Abstract
Invasion of trophoblasts into maternal uterine tissue is essential for establishing mature feto-maternal circulation. The trophoblast invasion associated with placentation is similar to tumor invasion. In this study, we investigated the role of KAI1, an anti-metastasis factor, at the maternal-fetal interface during placentation. Mouse embryos were obtained from gestational days 5.5 (E5.5) to E13.5. Immunohistochemical analysis revealed that KAI1 was expressed on decidual cells around the track made when a fertilized ovum invaded the endometrium, at days E5.5 and E7.5, and on trophoblast giant cells, along the central maternal artery of the placenta at E9.5. KAI1 in trophoblast giant cells was increased at E11.5, and then decreased at E13.5. Furthermore, KAI1 was upregulated during the forskolinmediated trophoblastic differentiation of BeWo cells. Collectively, these results indicate that KAI1 is differentially expressed in decidual cells and trophoblasts at the maternal-fetal interface, suggesting that KAI1 prevents trophoblast invasion during placentation. [BMB Reports 2013; 46(10): 507-512]
Keywords: Decidual cells, KAI1, Placenta, Trophoblast giant cells
INTRODUCTION
The successful formation of a placenta is essential for the maintenance of pregnancy. In humans, the maternal endometrium changes into decidua in pregnancy, even without contact with blastocysts. Decidualization transforms fibroblast-like endometrial stromal cells into decidual cells in preparation for blastocyst implantation, and blastocysts are readied for docking with the endometrium. After blastocysts make contact with the epithelium of the uterus, blastocysts and the epithelium dramatically change into invasive trophoblasts and a protective deciduas, respectively (1,2).
The process of mature placenta formation follows different time schedules in different species. The mature circulation of maternal blood vessels through the human placenta is not established until approximately the 12th-week of gestation (3), and oxygen concentration in the intervillous space is very low before 10 weeks, rising approximately 3-fold thereafter (3). Mature placenta formation in mice is established between embryonic days 10 (E10) and E12.5, which means that three principal layers are fully formed for the mature circulation of maternal blood vessels through the placenta. These three layers are composed of an outer trophoblast giant cell layer, a middle spongiotrophoblast layer, and an innermost labyrinth (4).
Cellular components containing high oxygen levels do not enter the intervillous space until a mature circulation has been established, and thus, feto-placental development probably occurs in a low-oxygen environment during early gestation. Accordingly, this process is similar to processes involved in tumor invasion and metastasis, which are also related to a low oxygen status. Therefore, it is plausible that trophoblast invasion is analogous to tumor invasion (5-7). For example, extravillous trophoblasts share several features with malignant tumors, in that they have high proliferative and invasive potentials, they are immunologically tolerated by the host, and they disseminate into the host’s vasculature. Thus, unsurprisingly, many genes related to metastasis have been found to be expressed in the feto- maternal interface during placentation (8). However, the rarity of malignant transformation and the metastasis of trophoblasts suggest that strict regulatory mechanisms function in the feto-maternal interface to ensure normal development.
Of the metastasis-associated genes, KAI1 (CD82) is a known tumor suppressor in prostate cancer and as a general suppressor of metastasis in several cancer types (9). Although KAI1 does not affect primary tumor growth, its loss of expression has been correlated with the metastatic progression of primary tumors (10). Reduced KAI1 expression is associated with increased motility, reduced cell-cell interactions, and decreased adhesion to extracellular matrix components (11). The restoration of KAI1 expression in a metastatic prostate cancer cell line was found to inhibit integrin-mediated cell migration, invasion, and activation (12). Interestingly, HIF-1α is a strong regulator of KAI1 expression (13). Recently, it was shown that KAI1 is expressed in decidual cells and not in trophoblasts during human placenta formation (14). This lack of KAI1 expression in trophoblasts is reminiscent of the loss of KAI1 expression that occurs during tumor cell invasion. However, this previous human study was performed using a limited number of time points, and thus, a more extensive investigation of KAI1 expression in the feto-maternal interface was required. Accordingly, the purpose of this study was to investigate the spatio-temporal expression of KAI1 at the feto-maternal interface in the uteri of pregnant mice around the time of mature placentation.
RESULTS
KAI1 was expressed in both decidua and trophoblast giant cells
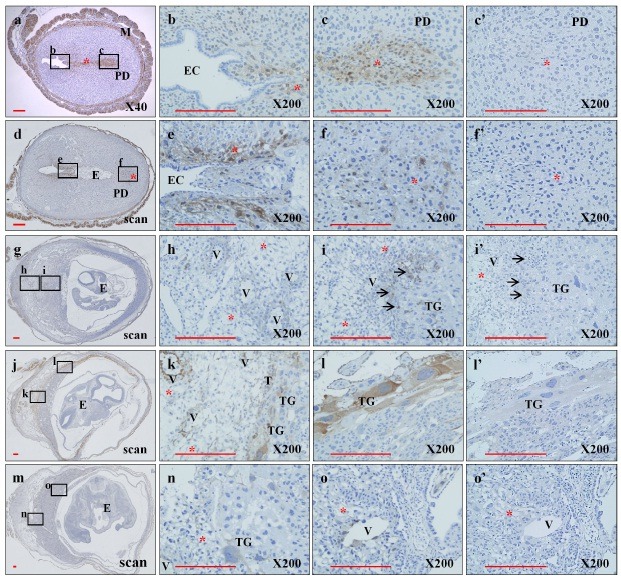
To explore the expression pattern of KAI1 during gestation, whole uteri and embryos of mice were studied immunohistochemically (Fig. 1). KAI1 expression was observed mainly on decidual cells along the embryo trace extending from the endometrial cavity to the final implantation site at E5.5 and E7.5. No expression of KAI1 on embryonic cells was observed during this period. Interestingly, faint KAI1 expression was seen on decidual cells surrounding central maternal arteries near the border between trophoblast giant cells and the decidua basalis at E9.5. At E11.5, the expression pattern of KAI1 was much clearer and could be seen along the feto-maternal interface exclusively on trophoblast giant cells and decidual cells, and strong KAI1 expression was observed around the central maternal artery in the decidua basalis. However, KAI1 expression was not detected at E13.5. These results indicate that KAI1 is expressed in decidual cells and trophoblas
Fig. 1. Differential expression of KAI1 at the feto-maternal interface during different embryonic periods. Paraffin sections of whole uteri and embryos of C57BL/6 mice at E5.5 (a), E7.5(d), E9.5(g), E11.5(j), and E13.5(m) were prepared to detect KAI1 protein with rabbit polyclonal anti-KAI1 antibody as described in “Materials and Methods.” Sections were treated with anti-KAI1 antibody (a, d, g, j, and m). Images b, c, e, f, h, i, k, l, n, and o are higher-magnification images corresponding to the small rectangles in a, d, g, j, and m, respectively. The sections of c’, f’, i’, l’, and o’ were stained with normal IgG as an internal control of c, f, i, l, and o, respectively. E, embryo; EC, endometrial cavity; M, myometrium; PD, predecidua; T, trophoblast; TG, trophoblast giant cell; V, Uterine spiral artery; *, decidua; →, glycogen trophoblast cell. Scale bar indicates 200 μm.
Expression of KAI1 mRNA during trophoblast differentiation
KAI1 was clearly expressed in trophoblasts (Fig. 1). To confirm the expression pattern of KAI1 and characteristic cell fusion during trophoblast differentiation, we used BeWo cells, a model commonly used to study the syncytialization of trophoblasts with forskolin (15,16). Forskolin, an adenylyl cyclase activator, stimulates syncytium formation and increases the expression of fusogenic genes, including human chorionic gonadotropin (hCG), and Syncytin 1and 2 through the cyclic AMP pathway (16).
KAI1 mRNA was well detected by RT-PCR, and real-time PCR and was higher at 72 h than at 24 h under normal culture conditions without forskolin (Fig. 2). Furthermore, its identity was confirmed by sequencing after TA cloning of the PCR products (data not shown). In contrast, KAI1 mRNA expression was strongly decreased during trophoblast differentiation 24 h and 72 h after treatment with forskolin. BeWo cells rapidly changed morphologically, and forskolin caused cell-to-cell fusion (data not shown). In addition, forskolin upregulated hCG mRNA, as has been previously reported (17), and this upregulaton was prominent at 72 h and paralleled trophoblast differentiation (Fig. 2B). These results confirmed the expression of KAI1 mRNA in BeWo cells, and the observed forskolin- induced reduction in KAI1 mRNA expression suggests that KAI1 expression is downregulated during trophoblastic BeWo cell differentiation.
Fig. 2. Expression pattern of KAI1 mRNA during trophoblast differentiation. BeWo cells were cultured and differentiated using forskolin (50 μM). After 24 or 72 h, cells were examined to determine mRNA expressions. KAI1 and β-Actin mRNA levels were determined by reverse-transcription PCR (A) and those of KAI1, hCG, and GAPDH were determined by real-time RT-PCR (B). Empty and black bars indicate control and forskolin, respectively. Two independent experiments were performed. Each sample was triplicated and represented as means ± SD. *P < 0.05 and **P < 0.01.

Expressions of different KAI1 isoforms during trophoblast differentiation
KAI1 protein expressions were also examined (Fig. 3). Interestingly, forskolin downregulated the 47 kDa large KAI1 isoform (KAI1-l), but upregulated the 23 kDa small KAI1 (KAI1-s) isoform by a factor of around two at 24 h and 72 h after forskolin treatment (Fig. 3). Furthermore, this upregulation of KAI1-s was in-line with immunofluorescent staining results (Fig. 4). In addition, E-cadherin, a positive trophoblast marker, was increased by forskolin at only 24 h, but was decreased to the basal level at 72 h (Fig. 3).
Fig. 3. KAI1 was increased during trophoblast differentiation. BeWo to trophoblast differentiation was induced with forskolin (50 μM). At 24 or 72 h after treatment, total proteins were prepared and KAI1 expressions were determined by Western blotting (A). KAI1-l and KAI1-s indicate large 47 kDa and small 23 kDa KAI1, respectively. The relative expression levels of KAI1-l, KAI1-s, and E-cadherin were quantified densitometrically and normalized versus β-actin (B). Empty and black bars indicate control and forskolin treatments, respectively. Graphs were presented as means ± SD of three independent experiments. *P < 0.05.
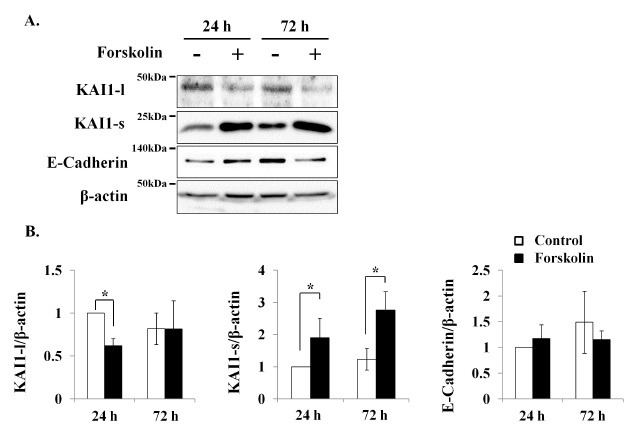
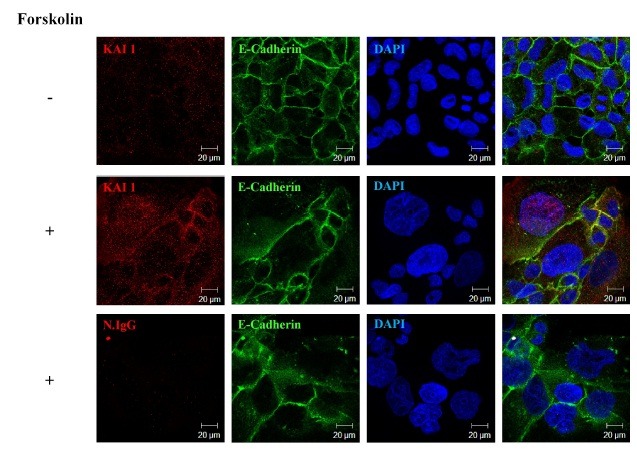
Fig. 4. Expression pattern of KAI1 by in situ immunofluorescent staining during trophoblast differentiation. BeWo cells were cultured and differentiated with forskolin (50 μM) for 72 h. Cells were then examined for morphological changes, protein expression patterns, and KAI1 localization. KAI1 and E-cadherin localizations were determined using Alexa fluor 555 (red) and FITC (green), respectively. Blue indicates DAPI staining. Normal rabbit or mouse IgG was used as internal controls. Photos were taken using a confocal microscope.
Subcellular distribution of KAI1 in BeWo cells
The forskolin-mediated upregulation of KAI1 expression was further confirmed by immunofluorescent staining (Fig. 4). Before forskolin treatment, KAI1 was weakly detected throughout cells without any particular localization. However, 50 μM forskolin increased the fusion of cells with large nuclei, which was clearly shown by DAPI staining. KAI1 protein expression was enhanced in the cytoplasm, nucleus, and cell membrane at 72 h after forskolin treatment. In addition, a speckled KAI1 pattern was clearly detected in DAPI/KAI1/E-Cadherin superimposed images. An expansion of membrane E-cadherin immunoreactivity was also found to represent cell fusion. More interestingly, KAI1 and E-cadherin were partially colocalized in the cell membrane. These results indicate that KAI1 expression is increased during BeWo cell differentiation, and that this enhanced expression localizes to the cytoplasm, nucleus, and cell membrane.
DISCUSSION
In this study, we found that KAI1 was expressed in both decidual cells and trophoblasts of the feto-maternal interface. KAI1 was expressed early on decidual cells at days E5.5 and E7.5, and appeared on trophoblast giant cells at E9.5, peaked at E11.5 with decidual cells, and then decreased to the basal level at E13.5. The expression of KAI1 was found to be upregulated during trophoblastic BeWo cell differentiation. Given the anti-metastatic effect of KAI1, these results suggest that KAI1 acts as a negative regulator of trophoblast invasion into the endometrium at the feto-maternal interface.
Previously, KAI1 was found to be expressed only in decidual cells, and not in trophoblasts (14,18). However, our results show that KAI1 is expressed in both decidua and trophoblasts at the feto-maternal interface. This discrepancy may be due to the narrow time window used to observe the expression of KAI1 in human term placenta (14), and the use of serial sections of whole embryos obtained at different time points in the present study. In a previous study, KAI1 expression in trophoblasts was not observed during the early and late implantation stages (14), and in the present study, we also found it difficult to detect KAI1 at E5.5 and E13.5. This means that the expression of KAI1 in trophoblasts is either transient, or that species differences caused the discrepancy. The latter explanation supports that the expressions of KAI1 in both decidua and trophoblasts of mice show substantially less invasion than the deep invasion shown by human trophoblasts.
The expression of KAI1 mRNA in BeWo cells was examined, because a previous study found that they did not express KAI1 (18). BeWo cells display most of the characteristics of villous trophoblasts, and secret hormones, such as hCG, progesterone, prolactin, and estradiol (19). Therefore, BeWo cells provide an excellent means of determining whether KAI1 is expressed. Consistent with our findings, KAI1 was found to be highly expressed during forskolin-induced trophoblast differentiation. Although KAI1 mRNA levels were not increased, KAI1 protein levels were increased two-fold compared to the control. KAI1 has two isoforms, KAI1-s and KAI1-l. The upregulated KAI1 observed by immunofluorescent staining appears to be KAI1-s, because forskolin decreased KAI1-l. Although we do not know the mechanism responsible for the posttranslational modification of KAI1, KAI1-l might be produced post-translationally, as suggested by the recent finding that KAI1 has three N-glycosylation sites, and exhibits a highly heterogeneous N-linked glycan pattern (20). Therefore, the two different isotypes of KAI1 expressed during trophoblastic differentiation may result from posttranslational changes, like glycosylation, in BeWo cells. The mechanism responsible for the aforementioned discrepancy and the differential regulation between KAI1 mRNA and protein levels should be determined by future study. Taken together, the high expression of KAI1 in trophoblast giant cells implies that KAI1 may act as an autonomous inhibitory brake of the invasion of trophoblasts into decidua at the feto-maternal interface.
Based on the biological function of KAI1 as a cell membrane binding protein that functions as a tumor suppressor, the expression of KAI1 in trophoblast giant cells may provide another barrier that prevents the fetus from being sensitized by maternal cells. Decidual cells express KAI1 along the track of an embryo implantation site and in the surrounding gestational sac during the early embryonic stage (E5.5 and E7.5). At this time, KAI1 expression on decidual cells may be due to a need to inhibit the invasion and destruction of embryonic tissue. When trophoblasts invade the endometrium, KAI1 expression on decidual cells gradually subsides, and under certain conditions, this process may allow trophoblast invasion. However, the expression of KAI1 at E11.5, which had been observed exclusively on trophoblast giant cells and decidual cells, was appeared along the feto-maternal interface, and strong expression was detected around the central maternal artery in the decidua basalis. The expression of KAI1 in decidua basalis cells around the central artery means that cells of two different origins confront each other in this region. The expression of KAI1 at the interface between two different tissues was not seen at E13.5, but was seen at E11.5. This observation appears to be related to the onset of hemotrophic exchange in the definitive placenta; in other words, KAI1 protein is no longer observed after mature placentation. Although we do not know the identity of the protein that partners with KAI1 in decidual cells or trophoblasts, KAI1 may participate in intercellular communication between the two. These results also suggest that KAI1 participates as a component of the regulatory circuit that prevents fetal invasion of the endometrium at the feto-maternal interface.
Collectively, this study shows that KAI1 is expressed in both decidual cells and trophoblast giant cells at the mouse feto-maternal interface. Furthermore, the differential expressions of KAI1 observed in decidual cells and trophoblast giant cells imply that KAI1 plays an important role in cell invasion and protection during placentation.
MATERIALS AND METHODS
Animals and tissues
All experiments were conducted in accordance with the guidelines issued by Kyungpook National University concerning the Care and Use of Laboratory Animals. C57BL6/N mice (Koatech, Gyeonggi-do, Korea) were housed at 22±1℃ and a relative humidity of 40-60% with 12-hour day/ 12-hour night cycles in a specific-pathogen-free environment with access to food and water ad libitum. Six to eight-week-old female mice were mated with male mice. Mating was confirmed by the presence of a vaginal plug. The mornings when vaginal plugs were found were designated 0.5 (E0.5) post-coitus. Gestational materials were obtained from pregnant mice sacrificed at 5.5, 7.5, 9.5, 11.5, and 13.5 days post-coitus.
Immunohistochemistry
Whole uteri and embryos were fixed in 0.1 M sodium phosphate buffer (PBS) containing 4% paraformaldehyde for 16 hours (h) at 4℃. After fixation, specimens were dehydrated in ethanol, embedded in paraffin, and sectioned at 3 μm. Deparaffinized sections were quenched in 3% H2O2 in methanol for 30 minutes (m), incubated in 10 mM sodium citrate buffer (pH 6.0) for 30 m in a 100℃, and then in 50 mM ammonium chloride for 30 m at room temperature (RT), and blocked with 1% bovine serum albumin (BSA) for 2 h at RT. The sections were incubated overnight at 4℃ with anti-KAI 1 antibody C-16 (0.2 μg/ml; Santa Cruz, CA, USA), rinsed 3 times in PBS, incubated with horse radish peroxidase (HRP)-conjugated anti-IgG (DAKO, Glostrup, Denmark), and developed using a Dab+ Substrate system (DAKO). As a control, normal IgG was used at the same dilution instead of the primary antibody.
Cell culture
Trophoblastic BeWo cells were obtained from Cell Bank (KCB, Seoul) and maintained in DMEM high-glucose medium (Lonza, Walkersville, MD, USA) supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen, Carlsbad, CA, USA ), 100 U/ml penicillin, and 100 μg/ml streptomycin at 37℃ in a 5% CO2 humidified atmosphere. To examine the KAI1 expression pattern during trophoblast differentiation, BeWo cells (1 × 105) were treated with 50 μM forskolin for 24 or 72 h in 6-well plates.
Western blot analysis
Western blot analysis was performed as described in previous method (21). Briefly, nuclear and cytoplasmic extracts were prepared using an NE-PERⓇ kit (PIERCE Biotechnology, Rockford, IL, USA). Cytoplasmic extracts (20 μg each) were separated by 10% SDS-PAGE and transferred onto nitrocellulose membranes, which were then blocked with 5% skim milk in TBS-T (TBS containing 0.1% Tween-20) for 1 h at RT. Membranes were then incubated for 1 h at RT with anti-KAI1 antibody (diluted 1:2000 in TBS-T) or anti-E-Cadherin antibody (diluted 1:2000; BD Biosciences, Franklin Lakes, NJ, USA), and then incubated for 1 h at RT with HRP-conjugated antibodies (diluted 1:2500 in TBS-T; Santa Cruz). Blots were developed using Amersham ECLTM (Bucks, UK) reagent. Anti-β-actin antibody (Santa Cruz) was used as internal controls.
Reverse transcription (RT) PCR and real-time RT-PCR analysis
Real-time RT-PCR was performed as described in previous method (22). Briefly, total RNA (1-5 μg) was extracted using Trizol reagent (Invitrogen) from cultured BeWo cells and reverse-transcribed using Superscript II RT (Invitrogen) according to the manufacturer’s instructions. PCR was performed at 95℃ for 5 m, and then 35 cycles of 95℃ for 30 seconds (s), 59℃ for 30 s, and 72℃ for 30 s. The primers used for RT-PCR were KAI1 5’-TTT GCT TTC CTG CTG CTC CTG AT-3’ (sense) and 5’-TTC ATG AGC TCA GCG TTG TC-3’ (antisense) and β-actin 5’-TTG TTA CAG GAA GTC CCT TGC C-3’ (sense) and 5’-ATG CTA TCA CCT CCC CTG TGR-3’ (antisense). Real-time RT-PCR was performed for the relative quantification of the mRNAs of KAI1 and human chorionic gonadotropin (hCG) using a LightCycler 480 (Roche, Indianapolis, IN, USA). SYBR green PCR master mix and specific primers were combined with the complementary DNA reverse transcribed from the total RNA of cultured BeWo cells. A negative control with no template was also included in each assay. Real-time PCR was performed at 95℃ for 10 m followed by 50 cycles of 95℃ for 15 s, 60℃ for 15 s, and 72℃ for 15 s. Primers for real-time RT-PCR were KAI1, 5’-TTC CTG CTC CTG ATC CTC ATT G-3’ (sense) and 5’-TCC TCG CGA CTG CTG TTG TAG T-3’ (antisense); hCG, 5’-TCA GCT GTC AAT GTG CAC TCT G-3’ (sense) and 5’-CCT TTG AGG AAG AGG AGT CCT G-3’ (antisense); GAPDH, 5’-GCA TCT CCC TCA CAA TTT CCA-3’ (sense) and 5’-GTG CAG CGA ACT TTA TTG ATG G-3’ (antisense). All primers were designed using ABI Primer Express version 2.0 (Carlsbad, CA, USA) and showed specific melting points.
TA-cloning and sequencing
Total RNA was extracted and reverse-transcribed as described above. Full-length KAI1 was obtained by PCR using the following primer set: KAI1 5’-AAA GCA GAA CCC GCA GAG T-3’ (sense) and 5’-GGA GAT GGG GAT AGC AGC T-3’ (antisense). RT-PCR products were cloned using a TOPcloner TA kit (Enzinomics, Daejeon, Korea), and the cloned products obtained were confirmed by sequencing using M13 forward primer (-20) and M13 F reverse primer (-40).
Immunofluorescence analysis
BeWo cells were seeded on an 8-chamber slide (Nalge Nunc International, Rochester, NY, USA), and after about 50% confluence, cells were treated with forskolin (50 μM) for 72 h. Cells were then washed with PBS, fixed with 4% paraformaldehyde in PBS (pH 7.4), blocked with 1% BSA in PBS containing 5% normal goat serum and 0.3% Triton X-100, and incubated with anti-KAI1 (1:200), anti-E-cadherin-FITC (1:200; BD Biosciences), normal antibodies (1:200, Santa Cruz) in 1% BSA in PBS, washed three times with PBS, and incubated with Alexa Fluor 555 antibody (1:200; Invitrogen) in PBS containing 1% BSA. Cells were then counter-stained with DAPI (1:10,000; Sigma, St. Louis, MO, USA), and mounted with anti-fade solution (Invitrogen).
Statistical analysis
The analysis was performed using the Student’s t-test. All data are presented as means ± standard deviations. P-values lower than 0.05 were considered statistically significant.
Acknowledgments
This study was supported by the Kyungpook National University Hospital Medical Research Institute in 2006, the WCU program of the National Research Foundation of Korea (grant no. R32-10064) and a grant of the Korean Health Technology R & D project (A111487), Republic of Korea.
References
- 1.Goldman-Wohl D., Yagel S. Regulation of trophoblast invasion: from normal implantation to preeclampsia. Mol. Cell. Endocrinol. (2002);187:233–238. doi: 10.1016/S0303-7207(01)00687-6. [DOI] [PubMed] [Google Scholar]
- 2.Koo T. B., Song H., Moon I., Han K., Chen C., Murphy K., Lim H. Differential expression of the PEA3 subfamily of ETS transcription factors in the mouse ovary and peri-implantation uterus. Reproduction. (2005);129:651–657. doi: 10.1530/rep.1.00656. [DOI] [PubMed] [Google Scholar]
- 3.Jauniaux E., Watson A. L., Hempstock J., Bao Y. P., Skepper J. N., Burton G. J. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am. J. Pathol. (2000);157:2111–2122. doi: 10.1016/S0002-9440(10)64849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cross J. C., Hemberger M., Lu Y., Nozaki T., Whiteley K., Masutani M., Adamson S. L. Trophoblast functions, angiogenesis and remodeling of the maternal vasculature in the placenta. Mol. Cell. Endocrinol. (2002);187:207–212. doi: 10.1016/S0303-7207(01)00703-1. [DOI] [PubMed] [Google Scholar]
- 5.Murray M. J., Lessey B. A. Embryo implantation and tumor metastasis: common pathways of invasion and angiogenesis. Semin. Reprod. Endocrinol. (1999);17:275–290. doi: 10.1055/s-2007-1016235. [DOI] [PubMed] [Google Scholar]
- 6.Soundararajan R., Rao A. J. Trophoblast 'pseudo- tumorigenesis': significance and contributory factors. Reprod. Biol. Endocrinol. (2004);2:15. doi: 10.1186/1477-7827-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strickland S., Richards W. G. Invasion of the trophoblasts. Cell. (1992);71:355–357. doi: 10.1016/0092-8674(92)90503-5. [DOI] [PubMed] [Google Scholar]
- 8.Lotem J., Netanely D., Domany E., Sachs L. Human cancers overexpress genes that are specific to a variety of normal human tissues. Proc. Natl. Acad. Sci. U. S. A. (2005);102:18556–18561. doi: 10.1073/pnas.0509360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong J. T., Lamb P. W., Rinker-Schaeffer C. W., Vukanovic J., Ichikawa T., Isaacs J. T., Barrett J. C. KAI1, a metastasis suppressor gene for prostate cancer on human chromosome 11p11.2. Science. (1995);268:884–886. doi: 10.1126/science.7754374. [DOI] [PubMed] [Google Scholar]
- 10.Kauffman E. C., Barocas D. A., Chen Y. T., Yang X. J., Scherr D. S., Tu J. J. Differential expression of KAI1 metastasis suppressor protein in renal cell tumor histological subtypes. J. Urol. (2009);181:2305–2311. doi: 10.1016/j.juro.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Jackson P., Marreiros A., Russell P. J. KAI1 tetraspanin and metastasis suppressor. Int. J. Biochem. Cell. Biol. (2005);37:530–534. doi: 10.1016/j.biocel.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Rinker-Schaeffer C. W., Hickson J. A. Stopping cancer before it colonizes. Nat. Med. (2006);12:887–888. doi: 10.1038/nm0806-887. [DOI] [PubMed] [Google Scholar]
- 13.Kim B., Boo K., Lee J. S., Kim K. I., Kim W. H., Cho H. J., Park Y. B., Kim H. S., Baek S. H. Identification of the KAI1 metastasis suppressor gene as a hypoxia target gene. Biochem. Biophys. Res. Commun. (2010);393:179–184. doi: 10.1016/j.bbrc.2010.01.118. [DOI] [PubMed] [Google Scholar]
- 14.Gellersen B., Briese J., Oberndorfer M., Redlin K., Samalecos A., Richter D. U., Loning T., Schulte H. M., Bamberger A. M. Expression of the metastasis suppressor KAI1 in decidual cells at the human maternal- fetal interface: Regulation and functional implications. Am. J. Pathol. (2007);170:126–139. doi: 10.2353/ajpath.2007.060175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kudo Y., Boyd C. A., Sargent I. L., Redman C. W., Lee J. M., Freeman T. C. An analysis using DNA microarray of the time course of gene expression during syncytialization of a human placental cell line (BeWo). Placenta. (2004);25:479–488. doi: 10.1016/j.placenta.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Delidaki M., Gu M., Hein A., Vatish M., Grammatopoulos D. K. Interplay of cAMP and MAPK pathways in hCG secretion and fusogenic gene expression in a trophoblast cell line. Mol. Cell. Endocrinol. (2011);332:213–220. doi: 10.1016/j.mce.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Orendi K., Gauster M., Moser G., Meiri H., Huppertz B. Effects of vitamins C and E, acetylsalicylic acid and heparin on fusion, beta-hCG and PP13 expression in BeWo cells. Placenta. (2010);31:431–438. doi: 10.1016/j.placenta.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 18.Li M. Q., Hou X. F., Shao J., Tang C. L., Li D. J. The DSCs-expressed CD82 controls the invasiveness of trophoblast cells via integrinbeta1/MAPK/MAPK3/1 signaling pathway in human first-trimester pregnancy. Biol. Reprod. (2010);82:968–979. doi: 10.1095/biolreprod.109.080739. [DOI] [PubMed] [Google Scholar]
- 19.Orendi K., Kivity V., Sammar M., Grimpel Y., Gonen R., Meiri H., Lubzens E., Huppertz B. Placental and trophoblastic in vitro models to study preventive and therapeutic agents for preeclampsia. Placenta. (2011);32(Suppl):S49–54. doi: 10.1016/j.placenta.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 20.Wang H., Zhang W., Zhao J., Zhang L., Liu M., Yan G., Yao J., Yu H., Yang P. N-Glycosylation pattern of recombinant human CD82 (KAI1), a tumor-associated membrane protein. J. Proteomics. (2012);75:1375–1385. doi: 10.1016/j.jprot.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Kim H. N., Min W. K., Jeong J. H., Kim S. G., Kim J. R., Kim S. Y., Choi J. Y., Park B. C. Combination of Runx2 and BMP2 increases conversion of human ligamentum flavum cells into osteoblastic cells. BMB Rep. (2011);44:446–451. doi: 10.5483/BMBRep.2011.44.7.446. [DOI] [PubMed] [Google Scholar]
- 22.Jang W. G., Kim E. J., Koh J. T. Tunicamycin negatively regulates BMP2-induced osteoblast differentiation through CREBH expression in MC3T3E1 cells. BMB Rep. (2011);44:735–740. doi: 10.5483/BMBRep.2011.44.11.735. [DOI] [PubMed] [Google Scholar]


