Abstract
In mammalian cells, aberrant transcripts harboring a premature termination codon (PTC) can be generated by abnormal or inefficient biogenesis of mRNAs or by somatic mutation. Truncated polypeptides synthesized from these aberrant transcripts could be toxic to normal cellular functions. However, mammalian cells have evolved sophisticated mechanisms for monitoring the quality of mRNAs. The faulty transcripts harboring PTC are subject to nonsense-mediated mRNA decay (NMD), nonsense-mediated translational repression (NMTR), nonsense-associated alternative splicing (NAS), or nonsense-mediated transcriptional gene silencing (NMTGS). In this review, we briefly outline the molecular characteristics of each pathway and suggest mRNA quality control mechanisms as a means to regulate normal gene expression. [BMB Reports 2013; 46(1): 9-16]
Keywords: NAS, NMD, NMTGS, NMTR, PTC
INTRODUCTION
Eukaryotic gene expression is tightly and precisely regulated at multiple steps. Disturbances of any of these steps can result in abnormal gene expression and abnormal cell function, consequently leading to genetic diseases and cancers. To increase the fidelity of each step of gene expression, eukaryotic cells have developed evolutionarily highly conserved surveillance mechanisms. In particular, when mRNAs contain premature termination codons (PTCs), which can arise in mRNAs in various ways (1-3), these aberrant mRNAs are subject to various mRNA quality control mechanisms to block expression of potentially deleterious truncated polypeptides.
Although there is growing appreciation for the importance of mRNA quality control mechanisms in diverse cellular events, how mRNA quality is precisely monitored and controlled remains elusive. In this review, we summarize the current molecular understanding of diverse mRNA quality control mechanisms which target PTC-containing mRNAs. We furthermore suggest mRNA quality control mechanisms as a means to regulate cellular gene expression.
OVERVIEW OF GENE EXPRESSION IN MAMMALIAN CELLS
A simplified outline of the gene expression in mammalian cells is depicted in Fig. 1. RNA polymerase II (Pol II) initiates the synthesis of pre-mRNA in the nucleus. Immediately after transcription initiation, the 5’-end of the mRNA undergoes a capping process that is mediated by nuclear capping enzyme (also called RNA guanylyltransferase and 5’ phosphatase) and mRNA (guanine-N7-)-methyltransferase (4). The cap structure is recognized by a nuclear cap-binding protein complex (CBC) composed of a heterodimer of cap-binding protein (CBP) 80 and CBP20.
Fig. 1. Simplified view of mammalian gene expression. Gene expression cascades of normal mRNAs (left) and PTC-containing mRNAs targeted for NMD (right) are depicted. The details are described in the text.
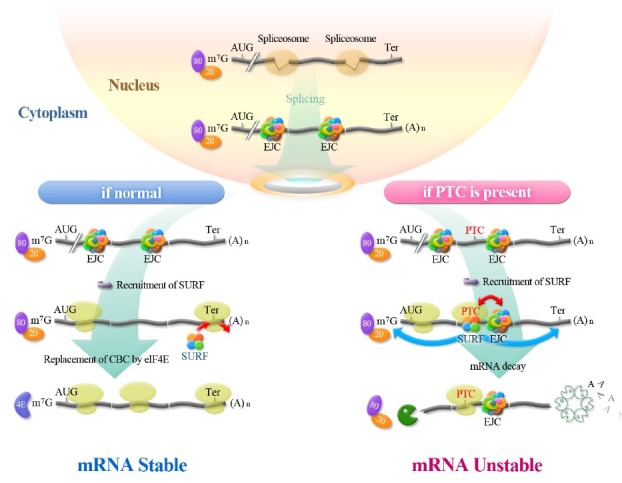
During transcription elongation, a spliceosome complex recognizes and removes introns from pre-mRNAs connecting the neighboring exons. During pre-mRNA splicing, a large protein complex, the so-called exon junction complex (EJC), is deposited 20-24 nucleotides upstream of the exon-exon junction. The EJC is composed of a core complex that includes the DEAD-box RNA helicase eIF4AIII, a heterodimer of MAGOH and Y14, Barentsz/MLN51, and other accessory proteins (5-8). The EJCs play multiple roles in various cellular processes including pre-mRNA splicing, mRNA export, mRNA localization, translation, mRNA stability, and even transcription (8-14) . Interestingly, recent cross-linking immunoprecipitationhigh-throughput sequencing (CLIP-Seq) experiments revealed that a loading efficiency of EJC on each exon-exon junction and composition of EJCs vary, even for the same mRNA (15,16). In addition, the same approaches showed that EJCs are loaded onto other sequences as well as the canonical exon-exon junction, suggesting additional regulatory roles of EJCs in gene expression (15,16).
Finally, during transcription termination, the 3’-end of the pre-mRNA is cleaved and polyadenylated. After completion of all nuclear processes, the properly processed mRNA is exported from the nucleus to the cytoplasm via the nuclear pore complex (NPC) and is then translated into polypeptide in the cytoplasm.
MOLECULAR MECHANISM OF NONSENSE-MEDIATED mRNA DECAY IN MAMMALIAN CELLS
Nonsense-mediated mRNA decay (NMD) is the best-characterized mRNA surveillance mechanism by which PTC-containing transcripts are selectively recognized and downregulated in abundance before the expression of truncated polypeptides (6,7,17-22). In the past decade, many cellular proteins and regulators for preferential selection of NMD substrates have been characterized. The current prevailing model is outlined below and is depicted in Fig. 1.
NMD is tightly coupled to translation, since NMD machinery should recognize the translation termination codon on the mRNA as a PTC before mRNA degradation (5-7,17-25). More specifically, NMD is tightly coupled to the so-called first (or pioneer) round of translation or CBP80/20-dependent translation (26-28). Newly synthesized mRNA is exported from the nucleus to the cytoplasm with CBP80/20 bound to the cap structure at the 5’-end of the mRNA. During the export of newly synthesized mRNAs, CBP80/20 at the 5’-end of the mRNA exposed to the cytoplasm recruits ribosome to direct the first round of translation. All types of mRNAs are believed to be subject to this mode of translation, because all mRNAs that are completely processed in the nucleus contain a cap structure bound by CBP80/20.
The determination of whether mRNA is normal or aberrant occurs during translation termination (5-7,17-29). In most normal mRNAs, the translation termination codon resides in the last exon of the gene. Consequently, all deposited EJCs would be dissociated from the mRNA during the elongation step of the pioneer round of translation, possibly with the help of PYM associated with the ribosome (30). In such cases, the mRNA would be stable due to the lack of EJCs downstream of the translation termination codon. However, in the case of mRNAs harboring PTCs more than 50-55 nucleotides upstream of the last exon-exon junction, EJCs will remain downstream of the PTC. This microenvironment, that is, the existence of EJCs downstream of the terminating ribosome, serves as a molecular marker to induce NMD. As a general rule, although many exceptions have been reported, the existence of a PTC at least 50-55 nucleotides upstream of the last exon-exon junction is sufficient to elicit mRNA degradation by NMD; this is referred to as the “50-55 nucleotide rule” (5,6,20,31).
The terminating ribosome at a PTC during the pioneer round of translation recruits the SURF complex, which is composed of suppressor with morphological effect on genitalia (SMG) 1 kinase, up-frameshift 1 (Upf1), eukaryotic translation release factor (eRF) 1, and eRF3 (32,33). The recruited SURF complex then communicates with the PTC-downstream EJC via an interaction between Upf1 in the SURF complex and Upf2 in the downstream EJC (32). The transient interaction between SURF and EJC then activates SMG1 kinase, triggering the hyperphosphorylation of Upf1 (32).
In mammalian cells, hyperphosphorylated Upf1 recruits several Upf1-interacting adaptors or effectors: SMG5, SMG6, SMG7, and proline-rich nuclear receptor co-regulatory protein 2 (PNRC2) (34-37) to trigger either decapping followed by 5’-to-3’exoribonucleolytic cleavage or endoribonucleolytic cleavage. However, how hyperphosphorylated Upf1 communicates with 3’-to-5’exoribonucleolytic cleavage remains unknown. Of note, it is likely that each NMD substrate has its own binding preference for Upf1-interacting adaptors or effectors (38,39). In addition, recent microarray results revealed that SMG5-dependent NMD substrates significantly overlap with PNRC2-dependent NMD substrates (39), implicating the possible cross-talks among Upf1-interacting adaptors or effectors.
NONSENSE-MEDIATED TRANSLATIONAL REPRESSION OF PTC-CONTAINING mRNAs
Whether transcripts that escape NMD, despite being expected to be degraded by NMD, are efficiently translated or not is another important issue. In the case of high microsatellite instability (MSI)-H tumors, a frameshift mutation introduces a PTC into the open reading frame (ORF) of the transforming growth factor-β receptor type 2 (TGFβR2) gene. A PTC-containing TGFβR2 mRNA has been shown to escape NMD, although a PTC is located sufficiently upstream of the last exon-exon junction (40,41). However, this NMD-escaped TGFβR2 mRNA is subject to another surveillance mechanism - nonsense-mediated translational repression (NMTR) - in which the PTC-containing mRNAs are translationally repressed. The use of different types of internal ribosome entry sites (IRESes) revealed that NMTR occurs after formation of 80S ribosome complex (40). It should be noted that, unlike NMD, NMTR does not require Upf1 and Upf2, both of which are key NMD factors (40,41).
The detailed molecular mechanism by which PTC-containing mRNAs escape NMD and are instead targeted for NMTR is still poorly understood. However, both NMTR is, at least in part, in common with EJC-independent NMD, in that both mechanisms depend on the length of 3’-untranslated region (3’UTR) or a putative cis-acting element residing in the 3’UTR. A PTC-containing β-globin mRNA, triosephosphate isomerase (TPI) mRNA, and immunoglobulin (Ig)-μ mRNA typify EJC-independent NMD (42-44). A PTC within the penultimate exon of these mRNAs and EJC downstream of PTC are sufficient to elicit NMD, as long as the position of PTC conforms to the 50-55 nucleotide rule. However, deletion of the last intron downstream of a PTC, which is expected to abolish NMD, still elicits NMD (42-44). These observations suggest that the last exon or the last exon-exon junction sequence may have a cis-acting ‘‘failsafe’’ sequence that helps PTC recognition and triggers NMD despite the lack of an EJC downstream of PTC.
Like EJC-independent NMD, efficient NMTR also requires an intron (or deposition of EJC after splicing) downstream of a PTC (40,41). However, the removal of all introns from sequences downstream of a PTC still elicits moderate NMTR, although the presence of introns more efficiently triggers NMTR (40,41). Therefore, it is possible that the sequences downstream of a PTC may contain a specific cis-acting sequence that forms a peculiar RNA structure and recruits cellular factors that allow for PTC recognition and then trigger NMTR. It is also plausible that the sequences downstream of a PTC anneal to specific microRNAs (miRNAs). If some miRNAs target the region spanning from the PTC to a normal termination codon, the recruited RNA-induced silencing complex (RISC) would silence the pioneer round of translation and thereby abolish NMD. These NMD-escaped mRNAs would still be silenced at the step of eIF4E-dependent translation, because miRNA/RISC also targets eIF4E-dependent translation. Indeed, the inhibition of the pioneer round of translation and NMD by microRNAs has been clearly determined (45,46).
Considering that NMTR does not require Upf1 (40,41), it is possible that NMTR machinery may not require the recruitment of SURF complex containing Upf1 as a component. Alternatively, although the SURF complex is properly recruited to a PTC, Upf1 may fail to trigger mRNA degradation due to the lack of NMD-stimulating signal. Consequently, PTC-containing mRNAs would escape NMD with the EJCs binding to the sequences downstream of PTC. The downstream EJC may actively inhibit the translation of these NMD-escaped PTC-containing mRNAs, possibly at the step after 80S ribosome formation, as evidenced by the use of different IRESes (40).
NMTR may not be confined to PTC-containing transcripts that escape NMD. Since NMD efficiency is known to vary among target transcripts and cell types and NMD machinery does not reduce the level of PTC-containing mRNA to 0% (23,47-54), a small fraction of PTC-containing mRNAs would be resistant to NMD and remain in the cytoplasm. For instance, many reports showed that the abundance of β-globin mRNAs harboring a PTC is reduced to 5-30% of normal level (27,28,34,52,55-61). The remaining NMD-resistant mRNAs, despite being targeted for NMD, would still have EJCs downstream of PTC. In this scenario, the remaining NMD-resistant mRNAs would be translationally repressed by NMTR, which does not require Upf1. Alternatively, these mRNA would be translationally repressed in an Upf1-dependent manner. The hyperphosphorylation of Upf1, which occurs during PTC recognition, may trigger translational repression, as evidenced by the recent report that hyperphosphorylated Upf1 directly interacts with eIF3 and that it inhibits the conversion of 40S/Met-tRNAiMet/mRNA to 80S/Met-tRNAiMet/mRNA (25). In addition, it has been shown in yeast that the abundance of PTC-containing mRNAs is increased upon Upf1 deletion and its translational efficiency (the level of protein produced from the remaining PTC-containing mRNAs) is also increased upon Upf1 deletion (62).
NONSENSE-ASSOCIATED ALTERNATIVE SPLICING OF PTC-CONTAINING mRNAs
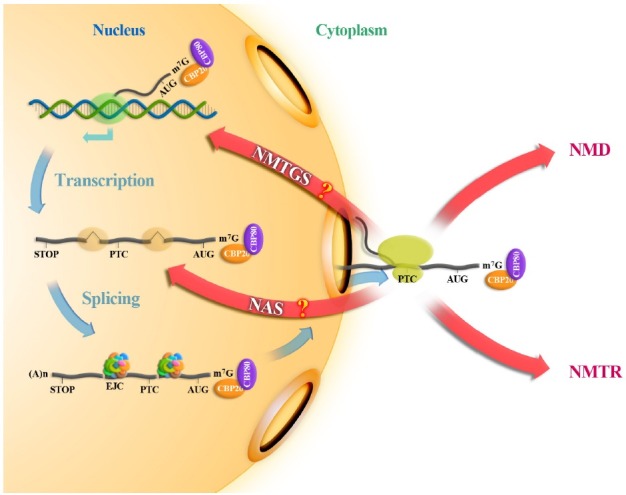
PTCs have been also shown to elicit alternative splicing called nonsense-associated alternative splicing (NAS) by a response to PTC recognition as depicted in Fig. 2. NAS is another posttranscriptional regulation mechanism that skips the offending PTCs and rearranges open reading frame of PTC-containing transcripts. NAS of PTC-containing transcripts is thought to be the second response to PTC recognition; i.e., after a translating ribosome meets a PTC.
Fig. 2. Different fates of PTC-containing mRNAs.
NAS shares a key NMD factor, Upf1. Downregulation of Upf1 reduces alternative splicing of PTC-containing transcripts and stabilizes the PTC-containing transcripts (63,64). However, overexpression of Upf1 mutant, which has a loss of function in NMD, fails to restore NMD, but successfully upregulates alternative splicing of PTC-containing transcripts in Upf1-depleted cells, indicating that NMD and NAS might be a separable mechanism by using a common factor, Upf1 (64). Downregulation of other NMD factors including SMG1, Upf2, Upf3a, and Upf3b showed no significant effects on NAS (63,65). Furthermore, an EJC-core protein, eIF4AIII, which is involved in NMD, had no effects on NAS (63), suggesting that EJC complex or downstream splicing event is not a critical factor in NAS. All these results suggest that NAS might be mechanistically different from NMD.
The effects of a PTC on NAS are controversial by conflicting results. A PTC-containing T cell receptor-β (TCRβ), which contains an alternative splicing acceptor and donor site in intron and exon, respectively, generates alternatively spliced transcript more efficiently than its PTC-free counterpart by skipping the offending PTC (64-66). In the experiments to test for NAS efficiency using various TCRβ variants containing different nonsense or missense codons introduced by point mutations, upregulation of NAS was observed in only position mutated to a PTC (64,66), suggesting that a PTC itself is responsible for efficient NAS. However, frame disruption by deletion of 1-nt, which did not generate a PTC in the transcript, increased the production of alternatively spliced transcripts and decreased the level of normal transcripts (63). In addition, the relative level of alternatively spliced transcripts of TCRβ pre-mRNA was higher in 9-nucleotide (nt) insertion (PTC-free transcript) than in 10-nt (PTC-containing transcript) (67). To rule out the possibility that nucleotide changes could elicit NAS, in-frame was maintained by insertion and deletion of 1-nt without generation of a PTC. In this construct, upregulation of alternative splicing did not happen (63). These conflicting observations indicate that frameshift per se without generation of a PTC can cause upregulation of NAS and that PTC may not be the only factor to elicit NAS.
To make the story more complicated, the level of alternatively spliced PTC-containing TCRβ transcript did not seem to be blocked by inhibition of translation, unlike NMD (65). Contradictory to irrelevance of translation-dependency, a strong Kozak consensus sequence augmented NAS in a response to a PTC (65), implicating the important role of translational reading frame of PTC-containing transcripts in NAS. However, translation processes, for example, translation elongation and termination, may not be responsible for NAS.
Another plausible suggestion in NAS is that alternative splicing in PTC-containing pre-mRNA may be achieved by rapid degradation of spliced PTC-containing mRNA in the nucleus (66). In support of this idea, PTC-containing mRNA was shown to be rapidly degraded in the nucleus (68). Inhibition of the export of PTC-containing transcript from the nucleus to the cytoplasm still reduced the amount of PTC-containing transcripts in the nucleus, suggesting that nuclear mRNA degradation machinery is involved in degradation of PTC-containing transcripts (68). In this scenario, rapid degradation of spliced PTC-containing mRNA in the nucleus may cause a rearrangement of splicing factors and consequently affect alternative splicing of PTC-containing pre-mRNA (66).
In situ hybridization to detect a PTC-containing Ig-μ premRNA in the nucleus showed the level of PTC-containing pre-mRNA was higher than the level of PTC-free pre-mRNA near the transcription site, although transcription rates were indistinguishable (69). These results were reproducible by photobleaching (FRAP) and photoconversion analyses, showing that unspliced PTC-containing transcripts were more condensed at the transcription site than unspliced PTC-free transcripts (70). Notably, downregulation of Upf1 or SMG6 released nuclear PTC-containing transcripts to cytoplasm, suggesting that Upf1, which is a NAS factor, and SMG6 play a key role in holding PTC-containing pre-mRNA in the nucleus. In summary, either an accumulation of PTC-containing pre-mRNA at the transcription site by Upf1 and SMG6 or rapid degradation of spliced PTC-containing mRNA in the nucleus may cause a rearrangement of splicing factors and consequently upregulate alternative splicing of PTC-containing transcripts.
NONSENSE-MEDIATED TRANSCRIPTIONAL GENE SILENCING OF PTC-CONTAINING mRNAs
PTCs have been also shown to elicit transcriptional inhibition (Fig. 2). A large number of studies about the first three nonsense-mediated posttranscriptional regulations have been done, but studies on nonsense-mediated transcriptional gene silencing (NMTGS) are rare. Chromatin immunoprecipitation (ChIP) assay of Ig-μ-encoding DNA revealed that, whereas PTC-free DNA was preferentially associated with transcriptionally active histone, PTC-containing DNA was preferentially associated with transcriptionally repressed chromatin (71). In addition, transcription rate of PTC-containing DNA was enhanced by histone deacetylase inhibitors (71). Taken together, these findings indicate that transcription of PTC-containing DNA is repressed although it is still not clear that repression of transcription in a specific gene results from PTC recognition or the accumulation of pre-mRNA. An important point in NMTGS is that NMTGS is not applied to other known NMD substrates, such as β-globin, glutathione peroxidase 1 (GPx1) and TCRβ (71). Therefore, some intrinsic factors in the transcript, i.e. specific sequences or mRNA structure generated during transcription would block a transcription of PTC-containing gene. Furthermore, it still remains unanswered how NMD factors including Upf or SMG proteins play a role in NMTGS in mammalian cells. In the future, studies about the effects of NMD factors on NMTGS, its mechanism and the coupling of transcription to posttranscriptional regulation will be critical.
CLOSING REMARKS
NMD has been considered an mRNA surveillance mechanism, since it targets faulty mRNAs generated by malfunctional cellular processes, such as aberrant transcription or inefficient splicing. However, the findings that NMD targets a variety of normal and cellular transcripts have changed this notion such that NMD is now viewed as a post-transcriptional regulatory mechanism (72-76). As long as cellular mRNAs are applicable to the 50-55 nucleotide rule of NMD, they would be subject to NMD-mediated gene regulation. The known features of natural NMD substrates are as follows: (i) the presence of upstream open reading frames (uORFs) in the 5’UTR, (ii) the presence of intron(s) in the 3’UTR, (iii) nonsense codon or frameshift generated by alternative splicing, (iv) UGA selenocysteine codon, and (v) nonfunctional pseudogenes that accumulate PTCs by genetic drift (72-77). Like NMD, NMTR, NAS, and NMTGS may also contribute to gene expression of normal and natural transcripts in mammalian cells. Except NMD, however, just few genes have been discovered yet to be regulated by NMTR, NAS or NMTGS. Future studies should reveal open genes regulated by them, putative their phenotypes and the detailed molecular mechanisms as to how PTCs are selectively recognized.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A2A1A01002469) to Y.K.K. and by the research fund of Hanyang University (HY-2011-N) to J. H.
References
- 1.Kuzmiak H. A., Maquat L. E. Applying nonsense-mediated mRNA decay research to the clinic: progress and challenges. Trends Mol. Med. (2006);12:306–316. doi: 10.1016/j.molmed.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Stoilov P., Meshorer E., Gencheva M., Glick D., Soreq H., Stamm S. Defects in pre-mRNA processing as causes of and predisposition to diseases. DNA Cell Biol. (2002);21:803–818. doi: 10.1089/104454902320908450. [DOI] [PubMed] [Google Scholar]
- 3.Caceres J. F., Kornblihtt A. R. Alternative splicing: multiple control mechanisms and involvement in human disease. Trends Genet. (2002);18:186–193. doi: 10.1016/S0168-9525(01)02626-9. [DOI] [PubMed] [Google Scholar]
- 4.Schoenberg D. R., Maquat L. E. Re-capping the message. Trends Biochem. Sci. (2009);34:435–442. doi: 10.1016/j.tibs.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behm-Ansmant I., Kashima I., Rehwinkel J., Sauliere J., Wittkopp N., Izaurralde E. mRNA quality control: an ancient machinery recognizes and degrades mRNAs with nonsense codons. FEBS Letters. (2007);581:2845–2853. doi: 10.1016/j.febslet.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 6.Chang Y. F., Imam J. S., Wilkinson M. F. The nonsense-mediated decay RNA surveillance pathway. Annu. Rev. Biochem. (2007);76:51–74. doi: 10.1146/annurev.biochem.76.050106.093909. [DOI] [PubMed] [Google Scholar]
- 7.Isken O., Maquat L. E. Quality control of eukaryotic mRNA: safeguarding cells from abnormal mRNA function. Genes Dev. (2007);21:1833–1856. doi: 10.1101/gad.1566807. [DOI] [PubMed] [Google Scholar]
- 8.Tange T. O., Nott A., Moore M. J. The ever-increasing complexities of the exon junction complex. Curr. Opin. Cell. Biol. (2004);16:279–284. doi: 10.1016/j.ceb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Reed R. Coupling transcription, splicing and mRNA export. Curr. Opin. Cell. Biol. (2003);15:326–331. doi: 10.1016/S0955-0674(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 10.Giorgi C., Moore M. J. The nuclear nurture and cytoplasmic nature of localized mRNPs. Semin. Cell. Dev. Biol. (2007);18:186–193. doi: 10.1016/j.semcdb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Le Hir H., Andersen G. R. Structural insights into the exon junction complex. Curr. Opin. Struct. Biol. (2008);18:112–119. doi: 10.1016/j.sbi.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Diem M. D., Chan C. C., Younis I., Dreyfuss G. PYM binds the cytoplasmic exon-junction complex and ribosomes to enhance translation of spliced mRNAs. Nat. Struct. Mol. Biol. (2007);14:1173–1179. doi: 10.1038/nsmb1321. [DOI] [PubMed] [Google Scholar]
- 13.Le Hir H., Seraphin B. EJCs at the heart of translational control. Cell. (2008);133:213–216. doi: 10.1016/j.cell.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Ma X. M., Yoon S. O., Richardson C. J., Julich K., Blenis J. SKAR links pre-mRNA splicing to mTOR/S6K1-mediated enhanced translation efficiency of spliced mRNAs. Cell. (2008);133:303–313. doi: 10.1016/j.cell.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 15.Singh G., Kucukural A., Cenik C., Leszyk J. D., Shaffer S. A., Weng Z., Moore M. J. The cellular ejc interactome reveals higher-order mRNP structure and an EJC-SR protein nexus. Cell. (2012);151:750–764. doi: 10.1016/j.cell.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sauliere J., Murigneux V., Wang Z., Marquenet E., Barbosa I., Le Tonqueze O., Audic Y., Paillard L., Roest Crollius H., Le Hir H. CLIP-seq of eIF4AIII reveals transcriptome-wide mapping of the human exon junction complex. Nat. Struct. Mol. Biol. (2012);19:1124–1131. doi: 10.1038/nsmb.2420. [DOI] [PubMed] [Google Scholar]
- 17.Silva A. L., Romão L. The mammalian nonsense-mediated mRNA decay pathway: to decay or not to decay! Which players make the decision? FEBS letters. (2009);583:499–505. doi: 10.1016/j.febslet.2008.12.058. [DOI] [PubMed] [Google Scholar]
- 18.Nicholson P., Yepiskoposyan H., Metze S., Zamudio Orozco R., Kleinschmidt N., Mühlemann O. Nonsense-mediated mRNA decay in human cells: mechanistic insights, functions beyond quality control and the double-life of NMD factors. Cell Mol. Life Sci. (2010);67:677–700. doi: 10.1007/s00018-009-0177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shyu A. B., Wilkinson M. F., van Hoof A. Messenger RNA regulation: to translate or to degrade. EMBO J. (2008);27:471–481. doi: 10.1038/sj.emboj.7601977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neu-Yilik G., Kulozik A. E. NMD: multitasking between mRNA surveillance and modulation of gene expression. Adv. Genet. (2008);62:185–243. doi: 10.1016/S0065-2660(08)00604-4. [DOI] [PubMed] [Google Scholar]
- 21.Mühlemann O., Eberle A. B., Stalder L., Zamudio Orozco R. Recognition and elimination of nonsense mRNA. Biochim. Biophys. Acta. (2008);1779:538–549. doi: 10.1016/j.bbagrm.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 22.McGlincy N. J., Smith C. W. Alternative splicing resulting in nonsense-mediated mRNA decay: what is the meaning of nonsense? Trends. Biochem. Sci. (2008);33:385–393. doi: 10.1016/j.tibs.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Linde L., Kerem B. Introducing sense into nonsense in treatments of human genetic diseases. Trends Genet. (2008);24:552–563. doi: 10.1016/j.tig.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Lejeune F., Maquat L. E. Mechanistic links between nonsense-mediated mRNA decay and pre-mRNA splicing in mammalian cells. Curr. Opin. Cell Biol. (2005);17:309–315. doi: 10.1016/j.ceb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Isken O., Maquat L. E. The multiple lives of NMD factors: balancing roles in gene and genome regulation. Nat. Rev. Genet. (2008);9:699–712. doi: 10.1038/nrg2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiu S. Y., Lejeune F., Ranganathan A. C., Maquat L. E. The pioneer translation initiation complex is functionally distinct from but structurally overlaps with the steady-state translation initiation complex. Genes Dev. (2004);18:745–754. doi: 10.1101/gad.1170204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishigaki Y., Li X., Serin G., Maquat L. E. Evidence for a pioneer round of mRNA translation: mRNAs subject to nonsense-mediated decay in mammalian cells are bound by CBP80 and CBP20. Cell. (2001);106:607–617. doi: 10.1016/S0092-8674(01)00475-5. [DOI] [PubMed] [Google Scholar]
- 28.Kim K. M., Cho H., Choi K., Kim J., Kim B. W., Ko Y. G., Jang S. K, Kim Y. K. A new MIF4G domain-containing protein, CTIF, directs nuclear cap-binding protein CBP80/20-dependent translation. Genes Dev. (2009);23:2033–2045. doi: 10.1101/gad.1823409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hwang J., Sato H., Tang Y., Matsuda D., Maquat L. E. UPF1 association with the cap-binding protein, CBP80, promotes nonsense-mediated mRNA decay at two distinct steps. Mol. Cell. (2010);39:396–409. doi: 10.1016/j.molcel.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gehring N. H., Lamprinaki S., Kulozik A. E., Hentze M. W. Disassembly of exon junction complexes by PYM. Cell. (2009);137:536–548. doi: 10.1016/j.cell.2009.02.042. [DOI] [PubMed] [Google Scholar]
- 31.Maquat L. E. Nonsense-mediated mRNA decay in mammals. J. Cell Sci. (2005);118:1773–1776. doi: 10.1242/jcs.01701. [DOI] [PubMed] [Google Scholar]
- 32.Kashima I., Yamashita A., Izumi N., Kataoka N., Morishita R., Hoshino S., Ohno M., Dreyfuss G., Ohno S. Binding of a novel SMG-1-Upf1-eRF1-eRF3 complex (SURF) to the exon junction complex triggers Upf1 phosphorylation and nonsense-mediated mRNA decay. Genes Dev. (2006);20:355–367. doi: 10.1101/gad.1389006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamashita A., Izumi N., Kashima I., Ohnishi T., Saari B., Katsuhata Y., Muramatsu R., Morita T., Iwamatsu A., Hachiya T., Kurata R., Hirano H., Anderson P., Ohno S. SMG-8 and SMG-9, two novel subunits of the SMG-1 complex, regulate remodeling of the mRNA surveillance complex during nonsense-mediated mRNA decay. Genes Dev. (2009);23:1091–1105. doi: 10.1101/gad.1767209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cho H., Kim K. M., Kim Y. K. Human proline-rich nuclear receptor coregulatory protein 2 mediates an interaction between mRNA surveillance machinery and decapping complex. Mol. Cell. (2009);33:75–86. doi: 10.1016/j.molcel.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 35.Fukuhara N., Ebert J., Unterholzner L., Lindner D., Izaurralde E., Conti E. SMG7 is a 14-3-3-like adaptor in the nonsense-mediated mRNA decay pathway. Mol. Cell. (2005);17:537–547. doi: 10.1016/j.molcel.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 36.Unterholzner L., Izaurralde E. SMG7 acts as a molecular link between mRNA surveillance and mRNA decay. Mol. Cell. (2004);16:587–596. doi: 10.1016/j.molcel.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 37.Cho H., Kim K. M., Han S., Choe J., Park S. G., Choi S. S., Kim Y. K. Staufen1-mediated mRNA decay functions in adipogenesis. Mol. Cell. (2012);46:495–506. doi: 10.1016/j.molcel.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 38.Yepiskoposyan H., Aeschimann F., Nilsson D., Okoniewski M., Mühlemann O. Autoregulation of the nonsense-mediated mRNA decay pathway in human cells. RNA. (2011);17:2108–2118. doi: 10.1261/rna.030247.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cho H., Han S., Choe J., Park S. G., Choi S. S., Kim Y. K. SMG5-PNRC2 is functionally dominant compared with SMG5-SMG7 in mammalian nonsense-mediated mRNA decay. Nucleic Acids Res. (2012) doi: 10.1093/nar/gks1222.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee H. C., Oh N., Cho H., Choe J., Kim Y. K. Nonsense-mediated translational repression involves exon junction complex downstream of premature translation termination codon. FEBS Letters. (2010);584:795–800. doi: 10.1016/j.febslet.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 41.You K. T., Li L. S., Kim N. G., Kang H. J., Koh K. H., Chwae Y. J., Kim K. M., Kim Y. K., Park S. M., Jang S. K., Kim H. Selective translational repression of truncated proteins from frameshift mutation-derived mRNAs in tumors. PLoS Biol. (2007);5:e109. doi: 10.1371/journal.pbio.0050109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang J., Sun X., Qian Y., LaDuca J. P., Maquat L. E. At least one intron is required for the nonsense-mediated decay of triosephosphate isomerase mRNA: a possible link between nuclear splicing and cytoplasmic translation. Mol. Cell Biol. (1998);18:5272–5283. doi: 10.1128/mcb.18.9.5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng J., Belgrader P., Zhou X., Maquat L. E. Introns are cis effectors of the nonsense-codon-mediated reduction in nuclear mRNA abundance. Mol. Cell Biol. (1994);14:6317–6325. doi: 10.1128/MCB.14.9.6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buhler M., Steiner S., Mohn F., Paillusson A., Mühlemann O. EJC-independent degradation of nonsense immunoglobulin-mu mRNA depends on 3' UTR length. Nat. Struct. Mol. Biol. (2006);13:462–464. doi: 10.1038/nsmb1081. [DOI] [PubMed] [Google Scholar]
- 45.Choe J., Cho H., Chi S. G., Kim Y. K. Ago2/miRISC-mediated inhibition of CBP80/20-dependent translation and thereby abrogation of nonsense-mediated mRNA decay require the cap-associating activity of Ago2. FEBS Letters. (2011);585:2682–2687. doi: 10.1016/j.febslet.2011.07.047. [DOI] [PubMed] [Google Scholar]
- 46.Choe J., Cho H., Lee H. C., Kim Y. K. microRNA/Argonaute 2 regulates nonsense-mediated messenger RNA decay. EMBO Rep. (2010);11:380–386. doi: 10.1038/embor.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kebaara B., Nazarenus T., Taylor R., Atkin A. L. Genetic background affects relative nonsense mRNA accumulation in wild-type and upf mutant yeast strains. Curr. Genet. (2003);43:171–177. doi: 10.1007/s00294-003-0386-3. [DOI] [PubMed] [Google Scholar]
- 48.Jensen L. R., Amende M., Gurok U., Moser B., Gimmel V., Tzschach A., Janecke A. R., Tariverdian G., Chelly J., Fryns J. P., Van Esch H., Kleefstra T., Hamel B., Moraine C., Gecz J., Turner G., Reinhardt R., Kalscheuer V. M., Ropers H. H., Lenzner S. Mutations in the JARID1C gene, which is involved in transcriptional regulation and chromatin remodeling, cause X-linked mental retardation. Am. J. Hum. Genet. (2005);76:227–236. doi: 10.1086/427563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bateman J. F., Freddi S., Nattrass G., Savarirayan R. Tissue-specific RNA surveillance? Nonsense-mediated mRNA decay causes collagen X haploinsufficiency in Schmid metaphyseal chondrodysplasia cartilage. Hum. Mol. Genet. (2003);12:217–225. doi: 10.1093/hmg/ddg054. [DOI] [PubMed] [Google Scholar]
- 50.Resta N., Susca F. C., Di Giacomo M. C., Stella A., Bukvic N., Bagnulo R., Simone C., Guanti G. A homozygous frameshift mutation in the ESCO2 gene: evidence of intertissue and interindividual variation in Nmd efficiency. J. Cell Physiol. (2006);209:67–73. doi: 10.1002/jcp.20708. [DOI] [PubMed] [Google Scholar]
- 51.Viegas M. H., Gehring N. H., Breit S., Hentze M. W., Kulozik A. E. The abundance of RNPS1, a protein component of the exon junction complex, can determine the variability in efficiency of the Nonsense Mediated Decay pathway. Nucleic Acids Res. (2007);35:4542–4551. doi: 10.1093/nar/gkm461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Linde L., Boelz S., Neu-Yilik G., Kulozik A. E., Kerem B. The efficiency of nonsense-mediated mRNA decay is an inherent character and varies among different cells. Eur. J. Hum. Genet. (2007);15:1156–1162. doi: 10.1038/sj.ejhg.5201889. [DOI] [PubMed] [Google Scholar]
- 53.Kerr T. P., Sewry C. A., Robb S. A., Roberts R. G. Long mutant dystrophins and variable phenotypes: evasion of nonsense-mediated decay? Hum. Genet. (2001);109:402–407. doi: 10.1007/s004390100598. [DOI] [PubMed] [Google Scholar]
- 54.Linde L., Boelz S., Nissim-Rafinia M., Oren Y. S., Wilschanski M., Yaacov Y., Virgilis D., Neu-Yilik G., Kulozik A. E., Kerem E., Kerem B. Nonsense-mediated mRNA decay affects nonsense transcript levels and governs response of cystic fibrosis patients to gentamicin. J. Clin. Invest. (2007);117:683–692. doi: 10.1172/JCI28523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maquat L. E., Kinniburgh A. J. A beta zero-thalassemic beta-globin RNA that is labile in bone marrow cells is relatively stable in HeLa cells. Nucleic Acids Res. (1985);13:2855–2867. doi: 10.1093/nar/13.8.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maquat L. E., Kinniburgh A. J., Rachmilewitz E. A., Ross J. Unstable beta-globin mRNA in mRNA-deficient beta o thalassemia. Cell. (1981);27:543–553. doi: 10.1016/0092-8674(81)90396-2. [DOI] [PubMed] [Google Scholar]
- 57.Zhang J., Sun X., Qian Y., Maquat L. E. Intron function in the nonsense-mediated decay of beta-globin mRNA: indications that pre-mRNA splicing in the nucleus can influence mRNA translation in the cytoplasm. RNA. (1998);4:801–815. doi: 10.1017/S1355838298971849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kugler W., Enssle J., Hentze M. W., Kulozik A. E. Nuclear degradation of nonsense mutated beta-globin mRNA: a post-transcriptional mechanism to protect heterozygotes from severe clinical manifestations of beta-thalassemia? Nucleic Acids Res. (1995);23:413–418. doi: 10.1093/nar/23.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lim S. K., Maquat L. E. Human beta-globin mRNAs that harbor a nonsense codon are degraded in murine erythroid tissues to intermediates lacking regions of exon I or exons I and II that have a cap-like structure at the 5' termini. EMBO J. (1992);11:3271–3278. doi: 10.1002/j.1460-2075.1992.tb05405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lim S. K., Sigmund C. D., Gross K. W., Maquat L. E. Nonsense codons in human beta-globin mRNA result in the production of mRNA degradation products. Mol. Cell Biol. (1992);12:1149–1161. doi: 10.1128/mcb.12.3.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Silva A. L., Ribeiro P., Inacio A., Liebhaber S. A., Romão L. Proximity of the poly(A)-binding protein to a premature termination codon inhibits mammalian nonsense-mediated mRNA decay. RNA. (2008);14:563–576. doi: 10.1261/rna.815108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muhlrad D., Parker R. Recognition of yeast mRNAs as "nonsense containing" leads to both inhibition of mRNA translation and mRNA degradation: implications for the control of mRNA decapping. Mol. Biol. Cell. (1999);10:3971–3978. doi: 10.1091/mbc.10.11.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chang Y. F., Chan W. K., Imam J. S., Wilkinson M. F. Alternatively spliced T-cell receptor transcripts are up-regulated in response to disruption of either splicing elements or reading frame. J. Biol. Chem. (2007);282:29738–29747. doi: 10.1074/jbc.M704372200. [DOI] [PubMed] [Google Scholar]
- 64.Mendell J. T., ap Rhys C. M., Dietz H. C. Separable roles for rent1/hUpf1 in altered splicing and decay of nonsense transcripts. Science. (2002);298:419–422. doi: 10.1126/science.1074428. [DOI] [PubMed] [Google Scholar]
- 65.Wang J., Chang Y. F., Hamilton J. I., Wilkinson M. F. Nonsense-associated altered splicing: a framedependent response distinct from nonsense-mediated decay. Mol. Cell. (2002);10:951–957. doi: 10.1016/S1097-2765(02)00635-4. [DOI] [PubMed] [Google Scholar]
- 66.Wang J., Hamilton J. I., Carter M. S., Li S., Wilkinson M. F. Alternatively spliced TCR mRNA induced by disruption of reading frame. Science. (2002);297:108–110. doi: 10.1126/science.1069757. [DOI] [PubMed] [Google Scholar]
- 67.Mohn F., Buhler M., Mühlemann O. Nonsenseassociated alternative splicing of T-cell receptor beta genes: no evidence for frame dependence. RNA. (2005);11:147–156. doi: 10.1261/rna.7182905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Buhler M., Wilkinson M. F., Mühlemann O. Intranuclear degradation of nonsense codon-containing mRNA. EMBO Rep. (2002);3:646–651. doi: 10.1093/embo-reports/kvf129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mühlemann O., Mock-Casagrande C. S., Wang J., Li S., Custodio N., Carmo-Fonseca M., Wilkinson M. F., Moore M. J. Precursor RNAs harboring nonsense codons accumulate near the site of transcription. Mol. Cell. (2001);8:33–43. doi: 10.1016/S1097-2765(01)00288-X. [DOI] [PubMed] [Google Scholar]
- 70.de Turris V., Nicholson P., Orozco R. Z., Singer R. H., Mühlemann O. Cotranscriptional effect of a premature termination codon revealed by live-cell imaging. RNA. (2011);17:2094–2107. doi: 10.1261/rna.02918111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Buhler M., Mohn F., Stalder L., Mühlemann O. Transcriptional silencing of nonsense codon-containing immunoglobulin minigenes. Mol. Cell. (2005);18:307–317. doi: 10.1016/j.molcel.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 72.Mendell J. T., Sharifi N. A., Meyers J. L., Martinez-Murillo F., Dietz H. C. Nonsense surveillance regulates expression of diverse classes of mammalian transcripts and mutes genomic noise. Nat. Genet. (2004);36:1073–1078. doi: 10.1038/ng1429. [DOI] [PubMed] [Google Scholar]
- 73.Mitrovich Q. M., Anderson P. mRNA surveillance of expressed pseudogenes in C. elegans. Curr. Biol. (2005);15:963–967. doi: 10.1016/j.cub.2005.04.055. [DOI] [PubMed] [Google Scholar]
- 74.He F., Li X., Spatrick P, Casillo R., Dong S., Jacobson A. Genome-wide analysis of mRNAs regulated by the nonsense-mediated and 5' to 3' mRNA decay pathways in yeast. Mol. Cell. (2003);12:1439–1452. doi: 10.1016/S1097-2765(03)00446-5. [DOI] [PubMed] [Google Scholar]
- 75.Weischenfeldt J., Damgaard I., Bryder D., Theilgaard-Monch K., Thoren L. A., Nielsen F. C., Jacobsen S. E., Nerlov C., Porse B. T. NMD is essential for hematopoietic stem and progenitor cells and for eliminating by-products of programmed DNA rearrangements. Genes Dev. (2008);22:1381–1396. doi: 10.1101/gad.468808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wittmann J., Hol E. M., Jack H. M. hUPF2 silencing identifies physiologic substrates of mammalian nonsense-mediated mRNA decay. Mol. Cell Biol. (2006);26:1272–1287. doi: 10.1128/MCB.26.4.1272-1287.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guan Q., Zheng W., Tang S., Liu X., Zinkel R. A., Tsui K. W., Yandell B. S., Culbertson M. R. Impact of nonsense-mediated mRNA decay on the global expression profile of budding yeast. PLoS Genet. (2006);2:e203. doi: 10.1371/journal.pgen.0020203. [DOI] [PMC free article] [PubMed] [Google Scholar]


