Abstract
Granulocyte colony-stimulating factor (G-CSF) is used for heart failure therapy and promotes myocardial regeneration by inducing mobilization of bone marrow stem cells to the injured heart after myocardial infarction; however, this treatment has one weakness in that its biological effect is transient. In our previous report, we generated 5 mutants harboring N-linked glycosylation to improve its antiapoptotic activities. Among them, one mutant (Phe140Asn) had higher cell viability than wild-type hG-CSF in rat cardiomyocytes, even after treatment with an apoptotic agent (H2O2). Cells treated with this mutant significantly upregulated the antiapoptotic proteins, and experienced reductions in caspase 3 activity and PARP cleavage. Moreover, the total number of apoptotic cells was dramatically lower in cultures treated with mutant hG-CSF. Taken together, these results suggest that the addition of an N-linked glycosylation was successful in improving the antiapoptotic activity of hG-CSF, and that this mutated product will be a feasible therapy for patients who have experienced heart failure. [BMB Reports 2012; 45(12): 742-747]
Keywords: Antiapoptotic activity, Heart failure, hG-CSF (Phe140Asn)
INTRODUCTION
G-CSF is a hematopoietic cytokine that induces proliferation and differentiation of neutrophil progenitors (1). After myocardial infarction, G-CSF promotes the mobilization of hematopoietic stem cells into heart tissue (2) and enhances the regeneration of cardiomyocytes and blood vessels by mobilizing and subsequently trans-differentiating bone marrow stem cells (3). Recently, it was reported that G-CSF averts H2O2-induced apoptosis of cardiomyocytes and facilitates cardiac remodeling after myocardial infarction (4). However, human G-CSF (hG-CSF) is expensive and has a short half life (5). Since a number of patients with heart failure suffer from physical and economic problems, there is increasing interest in developing a new long-lasting G-CSF with higher biological activity.
Oxidative stress, including that caused by exposure to H2O2, is increased in the myocardia of patients with chronic left ventricular systolic failure (6), and in animal models with heart failure after myocardial infarction (7). Therefore, in this study, H2O2 was used for mimicking in vivo heart failure. This chemical also induces apoptosis in H9c2 cells by upregulating active caspase 3, which stimulates poly(ADP-ribose) polymerase (PARP)- cleavage (8,9). Thus, H2O2 can be used to experimentally induce cell death in H9c2 rat cardiomyocytes.
The biological activity of Lenograstim is indistinguishable from that of endogenous hG-CSF, though its half-life in serum, 3-4 hours, is relatively short (5). Lenograstim has a single O-linked glycosylation at Thr133, the chemical structure of which is NeuNAc(α2-3)Gal(/ll-3)NeuNAc(α2-6)l (10-12). The carbohydrate chain of the glycoprotein is critical for its biological activities such as stability, half-life, and solubility (12,13). In our previous report, a novel N-linked hG-CSF (Phe140Asn) had enhanced biological activity in HL60 cells (14). Herein, we investigate another function of this mutant glycosylation in heart failure.
Human G-CSF has five cysteines (Cys, C) and two disulfide bonds (Cys36-Cys42 and Cys64-Cys74)(15). hG-CSF in which the free cysteine (Cys17) was mutated to alanine (Ala, A) was more stable than the wild type (WT) (16). A 7-point mutation in which glycines (Gly, G) were changed to Ala (17) yielded 4 versions of hG-CSF that had significantly more thermodynamic stability than WT. However, no work has been undertaken to determine whether these mutants had antiapoptotic effects in cardiomyocytes.
Here, we compared the antiapoptotic activity of previously generated mutant and WT hG-CSF by evaluating their abilities to upregulate antiapoptotic molecules, downregulate factors involved in the apoptotic process, and decrease the total number of apoptotic cells in cultures treated with H2O2. Our findings suggest that the novel mutant can be used to treat heart failure patients, including those who have experienced myocardial infarction.
RESULTS
Generation of mutant hG-CSFs via site-directed mutagenesis
Mutant candidates were selected as target products of site-directed mutagenesis if they exceeded the threshold for N-glycosylation potential (Supplemental Fig. S2). To develop hG-CSF with enhanced biological activity, 5 mutants for hG-CSF were produced by exchanging the target residue with Asn (N). Human G-CSF has 14 Ser and 7 Thr residues. Of these, residues within the 4 α-helices (Ser12, Ser76, Ser80, Ser155, Ser159, Ser164, Thr38, Thr102, Thr105, Thr115, and Thr116) were eliminated as possible mutagenesis candidates because exchanging them with Asn could have disrupted the structure of hG-CSF. This left 5 serines that were potential candidates for mutagenesis (Ser7, Ser8, Ser53, Ser62, and Ser142). The residues positioned prior to these amino acids were replaced with Asn, generating 5 mutants: Pro5Asn (Mut #1), Ala6Asn (Mut #2), Gly51Asn (Mut #3), Pro60Asn (Mut #4) and Phe140Asn (Mut #5) (Fig. 1A).
Fig. 1. Expression of mutant hG-CSFs in CHO cells. (A) Sequences of hG-CSF. Human G-CSF is composed of 204 amino acids containing 30 residues of signal peptides (gray circles) and four α-helices (black circles). The single O-linked glycosylation occurs at Thr133. Mutagenesis was used to create 5 mutants with different N-linked glycosylation sites (gray squares). (B) Results from ELISA investigating expression levels of mutant hG-CSFs. The data reflect means calculated from 2 sets of experiments performed in triplicate; error bars indicate the standard deviation. (C) Results from Western blot comparing the control (M), WT, mutant hG-CSFs, and Lenograstim (L). The 3 mutants (Mut #3, #4, and #5) had the additional upper bands regarding N-linked glycosylation. (D) Proteins (300 μg) from culture media transfected with mutant hG-CSFs were incubated with 10 units of N-glycosidase F for 20 h at 37℃. For WT hG-CSF, 300 μg total protein was incubated with 10 units of sialidase (Roche) and 5× reaction buffer (250 mM sodium phosphate, pH 5.0) for 1 h at 37℃ and mixed with 30 mU O-glycosidase (Roche) and incubated at 37℃ overnight. The control is WT hG-CSF treated with O-glycosidase. M means the culture media transfected with empty vector (pCMV-Tag4A). L is Lenograstim (GranocyteⓇ) and F is Filgrastim (NEUPOGENⓇ); these were used for size control of glycosylated and non-glycosylated hG-CSF, respectively.
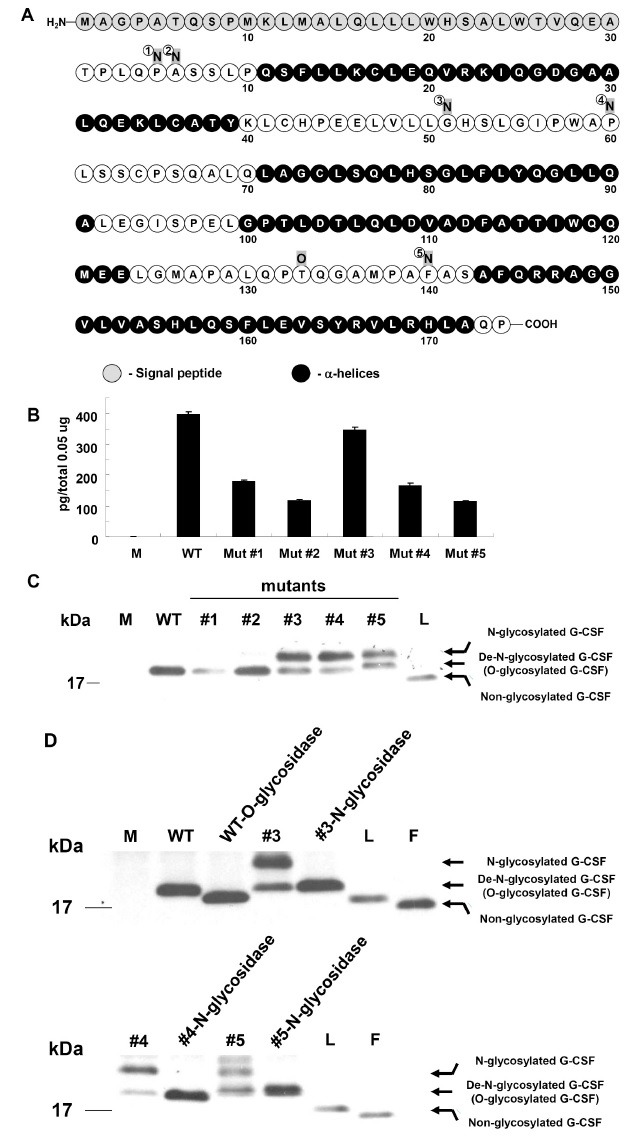
Expression and N-linked glycosylation of hG-CSF mutants in CHO cells
To determine the expression of the generated hG-CSF, WT and mutant hG-CSFs cloned to pCMV-Tag4A were transiently transfected into CHO cells. WT hG-CSF was expressed at a higher level than mutant hG-CSFs (approximately 0.4 ng per 0.05 μg of total protein) (Fig. 1B). Western blot analysis showed that N-linked glycosylation had successfully been introduced to 3 of the mutants (Mut #3, #4, and #5); this element can be visualized as an additional (upper) band (Fig. 1C). The presence of the glycosylation was confirmed by treatment with N-glycosidase F (Fig. 1D). N-glycosidase F cleaves all types of Asn bound N-glycans provided that the amino-group as well as the carboxyl group are present in a peptide linkage, and that the oligosaccharide has the minimum length of the chitobiose core unit (18,19). As a result, N-glycosylated bands of mutant hG-CSFs were identified by detecting the down-developed bands. As a positive control, O-glycosidase was also applied to the culture media transfected with WT hG-CSF. O-glycosidase is used to release the Gal β (1-3) GalNAc unit from O-glycans linked to the hydroxyls of Ser or Thr (20). Consequently, the down bands of non-glycosylated WT were detected (upper panel in Fig. 1D). These data indicate that the mutants (#3, #4, and #5) for hG-CSF have N-linked glycosylation.
Enhanced viability of H9c2 cells upon Mut #5 treatments
Cell viabilities of N-glycosylated mutants were tested using an MTT cell proliferation assay with rat cardiomyocytes (H9c2 cells). The overall scheme for cell viability and apoptosis analysis of the hG-CSFs generated is illustrated in Fig. 2A. Mutants #3, #4, and #5 had N-linked glycosylation (Fig. 1D); however, only cells treated with Mut #5 had statistically higher proliferation rates than cells treated with WT hG-CSF (P < 0.05, Fig. 2B). H2O2 treatment induced apoptosis in H9c2 cells (8,21). Consistent with the cell proliferation results, Mut #5 maintained a statistically higher level of cell proliferation after addition of H2O2 (P<0.05) (Fig. 2B). To verify the dosage effects of WT and Mut #5 hG-CSF, they were treated with three different doses. As a result, dosage effects for WT and Mut #5 hG-CSF were seen upon H2O2 treatment (Fig. 2D); however, the effects were not seen in normal condition (Fig. 2C). This suggests that Mut #5 is better than WT hG-CSF at protecting cardiac myocytes from H2O2-induced apoptosis. In this regard, we further analyzed the antiapoptotic effects for Mut #5 hG-CSF in H9c2 cells.
Fig. 2. Enhanced viability of H9c2 cells upon mutant hG-CSFs treatments. (A) Overall scheme for the enhanced biological activity test of the generated hG-CSF. (B) H9c2 cell proliferation assay in mutant hG-CSFs treatments after 24 h (white bar) and upon 1 mM H2O2 treatment (black bar). Absorbance for MTT assay was evaluated at 550 nm and the reference filter was 655 nm. The data represent the means from 3 sets of experiments performed in triplicate; error bars indicate standard deviation. Asterisks (*) indicate values that were significantly different (P < 0.05) from those of WT. M: control (CHO cell culture media transfected with an empty vector, pCMV-Tag4); F.I.: fold increase compared to M. (C) Cell proliferation assay for differential doses of hG-CSF WT and mutant #5. Cells were treated with the designated concentration for 48 h. (D) Cell viability test for differential doses of hG-CSF WT and mutant #5 upon 1 mM H2O2 treatment. Asterisks (* and **) indicate values that were significantly different (P < 0.05) from those of M and WT in the same concentration, respectively. Hashes (# and ##) indicate P < 0.05 in a comparison with 20 ng/ml and 100 ng/ml treatments, respectively.
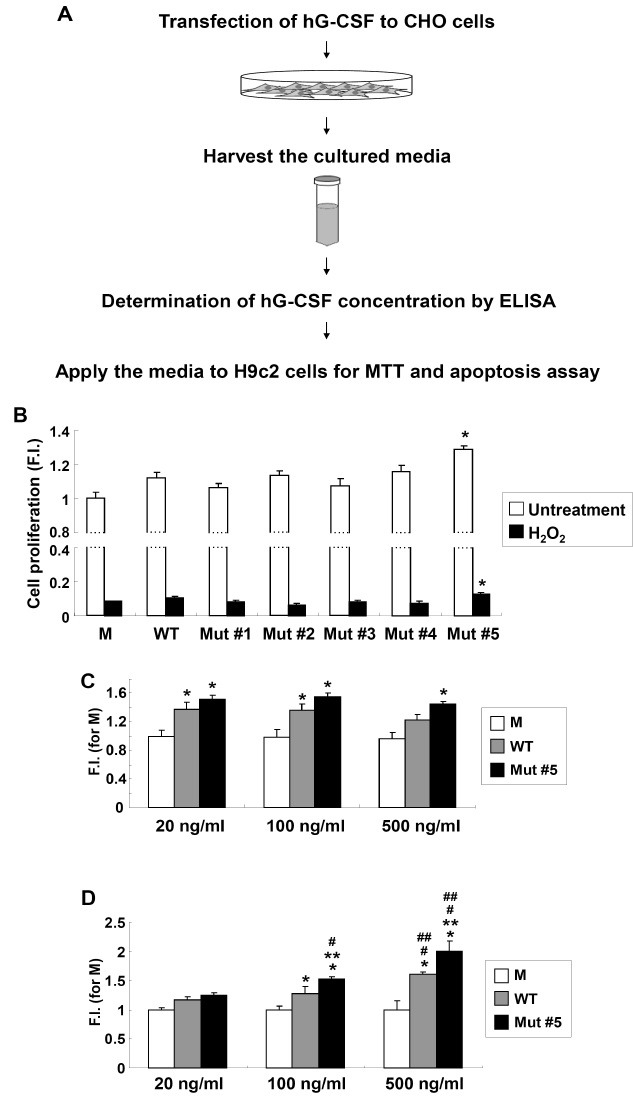
Anti-apoptotic effects of Mut #5 in H9c2 cells
To explore the enhanced anti-apoptotic effects of Mut #5, the expression of antiapoptotic proteins Bcl-xL and Bcl-2 upon Mut #5 was examined. Bcl-xL and Bcl-2 expression in H9c2 cells was upregulated in a time-dependent manner after treatment with hG-CSF (Fig. 3A and 3B). Additionally, these effects were more presented in Mut #5 than WT hG-CSF (P < 0.05 at both time points for Bcl-xL and Bcl-2). These same patterns were also found in cells treated with H2O2 (Fig. 3C). In H2O2-treated cells, the upregulation of Bcl-2 by Mut #5 was particularly strong (Fig. 3C), suggesting that this mutant is more efficient for stimulating anti-apoptotic defense mechanisms.
Fig. 3. Regulation of apoptotic proteins in H9c2 cells treated with Mut #5. (A) Comparison of Bcl-xL expression at 24 and 48 h upon 400 ng/ml WT and Mut #5 hG-CSF; histogram showed the band intensities in the lower panel. M indicates H9c2 cells treated with CHO cell culture media transfected with empty vector. Asterisks (*) indicate values that were significantly different (P < 0.05) from those of WT. (B) Results from real-time RT-PCR investigating expression of Bcl-2 and Bcl-xL at 24 h (white) and 48 h (black). Single symbols (# for 24 h and * for 48 h) indicate P < 0.05 in a comparison with M. Double symbols (**) indicate P < 0.05 in a comparison with WT and Mut #5. (C) Results from real-time RT-PCR for Bcl-2 and Bcl-xL expression in cells that were (black) or were not (white) treated with H2O2. Single symbols (#untreatment; *, H2O2) indicate P < 0.05 in a comparison with M. Double symbol (**) indicates P < 0.05 in a comparison with WT and Mut #5. GAPDH and β-actin were used as internal controls. (D) Results from H2O2-induced caspase 3 activity in the control (M) and in cells treated with either 400 ng/ml WT or Mut #5 hG-CSF. M means H9c2 cells treated with the culture media transfected with empty vector. F.I. means fold increase compared to M. Single asterisks (*) indicate P < 0.05 in a comparison with M, and double asterisks (**) indicate P < 0.05 in a comparison between WT and Mut #5. (E) Identification of PARP cleavage (cleaved fragment: 89 kDa) upon 400 ng/ml WT and Mut #5 hG-CSF. Numbers below the bands are ratios indicating the intensities between upper and lower bands. Actin was used as the loading control.
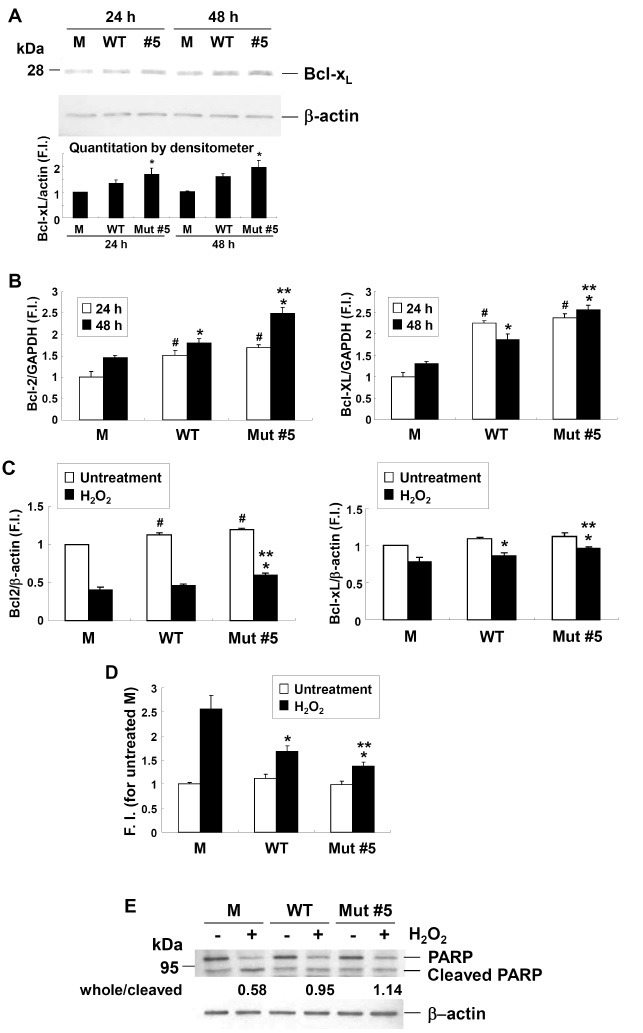
To determine whether these mechanisms actually reduced apoptosis in rat cardiomyocytes, caspase 3 activity, an indicator of apoptosis, was evaluated in cells treated with H2O2 (Fig. 3D). Both WT and Mut #5 treatments significantly decreased apoptotic activity (P < 0.05) in comparison to the control treatment, but Mut #5 had a significantly higher antiapoptotic effect than WT hG-CSF (P < 0.05) (Fig. 3D). Furthermore, a Western blot analysis demonstrated that active caspase 3 induced PARP cleavage (Fig. 3E). Control cells (M) contained the highest levels of cleaved PARP (whole/cleaved PARP ratio: 0.58), while cells treated with WT hG-CSF had intermediate levels of PARP cleavage (ratio: 0.95), and PARP cleavage was lowest in cells treated with Mut #5 (ratio: 1.14) (Fig. 3E).
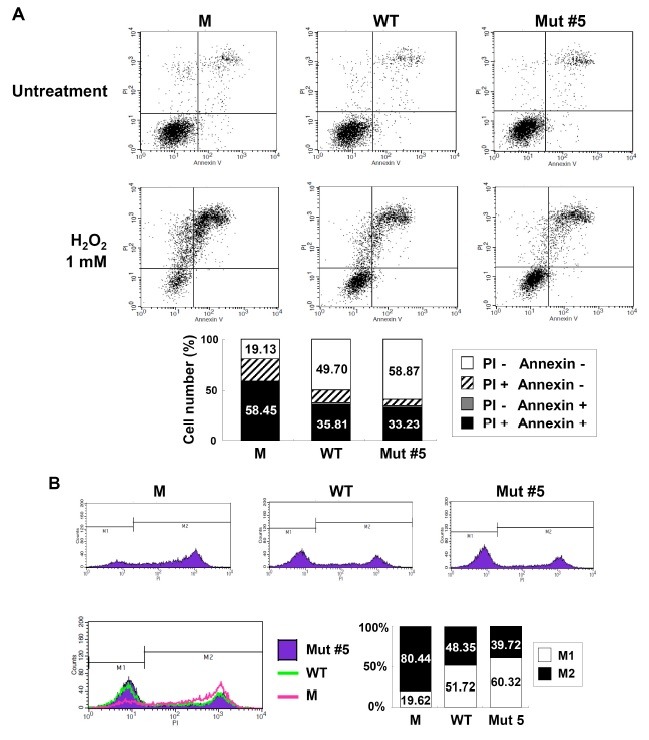
The anti-apoptotic effect of Mut #5 in H2O2-treated H9c2 cells was further demonstrated by FACS analysis, which showed that cell death (as demonstrated by testing positive for Annexin V and PI) occurred at a lower rate in Mut #5- than WT hG-CSF-treated cells (33.23% vs. 35.81%); conversely, survival was higher in Mut #5-treated than WT hG-CSF-treated cells (58.87% vs. 49.70%, respectively) (Fig. 4A). Additionally, the histogram data showed that apoptotic cells (PI positive; M2) were more common in the WT group (48.35%) than the Mut #5 group (39.72%) (Fig. 4B). Taken together, these data suggest that Mut #5 has potential to act as a therapeutic drug for patients who have experienced a myocardial infarction.
Fig. 4. Visualization of apoptotic activity in cells treated with Mut #5. (A) Results from flow cytometry analysis used to visualize the amount of apoptotic activity in the control (M), WT, and Mut #5 hG-CSF cells stained with Annexin V and PI. The histogram in the lower panel provides a comparison between the total number of apoptotic cells (black and gray bars) and surviving cells (white bars). (B) Results from the FACS analysis comparing the number of live (M1, white bars) and dead (M2, black bars) cells among the 3 treatments.
DISCUSSION
The number of heart failure patients has risen over the past several decades (22), over which time a variety of promising therapies has been developed to reduce the damage caused by myocardial infarction. Among them, treatment with G-CSF, which in combination with stem cell factor (SCF), significantly enhances cardiac function and decreases fatalities among mice that have experienced acute myocardial infarction (3). However, G-CSF has a short life in serum, requiring patients to receive multiple, and often expensive injections. Thus, we used N-linked glycosylation to develop a novel hG-CSF that would have stronger anti-apoptotic activities in injured cardiomyocytes.
The mutagenesis process produced 2 types of mutant hG-CSFs (those with only 1 band, like WT, and those with 2 bands, indicating the presence of N-linked glycosylation) (Fig. 1C). This suggests that the N-linked glycosylation event for the single-band mutants, #1 and #2, was not complete. Processing of N-glycans occurs mainly in the endoplasmic reticulum (ER), whereas O-linked glycosylation is initiated in the Golgi apparatus (23-26). Therefore, it is possible that there may be some polypeptides that have not undergone ER processing. Moreover, the bands without N-glycosidase treatment were 2 different sizes (Fig. 1D, lower panel). Lenograstim, expressed in CHO cells, is a 50:50 mixture of 2 glycosylated G-CSF forms containing sialic acid, galactose (Gal), and galactosamine (27,28). Additionally, CHO cells contain various glycans, including sialic acids. The size of glycoprotein molecules varies depending on how many sialic acids they contain. It would appear that most glycoproteins, including hG-CSF, can achieve glycosylation through multiple pathways. Therefore, it is not surprising that Mut #5 was so different from the other mutant hG-CSFs.
Despite the fact that N-linked glycosylation plays a pivotal role in enhancing enzymatic activity (13), Mut #3 and #4 hG-CSFs with this feature did not perform significantly different from WT hG-CSF (Fig. 1C and 2B). Human G-CSF has another short helix structure, but with 4 anti-parallel helices. The short helix is involved in major binding with G-CSF receptor (G-CSFR) (29); therefore, a mutation in this region disrupts the interaction between G-CSF and G-CSFR. Mut #3 (Gly51Asn) may have failed to promote proliferation in H9c2 cells (Fig. 2) because it was positioned within this short helix structure. Mut #4 (Pro60Asn), on the other hand, appears to have changed the structure of hG-CSF by removing a proline (Pro, P), which has an aliphatic side chain that is vital for determining the proper folding structure of the protein. Therefore, a mutation in this residue (Mut #4: Pro60Asn) may abolish the original structure of hG-CSF such that the changed hG-CSF does not bind to G-CSFR. This would explain why Mut #4 did not significantly increase the viability of H9c2 cells (Fig. 2).
Mut #5 was associated with higher levels of Bcl-xL and Bcl-2 in both the presence and absence of H2O2 (Fig. 3). Bcl-xL is a downstream molecule in the ERK and AKT pathway activated by the hematopoietic growth factor macrophage-CSF (M-CSF) (30). Upregulation of this molecule is associated with the inhibition of apoptosis. Similarly, Bcl-2 also has anti-apoptotic activities and, like Bcl-xL, can be activated via the JAK/STAT3 pathway (4). Although the current study did not explicitly show activation of JAK/STAT pathways, the upregulation of both Bcl-xL and Bcl-2 in the presence of Mut #5 suggests that this mutant is capable of inhibiting myocardial cell death.
Reperfusion is a reliable treatment to diminish heart injury caused by myocardial ischemia (31). However, this treatment induces side effects mediated by reactive oxygen species (ROS) such as hydrogen peroxide, and activates the transcription of proinflammatory genes (e.g., PARP-1) that are cleaved by caspase 3 (32). The PARP inhibitor PJ-34 can alleviate problems associated with heart disease, and reduces doxorubicin-induced apoptosis of cardiomyocytes (33). Because of this, PARP-1 null mice have been used as an animal model in numerous experiments investigating heart failure (31). We found that Mut #5 also down-regulates caspase 3 activity and PARP cleavage in response to H2O2 treatment (Fig. 3). Therefore, a newly developed hG-CSF (Phe140Asn) will be further employed for studying heart failure using this animal model.
In this study, we have shown that Mut #5 exerts higher anti-apoptotic effect than WT hG-CSF against H2O2-induced apoptosis in H9c2 cardiomyocytes. This illustrates the significance of N-glycosylation in determining the functionality of cytokines and glycoproteins. In the future, we hope to provide a more detailed characterization of the glycans present in Phe140Asn. For now, however, we conclude that Mut #5 can be used to effectively and economically treat patients with heart failure.
MATERIALS AND METHODS
Detailed information is described in Supplementary Material.
Acknowledgments
This work received grant support from the Agenda Program (No.PJ006702) and Next-Generation BioGreen21 Program (PJ008169), Rural Development Administration, Republic of Korea. This study was supported by the 2010 Post-Doctoral Course Program of National Institute of Animal Science, Rural Development Administration, Republic of Korea.
References
- 1.Takano H., Qin Y., Hasegawa H., Ueda K., Niitsuma Y., Ohtsuka M., Komuro I. Effects of G-CSF on left ventricular remodeling and heart failure after acute myocardial infarction. J. Mol. Med. (2006);84:185–193. doi: 10.1007/s00109-005-0035-z. [DOI] [PubMed] [Google Scholar]
- 2.Minatoguchi S., Takemura G., Chen X. H., Wang N., Uno Y., Koda M., Arai M., Misao Y., Lu C., Suzuki K., Goto K., Komada A., Takahashi T., Kosai K., Fujiwara T., Fujiwara H. Acceleration of the healing process and myocardial regeneration may be important as a mechanism of improvement of cardiac function and remodeling by postinfarction granulocyte colony-stimulating factor treatment. Circulation. (2004);109:2572–2580. doi: 10.1161/01.CIR.0000129770.93985.3E. [DOI] [PubMed] [Google Scholar]
- 3.Orlic D., Kajstura J., Chimenti S., Limana F., Jakoniuk I., Quaini F., Nadal-Ginard B., Bodine D. M., Leri A., Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc. Natl. Acad. Sci. U.S.A. (2001);98:10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harada M., Qin Y., Takano H., Minamino T., Zou Y., Toko H., Ohtsuka M., Matsuura K., Sano M., Nishi J., Iwanaga K., Akazawa H., Kunieda T., Zhu W., Hasegawa H., Kunisada K., Nagai T., Nakaya H., Yamauchi-Takihara K., Komuro I. G-CSF prevents cardiac remodeling after myocardial infarction by activating the Jak-Stat pathway in cardiomyocytes. Nat. Med. (2005);11:305–311. doi: 10.1038/nm1199. [DOI] [PubMed] [Google Scholar]
- 5.Martin-Christin F. Granulocyte colony stimulating factors: how different are they? How to make a decision? Anticancer. Drugs. (2001);12:185–191. doi: 10.1097/00001813-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Mallat Z., Philip I., Lebret M., Chatel D., Maclouf J., Tedgui A. Elevated levels of 8-iso-prostaglandin F2alpha in pericardial fluid of patients with heart failure: a potential role for in vivo oxidant stress in ventricular dilatation and progression to heart failure. Circulation. (1998);97:1536–1539. doi: 10.1161/01.CIR.97.16.1536. [DOI] [PubMed] [Google Scholar]
- 7.Hill M. F., Singal P. K. Antioxidant and oxidative stress changes during heart failure subsequent to myocardial infarction in rats. Am. J. Pathol. (1996);148:291–300. [PMC free article] [PubMed] [Google Scholar]
- 8.Eguchi M., Liu Y., Shin E. J., Sweeney G. Leptin protects H9c2 rat cardiomyocytes from H2O2-induced apoptosis. FEBS J. (2008);275:3136–3144. doi: 10.1111/j.1742-4658.2008.06465.x. [DOI] [PubMed] [Google Scholar]
- 9.Liu J., Mao W., Ding B., Liang C. S. ERKs/p53 signal transduction pathway is involved in doxorubicin-induced apoptosis in H9c2 cells and cardiomyocytes. Am. J. Physiol. Heart. Circ. Physiol. (2008);295:H1956–1965. doi: 10.1152/ajpheart.00407.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Souza L. M., Boone T. C., Gabrilove J., Lai P. H., Zsebo K. M., Murdock D. C., Chazin V. R., Bruszewski J., Lu H., Chen K. K., Barendt J., Platzer E., Moore M. A. S., Mertelsmann R., Welte K. Recombinant human granulocyte colony-stimulating factor: effects on normal and leukemic myeloid cells. Science. (1986);232:61–65. doi: 10.1126/science.2420009. [DOI] [PubMed] [Google Scholar]
- 11.Kubota N., Orita T., Hattori K., Oh-eda M., Ochi N., Yamazaki T. Structural characterization of natural and recombinant human granulocyte colony-stimulating factors. J. Biochem. (1990);107:486–492. doi: 10.1093/oxfordjournals.jbchem.a123072. [DOI] [PubMed] [Google Scholar]
- 12.Gervais V., Zerial A., Oschkinat H. NMR investigations of the role of the sugar moiety in glycosylated recombinant human granulocyte-colony-stimulating factor. Eur. J. Biochem. (1997);247:386–395. doi: 10.1111/j.1432-1033.1997.00386.x. [DOI] [PubMed] [Google Scholar]
- 13.Cumming D. A. Glycosylation of recombinant protein therapeutics: control and functional implications. Glycobiology. (1991);1:115–130. doi: 10.1093/glycob/1.2.115. [DOI] [PubMed] [Google Scholar]
- 14.Chung H. K., Kim S. W., Byun S. J., Ko E. M., Chung H. J., Woo J. S., Yoo J. G., Lee H. C., Yang B. C., Kwon M., Park S. B., Park J. K., Kim K. W. Enhanced biological effects of Phe140Asn, a novel human granulocyte colony- stimulating factor mutant, on HL60 cells. BMB Rep. (2011);44:686–691. doi: 10.5483/BMBRep.2011.44.10.686. [DOI] [PubMed] [Google Scholar]
- 15.Lu H. S., Boone T. C., Souza L. M., Lai P. H. Disulfide and secondary structures of recombinant human granulocyte colony stimulating factor. Arch. Biochem. Biophys. (1989);268:81–92. doi: 10.1016/0003-9861(89)90567-5. [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa M., Iijima H., Satake-Ishikawa R., Tsumura H., Iwamatsu A., Kadoya T., Shimada Y., Fukamachi H., Kobayashi K., Matsuki S., Asano K. The substitution of cysteine 17 of recombinant human G-CSF with alanine greatly enhanced its stability. Cell Struct. Funct. (1992);17:61–65. doi: 10.1247/csf.17.61. [DOI] [PubMed] [Google Scholar]
- 17.Bishop B., Koay D. C., Sartorelli A. C., Regan L. Reengineering granulocyte colony-stimulating factor for enhanced stability. J. Biol. Chem. (2001);276:33465–33470. doi: 10.1074/jbc.M104494200. [DOI] [PubMed] [Google Scholar]
- 18.Tarentino A. L., Gomez C. M., Plummer T. H. Jr. Deglycosylation of asparagine-linked glycans by peptide: N-glycosidase F. Biochemistry. (1985);24:4665–4671. doi: 10.1021/bi00338a028. [DOI] [PubMed] [Google Scholar]
- 19.Chu F. K. Requirements of cleavage of high mannose oligosaccharides in glycoproteins by peptide N-glycosidase F. J. Biol. Chem. (1986);261:172–177. [PubMed] [Google Scholar]
- 20.Umemoto J., Bhavanandan V. P., Davidson E. A. Purification and properties of an endo-alpha-N-acetyl-D-galactosaminidase from Diplococcus pneumoniae. J. Biol. Chem. (1977);252:8609–8614. [PubMed] [Google Scholar]
- 21.Wang Z., Cui M., Sun L., Jia Z., Bai Y., Ma K., Chen F., Zhou C. Angiopoietin-1 protects H9c2 cells from H2O2-induced apoptosis through AKT signaling. Biochem. Biophys. Res. Commun. (2007);359:685–690. doi: 10.1016/j.bbrc.2007.05.172. [DOI] [PubMed] [Google Scholar]
- 22.Takano H., Ueda K., Hasegawa H., Komuro I. G-CSF therapy for acute myocardial infarction. Trends. Pharmacol. Sci. (2007);28:512–517. doi: 10.1016/j.tips.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu. Rev. Biochem. (1985);54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- 24.Van den Steen P., Rudd P. M., Dwek R. A., Opdenakker G. Concepts and principles of O-linked glycosylation. Crit. Rev. Biochem. Mol. Biol. (1998);33:151–208. doi: 10.1080/10409239891204198. [DOI] [PubMed] [Google Scholar]
- 25.Burda P., Aebi M. The dolichol pathway of N-linked glycosylation. Biochim. Biophys. Acta. (1999);1426:239–257. doi: 10.1016/S0304-4165(98)00127-5. [DOI] [PubMed] [Google Scholar]
- 26.Munro S. What can yeast tell us about N-linked glycosylation in the Golgi apparatus? FEBS Lett. (2001);498:223–227. doi: 10.1016/S0014-5793(01)02488-7. [DOI] [PubMed] [Google Scholar]
- 27.Oheda M., Hase S., Ono M., Ikenaka T. Structures of the sugar chains of recombinant human granulocyte-colony-stimulating factor produced by Chinese hamster ovary cells. J. Biochem. (1988);103:544–546. doi: 10.1093/oxfordjournals.jbchem.a122305. [DOI] [PubMed] [Google Scholar]
- 28.Clogston C. L., Hu S., Boone T. C., Lu H. S. Glycosidase digestion, electrophoresis and chromatographic analysis of recombinant human granulocyte colony-stimulating factor glycoforms produced in Chinese hamster ovary cells. J. Chromatogr. (1993);637:55–62. doi: 10.1016/0021-9673(93)83098-D. [DOI] [PubMed] [Google Scholar]
- 29.Tamada T., Honjo E., Maeda Y., Okamoto T., Ishibashi M., Tokunaga M., Kuroki R. Homodimeric cross-over structure of the human granulocyte colony-stimulating factor (GCSF) receptor signaling complex. Proc. Natl. Acad. Sci. U.S.A. (2006);103:3135–3140. doi: 10.1073/pnas.0511264103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okazaki T., Ebihara S., Asada M., Yamanda S., Saijo Y., Shiraishi Y., Ebihara T., Niu K., Mei H., Arai H., Yambe T. Macrophage colony-stimulating factor improves cardiac function after ischemic injury by inducing vascular endothelial growth factor production and survival of cardiomyocytes. Am. J. Pathol. (2007);171:1093–1103. doi: 10.2353/ajpath.2007.061191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pacher P., Szabo C. Role of poly(ADP-ribose) polymerase 1 (PARP-1) in cardiovascular diseases: the therapeutic potential of PARP inhibitors. Cardiovasc. Drug. Rev. (2007);25:235–260. doi: 10.1111/j.1527-3466.2007.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ungvari Z., Gupte S. A., Recchia F. A., Batkai S., Pacher P. Role of oxidative-nitrosative stress and downstream pathways in various forms of cardiomyopathy and heart failure. Curr. Vasc. Pharmacol. (2005);3:221–229. doi: 10.2174/1570161054368607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pacher P., Liaudet L., Bai P., Virag L., Mabley J. G., Hasko G., Szabo C. Activation of poly(ADP-ribose) polymerase contributes to development of doxorubicin-induced heart failure. J. Pharmacol. Exp. Ther. (2002);300:862–867. doi: 10.1124/jpet.300.3.862. [DOI] [PubMed] [Google Scholar]


