Abstract
FK506 binding protein 12 (FK506BP) belongs to a family of immunophilins, and is involved in multiple biological processes. However, the function of FK506BP in corneal disease remains unclear. In this study, we examined the protective effects on dry eye disease in a Botulinum toxin A (BTX-A) induced mouse model, using a cell-permeable PEP-1-FK506BP protein. PEP-1-FK506BP efficiently transduced into human corneal epithelial cells in a time- and dose-dependent manner, and remained stable in the cells for 48 h. In addition, we demonstrated that topical application of PEP-1-FK506BP was transduced into mouse cornea and conjunctiva by immunohistochemistry. Furthermore, topical application of PEP-1-FK506BP to BTX-A-induced mouse model markedly inhibited expression levels of pro-inflammatory cytokines such as interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α) and macrophage inhibitory factor (MIF) in corneal and conjunctival epithelium. These results suggest PEP-1-FK506BP as a potential therapeutic agent for dry eye diseases. [BMB Reports 2013; 46(2): 124-129]
Keywords: Cytokines, Dry eye disease, Inflammation, PEP-1-FK506BP, Protein therapy
INTRODUCTION
Dry eye disease is well known as one of the most prevalent ocular surface diseases among the elderly, and it leads to potential damage to the ocular surface (1). In the United States, approximately 5 million people are affected by the condition, being estimated to have moderate to severe dry eye (2). Although the pathogenesis of dry eye disease is not fully understood, various risk factors, including aging, hormonal change, environmental factors, and inflammation, contribute. Treatments involving various drugs have been applied in cases of dry eye disease (3-7), however, there is no ideal or satisfactory therapeutic treatment at present. Of the various risk factors, inflammation is considered to play an important role in dry eye diseases (8,9). Several studies have shown that those with dry eye disease exhibit increased expression levels of pro-inflammatory cytokines, such as interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α) and macrophage inhibitory factor (MIF) in corneal and conjunctival epithelium. Additionally, it is well known that cyclooxygenase-2 (COX2) and matrix metalloproteinase-9 (MMP-9) expression levels are increased in conjunction with dry eye syndrome (9-13). Others studies have shown that increased pro-inflammatory cytokines were decreased in dry eye syndrome, when treated with various potential drugs, such as cyclosporine, corticosteroid and doxycycline (14-17).
FK506 binding proteins (FK506BPs) belong to a family of immunophilins that were named for their ability to bind to immunosuppressive drugs. FK506 binding protein 12 (FK506BP) is a small peptide with a single FK506BP domain that is involved in multiple biological processes (18). In a previous inflammation animal model, we showed that transduced PEP-1-FK506BP protein inhibits inflammatory response of cytokines and enzymes by blocking of NF-κB and MAPK kinase in Raw 264.7 cells. In addition, transduced PEP-1-FK506BP protein inhibits the inflammatory response in HaCaT cells. Furthermore, topical application of PEP-1-FK506BP to atopic dermatitis in NC/Nga mice was markedly inhibited by reducing the expression level of cytokines and chemokines (19,20).
Protein transduction domains (PTDs) can deliver various exogenous molecules into living cells and tissues. Although the exact mechanism of transduction is unclear, many studies have demonstrated that protein transduction allows the delivery of therapeutic proteins both in vitro and in vivo (21-26).
In the present study, we demonstrate that a PEP-1-FK506BP protein can be directly transduced into human corneal epithelial cells (HCE-2) as well as mice corneal and conjunctival tissue. Also, topical application of PEP-1-FK506BP to Botulinum toxin A (BTX-A)-induced dry eye mice significantly inhibits dry eye disease and cytokine expression levels. Therefore, we suggest that the topical application of PEP-1-FK506BP protein might be a suitable therapeutic treatment for dry eye diseases.
RESULTS AND DISCUSSION
Transduction of PEP-1-FK506BP into HCE-2 cells and cornea tissue
The construction of PEP-1-FK506BP, as well as its expression and purification have been previously described. Purified PEP-1-FK506BP was efficiently transduced into Raw 264.7 cells (19). Also, PEP-1-FK506BP was transduced into animal skin tissue (19,20). However, protein transduction efficiency by protein transduction domain (PTD) depends on various factors such as target proteins, cell type and nature of the PTD (27,28).
To assess the transduction ability of PEP-1-FK506BP into HCE-2 cells, the cells were incubated with various concentrations of PEP-1-FK506BP (0.5-5 μM) for 1 h, or were treated with PEP-1-FK506BP (5 μM) for various duration (5-60 min). As shown in Fig. 1A, PEP-1-FK506BP transduced to the cells in a dose- and time-dependent manner. However, control FK506BP did not transduce into the cells. In addition, the intracellular stability of PEP-1-FK506BP was evaluated after treatment with 5 μM PEP-1-FK506BP. The transduced PEP-1-FK506BP was maintained in the cells for nearly 48 h.
Fig. 1. Transduction of PEP-1-FK506BP into HCE-2 cells. (A) To assess the dose- and time-dependent transduction of PEP-1-FK506BP and FK506BP, HCE-2 cells were treated with various concentrations (0.5-5 μM) and times (5-60 min) of each protein (5 μM). To assess the intracellular stability of PEP-1-FK506BP, the cells were pretreated with PEP-1-FK506BP (5 μM) for 1 h and the intracellular level of the transduced PEP-1-FK506BP was measured by Western blot analysis. (B) The distribution of transduced PEP-1-FK506BP and FK506BP in HCE-2 cells. Cells were treated with PEP-1-FK506BP and FK506BP, and the intracellular localization of each protein was then visualized using Alexa488 and DAPI staining.
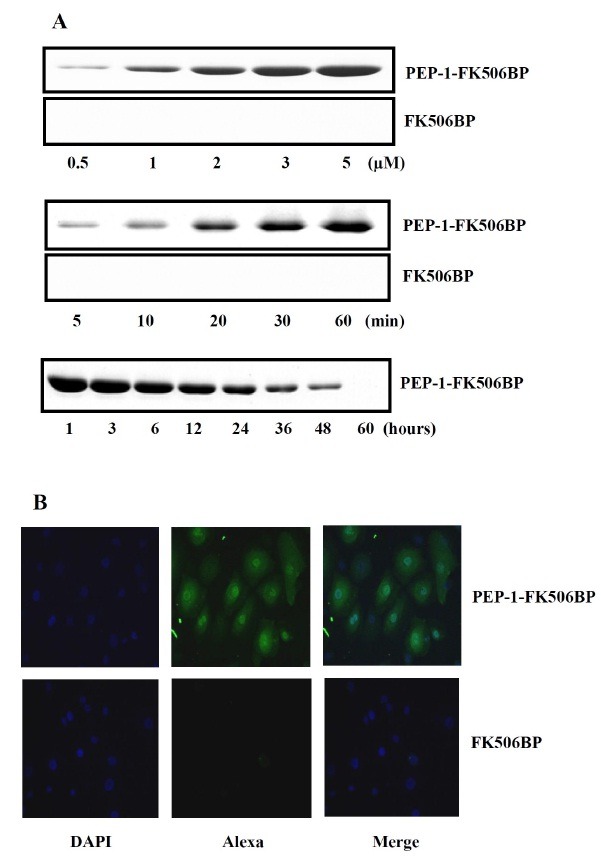
We also attempted to confirm the intracellular transduction of PEP-1-FK506BP into HCE-2 cells using fluorescence microscopy. Cells were treated with PEP-1-FK506BP and the intracellular localization of PEP-1-FK506BP was visualized using fluorescence, Alexa and DAPI, staining (Fig. 1B). In cells treated with PEP-1-FK506BP, fluorescence was significantly detected in the cytoplasm. However, the fluorescent signals of FK506BP treated cells were similar to those of control cells. These results indicate that PEP-1-FK506BP was efficiently transduced into HCE-2 cells.
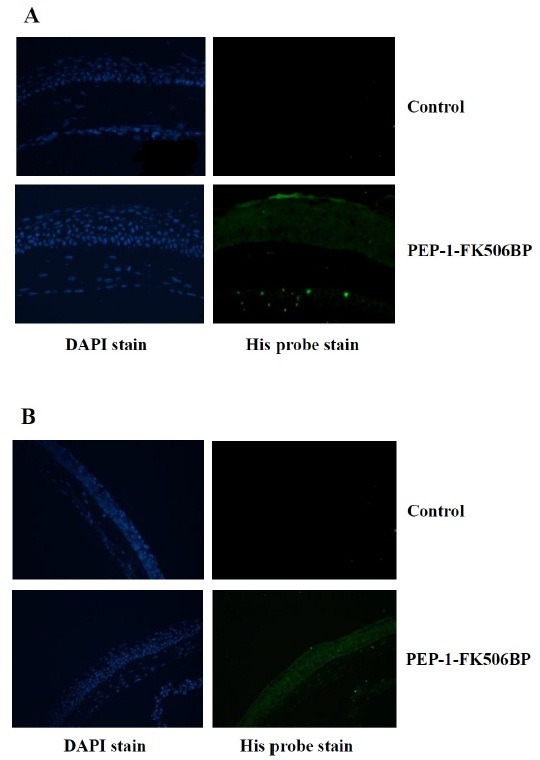
To examine whether PEP-1-FK506BP transduced into the cornea and conjunctiva of mice, we performed immunohistochemistry on cornea sections of PEP-1-FK506BP treated mice. As shown in Fig. 2, transduced PEP-1-FK506BP levels were markedly increased throughout the cornea and conjunctiva of PEP-1-FK506BP treated mice. However, control FK506BP did not transduce into the cornea and conjunctiva (data not shown). These results indicate that PEP-1-FK506BP can be efficiently transduced into HCE-2 cells, as well as into mouse cornea and conjunctiva.
Fig. 2. Transduction of PEP-1-FK506BP in cornea and conjunctiva. Representative images of the immunofluorescent staining of PEP-1-FK506BP-treated mice cornea (A) and conjunctiva (B) using anti 6-His antibody. The staining was strong in PEP-1-FK506BP-treated mice compared to that in control mice.
Inhibitory effect of PEP-1-FK506BP against corneal injury
It was reported that corneal injury is a common ophthalmologic disease, and corneal injury mice showed a markedly increased fluorescein staining score and inflammatory cytokine expression (15-17,29). Furthermore, we showed that transduced PEP-1-FK506BP has anti-inflammatory effects in macrophage cells and animal inflammation models (19,20). However, little is known about the potential application of PEP-1-FK506BP in corneal injury. Thus, we investigated the effect of PEP-1-FK506BP on corneal injury in a BTX-A induced animal model using fluorescein staining and immunohistochemistry.
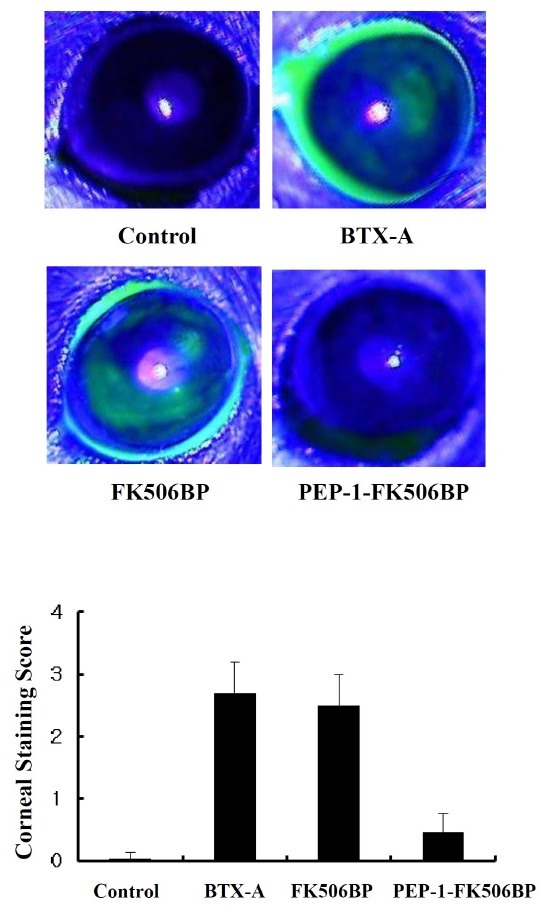
As shown in Fig. 3, BTX-A injected mice groups showed markedly increased amounts of corneal fluorescein staining throughout the cornea. The extent of corneal fluorescein staining in FK506BP treated groups was similar to that in the BTX-A treated control. By comparison, PEP-1-FK506BP treated mice showed a significantly decreased amount of corneal fluorescein staining compared with BTX-A or FK506BP treated mice. In addition, there were few differences in the corneal fluorescein staining between PEP-1-FK506BP treated mice and the control group.
Fig. 3. Effects of PEP-1-FK506BP on mouse corneal staining. Representative images of corneal fluorescein staining and corneal fluorescein staining score in BTX-A-injected, FK506BP-treated, and PEP-1-FK506BP-treated mice. The corneal injury was significantly reduced in the PEP-1-FK506BP-treated group, compared with the BTX-A-injected groups.
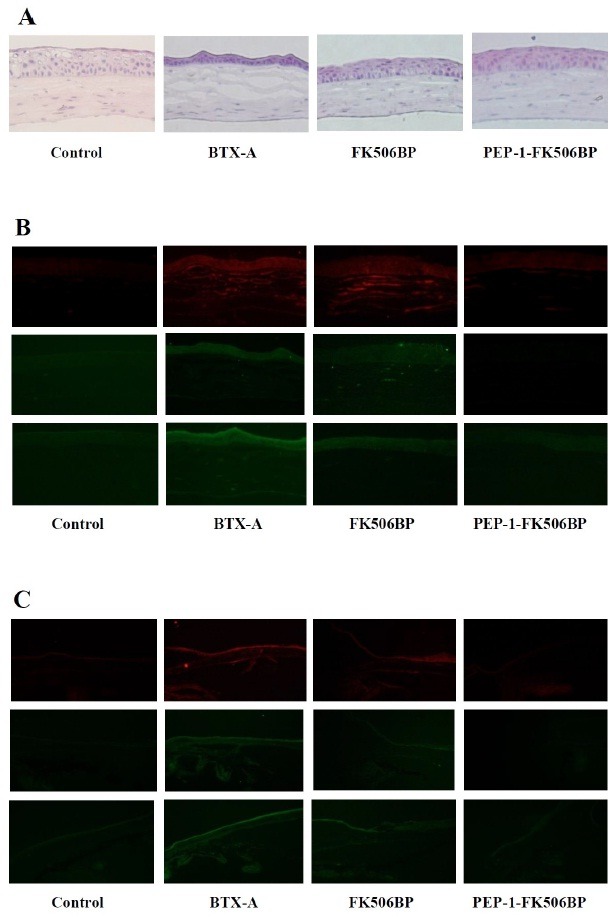
We also determined whether PEP-1-FK506BP affected the levels of pro-inflammatory cytokines, TNF-α, IL-1β and MIF. Hematoxylin and eosin (H&E) staining showed that the PEP-1-FK506BP treated group had significantly preserved corneal epithelium cell layers, as well as a preserved thicknesses of cornea stroma compared with BTX-A or FK506BP treated groups (Fig. 4A). Furthermore, PEP-1-FK506BP significantly reduced levels of TNF-α, IL-1β and MIF in the cornea (Fig. 4B) and conjunctiva (Fig. 4C). However, FK506BP failed to suppress the elevated expression of cytokines in a BTX-A-induced dry eye mouse model. These results indicate that cornea transduced PEP-1-FK506BP has anti-inflammatory effects and potent therapeutic efficacy against dry eye disease. Although further studies are needed to understand the exact mechanism, the present study revealed that PEP-1-FK506BP has anti-inflammatory effects, as it inhibited the expression of cytokines in BTX-A-induced dry eye mice.
Fig. 4. Anti-inflammatory effects of PEP-1-FK506BP in corneal and conjunctival epithelia. (A) Images of H&E staining after BTX-A-injection, FK506BP-treatment, and PEP-1-FK506BP-treatment. Immunofluorescent staining of corneal (B) and conjunctival epithelia (C) using indicated antibody. The staining for cytokines was strong in BTX-A-injected mice. The PEP-1-FK506BP treated mice group demonstrated significantly decreased cytokine expression compared with BTX-A or FK506BP treated groups.
The major feature of BTX-induced dry eye diseases is well known to be elevated levels of inflammatory cytokines, such as TNF-α and IL-1β in the cornea and conjunctival epithelia. Also, mitogen-activated protein kinase (MAPK) signal pathways play an important role in the inflammatory response (8,9,30). Previously we showed that transduced PEP-1-FK506BP and PTD-fusion proteins inhibit the production of COX-2 and inflammatory cytokines, as well as the activation of NF-κB and MAPKs in LPS-stimulated macrophage cells, and in TPA-treated inflammation animal models (19,20,24,31). Furthermore, Kubo et al. (2008) demonstrated that transduced TAT-peroxiredoxin 6 (PRDX6) protects against eye lens epithelial cell death and delays lens opacity, and suggest that transduced TAT-PRDX6 can prevent or delay the progression of cataractogenesis. This should provide an effective approach towards delaying cataracts (32).
In summary, it has been demonstrated that PEP-1-FK506BP can be efficiently transduced into corneal cells and tissues. Furthermore, topical application of PEP-1-FK506BP in a dry eye mice model markedly inhibits lens opacity and the expression of cytokines. Therefore, PEP-1-FK506BP may be relevant for clinical use against dry eye diseases, including eye inflammation and cataracts.
MATERIALS AND METHODS
Materials
A Ni2+-nitrilotriacetic acid Sepharose Superflow column was purchased from Qiagen (Valencia, CA, USA). Human corneal epithelial cells (HCE-2) were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Primary antibodies against interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α) and macrophage inhibitory factor (MIF) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). All other chemicals and reagents, unless otherwise stated, were obtained from Sigma-Aldrich (St. Louis, MO, USA), and were the highest analytical grade available.
Expression and purification of PEP-1-FK506BP proteins
Expression and purification of PEP-1-FK506BP protein was carried out as previously described (19,20). To produce the PEP-1-FK506BP, the plasmid was transformed into E. coli BL21 cells. The transformed bacterial cells were grown in 100 ml of LB media at 37℃ to a D600 value of 0.5-1.0, and were then induced with 0.5 mM IPTG at 37℃ for 4 h. Harvested cells were lysed by sonication and the recombinant PEP-1-FK506BP was purified using a Ni2+-nitrilotriacetic acid Sepharose affinity column (Qiagen) and PD-10 column chromatography (Amersham, Braunschweig, Germany). To remove endotoxin, purified PEP-1-FK506BP was treated using Detoxi-GelTM endotoxin removing gel (Pierce, Rockford, IL, USA). Endotoxin levels for PEP-1-FK506BP were below the detection limit (< 0.1 EU/ml) as tested using a Limulus amoebocyte lysate assay (Bio-Whitaker, Walkersville, MD, USA). The purified protein concentration was estimated by the Bradford procedure, using bovine serum albumin as a standard (33).
Transduction of PEP-1-FK506BP into human corneal epithelial cells
HCE-2 cells were maintained in Corneal Epithelial Cell Medium (ScienCellTM, Carlsbad, CA, USA) containing corneal epithelial cell growth supplement (CEpiCGS) and P/S solution (10,000 μg/ml streptomycin, 10,000 U/ml penicillin) at 37℃ in a humidified atmosphere of 95% air/5% CO2.
To detect the transduction of PEP-1-FK506BP proteins into HCE-2 cells, the cells were grown to confluence in wells of 6-well plates, and were incubated with various concentrations (0.5-5 μM) and times (5-60 min) of PEP-1-FK506BP proteins. The cells were harvested and cell extracts were prepared for Western blot analysis.
Fluorescence microscopy
HCE-2 cells were seeded on glass coverslips and were then incubated with PEP-1-FK506BP proteins (5 μM) at 37℃ for 1 h. The cells were washed with PBS twice and were then fixed with 4% paraformaldehyde at room temperature for 10 min. The anti-histidine primary antibody (Santa Cruz Biotechnology, Santa Cruze, CA, USA) was diluted 1:2,000, and was then incubated for 3 h at room temperature. Alexa fluor 488-conjugated secondary antibody (Invitrogen, Carlsbad, CA, USA) was then diluted 1:15,000, and was incubated for 45 min at room temperature in the dark. Nuclei were stained for 30 min with 1 μg/ml 4’6-diamidino-2-phenylindole (Roche Applied Science, Basel, Switzerland). The fluorescence was analyzed using an ELIPSE 80i fluorescence microscope (Nikon, Tokyo, Japan).
Western blot analysis
Equal amounts of proteins from each cell lysate were resolved by 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The resolved proteins were electrotransferred to a nitrocellulose membrane, which was then blocked with 5% non-fat dry milk in PBS. The membrane was probed with a rabbit anti-histidine polyclonal antibody (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), followed by detection with horseradish peroxidase-conjugated goat anti-rabbit immunoglobulins (dilution 1:10,000; Sigma-Aldrich). The bound antibody complexes were then visualized with enhanced chemiluminescence reagents, according to the manufacturer’s instructions (Amersham, Franklin Lakes, NJ, USA).
Transduction of PEP-1-FK506BP into mouse corneal and conjunctival epithelium
Male C57BL/6 (6-8 weeks; 20-25 g) mice were obtained from the Experimental Animal Center, at Hallym University. The animals were housed at a constant temperature (23℃) and relative humidity (60%) with a fixed 12 h light/dark cycle, and were provided free access to food and water. All experimental procedures involving animals and their care were in accordance with the Guide for the Care and Use of Laboratory Animals of the National Veterinary Research and Quarantine Service of Korea, and were approved by the Hallym Medical Center Institutional Animal Care and Use Committee.
To determine whether PEP-1-FK506BP was transduced into corneal and conjunctival epithelium, we administrated the proteins topically. The eyes of each mouse was treated with PEP-1-FK506BP (20 μg of protein in 10 μl of saline) and control FK506BP. Treatment was applied once. After 30 minutes of treatment, corneal and conjunctivas were isolated from the mouse eyes and were photographed, after which the level of transduced protein was determined by immunohistochemistry using anti-His antibody.
Botulinum toxin A-induced dry eye model
The mice (male C57BL/6; 6-8 weeks; 20-25 g) were divided into four groups, each containing seven mice. Group 1 was used as a control without any injection into lacrimal glands. Group 2 was injected with BTX-A (30 μl, 20 mU) into the lacrimal glands. Group 3 was treated with BTX-A + control FK506BP (10 μg). Group 4 was treated with BTX-A + PEP-1-FK506BP (10 μg). Control FK506BP and PEP-1-FK506BP were topically applied on the cornea four times at intervals of 30 min per day. The treatments were repeated every two days for 10 days.
Measurement of corneal injury and immunohistochemistry
Immunofluorescent staining (1 μl of 2% sodium fluorescein, Sigma-Aldrich, St. Louis, MO, USA) was performed as previously reported (17, 34). The area of punctate staining was determined using a grading system. Grade 0 was determined when there was no punctate staining, grade 1 was determined when less than on eighth was stained, grade 2 was determined when less than one fourth was stained, grade 3 was determined when less than one half was stained, and grade 4 was determined when greater than one half was stained (35).
For histological analysis, the cornea and conjunctiva from each group were collected by dissection, and biopsy samples were fixed, embedded in liquid OCT compound (Sakura FineTek, Torrance, CA, USA), sectioned at a thickness of 4 μm, and stained with hematoxylin and eosin (H&E) and pro-inflammatory cytokine (TNF-α, IL-1β and MIF) immunofluorescent staining was applied for the indicated specific antibody.
Acknowledgments
This work was supported by a Next Generation Growth Engine Program grant (2010K001266) and by a Priority Research Centers Program grant (2009-0093812) through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology and in part by a Kyungpook National University Research Fund (2012).
References
- 1.Schaumberg D. A., Sullivan D. A., Buring J. E., Dana M. R. Prevalence of dry eye syndrome among US women. Am. J. Ophthalmol. (2003);136:318–326. doi: 10.1016/S0002-9394(03)00218-6. [DOI] [PubMed] [Google Scholar]
- 2.Schaumberg D. A., Sullivan D. A., Dana M. R. Epidemiology of dry eye syndrome. Ade. Exp. Med. Biol. (2002);506:989–998. doi: 10.1007/978-1-4615-0717-8_140. [DOI] [PubMed] [Google Scholar]
- 3.Behrens A., Doyle J. J., Stern L., Chuck R. S., McDonnell P. J., Azar D. T., Dua H. S., Hom M., Karpecki P. M., Laibson P. R., Lemp M. A., Meisler D. M., Del Castillo J. M., O’Brien T. P., Pflugfelder S. C., Rolando M., Schein O. D., Seitz B., Tseng S. C., van Setten G., Wilson S. E., Yiu S. C. Dysfunctional tear syndrome study group. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea. (2006);25:900–907. doi: 10.1097/01.ico.0000214802.40313.fa. [DOI] [PubMed] [Google Scholar]
- 4.Gupta H., Jain S., Mathur R., Mishra P., Mishra A. K., Velpandian T. Sustained ocular drug delivery from a temperature and pH triggered novel in situ gel system. Drug Deliv. (2007);14:507–515. doi: 10.1080/10717540701606426. [DOI] [PubMed] [Google Scholar]
- 5.Khaw P. T., Shah P., Elkington A. R. Injury to the eye. BMJ. (2004);328:36–38. doi: 10.1136/bmj.328.7430.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hosseini H., Nejabat M. A potential therapeutic strategy for inhibition of corneal neovascularization with new anti-VEGF agents. Med. Hypotheses. (2007);68:799–801. doi: 10.1016/j.mehy.2006.06.063. [DOI] [PubMed] [Google Scholar]
- 7.Schaumberg D. A., Buring J. E., Sullivan D. A., Dana M. R. Hormone replacement therapy and dry eye syndrome. JAMA. (2001);286:2114–2119. doi: 10.1001/jama.286.17.2114. [DOI] [PubMed] [Google Scholar]
- 8.Narayanan S., Miller W. L., McDermott A. M. Conjunctival cytokine expression in symptomatic moderate dry eye subjects. Invest. Ophthalmol. Vis. Sci. (2006);47:2445–2450. doi: 10.1167/iovs.05-1364. [DOI] [PubMed] [Google Scholar]
- 9.Luo L., Li D.Q., Doshi A., Farley W., Corrales R. M., Pflugfelder S. C. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest. Ophthalmol. Vis. Sci. (2004);45:4293–4301. doi: 10.1167/iovs.03-1145. [DOI] [PubMed] [Google Scholar]
- 10.Dana M. R., Hamrah P. Role of immunity and inflammation in corneal and ocular surface diseases associated with dry eye. Ade. Exp. Med. Biol. (2002);506:729–738. doi: 10.1007/978-1-4615-0717-8_103. [DOI] [PubMed] [Google Scholar]
- 11.Gulati A., Sacchetti M., Bonini S., Dana R. Chemokine receptor CCR5 expression in conjunctival epithelium of patients with dry eye syndrome. Arch. Ophthalmol. (2006);124:710–716. doi: 10.1001/archopht.124.5.710. [DOI] [PubMed] [Google Scholar]
- 12.Solomon A., Dursun D., Liu Z., Xie Y., Macri A., Pflugfelder S. C. Pro- and anti-inflammatory forms of inerleukin-1 in the tear fluid and conjunctiva of patients with dry eye diseases. Invest. Ophthalmol. Vis. Sci. (2001);42:2283–2293. [PubMed] [Google Scholar]
- 13.Perry H. D., Solomon R., Donnenfeld E. D., Perry A. R., Wittpenn J. R., Greenman H. E., Savage H. E. Evaluation of topical cyclosporine for the treatment of dry eye diseases. Arch. Ophthalmol. (2008);126:1046–1050. doi: 10.1001/archopht.126.8.1046. [DOI] [PubMed] [Google Scholar]
- 14.De Paiva C. S., Corrales R. M., Villarrea A. L. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp. Eye Res. (2006);83:526–535. doi: 10.1016/j.exer.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Li N., He J., Schwartz C. E., Gjorstrup P., Bazan H. E. Resolvin E1 improves tear production and decreases inflammation in a dry eye mouse model. J. Ocul. Pharmacol. Ther. (2010);26:431–439. doi: 10.1089/jop.2010.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hongyok T., Chae J. J., Shin Y. J., Na D., Li L., Chuck R. S. Effect of chitosan-N-acetylcysteine conjugate in a mouse model of Botulium toxin B-induced dry eye. Arch. Ophthalmol. (2009);127:525–532. doi: 10.1001/archophthalmol.2009.52. [DOI] [PubMed] [Google Scholar]
- 17.Zhu L., Shen J., Zhang C., Park C. Y., Kohanim S., Yew M., Parker J. S., Chuck R. S. Inflammatory cytokine expression on the ocular surface in the Botulium toxin B induced murine dry eye model. Mol. Vis. (2009);15:250–258. [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang W., Cazacu S., Xiang C., Zenklusen J. C., Fine H. A., Berens M., Armstrong B., Brodie C., Mikkelsen T. FK506 binding protein mediates glioma cell growth and sensitivity to rapamycin treatment by regulating NF-κB signaling pathway. Neoplasis. (2008);10:235–243. doi: 10.1593/neo.07929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim S. Y., Jeong H. J., Kim D. W., Kim M. J., An J. J., Sohn E. J., Kang H. W., Shin M. J., Ahn E. H., Kwon S. W., Kim D. S., Cho S. W., Park J., Eum W. S., Choi S. Y. Transduced PEP-1-FK506BP inhibits the inflammatory response in the Raw 264.7 cells and mouse models. Immunobiol. (2011);216:771–781. doi: 10.1016/j.imbio.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Kim S. Y., Sohn E. J., Kim D. W., Jeong H. J., Kim M. J., Kang H. W., Shin M. J., Ahn E. H., Kwon S. W., Kim Y. N., Kwon H. J., Kim T. Y., Lee K. S., Park J., Eum W. S., Choi S. Y. Transduced PEP-1-FK506BP ameliorates atopic dermatitis in NC/Nga mice. J. Invest. Dermatol. (2011);131:1477–1485. doi: 10.1038/jid.2011.49. [DOI] [PubMed] [Google Scholar]
- 21.Dietz G. P. Cell-penetrating peptide technology to deliver chaperones and associated factors in diseases and basic research. Curr. Pharm. Biotechol. (2010);11:167–174. doi: 10.2174/138920110790909731. [DOI] [PubMed] [Google Scholar]
- 22.Wadia J. S., Dowdy S. F. Protein transduction technology. Curr. Opin. Biotechnol. (2002);13:52–56. doi: 10.1016/S0958-1669(02)00284-7. [DOI] [PubMed] [Google Scholar]
- 23.Ahn E. H., Kim D. W., Kang H. W., Shin M. J., Won M. H., Kim J., Kim D. S., Kwon O. S., Kang T. C., Han K. H., Park J., Eum W. S., Choi S. Y. Transduced PEP-1-ribosomal protein S3 (rpS3) ameliorates ameliorates 12-O-tetradecanoyl phorbol-13-acetate-induced inflammation in mice. Toxicol. (2010);276:192–197. doi: 10.1016/j.tox.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Shin M. J., Lee Y. P., Kim D. W., An J. J., Jang S. H., Cho S. M., Sheen S. H., Lee H. R., Kweon H. Y., Kang S. W., Lee K. G., Park J., Eum W. S., Cho Y. J., Choi S. Y. Transduced PEP-1-AMPK inhibits LPS-induced COX-2 and iNOS expression in Raw264.7 cells. BMB Rep. (2010);43:40–45. doi: 10.5483/BMBRep.2010.43.1.040. [DOI] [PubMed] [Google Scholar]
- 25.Eum W. S., Kim D. W., Hwang I. K., Yoo K. Y., Kang T. C., Jang S. H., Choi H. S., Choi S. H., Kim Y. H., Kim S. Y., Kwon H. Y., Kang J. H., Kwon O. S., Cho S. W., Lee K. S., Park J., Won M. H., Choi S. Y. In vivo protein transduction: Biologically active intact PEP-1-superoxide dismutase fusion protein efficiently protects against ischemic insult. Free Radic. Biol. Med. (2004);37:1656–1669. doi: 10.1016/j.freeradbiomed.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 26.Kwon S. W., Sohn E. J., Kim D. W., Jeong H. J., Kim M. J., Ahn E. H., Kim Y. N., Dutta S., Kim D. S., Park J., Eum W. S., Hwang H. S., Choi S. Y. Anti-inflammatory effect of transduced PEP-1-heme oxygenase- 1 in Raw 264.7 cells and a mouse edema model. Biochem. Biophys. Res. Commun. (2011);411:354–359. doi: 10.1016/j.bbrc.2011.06.147. [DOI] [PubMed] [Google Scholar]
- 27.Lim K. S., Won Y. W., Park Y. S., Kim Y. H. Preparation and functional analysis of recombinant protein transduction domain-metallothionein fusion proteins. Biochimie. (2010);92:964–970. doi: 10.1016/j.biochi.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Wang H., Zhong C. Y., Wu J. F., Huang Y. B., Liu C. B. Enhancement of TAT cell membrane penetration efficiency by dimethyl sulphoxide. J. Con. Rel. (2010);143:64–70. doi: 10.1016/j.jconrel.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Liu X., Lin Z., Zhou T., Zong R., He H., Liu Z., Ma J. X., Liu Z., Zhou Y. Anti-angiogenic and anti- inflammatory effects of SERPINA3K on corneal injury. PLoS ONE. (2011);6:e16712. doi: 10.1371/journal.pone.0016712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stern M. E., Pflugfelder S. C. Inflammation in dry eye. Ocul. Surf. (2004);2:124–130. doi: 10.1016/S1542-0124(12)70148-9. [DOI] [PubMed] [Google Scholar]
- 31.Lee S. H., Kim D. W., Eom S. A., Jun S. Y., Park M., Kim D. S., Kwon H. J., Kwon H. Y., Han K. H., Park J., Hwang H. S., Eum W. S., Choi S. Y. Suppression of 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced skin inflammation in mice by transduced Tat-Annexin protein. BMB Rep. (2012);45:354–359. doi: 10.5483/BMBRep.2012.45.6.036. [DOI] [PubMed] [Google Scholar]
- 32.Kubo E., Fatma N., Akagi Y., Beier D. R., Singh S. P., Singh D. P. TAT-mediated PRDX6 protein transduction protects against eye lens epithelial cell death and delays lens opacity. Am. J. Physiol. Cell Physiol. (2008);294:C842–C855. doi: 10.1152/ajpcell.00540.2007. [DOI] [PubMed] [Google Scholar]
- 33.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities utilizing the principle of protein-dye binding. Anal. Biochem. (1976);72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 34.Suwan-apichon O., Rizen M., Rangsin R., Herretes S., Reyes J. M., Lekhanont K., Chuck R. S. Botulium toxin B-induced mouse model of keratoconjunctivitis sicca. Invest. Ophthalmol. Vis. Sci. (2006);47:133–139. doi: 10.1167/iovs.05-0380. [DOI] [PubMed] [Google Scholar]
- 35.Lekhanont K., Ilya L., Suwan-apichon O., Rangsin R., Chuck R. S. Comparison of topical dry eye medications for the treatment of keratoconjunctivitis sicca in a botulium toxin B-induced mouse model. Cornea. (2007);26:84–89. doi: 10.1097/01.ico.0000240079.24583.a1. [DOI] [PubMed] [Google Scholar]


