Abstract
Tissue inhibitor of metalloproteinases (TIMPs; TIMP-1, -2, -3 and -4) are endogenous inhibitor for matrix metalloproteinases (MMPs) that are responsible for remodeling the extracellular matrix (ECM) and involved in migration, invasion and metastasis of tumor cells. Unlike under normal conditions, the imbalance between MMPs and TIMPs is associated with various diseased states. Among TIMPs, TIMP-1, a 184-residue protein, is the only N-linked glycoprotein with glycosylation sites at N30 and N78. The structural analysis of the catalytic domain of human stromelysin-1 (MMP-3) and human TIMP-1 suggests new possibilities of the role of TIMP-1 glycan moieties as a tuner for the proteolytic activities by MMPs. Because the TIMP-1 glycosylation participate in the interaction, aberrant glycosylation of TIMP-1 presumably affects the interaction, thereby leading to pathogenic dysfunction in cancer cells. TIMP-1 has not only the cell proliferation activities but also anti-oncogenic properties. Cancer cells appear to utilize these bilateral aspects of TIMP-1 for cancer progression; an elevated TIMP-1 level exerts to cancer development via MMP-independent pathway during the early phase of tumor formation, whereas it is the aberrant glycosylation of TIMP-1 that overcome the high anti-proteolytic burden. The aberrant glycosylation of TIMP-1 can thus be used as staging and/or prognostic biomarker in colon cancer. [BMB Reports 2012; 45(11): 623-628]
Keywords: Aberrant glycosylation, Cancer progression, MMP, TIMP-1, Tumor microenvironment
INTRODUCTION
Cancer progression is a very complex process during which various cells including malignant cells, surrounding stromal cells and inflammatory cells communicate each other in the tumor microenvironment. Extracellular matrix (ECM) that is formed by structural proteins such as collagen, elastin, fibronectin, and laminin also acts as a barrier or promoting environment (1,2). Various proteases are involved in the remodeling of the extracellular matrix (ECM) to allow dissemination and metastasis of tumor cells including serine proteases, cysteine cathepsins and matrix metalloproteinases (MMPs).
MMPs are among the best characterized proteases and a family of zinc endopeptidases sharing homologous catalytic domains. These enzymes catalyze the cleavage the protein components of the ECM and play a critical role in tissue remodeling during development, wound healing, cancer invasion and metastasis as well as other physiological processes (3). There are a total of 24 MMPs in human cells that have broad and overlapping specificities. The collagenases, a subgroup of MMPs that cleave collagens type I, II, and III, include MMP-1 (fibroblast collagenase), MMP-8 (neutrophil collagenase), and MMP-13 (collagenase-3) (4-6). MMP-1 is considered a prototype for all interstitial collagenases and plays a pivotal role in the turnover of collagen fibrils in the extracellular matrix. The degradation of collagen is evidently an important event in diverse physiological processes such as development, tissue morphogenesis, and wound healing (7), whereas unregulated collagenolysis is associated with a variety of human diseases including cancer, rheumatoid arthritis, pulmonary emphysema, and fibrotic disorders (8).
Tissue inhibitors of metalloproteinases (TIMPs) are endogenous inhibitors for MMPs and also exhibit an MMP-independent biological function. TIMP-1, -2, -3, and -4 comprise the TIMP family, have their binding MMP partners, and regulates remodeling and turnover of the ECM during normal development and pathological conditions (9). Among the TIMP members, TIMP-1 is the only protein that is glycosylated. In this review, TIMP-1 will be highlighted to describe the effects of aberrant glycosylation on cancer progression.
Glycosylation is an enzymatic modification and is among the most common post-translational protein modifications to be found in eukaryotic cells. The biological roles of glycosylation are extremely diverse including protein stability, cellular interaction, development and growth (10,11). However, alterations of glycan structures are known to be associated with the development and progression of several diseases, such as inflammatory diseases, cancers, and congenital disorders (12-14).
Interestingly, TIMP-1 shows a dual role in cancer progression. TIMP-1 has been reported to interact with several MMPs and the matrix-degrading properties of the MMPs, which play a central role in the growth and spread of cancer, are inhibited by TIMP-1 binding (15). Besides the MMP-inhibitory function, TIMP-1 has been shown to stimulate cell growth (16) and exhibits an anti-apoptotic activity (17). These two functions appear to be contradictory, but the plausible explanation will be given by considering an aberrant glycosylation of TIMP-1 along with variation of TIMP-1 level in cancer.
Several different enzyme-linked immunosorbent assays have been described for the measurement of TIMP-1 in blood (18) and it has been shown that patients with colorectal cancer showed significantly increased plasma levels compared to healthy donors and patients with inflammatory bowel disease (19). However, a major hurdle resides in a perspective of biomarker discovery that some intermediate levels of TIMP-1 can be associated with both the healthy and diseased scenario. This shortfall of TIMP-1 as a cancer biomarker prompted to investigate the glycan status of TIMP-1 and its effect on the cellular functions under pathological conditions. In this review, a more comprehensive understanding of biological functions for TIMP-1 is to be presented by considering the glycan status of TIMP-1. In addition, we review the possible role of TIMP-1 glycosylation in cancer and consider the promising and limiting aspects associated with the use of glycosylation status as a companion biomarker.
PROTEIN STRUCTURE OF TIMP-1
TIMPs show 40-50% sequence identity with molecular mass ranging from 22 to 30 kDa (20). The members regulate MMPs activities with relatively low selectivity, forming a tight non-covalent complex with a 1:1 stoichiometry. Native TIMP-1 contains two glycosylation sites at Asn53 and Asn101 (21). A C-terminally truncated form of TIMP-1 has been shown to bind to MMP-3 with a slightly reduced affinity compared with full-length TIMP-1 (22,23), suggesting that the N-terminal region is critical for their binding.
The nature of TIMP-1 binding with MMPs was revealed basically through analysis of an X-ray crystal structure of the complex formed between unglycosylated human TIMP-1 and the catalytic domain of MMP-3 (9). The structural analysis of this complex revealed that TIMP-1 shows a wedge-shaped structure with its edge into the active-site cleft of the MMP-3 catalytic domain. With its long edge, consisting of five different chain regions, it occupies the entire length of the active-site cleft of MMP-3. Cys 1-Thr 2-Cys 3-Val 4 and Ser 68-Val 69 form a central disulfide segment to bind to either side of the catalytic zinc. Cys 1 is involved in the bidental coordination of zinc ion, and the Thr-2 side chain extends into the large slot of MMP-3. However, the complex structure of the catalytic domain of fibroblast collagenase (MMP-1) and the N-terminal inhibitory domain of hTIMP-1 showed a slightly different pattern when compared to that of a complex with MMP-3. It turned out that TIMP-1 inhibits MMP-1 in a similar manner, but there are significant differences in the protein-protein interfaces in the two complexes. Specifically, the loop between beta-strands A and B of TIMP-1 that is involved in the contact with MMP-3 does not participate in the binding with MMP-1. Besides, there are marked differences in the roles of individual residues in the C-D connector of TIMP-1 in binding to the two MMPs. Considering the fact that glycosylation contributes to the proper folding of a protein, a more complete structural analysis of TIMP-1 and the complex could be possible by using glycosylated TIMP-1 (9).
GLYCOPROFILES OF TIMP-1
Recently, a complete characterization of N-glycan structures has been made possible through the development of a combination of techniques. One standard approach is to detach the glycans from the peptide backbone using PNGase F, to resolve the heterogeneous glycans on a PGC column and to analyze by MS and MS/MS (24-26). A targeted approach for glycol-profiling has been attempted to focus on selective glycoproteins following enrichment from complex mixtures using lectin and group-specific binding molecules. An identification using LC-ESI-MS allows an in-depth analysis of heterogeneity of glycans (27,28).
Alternatively, glycol-peptides can be separated on an LC column following a proteolytic digestion, and a small fraction of the extracted peptides was used for the identification of the protein. The peptide mass fingerprinting and the characterization of the glycan structures can be performed using MALDI-TOF and Q-TOF MS and MS/MS. The method for glycoprofiling developed was tested for characterization of rhTIMP-1 from two different sources, produced by Chinese hamster ovary cells and NS0 cells. The method was also applied to the analysis of TIMP-1 which is present in approximately low amounts (50-80 μg/ml) (29).
TIMP-1 glycans showed, similar to other glycoproteins, heterogeneity in the glycan composition at both glycosylation sites. A total number of 30 glycopeptides from CHO cells were characterized, and their relative quantities agreed reasonably well with the reference study (30), proving a reliable glycoprofiling. The heterogeneity was also observed for TIMP-1 from NS0 cells. Although TIMP-1 from NS0 cells was found to have N-glycans with more branches, the glycans from both cells were all fucosylated bi-, tri-, or tetra-antennary. Apparently sialylated glycan structures were exclusively observed on the TIMP-1 from CHO cells. It is noteworthy that the proportions of each glycan were found to vary considerably between the two sites and the proportions varied even more when comparing TIMP-1 between the two recombinant species.
A total number of 38 glycopeptides were used from TIMP-1 from plasma and characterization characterized revealed that most of glycan have biantennary structures with some of them decorated with sialic acids. And the glycol-patterns were found to be similar among normal subjects indicating a minimal person-specific variation. Besides, TIMP-1s from plasma and platelets were uniformly glycosylated (31).
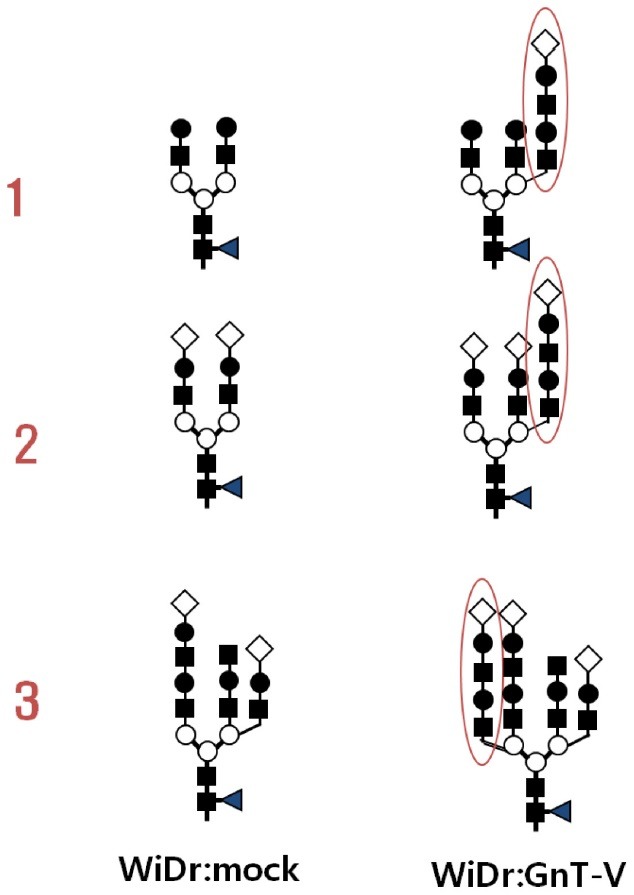
In our previous study (32), glycosylation mutants of TIMP-1 were generated using site-directed mutagenesis to produce three mutated genes, T-N53Q, T-N101Q, and T-N53/101Q. The mutant genes were transfected into control colorectal cancer WiDr cells (WiDr:Mock) and GnT-V transfected WiDr cells (WiDr:GnT-V). To deduce the structure of aberrant glycan of TIMP-1, the cognate TIMP-1 was purified both from WiDr: mock and WiDr:GnT-V and subjected to two-dimensional electrophoresis followed by immunoblot using an anti-TIMP-1 antibody or a lectin blot analysis using L-PHA, a lectin recognizing beta 1,6 N-acetylglucosamine residue and Datura stramonium agglutinin (DSA), a lectin recognizing lactosamine moiety. The result indicate that TIMP-1 from WiDr:GnT-V showed a more heterogenous glycol-pattern compared to that from mock cells (Fig. 1) with more branches, elongation with polylactosamine moiety and sialic acid. The microheterogeneity was also confirmed by lectinblot analysis, where GnT-V attacked TIMP-1 showed a multi-spot pattern as assessed by L-PHA and DSA. The deduced glycan structures were confirmed by a mass analysis of each glycan that had been treated with sialidase and sialidase plus beta-galactosidase. The results imply that heterogeneity of TIMP-1 glycan may affect interactions with MMPs through electrostatic repulsion and/or steric hindrance at the contact points (32).
Fig. 1. Changes in N-glycans of TIMP-1 by overexpression of N-acetylglucosaminyltransferase V. Three representative glycans from WiDr and their counterparts from GnT-V overexpressing WiDr cells were subjected to structural analysis. Additional branch was formed consisting of lactosamine and sialic acids. ■: N-acetylglucosamine, ○: mannose, ●: galactose, ◀: fucose, ◇: sialic acid.
DEBATES SURROUNDING BIOLOGICAL FUNCTIONS OF TIMP-1
Evidence is accumulating that indicates the important role of ECM in cancer development and progression (33-35). Deregulated ECM dynamics are observed in the tumor microenvironment and interacting stromal cells and non-cellular components help tumor cells evolve in the niche (36). The bilateral interaction guides cancer progression. MMPs play a pivotal role in the deregulation of the tumor microenvironment, which are precisely regulated under normal conditions, but an imbalance between MMPs and their regulators like TIMPs is thought to lead to cancer progression (37). TIMP-1 is not only a key regulator for several MMPs but has also an MMP-independent cell growth-stimulating activity (38). Because of these dual roles of TIMP-1, it is difficult to define the net outcome of TIMP-1 action during cancer progression. TIMP-1 stimulates proliferation of transformed cells of different tissue origin and tumor cell lines (39-42) and is responsible for resistance against apoptotic cues (43,44). However, a quite opposite function was observed through an in vivo study that points to an inhibitory function of TIMP-1 during cancer progression (45,46). Additionally, the in vivo study is apparently contradictory to the reports that TIMP-1 is accumulated in tumor tissues (47,48), and the accumulated TIMP-1 is associated with poor prognosis (49).
The role of TIMP-1 in tumor formation and proliferation was also investigated in an in vivo nude mouse model. After transfectants of mutant TIMP-1 genes along with a wild-type was subcutaneously injected into nude mice, the tumor volumes were measured daily up to 60 days after injection. Transfection of wild-type TIMP-1 resulted in a high tumor growth in an early phase, whereas it inhibits further growth in a later phase. This biphasic growth curve was also observed for other mutant TIMP-1 transfectants. Although the expression level and the glycan structure of TIMP-1 governed the tumor proliferation, they exerted their effects differently between the early and late phase. Tumor growth rate in the early phase was proportional to the expression level of glycosylated form of TIMP-1. Although GnT-V expression contributed to the higher tumor proliferation rate, dependency on TIMP-1 level was identical for the mock and GnT-V cells. However, the tumor growth rate in vivo was independent of an overexpression level of aglycosylated mutant of TIMP-1, which suggests that TIMP-1 glycan is essential to exert TIMP-1 effects on tumor formation and growth in an early phase. It was in the late phase of tumor growth that an overexpression of aglycosylated TIMP-1 affects the tumor growth rate. It was responsible for a slow growth of tumor legions, compared to the control cells. TIMP-1 plays a role as a regulatory molecule via an MMP-independent or an MMP-dependent mechanism. TIMP-1 expression also stimulated the resistance against the apoptotic activity by TNF-alpha and IL-2 in WiDr cells without affecting the expression levels of the receptors for TNF-alpha and IL-2. Expression of GnT-V also stimulated the resistance against the cytotoxicity triggered by the cytokines. RNA interference was applied to confirm the apoptotic resistance by TIMP-1 secretion (50).
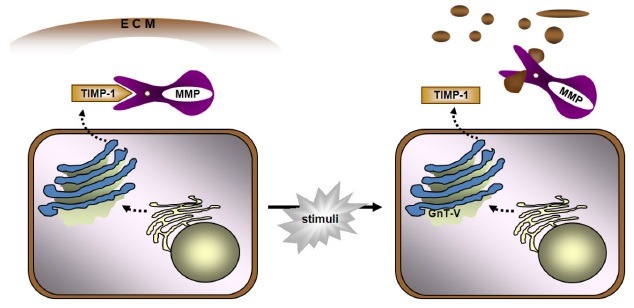
Taken together, these reports indicate that TIMP-1 contributes to cancer cell proliferation in early stage and this high anti-proteolytic burden can be overcome by an aberrant glycosylation catalyzed by relevant glycosyltransferases. An aberrant glyco-form of TIMP-1 loses a portion of MMP-inhibitory activity, contributing to high invasive potential of cancer cells in the tumor microenvironment (Fig. 2). Three-dimensional culture system was applied to confirm the dependence of cancer progression on the glycan status of TIMP-1. This result provides a plausible explanation to the enigmatic nature of TIMP-1 that has remained questionable.
Fig. 2. Schematic diagram of aberrant glycosylation of TIMP-1 and the effect on increased invasive potential of cancer cells. MMP is tightly regulated by TIMP-1 under normal conditions, but in GnT-V overexpressing cells, TIMP-1 becomes aberrant in glycosylation and loses significant portion of MMP-inhibitory capability. This results in a net increase in the availability of MMPs that are free from the regulation by TIMP-1 and a stimulated degradation of the extracellular matrix.
DETECTION OF ABERRANT GLYCOFORM OF TIMP-1 BY MASS SPECTROMETRY
An analysis of TIMP-1 alone appears to provide comprehensive information on the existence or status of cancer cells. Considering the fact that blood is a most favorable source of biomarkers and TIMP-1 from every tissue circulates in blood, it may not be easy to pinpoint the existence or growth of tumor cells. Actually, the amount of TIMP-1 in human serum is ∼100 ng/ml, which is the sum of secreted TIMP-1 levels from every tissue source and the tumor legions are negligible among the volumes of TIMP-1 secretory tissues. However, because an aberrantly glycosyled TIMP-1 is limitedly expressed under normal conditions, aberrant TIMP-1 can be indicative of cancer progression. Enrichment step using lectin or antibody can be an excellent approach to detect a low level of TIMP-1 molecules. In fact, it was not easy to detect TIMP-1 by using an LC/FTICR/LTQ mass spectrometer without any enrichment step. However, it was made possible by enrichment with L-PHA lectin, further enrichment or extensive separation of the sample prior to analysis.
SISCAPA method can be applied for efficient detection of TIMP-1. The tryptic peptides were consistently identified with relatively strong signal intensity by ESI mass analysis (32). Among the peptides, GFQALGDAADIR that contains no cysteine residue and posttranslational modification site reported within its sequence was selected as a target peptide for MRM analysis. Although the total TIMP-1 level did not show a drastic increase by GnT-V expression, the aberrant TIMP-1 as assessed by enrichment using L-PHA turned out to drastically increase by GnT-V expression. Through this approach, TIMP-1 could be quantified at an atto-mole level with good reproducibility (51). The total level of TIMP-1 was nearly identical, whereas aberrant TIMP-1 showed 11.7-fold increase in GnT-V overexpression cells, compared to mock cells. This tendency was also confirmed qualitatively by immunoblot analysis as described elsewhere (32), where the aberrant TIMP1 glycoform induced by GnT-V plays important roles in invasive and metastatic colon cancer cells. The comparative monitoring method for aberrant glycosylation of glycoproteins may also be applied to the analysis of more complex samples, like serum, as well as to the qualification and validation of newly identified and unproven biomarker candidates whose aberrant glycosylation is implicated in cancer development and progression.
CONCLUSION
TIMP-1 has a contradictory, dual function, one of which is based on an MMP-dependent anti-proteolytic activity with the other MMP-independent cell growth activity. This contradictory function has been hampered comprehesive understanding of a true role of TIMP-1 in cancer progression. An additional information obtained from the analysis of TIMP-1 glycan and its effect on the MMP interaction provided basis on the plausible description of interplay between TIMP-1 and MMPs and on the deregulated cancer invasion in the tumor microenvironment. The limitation in the detection of aberrant TIMP-1 could be overcome by combining lectin enrichment tools with SISCAPA. Advancements in both understanding of the biological role of TIMP-1 and mass analysis provide an opportunity that a precise analysis of TIMP-1 and the glycan structure may provide diagnostic options about using TIMP-1 as stage or diagnostic biomarker in colon cancer.
Acknowledgments
This work was supported by the ‘Convergence Research Center Program (2011K000891)’ of the Ministry of Education, Science and Technology and grants from the KRIBB Research Initiative Program (KGM3231211).
References
- 1.Bhowmick N. A., Neilson E. G., Moses H. L. Stromal fibroblasts in cancer initiation and progression. Nature. (2004);432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stetler-Stevenson W. G., Aznavoorian S., Liotta L. A. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu. Rev. Cell Biol. (1993);9:541–573. doi: 10.1146/annurev.cb.09.110193.002545. [DOI] [PubMed] [Google Scholar]
- 3.Nagase H., Woessner J. F. Jr. Matrix metalloproteinases. J. Biol. Chem. (1999);274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 4.Welgus H. G., Jeffrey J. J., Eisen A. Z. The collagen substrate specificity of human skin fibroblast collagenase. J. Biol. Chem. (1981);256:9511–9515. [PubMed] [Google Scholar]
- 5.Hasty K. A., Jeffrey J. J., Hibbs M. S., Welgus H. G. The collagen substrate specificity of human neutrophil collagenase. J. Biol. Chem. (1987);262:10048–10052. [PubMed] [Google Scholar]
- 6.Birkedal-Hansen H., Moore W. G., Bodden M. K., Windsor L. J., Birkedal-Hansen B., DeCarlo A., Engler J. A. Matrix metalloproteinases: a review. Crit. Rev. Oral Biol. Med. (1993);4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 7.Greene J., Wang M., Liu Y. E., Raymond L. A., Rosen C., Shi Y. E. Molecular cloning and characterization of human tissue inhibitor of metalloproteinase 4. J. Biol. Chem. (1996);271:30375–30380. doi: 10.1074/jbc.271.48.30375. [DOI] [PubMed] [Google Scholar]
- 8.Laurent G. J., Tetley T. D. Pulmonary fibrosis and emphysema: connective tissue disorders of the lung. Eur. J. Clin. Investig. (1984);14:411–413. doi: 10.1111/j.1365-2362.1984.tb01204.x. [DOI] [PubMed] [Google Scholar]
- 9.Gomis-Rueth F., Maskos K., Michael Betz M., Bergner A., Huber R., Suzuki K., Yoshida N., Nagase H., Brew K., Bourenkovk G. P., Bartunikk H., Bode W. Mechanism of inhibition of the human matrix metalloproteinase stromelysin-1 by TIMP-1. Nature. (1997);389:77–81. doi: 10.1038/37995. [DOI] [PubMed] [Google Scholar]
- 10.Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. (1995);3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moens S., Vanderleyden J. Glycoproteins in prokaryotes. Arch. Microbiol. (1997);168:169–175. doi: 10.1007/s002030050484. [DOI] [PubMed] [Google Scholar]
- 12.Daniels M. A., Hogquist K. A., Jameson S. C. Sweet ‘n’ sour: the impact of differential glycosylation on T cell responses. Nat. Immunol. (2002);3:903–910. doi: 10.1038/ni1002-903. [DOI] [PubMed] [Google Scholar]
- 13.Dwek M. V., Ross H. A., Leathem A. J. Proteome and glycosylation mapping identifies post-translational modifications associated with aggressive breast cancer. Proteomics. (2001);1:756–762. doi: 10.1002/1615-9861(200106)1:6<756::AID-PROT756>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 14.Freeze H. H. Update and perspectives on congenital disorders of glycosylation. Glycobiology. (2001);11:129R–143R. doi: 10.1093/glycob/11.12.129R. [DOI] [PubMed] [Google Scholar]
- 15.Thaysen-Andersen M., Thøgersen I. B., Lademann U., Offenberg H., Giessing A. M. B., Enghild J. J., Nielsen H. J., Brünner N., Højrup P. Investigating the biomarker potential of glycoproteins using comparative glycoprofiling - application to tissue inhibitor of metalloproteinases-1. Biochim. Biophys. Acta. (2008);1784:455–463. doi: 10.1016/j.bbapap.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Luparello C., Avanzato G., Carella C., Pucci-Minafra I. Tissue inhibitor of metalloproteinases (TIMP-1) and proliferative behaviour of clonal breast cancer cells. Breast Cancer Res. Treat. (1999);54:235–244. doi: 10.1023/A:1006121129382. [DOI] [PubMed] [Google Scholar]
- 17.Guedez L., Stetler-Stevenson W. G., Wolff L., Wang J., Fukushima P., Mansoor A., Stetler-Stevenson M. In vitro suppression of programmed cell death of B cells by tissue inhibitor of metalloproteinases-1. J. Clin. Invest. (1998);102:2002–2010. doi: 10.1172/JCI2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li G., Fridman R., Kim H. R. Tissue inhibitor of metalloproteinases-1 inhibits apoptosis of human breast epithelial cells. Cancer Res. (1999);59:6267–6275. [PubMed] [Google Scholar]
- 19.Jung K., Nowak L., Lein M., Henke W., Schnorr D., Loening S. A. What kind of specimen should be selected for determining tissue inhibitor of metalloproteinase-1 (TIMP-1) in blood? Clin. Chim. Acta. (1996);254:97–100. doi: 10.1016/0009-8981(96)06367-X. [DOI] [PubMed] [Google Scholar]
- 20.Greene J., Wang M., Liu Y. E., Raymond L. A., Rosen C., Yuenian E., Shi Y. E. Molecular cloning and characterization of human tissue inhibitor of metalloproteinase4. J. Biol. Chem. (1996);271:30375–30380. doi: 10.1074/jbc.271.48.30375. [DOI] [PubMed] [Google Scholar]
- 21.Docherty A. J., Lyons A., Smith B. J., Wright E. M., Stephens P. E., Harris T. J., Murphy G., Reynolds J. J. Sequence of human tissue inhibitor of metalloproteinases and its identity to erythroid-potentiating activity. Nature. (1985);318:66–69. doi: 10.1038/318066a0. [DOI] [PubMed] [Google Scholar]
- 22.Murphy G., Houbrechts A., Cockett M. I., Williamson R. A., O'Shea M., Docherty A. J. P. The N-terminal domain of human tissue inhibitor of metalloproteinases retains metalloproteinase inhibitory activity. Biochemistry. (1991);30:8097–8102. doi: 10.1021/bi00247a001. [DOI] [PubMed] [Google Scholar]
- 23.Huang W., Suzuki K., Nagase H., Arumugan S., Van Doren S. R., Brew K. Folding and characterization of the amino-terminal domain of human tissue inhibitor of metalloproteinase-1 (TIMP-1) expressed at high yield in E. coli. FEBS Lett. (1996);384:155–161. doi: 10.1016/0014-5793(96)00304-3. [DOI] [PubMed] [Google Scholar]
- 24.Stahl B., Klabunde T., Witzel H., Krebs B., Steup M., Karas M., Hillenkamp F. The oligosaccharides of the Fe(III)-Zn(II) purple acid-phosphatase of the red kidney bean-determination of the structure by a combination of matrix-assisted laser-desorption ionization mass spectrometry and selective enzymatic degradation. Eur. J. Biochem. (1994);220:321–330. doi: 10.1111/j.1432-1033.1994.tb18628.x. [DOI] [PubMed] [Google Scholar]
- 25.Mortz E., Sareneva T., Haebel S., Julkunen I., Roepstorff P. Mass spectrometric characterization of glycosylated interferon-variants separated by gel electrophoresis. Electrophoresis. (1996);17:925–931. doi: 10.1002/elps.1150170514. [DOI] [PubMed] [Google Scholar]
- 26.Garner B., Merry A. H., Royle L., Harvey D. J., Rudd P. M., Thillet J. Structural elucidation of the Nand O-glycans of human apolipoprotein(a): role of O-glycans in conferring protease resistance. J. Biol. Chem. (2001);276:200–208. doi: 10.1074/jbc.M102150200. [DOI] [PubMed] [Google Scholar]
- 27.Larsen M. R., Højrup P., Roepstorff P. Characterization of gel-separated glycoproteins using two-step proteolytic digestion combined with sequential microcolumns and mass spectrometry. Mol. Cell. Proteomics. (2005);4:107–119. doi: 10.1074/mcp.M400068-MCP200. [DOI] [PubMed] [Google Scholar]
- 28.Bunkenborg J., Pilch B. J., Podtelejnikov A. V., Wisniewski J. R. Screening for N-glycosylated proteins by liquid chromatography mass spectrometry. Proteomics. (2004);4:454–465. doi: 10.1002/pmic.200300556. [DOI] [PubMed] [Google Scholar]
- 29.Holten-Andersen M. N., Murphy G., Nielsen H. J., Pedersen A. N., Christensen I. J., Høyer-Hansen G., Bruenner N., Stephens R. W. Quantitation of TIMP-1 in plasma of healthy blood donors and patients with advanced cancer. Br. J. Cancer. (1999);80:495–503. doi: 10.1038/sj.bjc.6690384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sutton C. W., O’Neill J. A., Cottrell J. S. Sitespecific characterization of glycoprotein carbohydrates by exoglycosidase digestion and laser desorption mass spectrometry. Anal. Biochem. (1994);218:34–46. doi: 10.1006/abio.1994.1138. [DOI] [PubMed] [Google Scholar]
- 31.Thaysen-Andersen M., Thøgersen I. B., Nielsen H. J., Lademann U., Nils Bruenner N., Enghild J. J., Peter Højrup P. Rapid and individualspecific glycoprofiling of a low-abundant N-glycosylated protein tissue inhibitor of metalloproteinases-1. Mol. Cell. Proteomics. (2007);6:638–647. doi: 10.1074/mcp.M600407-MCP200. [DOI] [PubMed] [Google Scholar]
- 32.Kim Y. S., Hwang S. Y., Kang H. Y., Sohn H., Oh S., Kim J. Y., Yoo J. S., Kim Y. H., Kim C. H., Jeon J. H., Lee J. M., Kang H. A., Miyoshi E., Taniguchi N., Yoo H. S., Ko J. H. Functional proteomics study reveals that N-acetylglucosaminyltransferase V reinforces the invasive/metastatic potential of colon cancer through aberrant glycosylation on tissue inhibitor of metalloproteinase-1. Mol. Cell. Proteomics. (2008);7:1–14. doi: 10.1074/mcp.M700084-MCP200. [DOI] [PubMed] [Google Scholar]
- 33.Whiteside T. L. The tumor microenvironment and its role in promoting tumor growth. Oncogene. (2008);27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu P., Weaver V. M., Werb Z. The extracellular matrix. A dynamic niche in cancer progression. J. Cell Biol. (2012);196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang J. G., Ko J. H., Kim Y. S. Pros and cons of using aberrant glycosylation as companion biomarkers for therapeutics in cancer. BMB Rep. (2011);44:765–771. doi: 10.5483/BMBRep.2011.44.12.765. [DOI] [PubMed] [Google Scholar]
- 36.Levental K. R., Yu H., Kass L., Lakins J. N., Egeblad M., Erler J. T., Fong S. F., Csiszar K., Giaccia A., Weninger W., Yamauchi M., Gasser D. L., Weaver V. M. Matrix cross-linking forces tumor progression by enhancing integrin signaling. Cell. (2009);139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Egeblad M., Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer. (2002);2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 38.Docherty A. J., Lyons A., Smith B. J., Wright E. M., Stephens P. E., Harris T. J., Murphy G., Reynolds J. J. Sequence of human tissue inhibitor of metalloproteinases and its identity to erythroid-potentiating activity. Nature. (1985);318:66–69. doi: 10.1038/318066a0. [DOI] [PubMed] [Google Scholar]
- 39.Taube M. E., Liu X. W., Fridman R., Kim H. R. TIMP-1 regulation of cell cycle in human breast epithelial cells via stabilization of p27KIP1 protein. Oncogene. (2006);25:3041–3048. doi: 10.1038/sj.onc.1209336. [DOI] [PubMed] [Google Scholar]
- 40.Bloomston M., Shafii A., Zervos E., Rosemurgy A. S. TIMP-1 antisense gene transfection attenuates the invasive potential of pancreatic cancer cells in vitro and inhibits tumor growth in vivo. Am. J. Surg. (2005);189:675–679. doi: 10.1016/j.amjsurg.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 41.Rho S. B., Chung B. M., Lee J. H. TIMP-1 regulates cell proliferation by interacting with the ninth zinc finger domain of PLZF. J. Cell. Biochem. (2007);101:57–67. doi: 10.1002/jcb.21127. [DOI] [PubMed] [Google Scholar]
- 42.Fowell A. J., Collins J. E., Duncombe D. R., Pickering J. A., Rosenberg W. M., Benyon R. C. Silencing tissue inhibitors of metalloproteinases (TIMPs) with short interfering RNA reveals a role for TIMP-1 in hepatic stellate cell proliferation. Biochem. Biophys. Res. Commun. (2011);407:277–282. doi: 10.1016/j.bbrc.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 43.Tsagaraki I., Tsilibary E. C., Tzinia A. K. TIMP-1 interaction with v3 integrin confers resistance to human osteosarcoma cell line MG-63 against TNF-α-induced apoptosis. Cell Tissue Res. (2010);342:87–96. doi: 10.1007/s00441-010-1025-1. [DOI] [PubMed] [Google Scholar]
- 44.Davidsen M. L., Wurtz S. Ø., Rømer M. U., Sørensen N. M., Johansen S. K., Christensen I. J., Larsen J. K., Offenberg H., Brunner N., Lademann U. TIMP-1 gene deficiency increases tumor cell sensitivity to chemotherapy-induced apoptosis. Br. J. Cancer. (2006);95:114–120. doi: 10.1038/sj.bjc.6603378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khokha R. Suppression of the tumorigenic and metastatic abilities of murine B16-F10 melanoma cells in vivo by the overexpression of the tissue inhibitor of the metalloproteinases-1. J. Natl. Cancer Inst. (1994);86:299–304. doi: 10.1093/jnci/86.4.299. [DOI] [PubMed] [Google Scholar]
- 46.Martin D. C., Ruther U., Sanchez-Sweatman O. H., Orr F. W., Khokha R. Inhibition of SV40 T antigen-induced hepatocellular carcinoma in TIMP-1 transgenic mice. Oncogene. (1996);13:569–576. [PubMed] [Google Scholar]
- 47.Aljada I. S., Ramnath N., Donohue K., Harvey S., Brooks J. J., Wiseman S. M., Khoury T., Loewen G., Slocum H. K., anderson T. M., Bepler G., Tan D. Up-regulation of the tissue inhibitor of metalloproteinase-1 protein is associated with progression of human non-small cell lung cancer. J. Clin. Oncol. (2004);22:3218–3229. doi: 10.1200/JCO.2004.02.110. [DOI] [PubMed] [Google Scholar]
- 48.Korpi J. T., Hagstrom J., Lehtonen N., Parkkinen J., Sorsa T., Salo T., Laitinen M. Expression of matrix metalloproteinases-2, -8, -13, -26 and tissue inhibitors of metalloproteinase-1 in human osteosarcoma. Surg. Oncol. (2011);20:e18–e22. doi: 10.1016/j.suronc.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 49.Inagaki D., Oshima T., Yoshihara K., Tamura S., Kanazawa A., Yamada T., Yamamoto N., Sato T., Shiozawa M., Morinaga S., Akaike M., Fujii S., Numata K., Kunisaki C., Rino Y., Tanaka K., Masuda M., Imada T. Overexpression of tissue inhibitor of metalloproteinase-1 gene correlates with poor outcomes in colorectal cancer. Anticancer Res. (2010);30:4127–4130. [PubMed] [Google Scholar]
- 50.Kim Y. S., Ahn Y. H., Song K. J., Jeong G., Kang J. G., Lee J. H., Jeon S. K., Kim H. C., Yoo J. S., Ko J. H. Overexpression and β-1,6-N-acetylglucosaminylation initiated aberrant glycosylation of TIMP-1. J. Biol. Chem. (2012);287:32467–32478. doi: 10.1074/jbc.M112.370064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahn Y. H., Kim Y. S., Ji E. S., Lee J. Y., Jung J. A., Ko J. H., Jong S. Y. Comparative quantitation of aberrant glycoforms by lectin-based glycoprotein enrichment coupled with multiple-reaction monitoring mass spectrometry. Anal. Chem. (2010);82:4441–4447. doi: 10.1021/ac1001965. [DOI] [PubMed] [Google Scholar]


