Abstract
To understand the effects of HCN as potential mediators in the pathogenesis of epilepsy that evoke long-term impaired excitability; the present study was designed to elucidate whether the alterations of HCN expression induced by status epilepticus (SE) is responsible for epileptogenesis. Although HCN1 immunoreactivity was observed in the hippocampus, its immunoreactivities were enhanced at 12 hrs following SE. Although, HCN1 immunoreactivities were reduced in all the hippocampi at 2 weeks, a re-increase in the expression at 2-3 months following SE was observed. In contrast to HCN1, HCN 4 expressions were un-changed, although HCN2 immunoreactive neurons exhibited some changes following SE. Taken together, our findings suggest that altered expressions of HCN1 following SE may be mainly involved in the imbalances of neurotransmissions to hippocampal circuits; thus, it is proposed that HCN1 may play an important role in the epileptogenic period as a compensatory response. [BMB Reports 2012; 45(11): 635-640]
Keywords: Epilepsy, HCN channels, Hippocampus, Immunohistochemistry, Status epilepticus
INTRODUCTION
Temporal lobe epilepsy (TLE), such as spontaneous seizure involving the hippocampal formation, is the most prevalent form of refractory epilepsy, and the mechanisms converting the normal hippocampus into an epileptic one are not fully understood. In laboratory rodents, status epilepticus (SE)-inducing insults such as continuous perforant path stimulation and administration of pilocarpine have been shown to produce a condition with spontaneous limbic seizures and hippocampal sclerosis (1). Numerous previous studies have focused on the neurodegeneration of the hippocampus in TLE rodent animal models, including pilocarpine-induced SE, since specific patterns of neuronal loss occur in both principal neurons and interneurons (2-5).
On the other hand, hyperpolarization-activated cyclic nucleotide-gate cation channel (HCN) is found in a variety of peripheral and central neurons (6,7). HCNs mediate hyperpolarization-activated cation currents (Ih) in the heart and brain (6,7). In the mature hippocampus, HCN is expressed in pyramidal and nonpyramidal neurons (8,9) where it contributes to the resting membrane potential, hyperpolarizing events and rebound excitation (7,10,11), thus regulating rhythmic electrical activity. Therefore, HCN plays an important role in regulating rhythmic electrical activity, and the abnormality of its function or expression is linked to pathological hyperexcitability (12).
With respect to the properties of HCNs, HCNs are thought to be potential mediators in the pathogenesis of epilepsy (13) that evoke long-term impaired excitability. However, controversy exists regarding the involvement of HCNs in“epileptogenic” or “compensatory” mechanisms in the epileptic hippocampus (14). Although some epilepsy models show alterations in the properties of Ih and long-lasting molecular changes in the HCNs (12,13,15,16), the spatio-temporal alterations in HCN expression during the epileptogenic period following SE are not fully understood in vivo. Therefore, to understand the roles of HCNs in epileptogenesis, we investigated HCN expression in the rat hippocampus during the epileptogenic periods.
RESULTS AND DISCUSSION
HCN1 immunoreactivity in the epileptic hippocampus
As shown in Fig. 1 and 2, HCN1 immunoreactivity was selectively detected in the stratum lacunosum-moleculare, some hilar neurons and CA2-3 pyramidal cells (Fig. 1A1-A4). However, an elevation in HCN1 immunoreactivity in all the hippocampal formation compared to the control was observed at 30 min following SE (Fig. 1B2, B3, and B4, 2A). Interestingly, at 12 hrs after pilocarpine treatment, HCN1 immunoreactivities were strongly enhanced throughout the hippocampus, particularly the somata and dendritic processes of the presumed interneurons, nerve fibers of CA1-3, and dentate hilar neurons (Fig. 1C1-C4, 2A, and 2B), but its immunoreactivity was un-changed in the granule cell layer of the dentate gyrus following the time course after SE (Fig. 2B). Moreover, HCN1 immunoreactivity in stratum lacunosum-molecular, which is the main excitatory input site to the hippocampal formation, was also markedly increased (Fig. 1C1). At this time point, quantificational analysis of immunoreactivities of HCN1 channel showed similar results (Fig. 2A and 2B).
Fig. 1. The HCN1 expressions in the hippocampus following pilocarpine-induced SE. HCN1 immunoreactivity is selectively detected in the stratum lacunosum-molecule and some hilar neurons (arrows in panel A1 and A4). However, HCN1 immunoreactivities at 30 min-12 hrs following SE are significantly enhanced in CA1-3 and the stratum lacunosum-molecule (B1-B3 and C1-C3). In addition, HCN1-positive interneurons and hilar neurons are increased (arrows and open arrows in panel B2, B4, C2, and C4). Nevertheless, at 2 weeks following SE, HCN1 immunoreactivities are down-regulated to levels similar to the control (D1-D4). At 11 weeks after SE, HCN1 expression in all hippocampal regions and its immunoreactive interneurons is re-enhanced, similar to 12 hrs following SE (E1-E4). Bar = 280 μm (panels A1, B1, C1, D1, and E1), 50 μm (panels A2-A4, B2-B4, C2-C4, D2-D4, and E2-E4).
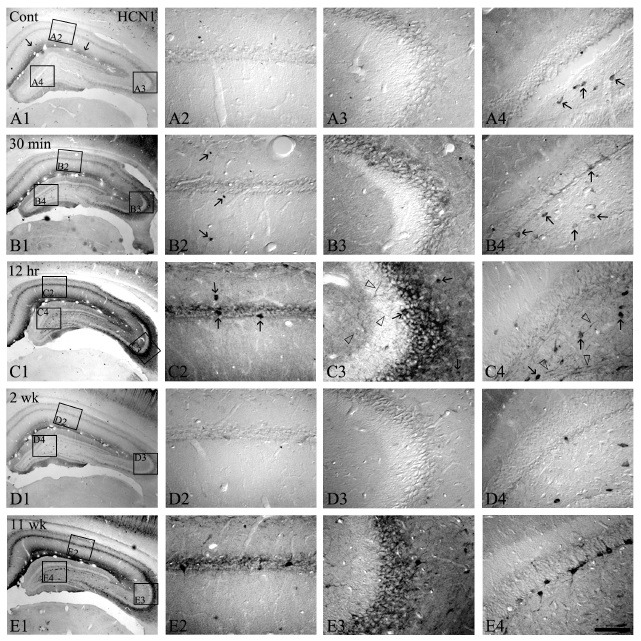
Fig. 2. Quantitative analyses of HCN1 immunoreactive interneurons (A) and immunodensity (B) in normal and epileptic hippocampi following pilocarpine-induced SE (mean ± S.E.M). Significant differences from the control group, *P < 0.05, **P < 0.01.
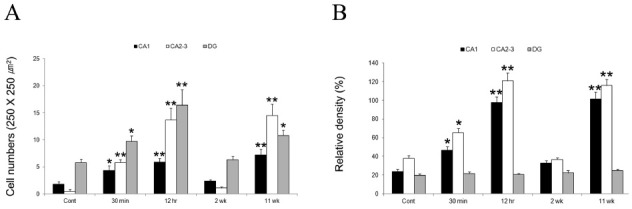
HCN1, which encodes fast-kinetics channels with modest cAMP gating, is highly expressed in hippocampal pyramidal cells and in CA1 interneurons (16-18). The Ih currents of these neurons have been implicated in the maintenance of the resting membrane potential, synchronized network activity, and the dampening effects of dendritic excitation such as depolarization, and on somatic output (13,19-22). In the present study, HCN1 immunoreactivities in the control animals were selectively detected throughout the hippocampus. These mutual discordances may be caused by the fact that mRNA expression does not always translate into functional proteins, and the distribution of the latter may differ from that of the cognate mRNA (18). However, at 30 min-12 hrs after pilocarpine treatment, HCN1 immunoreactivity in the SE hippocampus was gradually up-regulated in the pyramidal cell layers and in the interneurons of CA1-3, dentate hilar neurons. The expression levels of HCN1 were peaked at 12 hrs following SE in the stratum lacunosum-molecule and in the CA2-3 region, which are the main sites of excitatory neuronal inputs from the entorhinal cortex and dentate gyrus, respectively. These findings may reflect a gradual elevation of neuronal excitabilities in hippocampal circuits and an enhancement in the excitatory neurotransmission through the stratum lacunosum-molecule and mossy fibers to the hippocampus during the time course after pilocarpine treatments, although unchanged HCN1 expression in the granule cell layer of the dentate gyrus may not have been affected. Thus, enhanced HCN1 immunoreactivities may result from elevated hippocampal excitability and act as compensatory responses to this phase. In fact, many previous studies have demonstrated that hippocampal circuit activity might be the key mechanism for a novel regulation of HCN expression in the rat hippocampus (16), and up-regulation of HCN1 mRNA expression may be driven by the altered inhibitory and excitatory circuitries of the dentate gyrus, perhaps as a compensatory response (13). Therefore, our findings indicated that elevated HCN1 immunoreactivity at early time stages following SE may be caused by the altered balance between inhibitory and excitatory inputs, such as excessive dendritic excitation, throughout hippocampal circuits and in consequence of down-regulation of inhibitory response in the SE hippocampal formats.
As shown in Fig. 1D, at 2 weeks after pilocarpine treatment, a gradual decrease in the HCN1 expression was observed as similar to the control levels (Fig. 1D1-D4 and Fig. 2). However, at 2-3 months following SE, HCN1 immunoreactivity was significantly and abundantly re-enhanced throughout the hippocampus similar to that of 12 hrs following SE (Fig. 1E1-4 and Fig. 2).
Since many previous studies have demonstrated that selective down-regulation of HCN1 mRNA expression occurs in response to increased hyperpolarizing input and consequent enhanced activation of this channel (13), this may be the consequence of an enhanced compensatory response to suppressing enhanced excitatory hippocampal circuitry. In addition, it is well documented that neurodegeneration of hippocampal principal cells and interneurons begins in the pilocarpine-induced SE hippocampus, and that mossy fiber sprouting, such as synaptic re-organization, at 2-3 months after pilocarpine treatments led to an elevation of recurrent excitatory input into the dentate gyrus. Similar to these studies, at 11 weeks following SE, HCN1 immunoreactivities were re-enhanced throughout the epileptic hippocampus. These elevated expression patterns were significantly increased in all the hippocampi as similar to the hippocampus 12 hrs after SE. In fact, previous studies have demonstrated that loss of interneurons and consequent reduced perisomatic inhibition in human epileptic hippocampus, perhaps in conjunction with enhanced dendritic excitation, triggered increased HCN1 mRNA expression. Additionally, because of the altered inhibition, the increased excitation in the dentate gyrus circuit as a reactive sprouting also contributed to HCN1 regulation (13). Moreover, the majority of sprouted mossy fiber contacts are onto neighboring granular (23-25) and primarily onto their dendrites cells (26), so the surviving epileptic granular cell at this time course is more subjected to enhanced dendritic excitation (27). Therefore, our findings may indicate that re-enhancement of HCN1 immunoreactivity at the later stage of the epileptogenic period following SE may be a compensatory response to the reduced inhibitory input and enhanced excitability to the dentate gyrus, since the interneuronal loss and the abnormality of synaptic re-organization induced by mossy fiber sprouting occurred at a later epileptogenic period in pilocarpine treatment animal models.
HCN2 and HCN4 immunoreactivities in the epileptic hippocampus
As shown in Fig. 3 and Fig. 4, HCN2 immunoreactivity was detected in some interneuronal populations in the control animal hippocampus (Fig. 3A2-A4). In addition, HCN2 immunoractivities were expressed in the granular cell layer of the dentate gyrus (Fig. 3A2-A4). At 12 hrs following SE, some changes in the HCN2 immunoreactive neurons in all the epileptic hippocampi were seen except in the CA2-3 region (Fig. 3B1-B4 and E). In addition, HCN2 immunoreactivity was also down-regulated in the granule cell layer of the dentate gyrus (Fig. 3B4). The immunoreactivities of HCN2 following SE were enhanced to control the levels in CA1 and dentate gyrus at day 7 (Fig. 3C1-C4 and E), although HCN2 expression in the CA2-3 region remained unchanged (Fig. 3C3). After this time window, until 5 weeks following SE, however, HCN2 immunoreactive neurons were re-declined in all the hippocampi as similar to the 12 hr groups (Fig. 3D1-D4 and E). Immunoreactivities of HCN4 resembled those of HCN2 expression in the SE hippocampus, but this isoform was less commonly observed within the interneurons of all the hippocampi. However, its immunoreactivity within the interneurons of all the epileptic hippocampi was un-changed following SE (Fig. 4).
Fig. 3. The HCN2 immunoreactivity in the hippocampus in the normal and epileptic animal models following SE. HCN2 expressions are detected in some interneurons of CA1-3 and the dentate gyrus (arrows in panel A2-A4), whereas its immunoreactivity is observed in the granule cell layer of the dentate gyrus (A1 and A4). At 12 hrs following SE, HCN2 immunoreactivities in interneuronal populations and the granule cell layer are reduced compared to the control (arrows in panel B2-B4). These expressions are enhanced in CA1 and the dentate gyrus at day 7 following SE (C1-C4). After this time window, HCN2 immunoreactive interneurons are re-declined until 5 weeks following SE (D1-D4). Bar = 280 μm (panels A1, B1, C1, and D1), 50 μm (panels A2-A4, B2-B4, C2-C4, and D2-D4). Quantitative analyses of HCN2 immunoreactivity in the normal and epileptic hippocampi following SE (E, mean ± S.E.M). Significant differences from the control group, *P < 0.05, **P < 0.01.
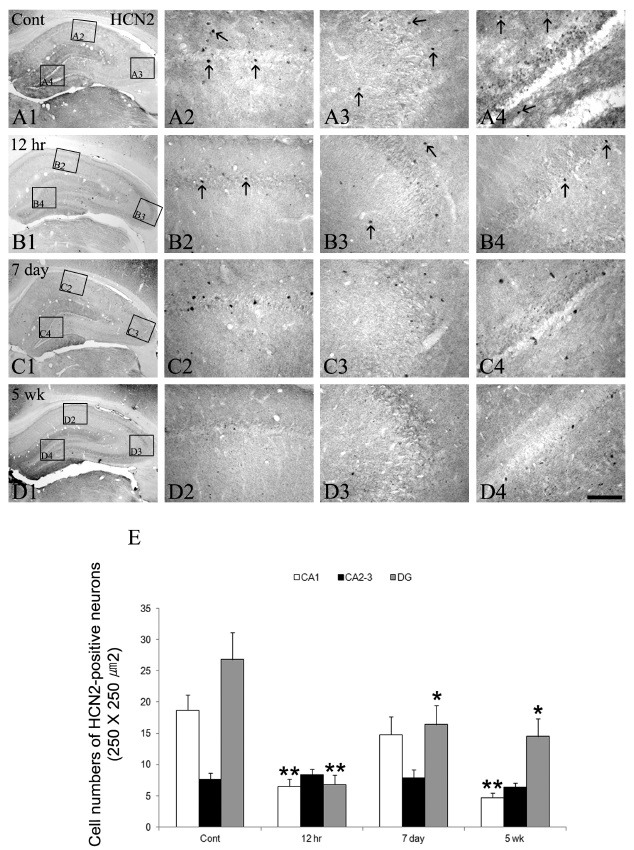
Fig. 4. The HCN4 expressions in the hippocampus of the normal and epileptic animal groups following SE. HCN4 immunoreactivities are rarely detected in all hippocampal regions, whereas its expression is observed in some interneurons (arrows in panels A2-A4). However, the immunoreactivities of HCN4 in the hippocampus following SE are unchanged depending on the time course after pilocarpine treatment (B1-D4). Bar = 280 μm (panels A1, B1, C1, and D1), 50 μm (panels A2-A4, B2-B4, C2-C4, and D2-D4). Quantitative analyses of HCN4 immunoreactivity in the normal and epileptic hippocampi following SE (E, mean ± S.E.M).
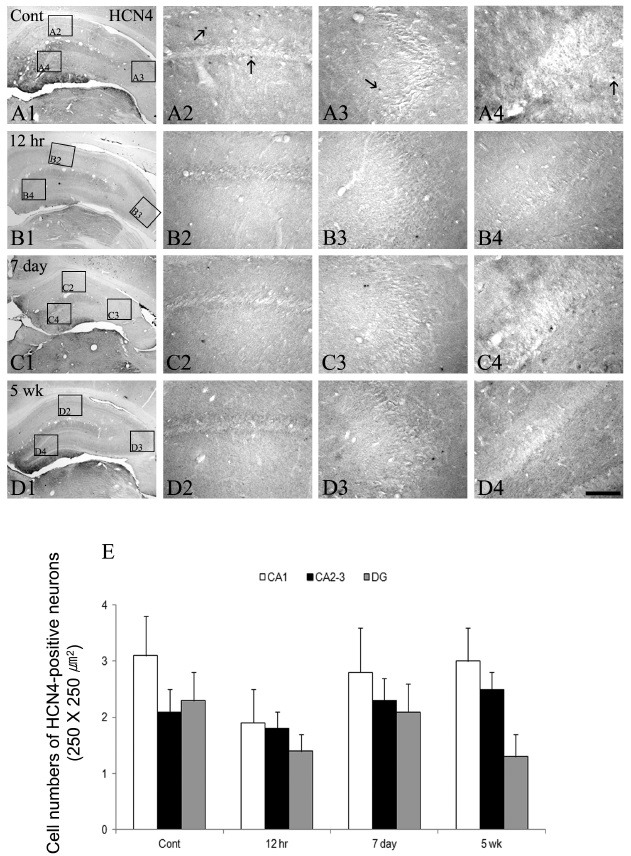
The HCN2 and HCN4 encoding channels with slower activation and deactivation kinetics are expressed in both the mature and developing rodent hippocampi (17,18,28). In addition, this channel is highly expressed in pacemaker cells, where it likely contributes critically to the provocation of repetitive neuronal firing (7,13,17,29). In our study results, HCN4 immunoreactivities were unaltered throughout the hippocampus during the epileptogenic period following SE. These immunoreactive patterns were maintained until 5 weeks after pilocarpine treatments. However, HCN2 immunoreactivity was slightly elevated in the interneuronal populations in all the epileptic hippocampi. These findings may indicate that, at a later stage of the epileptogenic period in the SE hippocampus, expression of HCN2 subtype somewhat affected the dendritic excitation of the dentate gyrus. In fact, a previous study demonstrated that increased abundance of slower-kinetic HCN2 channels in affected neurons promoted neuronal activity-dependent depolarization and firing and enhanced excitation in the hippocampal circuit (12,16). However, their contribution to the overall cellular HCN physiology of hippocampal neurons appears to be relatively minor (16). Therefore, these results may reflect that altered expressions of HCN2, including HCN4, in the SE hippocampus may not be the main factors but minor factors of the enhanced excitation of hippocampal circuits in the epileptogenic period.
In conclusion, the present results reveal that major spatio-temporal alterations of HCN1, but minor alterations of HCN2 and 4, occurred in the epileptic hippocampus following pilocarpine-induced SE. These changes may contribute to the imbalance between inhibitory and excitatory inputs to hippocampal circuits; thus HCN1 may mainly play an important role in the epileptogenic period after SE as a compensatory response.
MATERIALS AND METHODS
Experimental animals
This study utilized the progeny of Sprague - Dawley (SD) rats (male, 9-11 weeks old) obtained from Experimental Animal Center, Soonchunhyang University (Cheonan, South Korea). The animals were provided with a commercial diet and water ad libitum under conditions of controlled temperature, humidity, and lighting conditions (22 ± 2℃, 55 ± 5%, and a 12:12 light/dark cycle). Procedures involving animals and their care were conducted in accordance with our institutional guidelines, which comply with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, 1996). In addition, we made all efforts to minimize the number of animals used and their suffering.
Seizure induction
Rats were intraperitoneally (i.p.) treated with pilocarpine (380 mg/kg) at 20 minutes after atropine methylbromide (5 mg/kg, i.p.). Approximately, 80% of pilocarpine-treated rats showed acute behavioral features of SE, including akinesia, facial automatisms, limbic seizures consisting of forelimb clonus with rearing, salivation, masticatory jaw movements, and falling. Diazepam (10 mg/kg, i.p.) was administered 2 hours after the onset of SE and repeated as needed. The rats were then observed for 3-4 hours a day in the vivarium for general behavior and occurrence of spontaneous seizures. Spontaneous recurrent seizures were seen at 3-4 weeks after SE. At designated time courses, animals were used for immunohistochemistry. Non-experienced SE rats (showing only acute seizure behaviors during 10-30 minutes, n = 22) and age-matched normal rats were used as controls (n = 15).
Tissue processing and Immunohistochemistry
At designated time courses (30 min, 3, 6, 12 hrs, 1, 2, 3, 4, 5, 6, 7 days, 2-4 weeks, and 2-3 months after SE), experimental animals (n = 3, respectively) were anesthetized (urethane, 1.5 g/kg, i.p.) and perfused transcardially with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde in 0.1 M PB (pH 7.4). The brains were removed, and postfixed in the same fixative for 4 hr. The brain tissues were cryoprotected by infiltration with 30% sucrose overnight. Thereafter the tissues were frozen and sectioned with a cryostat at 30 μm and consecutive sections were collected in six-well plates containing PBS. The sections were first incubated with 3% bovine serum albumin in PBS for 30 min at room temperature. Sections were then incubated in below mentioned primary antibodies in PBS containing 0.3% triton X-100 overnight at room temperature; the antibodies employed were rabbit anti-HCN1, HCN2, and HCN4 IgG (Alomone labs, Israel, diluted 1:200). The sections were washed three times for 10 min with PBS, incubated sequentially in biotinylated goat anti-rabbit IgG (Vector, USA) and Avidin-Biotin Complex (ABC; Vector, USA), and diluted 1:200 in the same solution as the primary antiserum. In-between the incubations, the tissues were washed thrice with PBS for 10 min each. The sections were visualized with 3,3’-diaminobenzidine (DAB) in 0.1 M Tris buffer and mounted on gelatin-coated slides. In order to establish the specificity of the immunostaining, a negative control test was carried out with pre-immune serum instead of the primary antibody. Preabsorption tests were also performed with control peptides. The control for immunohistochemistry resulted in the absence of immunoreactivity in any structure (data not shown). The immunoreactions were observed under an Olympus BX50 microscope (Japan), and the images were captured using Olympus DP72 digital camera and DP2-BSW microscope digital camera software.
Densitometry analysis of data
The immunohistochemical data were quantified as previously described (30-32). Briefly, the images of each section on the monitor were captured (15 sections per animal). The mean gray value and standard deviation were obtained from the selected images using Adobe PhotoShop, version. 8.0. Each image was normalized by assessing the mean gray value. After the regions (CA1-3 pyramidal cell layers) were outlined, 10 areas/rat (500 μm2/area) were selected from the hippocampus, and the gray values were measured. The intensity measurements were represented as the mean number of a 256-gray scale using NIH Image 1.59 software. The values of background staining were obtained from the corpus callosum. Optical density values were then corrected by subtracting the average values of background noise obtained from 15 image inputs.
Cell count
Cell counts were carried out with a microscope connected via a CCD camera to a PC monitor. At a magnification of 25-50×, the hippocampal regions were outlined and the surface areas measured. The HCN1, HCN2 and HCN4-positive cells were counted by clicking on the monitor, at a magnification of 100×. All the HCN subtypes-positive cells were counted regardless of the intensity of labeling. Based on the localization and the morphology, HCN1, HCN2 and HCN4-positive neurons were identified as interneurons (CA1-3 regions and dentate gyrus). Cell counts were performed by two different investigators who were blinded to the classification of tissues. The estimated cell number (n) was the average of values obtained from three adjacent sections. Since the measurement of nucleus size was used to correct the potential sampling bias, the diameter for each nucleus in the sample population was also measured at a magnification of 200 × and reduced to a mean diameter (D). The true estimate of cell number was then calculated using the Abercrombie correction method, as follows: N (per 250 × 250 μm2) = n (T/T + D)/A, where N is the true cell number, T is the section thickness, and A is the measured area (per 250 × 250 μm2) of each hippocampal region (30-34).
Statistical analysis
All data obtained from the quantitative measurements were analyzed using one-way analysis of variance (ANOVA) to determine statistical significance. Bonferroni’s test was used for post-hoc comparisons. A P-value < 0.01 or 0.05 was considered statistically significant (30-34).
Acknowledgments
This work was supported in part by the Soonchunhyang University Research Fund.
References
- 1.Dalby N. O., Mody I. The process of epileptogenesis; a pathophysiological approach. Curr. Opin. Neurol. (2001);14:187–192. doi: 10.1097/00019052-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Margerison J. H., Corsellis J. A. Epilepsy and the temporal lobes. A clinical, electroencephalographic and neuropathological study of the brain in epilepsy, with particular reference to the temporal lobes. Brain. (1966);89:499–530. doi: 10.1093/brain/89.3.499. [DOI] [PubMed] [Google Scholar]
- 3.De Lanerolle N. C., Kim J. H., Robbins R. J., Spencer D. D. Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res. (1989);495:389–395. doi: 10.1016/0006-8993(89)90234-5. [DOI] [PubMed] [Google Scholar]
- 4.Mathern G. W., Babb T. L., Pretorius J. K., Melendez M., Levesque M. F. The pathophysiologic relationships between lesion pathology, intracranial ictal EEG onsets, and hippocampal neuron losses in temporal lobe epilepsy. Epilepsy Res. (1995);21:133–147. doi: 10.1016/0920-1211(95)00014-2. [DOI] [PubMed] [Google Scholar]
- 5.Wittner L., Magloczky Z., Borhegyi Z., Halasz P., Toth S., Eross L., Szabo Z., Freund T.T. Preservation of perisomatic inhibitory input of granule cells in the pileptic human dentate gyrus. Neuroscience. (2001);108:587–600. doi: 10.1016/S0306-4522(01)00446-8. [DOI] [PubMed] [Google Scholar]
- 6.DiFrancesco D. Pacemaker mechanisms in cardiac tissue. Annu. Rev. Physiol. (1993);55:455–472. doi: 10.1146/annurev.ph.55.030193.002323. [DOI] [PubMed] [Google Scholar]
- 7.Pape H. C. Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu. Rev. Physiol. (1996);58:299–327. doi: 10.1146/annurev.ph.58.030196.001503. [DOI] [PubMed] [Google Scholar]
- 8.Maccaferri G., Mangoni M., Lazzari A., DiFrancesco D. Properties of the hyperpolarization-activated current in rat hippocampal CA1 pyramidal cells. J. Neurophysiol. (1993);69:2129–2136. doi: 10.1152/jn.1993.69.6.2129. [DOI] [PubMed] [Google Scholar]
- 9.Richter H., Heinemann U., Eder C. Hyperpolarization-activated cation currents in stellate and pyramidal neurons of rat entorhinal cortex. Neurosci. Lett. (2000);281:33–36. doi: 10.1016/S0304-3940(00)00794-1. [DOI] [PubMed] [Google Scholar]
- 10.Dickson C. T., Magistretti J., Shalinsky M. H., Fransen E., Hasselmo M. E., Alonso A. Properties and role of I(h) in the pacing of subthreshold oscillations in entorhinal cortex layer II neurons. J. Neurophysiol. (2000);83:2562–2579. doi: 10.1152/jn.2000.83.5.2562. [DOI] [PubMed] [Google Scholar]
- 11.Williams S. R., Stuart G. J. Site independence of EPSP time course is mediated by dendritic I(h) in neocortical pyramidal neurons. J. Neurophysiol. (2000);83:3177–3182. doi: 10.1152/jn.2000.83.5.3177. [DOI] [PubMed] [Google Scholar]
- 12.Chen K., Aradi I., Thon N., Eghbal-Ahmadi M., Baram T. Z., Soltesz I. Persistently modified h-channels after complex febrile seizures convert the seizure-induced enhancement of inhibition to hyperexcitability. Nat. Med. (2001);7:331–337. doi: 10.1038/85480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bender R. A., Soleymani S. V., Brewster A. L., Nguyen S. T., Beck H., Mathern G. W., Baram T. Z. Enhanced expression of a specific hyperpolarization-activated cyclic nucleotide-gated cation channel (HCN) in surviving dentate gyrus granule cells of human and experimental epileptic hippocampus. J. Neurosci. (2003);23:6826–6836. doi: 10.1523/JNEUROSCI.23-17-06826.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker M. C., Kullmann D. M. Febrile convulsions; a ‘benign’ condition? Nat. Med. (1999);5:871–872. doi: 10.1038/11308. [DOI] [PubMed] [Google Scholar]
- 15.Chen K., Baram T. Z., Soltesz I. Febrile seizures in the developing brain result in persistent modification of neuronal excitability in limbic circuits. Nat. Med. (1999);5:888–894. doi: 10.1038/70932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brewster A., Bender R. A., Chen Y., Dubé C., Eghbal-Ahmadi M., Baram T. Z. Developmental febrile seizures modulate hippocampal gene expression of hyperpolarization-activated channels in an isoform- and cell-specific manner. J. Neurosci. (2002);22:4591–4599. doi: 10.1523/JNEUROSCI.22-11-04591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santoro B., Chen S., Lüthi A., Pavlidis P., Shumyatsky G. P., Tibbs G. R., Siegelbaum S. A. Molecular and functional heterogeneity of hyperpolarization- activated pacemaker channels in the mouse CNS. J. Neurosci. (2000);20:5264–5275. doi: 10.1523/JNEUROSCI.20-14-05264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bender R. A., Brewster A., Santoro B., Ludwig A., Hofmann F., Biel M., Baram T. Z. Differential and age-dependent expression of hyperpolarization-activated, cyclic nucleotide-gated cation channel isoforms 1-4 suggests evolving roles in the developing rat hippocampus. Neuroscience. (2001);106:689–698. doi: 10.1016/S0306-4522(01)00314-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lupica C. R., Bell J. A., Hoffman A. F., Watson P. L. Contribution of the hyperpolarization-activated current (Ih) to membrane potential and GABA release in hippocampal interneurons. J. Neurophysiol. (2001);86:261–268. doi: 10.1152/jn.2001.86.1.261. [DOI] [PubMed] [Google Scholar]
- 20.Magee J. C. Dendritic Ih normalizes temporal summation in hippocampal CA1 neurons. Nat. Neurosci. (1999);2:508–514. doi: 10.1038/9158. [DOI] [PubMed] [Google Scholar]
- 21.Magee J. C. Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. J. Neurosci. (1998);18:7613–7624. doi: 10.1523/JNEUROSCI.18-19-07613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poolos N. P., Migliore M., Johnston D. Pharmacological upregulation of h-channels reduces the excitability of pyramidal neuron dendrites. Nat. Neurosci. (2002);5:767–774. doi: 10.1038/nn891. [DOI] [PubMed] [Google Scholar]
- 23.Okazaki M. M., Evenson D. A., Nadler J. V. Hippocampal mossy fiber sprouting and synapse formation after status epilepticus in rats; visualization after retrograde transport of biocytin. J. Comp. Neurol. (1995);352:515–534. doi: 10.1002/cne.903520404. [DOI] [PubMed] [Google Scholar]
- 24.Wenzel H. J., Woolley C. S., Robbins C. A., Schwartzkroin P. A. Kainic acid-induced mossy fiber sprouting and synapse formation in the dentate gyrus of rats. Hippocampus. (2000);10:244–260. doi: 10.1002/1098-1063(2000)10:3<244::AID-HIPO5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 25.Buckmaster P. S., Zhang G. F., Yamawaki R. Axon sprouting in a model of temporal lobe epilepsy creates a predominantly excitatory feedback circuit. J. Neurosci. (2002b);22:6650–6658. doi: 10.1523/JNEUROSCI.22-15-06650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buckmaster P. S., Yamawaki R., Zhang G. F. Axon arbors and symaptic connections of a vulnerable population of interneurons in the dentate gyrus in vivo. J. Comp. Neurol. (2002a);445:360–373. doi: 10.1002/cne.10183. [DOI] [PubMed] [Google Scholar]
- 27.Isokawa M., Levesque M., Fried I., Engel J.Jr. Glutamate currents in morphologically identified human dentate granule cells in temporal lobe epilepsy. J. Neurophysiol. (1997);77:3355–3369. doi: 10.1152/jn.1997.77.6.3355. [DOI] [PubMed] [Google Scholar]
- 28.Moosmang S., Biel M., Hofmann F., Ludwig A. Differential distribution of four hyperpolarizationactivated cation channels in mouse brains. J. Biol. Chem. (1999);380:975–980. doi: 10.1515/BC.1999.121. [DOI] [PubMed] [Google Scholar]
- 29.Ludwig A., Budde T., Stieber J., Moosmang S., Wahl C., Holthoff K., Langebartels A., Wotjak C., Munsch T., Zong X., Feil S., Feil R., Lancel M., Chien K. R., Konnerth A., Pape H. C., Biel M., Hofmann F. Absence epilepsy and sinus dysrhythmia in mice lacking the pacemaker channel HCN2. EMBO J. (2003);22:216–224. doi: 10.1093/emboj/cdg032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim D. S., Kim J. E., Kwak S. E., Won M. H., Kang T. C. Seizure activity affects neuroglial Kv1 channel immunoreactivities in the gerbil hippocampus. Brain Res. (2007);1151:17–187. doi: 10.1016/j.brainres.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 31.Kim D. S., Kim J. E., Kwak S. E., Choi K. C., Kim D. W., Kwon O. S., Choi S. Y., Kang T. C. Spatiotemoral characteristics of astroglial death in the rat hippocampo-entorhinal complex following pilocarpine-induced status epilepticus. J. Comp. Neurol. (2008);511:581–598. doi: 10.1002/cne.21851. [DOI] [PubMed] [Google Scholar]
- 32.An S. J., Kim D. S. Alterations in serotonin receptors and transporter immunoreactivities in the hippocampus in the rat unilateral hypoxic-induced epilepsy model. Cell Mol. Neurobiol. (2011);31:1245–1255. doi: 10.1007/s10571-011-9726-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park D. K., Park K. H., Ko J. S., Kim D. S. Alteration in NCX-3 immunoreactivity within the gerbil hippocampus following spontaneous seizure. BMB Rep. (2011);44:306–311. doi: 10.5483/BMBRep.2011.44.5.306. [DOI] [PubMed] [Google Scholar]
- 34.Lee S. B., Oh Y. J., Chung J. K., Jeong J. H., Lee S. D., Park D. K., Park K. H., Ko J. S., Kim D. S. Altered PLCbeta-1 expression in the gerbil hippocampal complex following spontaneous seizure. BMB Rep. (2011);44:566–571. doi: 10.5483/BMBRep.2011.44.9.566. [DOI] [PubMed] [Google Scholar]


