Abstract
The balance between osteoblast-dependent bone formation and osteoclast-dependent bone resorption maintains bone homeostasis. In inflammatory conditions, this balance shifts toward bone resorption, causing osteolytic bone lesions observed in rheumatoid arthritis and periodontitis. A recently discovered family of cytokine IL-17 is widely reported to mediate diverse inflammatory processes. During the last decade, novel roles for IL-17 in skeletal homeostasis have been discovered indicating the potential importance of this cytokine in bone metabolism. This review will summarize and discuss the involvement of IL-17 during bone homeostasis in both physiologic and pathologic conditions. A better understanding of the role of IL-17 in skeletal systems warrants an advance in bone biology, as well as development of therapeutic strategies against bone-lytic diseases, such as rheumatoid arthritis and periodontitis. [BMB Reports 2013; 46(10): 479-483]
Keywords: Bone metabolism, IL-17, Periodontitis, Rheumatoid arthritis
INTRODUCTION
IL-17 is a recently discovered family of cytokines composed of six members (1). IL-17A was cloned in T cell hybridoma, as the first member of the new class of cytokine and generally entitled as IL-17 (2). Additional isoforms homologous to IL-17A designated as IL-17B, IL-17C, IL-17D, IL-17E, and IL-17F were discovered afterwards (3). IL-17 is produced by a specialized subset of CD4+ T cells, called Th17 cells (4). It is likely that the primary function of Th17 cells is to eliminate pathogens and IL-17 is a potent inducer of inflammation. The receptors for IL-17, IL-17R, constitute a distinct family of cytokine receptor (3). In contrast to IL-17, IL17 receptor expression is ubiquitous, suggesting a possibility that IL-17 might affect the function of a wide variety of target cells. Until now, five members including IL-17RA, IL-17RB, IL-17RC, IL-17RD, and IL-17RE had been identified. IL-17RA is the founding member of this receptor family and binds to IL-17A (5). The ligand-receptor specificity of IL-17-IL-17R interaction is yet to be fully unveiled. However, it has been demonstrated that IL-17RA and IL-17RC bind to IL-17A and IL-17F (6,7).
IL-17 AND PRODUCTION OF INFLAMMATORY MEDIATORS
It has been shown that IL-17 can induce a wide variety of pro-inflammatory mediators in various types of cells involved in tissue damage, including macrophages. For example, IL-17 promoted the production of cytokines, such as IL-6, IL-1β, and TNF-α in mouse Kupffer cells (8). IL-17 stimulated the production of IL-6 and TNF-α in human macrophages obtained from peripheral blood (9). The increase of IL-6 following IL-17 treatment has also been reported in mouse microglia (10). Similar induction of IL-6 was also reported in IL-17-stimulated human gingival fibroblasts (11). Human peripheral blood mononuclear cell-derived macrophages responded to IL-17 to greatly enhance the production of IL-1β and TNF-α (11,12). IL-17 is also known to trigger chemokine production. The most frequently reported chemokine instigated by IL-17 is IL-8, which was observed in human gingival fibroblasts (11,13) and human macrophages (9). In mouse microglia, IL-17 also induced CXCL2 production (10). In addition, IL-17 significantly elevated the expression of CCL2 in human macrophages (14), CCL4 and CCL5 in mouse macrophages (15), and CCL20 in human gingival fibroblasts (16). IL-17 stimulated the production of prostaglandin E2 in MC3T3-E1 pre-osteoblasts (17,18). Finally, IL-17 induced nitric oxide eneration in MC3T3-E1 cells (19) and in mouse astrocytes (20).
IL-17 AND BONE METABOLISM
Bone homeostasis is intricately maintained by the coordination of bone formation by osteoblasts and bone resorption by osteoclasts. The role of IL-17 in the process of bone remodeling was first demonstrated in a study performed by Kotake et al. that showed IL-17, abundant in synovial fluids of rheumatoid arthritis patients, stimulated osteoclastogenesis in an osteo-blast-dependent manner (21). Numerous following studies corroborated the pro-osteoclastogenic role of IL-17 both in vitro and in vivo. IL-17 stimulated bone resorption in combination with TNF-α in fetal mouse long bones (22). However, whether IL-17 is directly working on osteoclast precursors or indirectly affecting osteoclast differentiation through stromal cells had not been clarified until Sato et al. revealed the role of Th17 cells on osteoclastogenesis (23). In an effort to dissect the role of T cells in arthritic bone destruction, the authors discovered that IL-17 only stimulated the osteoclastogenesis in a co-culture of mouse osteoclasts and bone marrow macrophages (osteoclast precursors), while having no effect on the differentiation of a macrophage-only culture, suggesting that IL-17 induces the expression of RANKL (the osteoclast differentiation factor) in osteoclast-supporting cells, such as osteoblasts. Yet, the direct effect of IL-17 on osteoclast precursors is still controversial. IL-17 induced osteoclast differentiation from human monocytes in the absence of osteoblasts (24). In contrast, Kitami et al. reported that IL-17 inhibited osteoclast differentiation from RAW264.7 cells (25). Recently, it was reported that IL-17 inhibits osteoclastogenesis in mouse osteoblast-bone marrow cell co-culture by inducing the release of GM-CSF, an anti-osteoclastogenesis cytokine (26). While the exact role of IL-17 in osteoclastogenesis still needs to be fully unveiled, it is likely that the effect of IL-17 on osteoclast differentiation is largely affected by multiple factors, such as the source of the osteoclast precursors, species, and culture conditions.
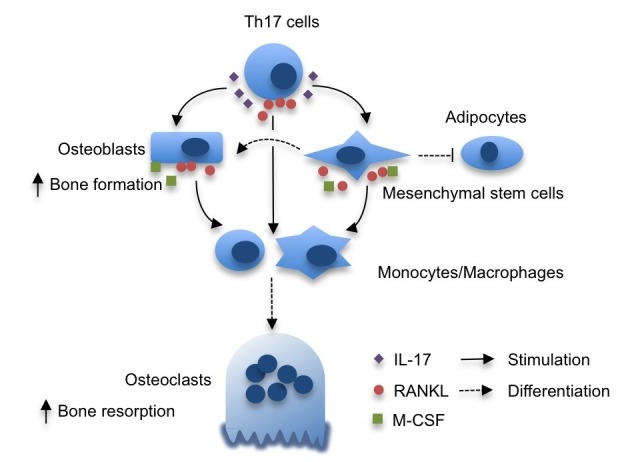
Little is known about the role of IL-17 in osteoblast differentiation and bone formation. Huang et al. published that IL-17 stimulated the formation of the colony-forming unit-fibroblast (CFU-f) from both human and mouse bone marrow stromal cells, suggesting that IL-17 is a growth factor for mesenchymal stem cells (27). Indeed, the CFU-f formation induced by CD4+ T cells was significantly reduced after bone marrow transplant in IL-17RA-deficient recipient mice. In line with these observations, IL-17 enhanced the proliferation, as well as osteogenic differentiation of human mesenchymal stem cells (28). The IL-17-induced mesenchymal stem cell proliferation was dependent upon the generation of reactive oxygen species (ROS) mediated by NADPH oxidase 1 downstream of TRAF6 and Act1. Then, ROS activated the MEK-ERK pathway to stimulate mesenchymal stem cell proliferation. Importantly, IL-17 induced the expression of M-CSF and RANKL, crucial cytokines required for osteoclast survival and differentiation, potentiating the role for IL-17 in bone remodeling. IL-17F also stimulated osteogenic differentiation of MC3T3-E1 mouse pre-osteoblast cells, as well as primary mouse mesenchymal stromal cells (29). In mouse myoblast cell line C2C12, IL-17 promoted osteogenic differentiation, while suppressing myogenic differentiation (30). Interestingly, IL-17 has been widely accepted to inhibit adipogenesis (31), suggesting that IL-17 may steer mesenchymal stem cells into an osteogenic fate (Fig. 1).
Fig. 1. The role of IL-17 in bone remodeling. IL-17, produced by Th17 cells, stimulate the production of MCSF and RANKL in osteoblasts and mesenchymal stem cells. These factors enhance the formation of bone-resorbing osteoclasts from monocyte/macrophage precursors. IL-17 not only accelerates the osteogenic differentiation of mesenchymal stem cells but also hampers adipogenic differentiation. Th17 cells are also RANKL-expressing T cells that support osteoclastogenesis.
IL-17 IN RHEUMATOID ARTHRITIS BONE DESTRUCTION
Since the first demonstration that IL-17 is crucially involved in bone resorption in rheumatoid arthritis patients (21), scores of papers during the last decade confirmed the role of IL-17. The treatment of mice with anti-IL-17 antibody dramatically reduced not only the joint inflammation but also cartilage and bone destruction in a collagen-induced arthritis model (32). The neutralization of endogenous IL-17 also significantly reduced bone erosion in a mouse methylated bovine serum albumin-induced experimental arthritis model by reducing the levels of RANKL, IL-1, and TNF-α (33). By the same token, IL-17RA-deficient mice were clearly protected from cartilage destruction following arthritis induction by bacterial cell wall challenge (34). These results strongly suggested that blocking the IL-17 signaling could be a strategy against rheumatoid arthritis. Indeed, Genovese et al. published that a humanized anti-IL-17 antibody successfully reduced the joint scores in a rheumatoid arthritis clinical study (35). The usefulness of the anti-IL-17 therapy was further supported by recent studies that revealed the bone-protective effect of IL-17 blockade (36-38). The aforementioned bone-destructive role of IL-17 is largely mediated by enhanced RANKL production by osteoblasts (21), synovial cells (33,39), and mesenchymal stem cells (28). In addition, the IL-17-producing Th17 cells were proven to be the RANKL-expressing T cells (23). In a recently published article, Kikuta et al. demonstrated that Th17 cells could activate mature osteoclasts into a bone-resorbing state (40). Thus it is likely that Th17 cells in rheumatoid synovium, not only stimulate osteoclast differentiation by M-CSF and RANKL production in osteoclast-supporting cells via IL-17 secretion, but also directly activate osteoclast bone resorption via cell-cell contact as RANKL-producing T cells.
IL-17 IN PERIODONTITIS
Periodontitis is a panel of inflammatory diseases of the tissues surrounding teeth that leads to the destruction of alveolar bone. The bone loss associated with periodontitis is also mediated by osteoclasts (41). In 2003, Oda et al. discovered that the surface antigens of Porphyromonas gingivalis, a gram-negative bacterium that causes periodontitis, significantly induced IL-17 expression in peripheral blood mononuclear cells (42). Indeed, IL-17 mRNA was readily detected in tissue samples from periodontitis patients (43). The increased amount of IL-17 protein was also detected in gingival crevicular fluid and cellular cultures of gingival tissues from periodontitis patients (44). These early studies suggested that IL-17 might be also linked to periodontal diseases in a similar fashion observed in rheumatoid arthritis. However, Yu et al. reported that IL-17RAdeficient mice exhibited more severe alveolar bone loss upon challenge by P. gingivalis, suggesting a bone-protective role for IL-17 signaling (45). The authors hypothesized that the IL-17 receptor-dependent signals are required for the neutrophil- mediated clearance of periodontal pathogens. Whether IL-17 stimulates bone destruction or protects bone in periodontitis is still an open question, although increasing evidence indicates that increased IL-17 expression in both chronic and aggressive periodontitis (46-48).
CONCLUSION
A newly identified family of cytokine IL-17 accelerates bone metabolism by stimulating osteogenic differentiation of mesenchymal stem cells and osteoblasts and promoting pro-osteoclastogenic molecules on these cells. In conjunction with the widely accepted pro-inflammatory role, numerous reports indicate that IL-17 is involved in inflammatory bone diseases, such as rheumatoid arthritis. Indeed anti-IL-17 therapy produced promising results in clinical trials among the rheumatoid arthritis patients. Several recent reports discovered potential association between IL-17 and periodontitis, although it is controversial whether IL-17 is a bone-protective or bone-destroying cytokine in alveolar bone during periodontitis. A better understanding on the physiologic, as well as pathologic role, for IL-17 in bone metabolism will provide greater insight into the osteolytic process during periodontitis and ensure future development of therapies against this bone-destructive disease.
Acknowledgments
This research was supported by the Kyungpook National University research fund, 2012.
References
- 1.Moseley T. A., Haudenschild D. R., Rose L., Reddi A. H. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. (2003);14:155–174. doi: 10.1016/S1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 2.Rouvier E., Luciani M. F., Mattei M. G., Denizot F., Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J. Immunol. (1993);150:5445–5456. [PubMed] [Google Scholar]
- 3.Witowski J., Ksiazek K., Jorres A. Interleukin-17: a mediator of inflammatory responses. Cell Mol. Life Sci. (2004);61:567–579. doi: 10.1007/s00018-003-3228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korn T., Bettelli E., Oukka M., Kuchroo V. K. IL-17 and Th17 Cells. Annu. Rev. Immunol. (2009);27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 5.Yao Z., Fanslow W. C., Seldin M. F., Rousseau A. M., Painter S. L., Comeau M. R., Cohen J. I., Spriggs M.K. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. (1995);3:811–821. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 6.Kuestner R. E., Taft D. W., Haran A., Brandt C. S., Brender T., Lum K., Harder B., Okada S, Ostrander C.D., Kreindler J. L., Aujla S. J., Reardon B., Moore M., Shea P., Schreckhise R., Bukowski T. R., Presnell S., Guerra-Lewis P., Parrish-Novak J., Ellsworth J. L., Jaspers S., Lewis K. E., Appleby M., Kolls J. K., Rixon M., West J. W., Gao Z., Levin S. D. Identification of the IL-17 receptor related molecule IL-17RC as the receptor for IL-17F. J. Immunol. (2007);179:5462–5473. doi: 10.4049/jimmunol.179.8.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toy D., Kugler D., Wolfson M., Vanden Bos T., Gurgel J., Derry J., Tocker J., Peschon J. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J. Immunol. (2006);177:36–39. doi: 10.4049/jimmunol.177.1.36. [DOI] [PubMed] [Google Scholar]
- 8.Meng F., Wang K., Aoyama T., Grivennikov S. I., Paik Y., Scholten D., Cong M., Iwaisako K., Liu X., Zhang M., Osterreicher C. H., Stickel F., Ley K., Brenner D. A., Kisseleva T. Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology. (2012);143:765–776. doi: 10.1053/j.gastro.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu Y., Hu X., Liu C., Qv X., Xu C. Interleukin (IL)-17 promotes macrophages to produce IL-8, IL-6 and tumour necrosis factor-alpha in aplastic anaemia. Br. J. Haematol. (2008);142:109–114. doi: 10.1111/j.1365-2141.2008.07161.x. [DOI] [PubMed] [Google Scholar]
- 10.Kawanokuchi J., Shimizu K., Nitta A., Yamada K., Mizuno T., Takeuchi H., Suzumura A. Production and functions of IL-17 in microglia. J. Neuroimmunol. (2008);194:54–61. doi: 10.1016/j.jneuroim.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Beklen A., Ainola M., Hukkanen M., Gurgan C., Sorsa T., Konttinen Y. T. MMPs, IL-1, and TNF are regulated by IL-17 in periodontitis. J. Dent. Res. (2007);86:347–351. doi: 10.1177/154405910708600409. [DOI] [PubMed] [Google Scholar]
- 12.Jovanovic D. V., Di Battista J. A., Martel-Pelletier J., Jolicoeur F. C., He Y., Zhang M., Mineau F., Pelletier J. P. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J. Immunol. (1998);160:3513–3521. [PubMed] [Google Scholar]
- 13.Mahanonda R., Jitprasertwong P., Sa-Ard-Iam N., Rerkyen P., Charatkulangkun O., Jansisyanont P., Nisapakultorn K., Yongvanichit K., Pichyangkul S. Effects of IL-17 on human gingival fibroblasts. J. Dent. Res. (2008);87:267–272. doi: 10.1177/154405910808700314. [DOI] [PubMed] [Google Scholar]
- 14.Shahrara S., Pickens S. R., Mandelin A. M. 2nd, Karpus W. J., Huang Q., Kolls J. K., Pope R. M. IL-17-mediated monocyte migration occurs partially through CC chemokine ligand 2/monocyte chemoattractant protein-1 induction. J. Immunol. (2010);184:4479–4487. doi: 10.4049/jimmunol.0901942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barin J. G., Baldeviano G. C., Talor M. V., Wu L., Ong S., Quader F., Chen P., Zheng D., Caturegli P., Rose N. R., Cihakova D. Macrophages participate in IL-17-mediated inflammation. Eur. J. Immunol. (2012);42:726–736. doi: 10.1002/eji.201141737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hosokawa Y., Hosokawa I., Ozaki K., Nakanishi T., Nakae H., Matsuo T. Catechins inhibit CCL20 production in IL-17A-stimulated human gingival fibroblasts. Cell. Physiol. Biochem. (2009);24:391–396. doi: 10.1159/000257431. [DOI] [PubMed] [Google Scholar]
- 17.Zhang F., Tanaka H., Kawato T., Kitami S., Nakai K., Motohashi M., Suzuki N., Wang C. L., Ochiai K., Isokawa K., Maeno M. Interleukin-17A induces cathepsin K and MMP-9 expression in osteoclasts via celecoxib-blocked prostaglandin E2 in osteoblasts. Biochimie. (2011);93:296–305. doi: 10.1016/j.biochi.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Zhang F., Koyama Y., Sanuki R., Mitsui N., Suzuki N., Kimura A., Nakajima A., Shimizu N., Maeno M. IL-17A stimulates the expression of inflammatory cytokines via celecoxib-blocked prostaglandin in MC3T3-E1 cells. Arch. Oral Biol. (2010);55:679–688. doi: 10.1016/j.archoralbio.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Van Bezooijen R. L., Papapoulos S. E., Lowik C. W. Effect of interleukin-17 on nitric oxide production and osteoclastic bone resorption: is there dependency on nuclear factor-kappaB and receptor activator of nuclear factor kappaB (RANK)/RANK ligand signaling? Bone. (2001);28:378–386. doi: 10.1016/S8756-3282(00)00457-9. [DOI] [PubMed] [Google Scholar]
- 20.Trajkovic V., Stosic-Grujicic S., Samardzic T., Markovic M., Miljkovic D., Ramic Z., Mostarica Stojkovic M. Interleukin-17 stimulates inducible nitric oxide synthase activation in rodent astrocytes. J. Neuroimmunol. (2001);119:183–191. doi: 10.1016/S0165-5728(01)00391-5. [DOI] [PubMed] [Google Scholar]
- 21.Kotake S., Udagawa N., Takahashi N., Matsuzaki K., Itoh K., Ishiyama S., Saito S., Inoue K., Kamatani N., Gillespie M. T., Martin T. J., Suda T. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J. Clin. Invest. (1999);103:1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van bezooijen R. L., Farih-Sips H. C., Papapoulos S. E., Lowik C. W. Interleukin-17: A new bone acting cytokine in vitro. J. Bone Miner. Res. (1999);14:1513–1521. doi: 10.1359/jbmr.1999.14.9.1513. [DOI] [PubMed] [Google Scholar]
- 23.Sato K., Suematsu A., Okamoto K., Yamaguchi A., Morishita Y., Kadono Y., Tanaka S., Kodama T., Akira S., Iwakura Y., Cua D. J., Takayanagi H. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J. Exp. Med. (2006);203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yago T., Nanke Y., Ichikawa N., Kobashigawa T., Mogi M., Kamatani N., Kotake S. IL-17 induces osteoclastogenesis from human monocytes alone in the absence of osteoblasts, which is potently inhibited by anti-TNF-alpha antibody: a novel mechanism of osteoclastogenesis by IL-17. J. Cell Biochem. (2009);108:947–955. doi: 10.1002/jcb.22326. [DOI] [PubMed] [Google Scholar]
- 25.Kitami S., Tanaka H., Kawato T., Tanabe N., Katono-Tani T., Zhang F., Suzuki N., Yonehara Y., Maeno M. IL-17A suppresses the expression of bone resorption-related proteinases and osteoclast differentiation via IL-17RA or IL-17RC receptors in RAW264.7 cells. Biochimie. (2010);92:398–404. doi: 10.1016/j.biochi.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 26.Balani D., Aeberli D., Hofstetter W., Seitz M. Interleukin-17A stimulates granulocyte-macrophage colony-stimulating factor release by murine osteoblasts in the presence of 1,25-dihydroxyvitamin D(3) and inhibits murine osteoclast development in vitro. Arthritis Rheum. (2013);65:436–446. doi: 10.1002/art.37762. [DOI] [PubMed] [Google Scholar]
- 27.Huang W., La Russa V., Alzoubi A., Schwarzenberger P. Interleukin-17A: a T-cell-derived growth factor for murine and human mesenchymal stem cells. Stem Cells (Dayton, Ohio) (2006);24:1512–1518. doi: 10.1634/stemcells.2005-0156. [DOI] [PubMed] [Google Scholar]
- 28.Huang H., Kim H. J., Chang E. J., Lee Z. H., Hwang S. J., Kim H. M., Lee Y., Kim H. H. IL-17 stimulates the proliferation and differentiation of human mesenchymal stem cells: implications for bone remodeling. Cell. Death. Differ. (2009);16:1332–1343. doi: 10.1038/cdd.2009.74. [DOI] [PubMed] [Google Scholar]
- 29.Nam D., Mau E., Wang Y., Wrigh D., Silkstone D., Whetstone H., Whyne C., Alman B. T-lymphocytes enable osteoblast maturation via IL-17F during the early phase of fracture repair. PloS One. (2012);7:e40044. doi: 10.1371/journal.pone.0040044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kocic J., Santibanez J. F., Krstic A., Mojsilovic S., Dordevic I. O., Trivanovic D., Ilic V., Bugarski D. Interleukin 17 inhibits myogenic and promotes osteogenic differentiation of C2C12 myoblasts by activating ERK1,2. Biochim. Biophys. Acta. (2012);1823:838–849. doi: 10.1016/j.bbamcr.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Ahmed M., Gaffen S. L. IL-17 in obesity and adipogenesis. Cytokine. Growth Factor Rev. (2010);21:449–453. doi: 10.1016/j.cytogfr.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lubberts E., Koenders M. I., Oppers-Walgreen B., van den Bersselaar L., Coenen-de Roo C. J., Joosten L. A., van den Berg W. B. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. (2004);50:650–659. doi: 10.1002/art.20001. [DOI] [PubMed] [Google Scholar]
- 33.Koenders M. I., Lubberts E., Oppers-Walgreen B., van den Bersselaar L., Helsen M. M., Di Padova F. E., Boots A. M., Gram H., Joosten L. A., van den Berg W. B. Blocking of interleukin-17 during reactivation of experimental arthritis prevents joint inflammation and bone erosion by decreasing RANKL and interleukin-1. Am. J. Pathol. (2005);167:141–149. doi: 10.1016/S0002-9440(10)62961-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koenders M. I., Kolls J. K., Oppers-Walgreen B., van den Bersselaar L., Joosten L. A., Schurr J. R., Schwarzenberger P., van den Berg W. B., Lubberts E. Interleukin-17 receptor deficiency results in impaired synovial expression of interleukin-1 and matrix metalloproteinases 3, 9, and 13 and prevents cartilage destruction during chronic reactivated streptococcal cell wall-induced arthritis. Arthritis Rheum. (2005);52:3239–3247. doi: 10.1002/art.21342. [DOI] [PubMed] [Google Scholar]
- 35.Genovese M. C., Van den Bosch F., Roberson S. A., Bojin S., Biagini I. M., Ryan P., Sloan-Lancaster J. LY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: A phase I randomized, double-blind, placebo-controlled, proof-of-concept study. Arthritis Rheum. (2010);62:929–939. doi: 10.1002/art.27334. [DOI] [PubMed] [Google Scholar]
- 36.Koenders M. I., Marijnissen R. J., Joosten L. A., Abdollahi-Roodsaz S., Di Padova F. E., van de Loo F. A., Dulos J., van den Berg W. B., Boots A. M. T cell lessons from the rheumatoid arthritis synovium SCID mouse model: CD3-rich synovium lacks response to CTLA-4Ig but is successfully treated by interleukin-17 neutralization. Arthritis Rheum. (2012);64:1762–1770. doi: 10.1002/art.34352. [DOI] [PubMed] [Google Scholar]
- 37.Zwerina K., Koenders M., Hueber A, Marijnissen R. J., Baum W., Heiland G. R., Zaiss M., McLnnes I., Joosten L., van den Berg W., Zwerina J., Schett G. Anti IL-17A therapy inhibits bone loss in TNF-alpha-mediated murine arthritis by modulation of the T-cell balance. Eur. J. Immunol. (2012);42:413–423. doi: 10.1002/eji.201141871. [DOI] [PubMed] [Google Scholar]
- 38.Chao C. C., Chen S. J., Adamopoulos I. E., Davis N., Hong K., Vu A., Kwan S, Fayadat-Dilman L., Asio A., Bowman E. P. Anti-IL-17A therapy protects against bone erosion in experimental models of rheumatoid arthritis. Autoimmunity. (2011);44:243–252. doi: 10.3109/08916934.2010.517815. [DOI] [PubMed] [Google Scholar]
- 39.Lubberts E., van den Bersselaar L., Oppers-Walgreen B., Schwarzenberger P., Coenen-de Roo C. J., Kolls J. K., Joosten L. A., van den Berg W. B. IL-17 promotes bone erosion in murine collagen-induced arthritis through loss of the receptor activator of NF-kappa B ligand/osteoprotegerin balance. J. Immunol. (2003);170:2655–2662. doi: 10.4049/jimmunol.170.5.2655. [DOI] [PubMed] [Google Scholar]
- 40.Kikuta J., Wada Y., Kowada T., Wang Z., Sun-Wada G. H., Nishiyama I., Mizukami S., Maiya N., Yasuda H., Kumanogoh A., Kikuchi K., Germain R. N., Ishii M. Dynamic visualization of RANKL and Th17-mediated osteoclast function. J. Clin. Invest. (2013);123:866–873. doi: 10.1172/JCI65054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bartold P. M., Cantley M. D., Haynes D. R. Mechanisms and control of pathologic bone loss in periodontitis. Periodontology 2000. (2010);53:55–69. doi: 10.1111/j.1600-0757.2010.00347.x. [DOI] [PubMed] [Google Scholar]
- 42.Oda T., Yoshie H., Yamazaki K. Porphyromonas gingivalis antigen preferentially stimulates T cells to express IL-17 but not receptor activator of NF-kappaB ligand in vitro. Oral Microbiol. Immunol. (2003);18:30–36. doi: 10.1034/j.1399-302X.2003.180105.x. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi K., Azuma T., Motohira H., Kinane D. F., Kitetsu S. The potential role of interleukin-17 in the immunopathology of periodontal disease. J. Clin. Periodentol. (2005);32:369–374. doi: 10.1111/j.1600-051X.2005.00676.x. [DOI] [PubMed] [Google Scholar]
- 44.Vernal R., Dutzan N., Chaparro A., Puente J., Antonieta Valenzuela M., Gamonal J. Levels of interleukin-17 in gingival crevicular fluid and in supernatants of cellular cultures of gingival tissue from patients with chronic periodontitis. J. Clin. Periodontol. (2005);32:383–389. doi: 10.1111/j.1600-051X.2005.00684.x. [DOI] [PubMed] [Google Scholar]
- 45.Yu J. J., Ruddy M. J., Wong G. C., Sfintescu C., Baker P. J., Smith J. B., Evans R. T., Gaffen S. L. An essential role for IL-17 in preventing pathogen-initiated bone destruction: recruitment of neutrophils to inflamed bone requires IL-17 receptor-dependent signals. Blood. (2007);109:3794–3802. doi: 10.1182/blood-2005-09-010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schenkein H. A., Koertge T. E., Brooks C. N., Sabatini R., Purkall D. E., Tew J. G. IL-17 in sera from patients with aggressive periodontitis. J. Dent. Res. (2010);89:943–947. doi: 10.1177/0022034510369297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duarte P. M., da Rocha M., Sampaio E., Mestnik M. J., Feres M., Figueiredo L. C., Bastos M. F., Faveri M. Serum levels of cytokines in subjects with generalized chronic and aggressive periodontitis before and after non-surgical periodontal therapy: a pilot study. J. Periodontol. (2010);81:1056–1063. doi: 10.1902/jop.2010.090732. [DOI] [PubMed] [Google Scholar]
- 48.Adibrad M., Deyhimi P., Ganjalikhani Hakemi M., Behfarnia P., Shahabuei M., Rafiee L. Signs of the presence of Th17 cells in chronic periodontal disease. J. Periodontal Res. (2012);47:525–531. doi: 10.1111/j.1600-0765.2011.01464.x. [DOI] [PubMed] [Google Scholar]


