Abstract
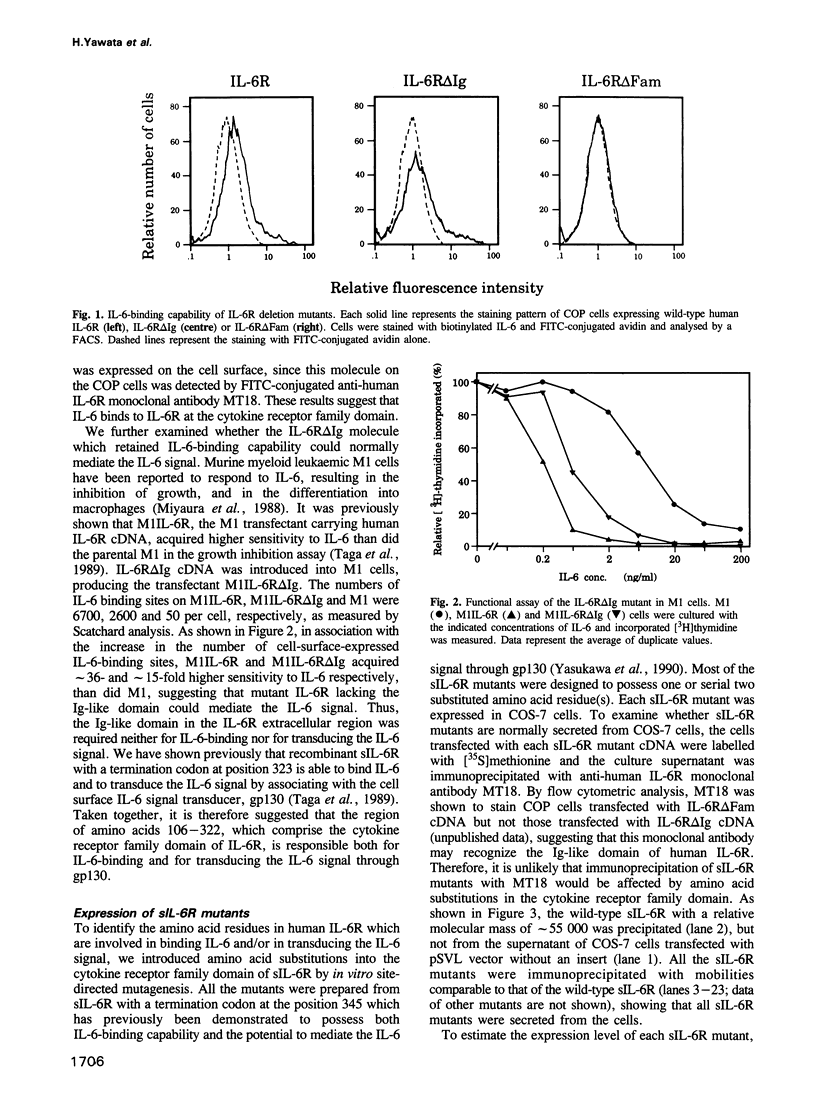
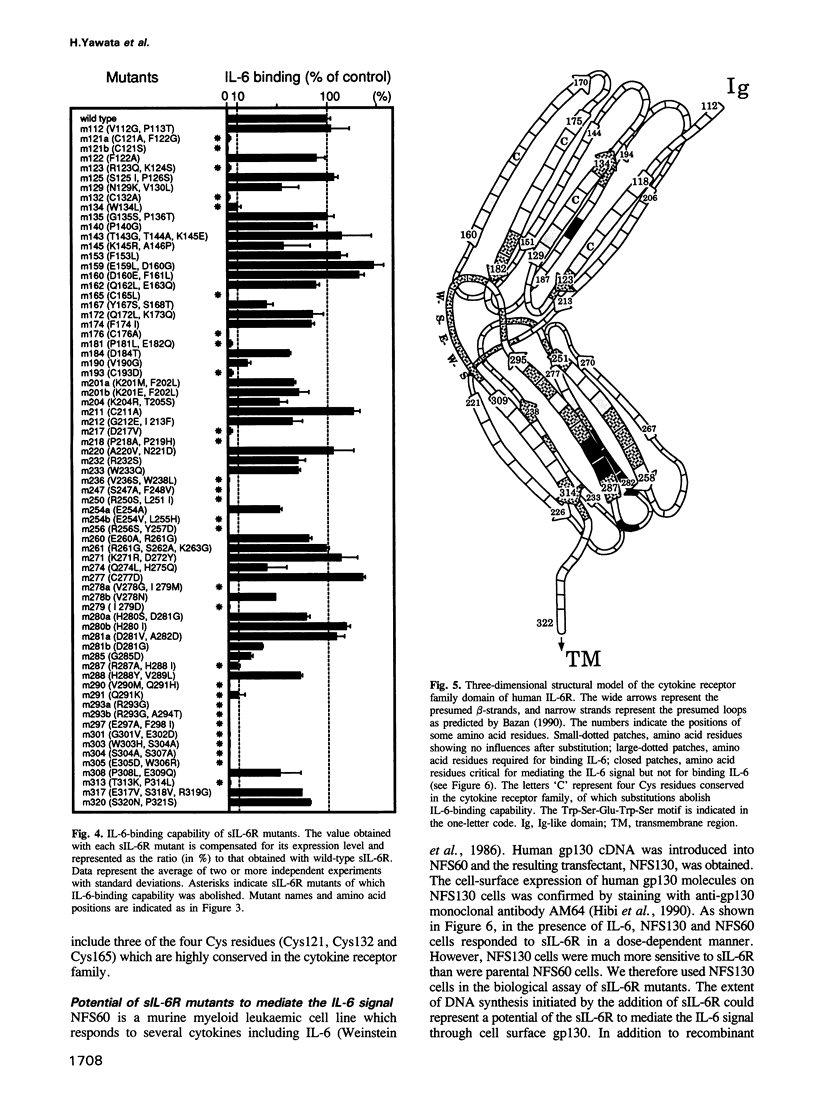
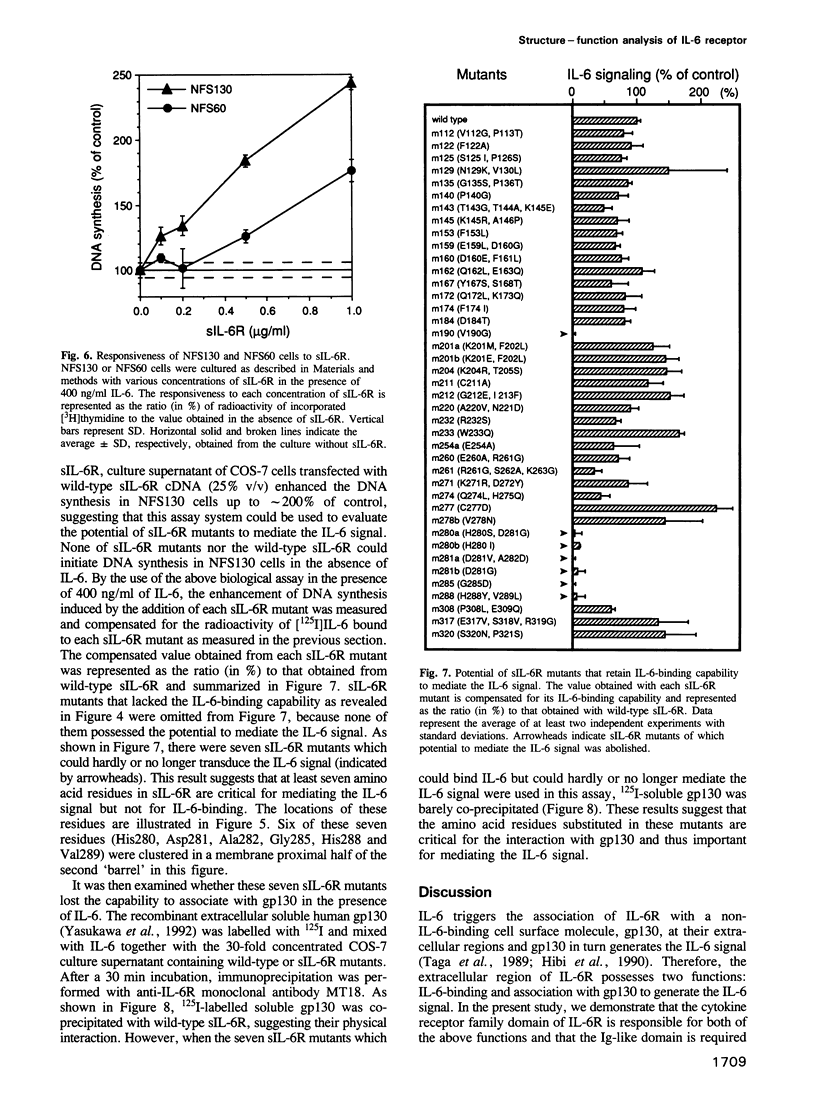
Here, we report the analysis of the structure-function relationship of the extracellular region of human interleukin 6 receptor (IL-6R). Upon binding of IL-6, IL-6R becomes associated extracellularly with a non-IL-6-binding but signal transducing molecule, gp130, and the IL-6 signal is generated. In this region, the cytokine receptor family domain, but not the immunoglobulin-like domain, was responsible both for IL-6 binding and for signal transduction through gp130. Because a soluble, extracellular portion of IL-6R (sIL-6R) could bind IL-6 and mediate IL-6 functions through gp130, amino acid substitutions were introduced into sIL-6R by site-directed mutagenesis. The results, together with the previously proposed tertiary structure model, suggested that the amino acid residues critical for IL-6 binding have a tendency to be distributed to the hinge region between the two 'barrel'-like fibronectin type III modules and to the same side of these two 'barrels'. Amino acid residues, of which substitutions barely affected the IL-6-binding but did abolish the IL-6 signalling capability of sIL-6R, were identified and found to be located mainly in the membrane proximal half of the second barrel. sIL-6R mutants carrying such substitutions lacked the capacity to associate with gp130 in the presence of IL-6.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bass S. H., Mulkerrin M. G., Wells J. A. A systematic mutational analysis of hormone-binding determinants in the human growth hormone receptor. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4498–4502. doi: 10.1073/pnas.88.10.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan J. F. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanslow W. C., Sims J. E., Sassenfeld H., Morrissey P. J., Gillis S., Dower S. K., Widmer M. B. Regulation of alloreactivity in vivo by a soluble form of the interleukin-1 receptor. Science. 1990 May 11;248(4956):739–742. doi: 10.1126/science.2139736. [DOI] [PubMed] [Google Scholar]
- Fukunaga R., Ishizaka-Ikeda E., Pan C. X., Seto Y., Nagata S. Functional domains of the granulocyte colony-stimulating factor receptor. EMBO J. 1991 Oct;10(10):2855–2865. doi: 10.1002/j.1460-2075.1991.tb07835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibi M., Murakami M., Saito M., Hirano T., Taga T., Kishimoto T. Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell. 1990 Dec 21;63(6):1149–1157. doi: 10.1016/0092-8674(90)90411-7. [DOI] [PubMed] [Google Scholar]
- Hirano T., Akira S., Taga T., Kishimoto T. Biological and clinical aspects of interleukin 6. Immunol Today. 1990 Dec;11(12):443–449. doi: 10.1016/0167-5699(90)90173-7. [DOI] [PubMed] [Google Scholar]
- Hirata Y., Taga T., Hibi M., Nakano N., Hirano T., Kishimoto T. Characterization of IL-6 receptor expression by monoclonal and polyclonal antibodies. J Immunol. 1989 Nov 1;143(9):2900–2906. [PubMed] [Google Scholar]
- Holmgren A., Bränden C. I. Crystal structure of chaperone protein PapD reveals an immunoglobulin fold. Nature. 1989 Nov 16;342(6247):248–251. doi: 10.1038/342248a0. [DOI] [PubMed] [Google Scholar]
- Imler J. L., Miyajima A., Zurawski G. Identification of three adjacent amino acids of interleukin-2 receptor beta chain which control the affinity and the specificity of the interaction with interleukin-2. EMBO J. 1992 Jun;11(6):2047–2053. doi: 10.1002/j.1460-2075.1992.tb05262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. E., Lee P. L., Lu J., Williams L. T. Diverse forms of a receptor for acidic and basic fibroblast growth factors. Mol Cell Biol. 1990 Sep;10(9):4728–4736. doi: 10.1128/mcb.10.9.4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T. The biology of interleukin-6. Blood. 1989 Jul;74(1):1–10. [PubMed] [Google Scholar]
- Klein B., Wijdenes J., Zhang X. G., Jourdan M., Boiron J. M., Brochier J., Liautard J., Merlin M., Clement C., Morel-Fournier B. Murine anti-interleukin-6 monoclonal antibody therapy for a patient with plasma cell leukemia. Blood. 1991 Sep 1;78(5):1198–1204. [PubMed] [Google Scholar]
- Klein B., Zhang X. G., Jourdan M., Boiron J. M., Portier M., Lu Z. Y., Wijdenes J., Brochier J., Bataille R. Interleukin-6 is the central tumor growth factor in vitro and in vivo in multiple myeloma. Eur Cytokine Netw. 1990 Oct-Nov;1(4):193–201. [PubMed] [Google Scholar]
- Miyaura C., Onozaki K., Akiyama Y., Taniyama T., Hirano T., Kishimoto T., Suda T. Recombinant human interleukin 6 (B-cell stimulatory factor 2) is a potent inducer of differentiation of mouse myeloid leukemia cells (M1). FEBS Lett. 1988 Jul 4;234(1):17–21. doi: 10.1016/0014-5793(88)81293-6. [DOI] [PubMed] [Google Scholar]
- Miyazaki T., Maruyama M., Yamada G., Hatakeyama M., Taniguchi T. The integrity of the conserved 'WS motif' common to IL-2 and other cytokine receptors is essential for ligand binding and signal transduction. EMBO J. 1991 Nov;10(11):3191–3197. doi: 10.1002/j.1460-2075.1991.tb04881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley B., Beckmann M. P., March C. J., Idzerda R. L., Gimpel S. D., VandenBos T., Friend D., Alpert A., Anderson D., Jackson J. The murine interleukin-4 receptor: molecular cloning and characterization of secreted and membrane bound forms. Cell. 1989 Oct 20;59(2):335–348. doi: 10.1016/0092-8674(89)90295-x. [DOI] [PubMed] [Google Scholar]
- Taga T., Hibi M., Hirata Y., Yamasaki K., Yasukawa K., Matsuda T., Hirano T., Kishimoto T. Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130. Cell. 1989 Aug 11;58(3):573–581. doi: 10.1016/0092-8674(89)90438-8. [DOI] [PubMed] [Google Scholar]
- Taga T., Kawanishi Y., Hardy R. R., Hirano T., Kishimoto T. Receptors for B cell stimulatory factor 2. Quantitation, specificity, distribution, and regulation of their expression. J Exp Med. 1987 Oct 1;166(4):967–981. doi: 10.1084/jem.166.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. M., Ogorochi T., Arai K., Miyajima A. Structure of mouse interleukin 3 (IL-3) binding protein (AIC2A). Amino acid residues critical for IL-3 binding. J Biol Chem. 1992 Jan 15;267(2):979–983. [PubMed] [Google Scholar]
- Weinstein Y., Ihle J. N., Lavu S., Reddy E. P. Truncation of the c-myb gene by a retroviral integration in an interleukin 3-dependent myeloid leukemia cell line. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5010–5014. doi: 10.1073/pnas.83.14.5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki K., Taga T., Hirata Y., Yawata H., Kawanishi Y., Seed B., Taniguchi T., Hirano T., Kishimoto T. Cloning and expression of the human interleukin-6 (BSF-2/IFN beta 2) receptor. Science. 1988 Aug 12;241(4867):825–828. doi: 10.1126/science.3136546. [DOI] [PubMed] [Google Scholar]
- Yasukawa K., Futatsugi K., Saito T., Yawata H., Narazaki M., Suzuki H., Taga T., Kishimoto T. Association of recombinant soluble IL-6-signal transducer, gp130, with a complex of IL 6 and soluble IL-6 receptor, and establishment of an ELISA for soluble gp130. Immunol Lett. 1992 Feb;31(2):123–130. doi: 10.1016/0165-2478(92)90138-e. [DOI] [PubMed] [Google Scholar]
- Yasukawa K., Saito T., Fukunaga T., Sekimori Y., Koishihara Y., Fukui H., Ohsugi Y., Matsuda T., Yawata H., Hirano T. Purification and characterization of soluble human IL-6 receptor expressed in CHO cells. J Biochem. 1990 Oct;108(4):673–676. doi: 10.1093/oxfordjournals.jbchem.a123261. [DOI] [PubMed] [Google Scholar]
- de Vos A. M., Ultsch M., Kossiakoff A. A. Human growth hormone and extracellular domain of its receptor: crystal structure of the complex. Science. 1992 Jan 17;255(5042):306–312. doi: 10.1126/science.1549776. [DOI] [PubMed] [Google Scholar]





