002/gcc.20928. [DOI] [PubMed] [Google Scholar]
- 58.Wu X., Ye Y., Kiemeney L. A., Sulem P., Rafnar T., Matullo G., Seminara D., Yoshida T., Saeki N., Andrew A. S., Dinney C. P., Czerniak B., Zhang Z. F., Kiltie A. E., Bishop D. T., Vineis P., Porru S., Buntinx F., Kellen E., Zeegers M. P., Kumar R., Rudnai P., Gurzau E., Koppova K., Mayordomo J. I., Sanchez M., Saez B., Lindblom A., de Verdier P., Steineck G., Mills G. B., Schned A., Guarrera S., Polidoro S., Chang S. C., Lin J., Chang D. W., Hale K. S., Majewski T., Grossman H. B., Thorlacius S., Thorsteinsdottir U., Aben K. K., Witjes J. A., Stefansson K., Amos C. I., Karagas M. R., Gu J. Genetic variation in the prostate stem cell antigen gene PSCA confers susceptibility to urinary bladder cancer. Nat. Genet. (2009);41:991–995. doi: 10.1038/ng.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matsuo K., Tajima K., Suzuki T., Kawase T., Watanabe M., Shitara K., Misawa K., Ito S., Sawaki A., Muro K., Nakamura T., Yamao K., Yamamura Y., Hamajima N., Hiraki A., Tanaka H. Association of prostate stem cell antigen gene polymorphisms with the risk of stomach cancer in Japanese. Int. J. Cancer. (2009);125:1961–1964. doi: 10.1002/ijc.24519. [DOI] [PubMed] [Google Scholar]
- 60.Kim S. Y., Yoo J. Y., Shin A., Kim Y., Lee E. S., Lee Y. S. Prostate stem cell antigen single nucleotide polymorphisms influence risk of estrogen receptor negative breast cancer in Korean females. Asian Pac. J. Cancer Prev. (2012);13:41–48. doi: 10.7314/APJCP.2012.13.1.041. [DOI] [PubMed] [Google Scholar]
- 61.Saffran D. C., Raitano A. B., Hubert R. S., Witte O. N., Reiter R. E., Jakobovits A. Anti-PSCA mAbs inhibit tumor growth and metastasis formation and prolong the survival of mice bearing human prostate cancer xenografts. Proc. Natl. Acad. Sci. U.S.A. (2001);98:2658–2663. doi: 10.1073/pnas.051624698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Feng H. C., Tsao S. W., Ngan H. Y., Xue W. C., Kwan H. S., Siu M. K., Liao X. Y., Wong E., Cheung A. N. Overexpression of prostate stem cell antigen is associated with gestational trophoblastic neoplasia. Histopathology. (2008);52:167–174. doi: 10.1111/j.1365-2559.2007.02925.x. [DOI] [PubMed] [Google Scholar]
- 63.Moore M. L., Teitell M. A., Kim Y., Watabe T., Reiter R. E., Witte O. N., Dubey P. Deletion of PSCA increases metastasis of TRAMP-induced prostate tumors without altering primary tumor formation. Prostate. (2008);68:139–151. doi: 10.1002/pros.20686. [DOI] [PubMed] [Google Scholar]
- 64.Marra E., Uva P., Viti V., Simonelli V., Dogliotti E., De Rinaldis E., Lahm A., La Monica N., Nicosia A., Ciliberto G., Palombo F. Growth delay of human bladder cancer cells by Prostate Stem Cell Antigen downregulation is associated with activation of immune signaling pathways. BMC Cancer. (2010);10:129. doi: 10.1186/1471-2407-10-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shetty J., Wolkowicz M. J., Digilio L. C., Klotz K. L., Jayes F. L., Diekman A. B., Westbrook V. A., Farris E. M., Hao Z., Coonrod S. A., Flickinger C. J., Herr J. C. SAMP14, a novel, acrosomal membrane-associated, glycosylphosphatidylinositol-anchored member of the Ly-6/urokinase-type plasminogen activator receptor superfamily with a role in sperm-egg interaction. J. Biol. Chem. (2003);278:30506–30515. doi: 10.1074/jbc.M301713200. [DOI] [PubMed] [Google Scholar]
- 66.Adermann K., Wattler F., Wattler S., Heine G., Meyer M., Forssmann W. G., Nehls M. Structural and phylogenetic characterization of human SLURP-1, the first secreted mammalian member of the Ly-6/uPAR protein superfamily. Protein. Sci. (1999);8:810–819. doi: 10.1110/ps.8.4.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fischer J., Bouadjar B., Heilig R., Huber M., Lefevre C., Jobard F., Macari F., Bakija-Konsuo A., Ait-Belkacem F., Weissenbach J., Lathrop M., Hohl D., Prud J. F. Mutations in the gene encoding SLURP-1 in Mal de Meleda. Hum. Mol. Genet. (2001);10:875–880. doi: 10.1093/hmg/10.8.875. [DOI] [PubMed] [Google Scholar]
- 68.Tsuji H., Okamoto K., Matsuzaka Y., Iizuka H., Tamiya G., Inoko H. SLURP-2, a novel member of the human Ly-6 superfamily that is up-regulated in psoriasis vulgaris. Genomics. (2003);81:26–33. doi: 10.1016/S0888-7543(02)00025-3. [DOI] [PubMed] [Google Scholar]
- 69.Arredondo J., Chernyavsky A. I., Webber R. J., Grando S. A. Biological effects of SLURP-1 on human keratinocytes. J. Invest. Dermatol. (2005);125:1236–1241. doi: 10.1111/j.0022-202X.2005.23973.x. [DOI] [PubMed] [Google Scholar]
- 70.Pettersson A., Nordlander S., Nylund G., Khorram-Manesh A., Nordgren S., Delbro D. S. Expression of the endogenous, nicotinic acetylcholine receptor ligand, SLURP-1, in human colon cancer. Auton Autacoid. Pharmacol. (2008);28:109–116. doi: 10.1111/j.1474-8673.2008.00424.x. [DOI] [PubMed] [Google Scholar]
- 71.Phan T. C., Ooi J., Goonewardene M. S. A novel molecule, SLURP-1, enhances the survival of periodontal ligament fibroblasts. J. Periodontal. Res. (2010);45:331–336. doi: 10.1111/j.1600-0765.2009.01240.x. [DOI] [PubMed] [Google Scholar]
- 72.Tjiu J. W., Lin P. J., Wu W. H., Cheng Y. P., Chiu H. C., Thong H. Y., Chiang B. L., Yang W. S., Jee S. H. SLURP1 mutation-impaired T-cell activation in a family with mal de Meleda. Br. J. Dermatol. (2011);164:47–53. doi: 10.1111/j.1365-2133.2010.10059.x. [DOI] [PubMed] [Google Scholar]
- 73.Arredondo J., Chernyavsky A. I., Jolkovsky D. L., Webber R. J., Grando S. A. SLURP-2: A novel cholinergic signaling peptide in human mucocutaneous epithelium. J. Cell. Physiol. (2006);208:238–245. doi: 10.1002/jcp.20661. [DOI] [PubMed] [Google Scholar]
- 74.Moriwaki Y., Yoshikawa K., Fukuda H., Fujii Y. X., Misawa H., Kawashima K. Immune system expression of SLURP-1 and SLURP-2, two endogenous nicotinic acetylcholine receptor ligands. Life Sci. (2007);80:2365–2368. doi: 10.1016/j.lfs.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 75.Anderson D. J., Johnson P. M., Alexander N. J., Jones W. R., Griffin P. D. Monoclonal antibodies to human trophoblast and sperm antigens: report of two WHO-sponsored workshops, June 30, 1986-Toronto, Canada. J. Reprod. Immunol. (1987);10:231–257. doi: 10.1016/0165-0378(87)90089-1. [DOI] [PubMed] [Google Scholar]
- 76.Herr J. C., Flickinger C. J., Homyk M., Klotz K., John E. Biochemical and morphological characterization of the intra-acrosomal antigen SP-10 from human sperm. Biol. Reprod. (1990);42:181–193. doi: 10.1095/biolreprod42.1.181. [DOI] [PubMed] [Google Scholar]
- 77.Kurth B. E., Klotz K., Flickinger C. J., Herr J. C. Localization of sperm antigen SP-10 during the six stages of the cycle of the seminiferous epithelium in man. Biol. Reprod. (1991);44:814–821. doi: 10.1095/biolreprod44.5.814. [DOI] [PubMed] [Google Scholar]
- 78.Vassalli J. D., Baccino D., Belin D. A cellular binding site for the Mr 55,000 form of the human plasminogen activator, urokinase. J. Cell Biol. (1985);100:86–92. doi: 10.1083/jcb.100.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mondino A., Resnati M., Blasi F. Structure and function of the urokinase receptor. Thromb Haemost. (1999);82(Supp 1):19–22. [PubMed] [Google Scholar]
- 80.Schlechte W., Murano G., Boyd D. Examination of the role of the urokinase receptor in human colon cancer mediated laminin degradation. Cancer Res. (1989);49:6064–6069. [PubMed] [Google Scholar]
- 81.Del Vecchio S., Stoppelli M. P., Carriero M. V., Fonti R., Massa O., Li P. Y., Botti G., Cerra M., D G., Esposito G., Salvatore M. Human urokinase receptor concentration in malignant and benign breast tumors by in vitro quantitative autoradiography: comparison with urokinase levels. Cancer Res. (1993);53:3198–3206. [PubMed] [Google Scholar]
- 82.de Vries T. J., Quax P. H., Denijn M., Verrijp K. N., Verheijen J. H., Verspaget H. W., Weidle U. H., Ruiter D. J., van Muijen G. N. Plasminogen activators, their inhibitors, and urokinase receptor emerge in late stages of melanocytic tumor progression. Am. J. Pathol. (1994);144:70–81. [PMC free article] [PubMed] [Google Scholar]
- 83.Mohanam S., Sawaya R. E., Yamamoto M., Bruner J. M., Nicholson G. L., Rao J. S. Proteolysis and invasiveness of brain tumors: role of urokinase-type plasminogen activator receptor. J. Neurooncol. (1994);22:153–160. doi: 10.1007/BF01052890. [DOI] [PubMed] [Google Scholar]
- 84.Pedersen H., Brunner N., Francis D., Osterlind K., Ronne E., Hansen H. H., Dano K., Grondahl- Hansen J. Prognostic impact of urokinase, urokinase receptor, and type 1 plasminogen activator inhibitor in squamous and large cell lung cancer tissue. Cancer Res. (1994);54:4671–4675. [PubMed] [Google Scholar]
- 85.Wagner S. N., Atkinson M. J., Thanner S., Wagner C., Schmitt M., Wilhelm O., Rotter M., Hofler H. Modulation of urokinase and urokinase receptor gene expression in human renal cell carcinoma. Am. J. Pathol. (1995);147:183–192. [PMC free article] [PubMed] [Google Scholar]
- 86.Morita Y., Hayashi Y., Wang Y., Kanamaru T., Suzuki S., Kawasaki K., Ohta K., Yamamoto M., Saitoh Y., Itoh H., Doe W. F. Expression of urokinase-type plasminogen activator receptor in hepatocellular carcinoma. Hepatology. (1997);25:856–861. doi: 10.1002/hep.510250412. [DOI] [PubMed] [Google Scholar]
- 87.Yonemura Y., Nojima N., Kawamura T., Ajisaka H., Taniguchi K., Fujimura T., Fujita H., Bandou E., Fushida S., Endou Y., Obata T., Sasaki T. Correlation between expression of urokinase-type plasminogen activator receptor and metastasis in gastric carcinoma. Oncol. Rep. (1997);4:1229–1234. doi: 10.3892/or.4.6.1229. [DOI] [PubMed] [Google Scholar]
- 88.Sier C. F., Stephens R., Bizik J., Mariani A., Bassan M., Pedersen N., Frigerio L., Ferrari A., Dano K., Brunner N., Blasi F. The level of urokinase-type plasminogen activator receptor is increased in serum of ovarian cancer patients. Cancer Res. (1998);58:1843–1849. [PubMed] [Google Scholar]
- 89.Schmidt M., Hoppe F. Increased levels of urokinase receptor in plasma of head and neck squamous cell carcinoma patients. Acta. Otolaryngol. (1999);119:949–953. doi: 10.1080/00016489950180342. [DOI] [PubMed] [Google Scholar]
- 90.Nozaki S., Endo Y., Nakahara H., Yoshizawa K., Hashiba Y., Kawashiri S., Tanaka A., Nakagawa K., Matsuoka Y., Kogo M., Yamamoto E. Inhibition of invasion and metastasis in oral cancer by targeting urokinase- type plasminogen activator receptor. Oral Oncol. (2005);41:971–977. doi: 10.1016/j.oraloncology.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 91.Wang J., Guo F., Wei H., Dong J., Wu J. Expression of urokinase-type plasminogen activator receptor is correlated with metastases of lingual squamous cell carcinoma. Br. J. Oral. Maxillofac. Surg. (2006);44:515–519. doi: 10.1016/j.bjoms.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 92.Hildenbrand R., Niedergethmann M., Marx A., Belharazem D., Allgayer H., Schleger C., Strobel P. Amplification of the urokinase-type plasminogen activator receptor (uPAR) gene in ductal pancreatic carcinomas identifies a clinically high-risk group. Am. J. Pathol. (2009);174:2246–2253. doi: 10.2353/ajpath.2009.080785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ossowski L., Aguirre-Ghiso J. A. Urokinase receptor and integrin partnership: coordination of signaling for cell adhesion, migration and growth. Curr. Opin. Cell Biol. (2000);12:613–620. doi: 10.1016/S0955-0674(00)00140-X. [DOI] [PubMed] [Google Scholar]
- 94.Nykjaer A., Moller B., Todd R. F. 3rd, Christensen T., Andreasen P. A., Gliemann J., Petersen C. M. Urokinase receptor. An activation antigen in human T lymphocytes. J. Immunol. (1994);152:505–516. [PubMed] [Google Scholar]
- 95.Gyetko M. R., Sitrin R. G., Fuller J. A., Todd R. F. 3rd, Petty H., Standiford T. J. Function of the urokinase receptor (CD87) in neutrophil chemotaxis. J. Leukoc. Biol. (1995);58:533–538. doi: 10.1002/jlb.58.5.533. [DOI] [PubMed] [Google Scholar]
- 96.Pedersen N., Schmitt M., Ronne E., Nicoletti M. I., Hoyer-Hansen G., Conese M., Giavazzi R., Dano K., Kuhn W., Janicke F., Blasi F. A ligand-free, soluble urokinase receptor is present in the ascitic fluid from patients with ovarian cancer. J. Clin. Invest. (1993);92:2160–2167. doi: 10.1172/JCI116817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rigolin G. M., Tieghi A., Ciccone M., Bragotti L. Z., Cavazzini F., Della Porta M., Castagnari B., Carroccia R., Guerra G., Cuneo A., Castoldi G. Soluble urokinase-type plasminogen activator receptor (suPAR) as an independent factor predicting worse prognosis and extra-bone marrow involvement in multiple myeloma patients. Br. J. Haematol. (2003);120:953–959. doi: 10.1046/j.1365-2141.2003.04176.x. [DOI] [PubMed] [Google Scholar]
- 98.Nijziel M. R., Van Oerle R., Hellenbrand D., Van Pampus E. C., Hillen H. F., Hamulyak K. The prognostic value of the soluble urokinase-type plasminogen activator receptor (s-uPAR) in plasma of breast cancer patients with and without metastatic disease. J. Thromb. Haemost. (2003);1:982–986. doi: 10.1046/j.1538-7836.2003.00207.x. [DOI] [PubMed] [Google Scholar]
- 99.Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. (1987);4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 100.Whelan S., Goldman N. A general empirical model of protein evolution derived from multiple protein families using a maximum-likelihood approach. Mol. Biol. Evol. (2001);18:691–699. doi: 10.1093/oxfordjournals.molbev.a003851. [DOI] [PubMed] [Google Scholar]


