Abstract
Glucagon like peptide-1 (GLP-1) regulates glucose mediated-insulin secretion, nutrient accumulation, and β-cell growth. Despite the potential therapeutic usage for type 2 diabetes (T2D), GLP-1 has a short half-life in vivo (t1/2 <2 min). In an attempt to prolong half-life, GLP-1 fusion proteins were genetically engineered: GLP-1 human serum albumin fusion (GLP-1/HSA), AGLP-1/HSA which has an additional alanine at the N-terminus of GLP-1, and AGLP-1-L/HSA, in which a peptide linker is inserted between AGLP-1 and HSA. Recombinant fusion proteins secreted from the Chinese Hamster Ovary-K1 (CHO-K1) cell line were purified with high purity (>96%). AGLP-1 fusion protein was resistant against the dipeptidyl peptidase-IV (DPP-IV). The fusion proteins activated cAMP-mediated signaling in rat insulinoma INS-1 cells. Furthermore, a C57BL/6N mice pharmacodynamics study exhibited that AGLP-1-L/HSA effectively reduced blood glucose level compared to AGLP-1/HSA. [BMB Reports 2013; 46(12): 606-610]
Keywords: Dipeptidyl peptidase-IV, Fusion protein, Glucagon like peptide-1, Human serum albumin, Type 2 diabetes
INTRODUCTION
Glucagon-like peptide-1 (GLP-1) is an insulinotropic hormone released in endocrine L-cells and is related to the regulation of glucose-dependent insulin secretion and glucagon (1). In addition, GLP-1 promotes insulin biosynthesis, pancreatic β-cell growth, and proliferation (2, 3). GLP-1 secreted from the small intestine is active as GLP-1 (7-36) amide and inactive as GLP-1 (1-39) amide. However, the bioactive GLP-1 (7-36) is converted to an inactive form of GLP-1, GLP-1 (9-36), within 2 minutes in vivo due to the rapid cleavage by dipeptidyl peptidase-IV (DPP-IV) (1, 2).
GLP-1 receptor (GLP-1R) agonists increase insulin biosynthesis and Ca2+ levels in cAMP-dependent and cAMP-independent pathways (4). GLP-1R agonists also mediate protein kinase A (PKA) and protein kinase C (PKC) signaling pathways to enhance β-cell mass via insulin secretion, β-cell proliferation, and anti-apoptosis (1,4). Furthermore, downstream targets of GLP-1R include important regulators such as cAMP response element binding protein (CREB), insulin receptor substrate-2 (IRS-2), and pancreatic duodenal homeobox-1 (PDX-1) on cytoprotective effects (5,6).
GLP-1R agonists have been recently developed for the treatment of type 2 diabetes (T2D) (7). Many approaches have been designed in order to increase the short in vivo half-life of the molecule. The modification of amino acids at the cleavage site of GLP-1 showed resistance to the activity by DPP-IV (8,9). In spite of this, the clinical limitation of these GLP-1 analogs remains due to the short duration of the peptides in the body. The conjugation of long-chain palmitic acid (PAA, C16 to GLP-1 increased the in vivo duration of GLP-1 substantially (10). The encapsulation of another GLP-1 analog, exendin-4 from the lizard salivary gland peptide also showed sustained circulation of the peptide in the body (11). However, it may be necessary to design the long-acting technology for a GLP-1 analog for the effective delivery of this important therapeutic peptide in order to improve the patient compliance significantly. Recent attempts have focused on GLP-1 or exendin-4 fusion proteins with human serum albumin, IgG-Fc, or transferrin (12-15) in order to improve the sustained circulation in the body.
We designed AGLP-1 albumin fusion proteins to develop the long-acting GLP-1R agonists. The biological activity and resistance to DPP-IV of the fusion proteins were examined in vitro and in vivo. The results indicated that the AGLP-1 albumin fusion protein could be an alternative agent for the treatment of T2D.
RESULTS
Construction, expression, and purification of recombinant fusion proteins
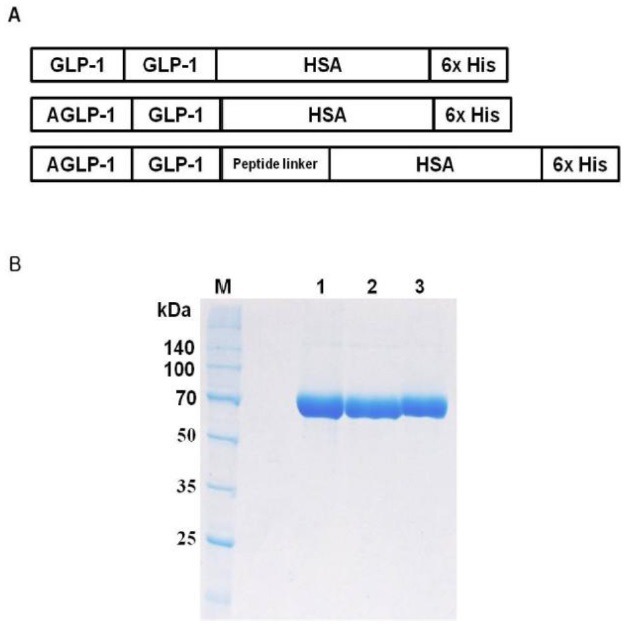
The recombinant fusion plasmids were genetically engineered for encoding proteins of GLP-1/HSA, AGLP-1/HSA, and AGLP-1-L/HSA to overcome the limitation of native GLP-1. GLP-1 or AGLP-1 that an alanine was added to N-terminus of GLP-1 were fused at the N-terminal region of HSA. AGLP-1-L/HSA was constructed by the insertion of a peptide linker between AGLP-1 and HSA (Fig. 1A). The recombinant fusion proteins were transiently overexpressed in CHO-K1 cells. The fusion proteins were purified using Ni-affinity chromatography, and SDS-PAGE showed that the proteins had a molecular weight of approximately 73 kDa (Fig. 1B). In further analysis, RP-HPLC and GP-HPLC determined that the purities of fusion proteins were more than 96 % (data not shown).
Fig. 1. Schematic diagram and purification of the recombinant albumin fusion proteins. (A) Fusion plasmids were genetically engineered: GLP-1 human serum albumin fusion (GLP-1/HSA), AGLP-1/HSA, which has an additional Ala at the N-terminal location of GLP-1, and AGLP-1-L/HSA in which a peptide linker is inserted. The plasmids for the fusion protein were transiently transfected into CHO-K1. (B) The fusion proteins were purified by Ni-affinity chromatography and analyzed using 10% (v/v) SDS-PAGE stained with Coomassie Brilliant Blue-G (CBB-G) under reducing conditions. M, Broad range protein marker; Lane 1, GLP-1/HSA; Lane 2, AGLP-1/HSA; Lane 3, AGLP-1-L/HSA.
Resistance to the enzymatic degradation by DPP-IV
Two amino acids (His-Ala) of the N-terminus in GLP-1 are enzymatically cleaved by DPP-IV in vivo and inactivated, leading to a short half-life (t1/2 < 2 min). Therefore, the N-terminus of the fusion proteins was analyzed via N-terminal sequencing equipment after treatment with DPP-IV in vitro. GLP-1/HSA was degraded by DPP-IV in the conditions described in the materials and methods while AGLP-1 albumin fusion protein was not (Table 1). The analysis of the N-terminal sequence demonstrated that the addition of an alanine to the N-terminus in GLP-1 prevents degradation by DPP-IV. Furthermore, the DPP-IV-resistant AGLP-1/HSA indicated that AGLP-1 albumin fusion proteins could have a longer half-life than native GLP-1 in vivo.
Table 1.
N-terminal sequences of GLP-1/HSA and AGLP-1/HSA with or without DPP-IV
| DPP-IV | N-terminal sequences | |
|---|---|---|
|
| ||
| GLP-1/HSA | AGLP-1/HSA | |
|
| ||
| − | HAEGTa | AHAEGb |
| + | EGTFTc | AHAEG |
aHAEGT is the N-terminal sequence of GLP-1/HSA. bAHAEG is the N-terminal sequence of AGLP-1/HSA with or without DPP-IV. cEGTFT is the N-terminal sequence after cleavage by DPP-IV in GLP-1/HSA.
In vitro activity of the albumin fusion proteins
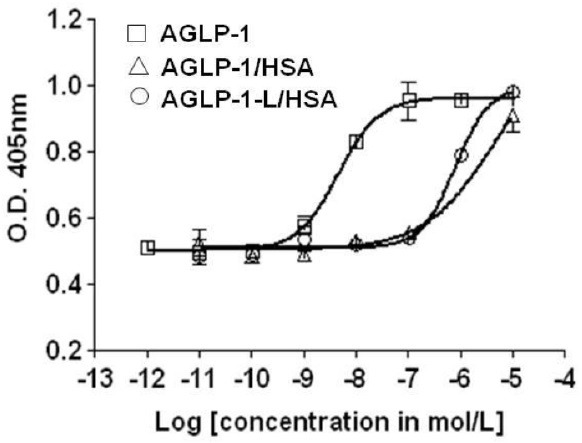
cAMP response element binding protein (CREB), a cellular transcription factor, is activated following cAMP/PKA dependent mechanisms, leading to the activation of insulin receptor substrate-2 (IRS-2). Therefore, the increase of CRE exhibits in vitro activity for AGLP-1 or AGLP-1 fusion proteins in the cAMP-mediated pathway. The activities of AGLP-1 fusion proteins analyzed by CRE-SEAP plasmid determined that the proteins stimulate the expression of CRE promoter in a dose-dependent manner, although AGLP-1 fusion proteins were less effective than AGLP-1 (EC50 = 5.881 ± 0.729 nM). The activity of AGLP-1/HSA was similar to that of AGLP-1-L/HSA (EC50 = 783.5 ± 18.735 nM) in the cAMP-dependent signaling pathway (Fig. 2).
Fig. 2. In vitro activity of the fusion proteins in INS-1 cells. The CRE-SEAP plasmid is transiently transfected into rat insulinoma INS-1 cells to compare with AGLP-1, AGLP-1/HSA, and AGLP-1-L/HSA. After serum deprivation, the cells were treated with the fusion proteins for 14 h at 37℃. The culture media were analyzed for CRE-SEAP using a chromogenic substrate, p-nitrophenyl phosphate (pNPP). Experiments were performed in triplicate and the data are expressed as the means ± standard error (n = 3).
Glucose-lowering effects of albumin fusion proteins
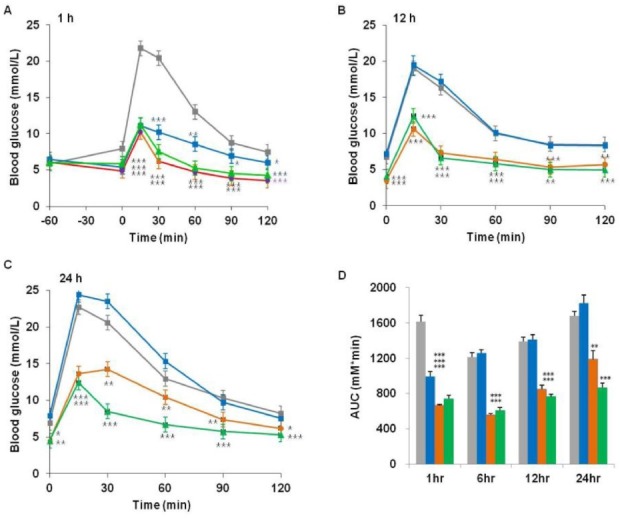
In order to investigate the in vivo activity for the DPP-IV-resistant fusion proteins, IPGTT was performed at 1, 12, 24 h post intra-peritoneal injection of the fusion proteins (0.1 μmol/kg) in C57BL/6N mice. Fig. 3 shows that AGLP-1 albumin fusion proteins reduced the glucose levels after administration of the proteins. AGLP-1 exerted blood glucose-lowering effect for up to 12 h. However, both AGLP-1/HSA and AGLP-1-L/HSA showed blood glucose-lowering effect at 24 h. In addition, the glucose-lowering potency of AGLP-1-L/HSA was interestingly more effective compared with that of AGLP-1/HSA (Fig. 3C, D). The pharmacodynamics study showed that AGLP-1-L/HSA inserted with linker peptides has a more sustained effect than AGLP-1/HSA in vivo.
Fig. 3. Blood glucose-lowering effect of the fusion proteins in vivo. C57BL/6N mice were intraperitoneally injected with PBS (gray), AGLP-1 (blue), AGLP-1/HSA (orange), or AGLP-1-L/HSA (green) fusion protein. Blood glucose levels were measured at (A) 1 h, (B) 12 h, and (C) 24 h after IPGTT in mice fasted overnight. (D) Blood glucose levels were expressed as the area under the curve (AUC). The values are expressed as the means ± standard error; n = 5 mice/group. *P < 0.05, **P < 0.01, ***P < 0.001 compared with PBS-treated animals.
DISCUSSION
GLP-1, a GLP-1R agonist, is known to be an important therapeutic agent for the treatment of type 2 diabetes. Despite the beneficial effects in incretin-based therapies (16), its small molecular weight and enzymatic degradation by DPP-IV lead to a short half-life (t1/2 < 2 min) in traditional treatments (13,17). Therefore, many approaches have been attempted for the pharmaceutical development of GLP-1R agonists and DPP-IV inhibitors (18). GLP-1 agonists should be resistant to DPP-IV, and should have long-acting capability (17).
In recent studies, few research groups focused on the addition of an amino acid to the N-terminus of GLP-1R agonists (8,9) and the fusion to HSA, IgG, or transferrin (8,14,15). In a previous study, we demonstrated that a modified GLP-1 peptide with additional alanine (AGLP-1) at the N-terminus was resistant to DPP-IV. AGLP-1 fusion protein with IgG-Fc was also prevented from rapidly degrading by the enzyme, and had biological activity which stimulated the expression levels of IRS-2 protein in vitro (9). Furthermore, the effect of AGLP-1/IgG-Fc fusion protein which suppressed the glucose levels was prolonged for up to 24 h in vivo (19). Although many approaches have been attempted to develop GLP-1R agonists showing prolonged pharmacokinetic properties, the development of long-lasting GLP-1R agonists is still needed for the treatment of type 2 diabetes.
Albumin fusion proteins were developed to show biologically active GLP-1 as a straightforward and economical method (8). In this study, we genetically constructed and purified novel albumin fusion proteins, AGLP-1/HSA and AGLP-1-L/HSA, which have an additional alanine of the N-terminus in GLP-1. AGLP-1 albumin fusion proteins were not cleaved by DPP-IV in vitro unlike GLP-1 albumin fusion protein. The resistance to DPP-IV of AGLP-1 albumin fusion protein is consistent with an earlier report (19).
GLP-1R agonists mediate the cAMP/PKA or PKC/protein kinase B (PKB) signaling pathways in pancreatic β–cells (1,4,20). In addition, GLP-1agonists activate the GLP-1 receptor and regulate CREB with a complicated molecular mechanism (1,5). In in vitro activity assay, AGLP-1 albumin fusion proteins increased the expression of CRE-SEAP in a cAMP-dependent signaling mechanism. The lower activity of either AGLP-1-HSA or AGLP-1-L/HSA may come from the blockage of receptor binding sites of the peptides due to the fusion with HSA. This phenomenon was frequently observed with fusion proteins due to the steric hindrance of fusion carrier with protein drugs (8,13).
In spite of the lower in vitro receptor binding activities of AGLP-1fusion molecules, the in vivo investigation showed that both AGLP-1/HSA and AGLP-1-L/HSA reduced blood glucose levels for up to 24 h after intraperitoneal injection in C57BL/6N mice. AGLP-1-L/HSA administration was significantly more effective at lowering the blood glucose level than AGLP-1/HSA. The biological property of AGLP-1-L/HSA was believed to be influenced by the insertion of linker peptide (GGGGSGGGGSGGGAS) between AGLP-1 and HSA, which might have a greater hydrodynamic volume in solution compared to the direct fusion between AGLP-1 and HSA.
In conclusion, we have designed AGLP-1 albumin fusion proteins. The AGLP-1 fusion protein was resistant to the proteolytic cleavage by DPP-IV in vitro, but GLP-1 fusion protein was not. AGLP-1 fusion proteins activated CREB in the cAMP-mediated pathway in vitro. AGLP-1-L/HSA fusion protein reduced the blood glucose levels in vivo for a substantial period. These findings suggest that AGLP-1 albumin fusion protein could be a potential therapeutic agent for T2D. In further research, pharmacokinetic study and the safety of AGLP-1-L/HSA require attention to develop it as a promising anti-diabetic agent.
MATERIALS AND METHODS
Construction of recombinant GLP-1 and AGLP-1 fusion plasmids
The plasmids encoding human serum albumin in pDNR-LIB (4724105, Open Biosystems) were purchased. The cDNA sequence for albumin was amplified by polymerase chain reaction (PCR) using forward primer (ACGT GGA TCC GAT GCA GCT AGC GAT GCA CAC AAG AGT GAG) and reverse primer (GGG A CCG CGG TCA GTG GTG GTG GTG GTG GTG AGC GGC CGC TAA GCC TAA GGC AGC). The PCR product was subcloned into the BamHI and NotI sites of the pSGHV0 vector, resulting in pN003 expression vector. Each of the genes GLP-1, AGLP-1, and AGLP-1L was amplified by PCR and subcloned into the XhoI and NheI sites of the pN003 vector, resulting in pGLP-1/HSA, pAGLP-1/HSA, and pAGLP-1-L/HSA.
Expression and purification of fusion proteins
CHO-K1 cells were cultured in DMEM medium with 10% (v/v) FBS and 1% (v/v) antibiotics at 37℃ with 5% CO2 air. The fusion plasmids were transiently transfected into CHO-K1 with polyethyleneimine (PEI). The medium was replaced with serum-free medium. Recombinant fusion proteins obtained from culture supernatants were purified by Ni-affinity chromatography. The purified proteins were analyzed using SDS-PAGE, Reverse phase (RP)-HPLC (Vydac 214TP54, C4, 5 μm, 4.6 × 250 mm), and Gel permeation (GP)-HPLC (TOSOH, TSK-gel G3000SWXL 7.8 × 300 mm (5)).
N-terminal sequencing
The fusion proteins (30 μg) were incubated for 4 h at 37℃ with or without 3 μl (0.0234 unit/μl) of human DPP-IV. The samples were loaded onto a ProsorbⓇ sample preparation cartridge according to the manufacturer’s instructions. The cartridge was placed in protein sequencing equipment (Applied Biosystems). The sequences of protein were read based on the Edman degradation principle using Procise software.
In vitro activity assay
INS-1 cells were grown in RPMI 1640 medium with 10% (v/v) FBS, 1% (v/v) antibiotics, and 50 μM β-mercaptoethanol at 37℃ with 5% CO2 air. To investigate in vitro activity, CRE (c-AMP responsive elements) -SEAP (Secreted alkaline phosphatase) plasmid is transiently transfected into rat insulinoma INS-1 cells with polyethyleneimine. The medium was replaced with serum-free medium. After 4 h of replacement, the INS-1 cells were treated with 0.001-1,000 nM or 0.01-10,000 nM of the fusion proteins with 10 nM intervals, and incubated for 14 h. Then, the media were mixed with SEAP assay buffer with 12 mM of a chromogenic substrate, p-nitrophenyl phosphate (pNPP). The absorbance was measured at O.D. 405 nm on a Microplate reader.
Intraperitoneal glucose tolerance test (IPGTT)
To perform IPGTT, C57BL/6N mice purchased from KOATECH were fasted for 10 h, and intraperitoneally injected with AGLP-1 peptide or purified fusion proteins (0.1 μmol/kg). IPGTT was conducted at 1, 12, and 24 h post injection of the fusion proteins. Blood samples were obtained from the tail vein at 0, 15, 30, 60, 90, and 120 min after glucose (1.5 g/kg) administration and blood glucose levels were measured by a glucometer (GlucoDrTM, Allmedicus).
Acknowledgments
This work was supported by Business for Cooperative R&D between Industry, Academy, and Research Institutes, funded by the Korea Small and Medium Business Administration in 2011 (Grants No. 00046320-2) and by the 2013 Hannam University Research Fund. The authors are grateful to Mr. H.S. Cho at Alteogen, Inc. for the animal study.
References
- 1.Baggio L. L., Drucker D. J. Biology of incretins: GLP-1 and GIP. Gastroenterology. (2007);132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 2.Holst J. J. The physiology of glucagon-like peptide 1. Physiol. Rev. (2007);87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 3.Quoyer J., Longuet C., Broca C., Linck N., Costes S., Varin E., Bockaert J., Bertrand G., Dalle S. GLP-1 mediates antiapoptotic effect by phosphorylating Bad through a β-arrestin 1-mediated ERK1/2 activation in pancreatic β-cells. J. Biol. Chem. (2010);285:1989–2002. doi: 10.1074/jbc.M109.067207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doyle M., Egan J. M. Mechanisms of action of GLP-1 in the pancreas. Pharmacol. Ther. (2007);113:546–593. doi: 10.1016/j.pharmthera.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jhala U. S., Canettieri G., Screaton R. A., Kulkarni R. N., Krajewski S., Reed J., Walker J., Lin X., White M., Montminy M. cAMP promotes pancreatic beta-cell survival via CREB-mediated induction of IRS2. Genes Dev. (2003);17:1575–1580. doi: 10.1101/gad.1097103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kodama S., Toyonaga T., Kondo T., Matsumoto K., Tsuruzoe K., Kawashima J., Goto H., Kume K., Kume S., Sakakida M., Araki E. Enhanced expression of PDX-1 and Ngn3 by exendin-4 during β cell regeneration in STZ-treated mice. Biochem. Biophysic. Res. Comm. (2005);327:1190–1196. doi: 10.1016/j.bbrc.2004.12.120. [DOI] [PubMed] [Google Scholar]
- 7.Zhou J., Chu J., Wang Y. H., Wang H., Zhuang Y. P., Zhang S. L. Purification and bioactivity of exendin-4, a peptide analogue of GLP-1, expressed in pichia pastoris. Biotechnol. Lett. (2008);30:651–656. doi: 10.1007/s10529-007-9610-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao Z., Bai G., Chen J., Zhang Q., Pan P., Bai F., Geng P. Development, characterization, and evaluation of a fusion protein of a novel glucagon-like peptide-1 (GLP-1) analog and human serum albumin in pichia pastoris. Biosci. Biotechnol. Biochem. (2009);73:688–694. doi: 10.1271/bbb.80742. [DOI] [PubMed] [Google Scholar]
- 9.Oh J. Y., Lee C. W., Jang S. H., Yoo S. B., Chung H. S. Novel DPP-IV-resistant analogs of GLP-1: The N-terminal extension of GLP-1 by a single amino acid. Bull. Korean Chem. Soc. (2009);30:2471–2474. doi: 10.5012/bkcs.2009.30.10.2471. [DOI] [Google Scholar]
- 10.Garber A. J. Long-acting glucagon-like peptide 1receptor agonists. Diabetes Care. (2011);34:S279–S284. doi: 10.2337/dc11-s231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu B., Dong Q., Wang M., Shi L., Wu Y., Yu X., Shi Y., Shan Y., Jiang C., Zhang X., Gu T., Chen Y., Kong W. Preparation, characterization, and pharmacodynamics of exenatide-loaded poly (DL-lactic-co-glycolic acid) microspheres. Chem. Pharm. Bull. (2010);58:1474–1479. doi: 10.1248/cpb.58.1474. [DOI] [PubMed] [Google Scholar]
- 12.Baggio L. L., Huang Q., Brown T. J., Drucker D. J. A recombinant human glucagon-like peptide (GLP)-1-albumin protein (Albugon) mimics peptidergic activation of GLP-1 receptor-dependent pathways coupled with satiety, gastrointestinal motility, and glucose homeostasis. Diabetes. (2004);53:2492–2500. doi: 10.2337/diabetes.53.9.2492. [DOI] [PubMed] [Google Scholar]
- 13.Huang Y. S., Chen Z., Chen Y. Q., Ma G. C., Shan J. F., Liu W., Zhou L. F. Preparation and characterization of a novel exendin-4 human serum albumin fusion protein expressed in Pichia pastoris. J. Pept. Sci. (2008);14:588–595. doi: 10.1002/psc.942. [DOI] [PubMed] [Google Scholar]
- 14.Kim B. J., Zhou J., Martin B., Carlson O. D., Maudsley S., Greig N. H., Mattson M. P., Ladenheim E. E., Wustner J., Turner A., Sadeghi H., Egan J. M. Transferrin fusion technology: A novel approach to prolonging biological half-life of insulinotropic peptides. J. Pharm. Exp. Ther. (2010);334:682–692. doi: 10.1124/jpet.110.166470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Q, Chen K., Liu R., Zhao F., Gupta S., Zhang N., Prud’homme G. J. Novel GLP-1 fusion chimera as potent long acting GLP-1 receptor agonist. PLoS One. (2010);5:e12734 1–9. doi: 10.1371/annotation/bc95caf3-62cf-4ecd-8a79-9116e62f4a50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bak E. J., Park H. G., Lee C. H., Lee T. I., Woo G. H., Na Y. W., Yoo Y. J., Cha J. H. Effects of novel chalcone derivatives on α-glucoside, dipeptidyl peptidase-4, and adipocyte differentiation in vitro. BMB Rep. (2011);44:410–414. doi: 10.5483/BMBRep.2011.44.6.410. [DOI] [PubMed] [Google Scholar]
- 17.Neumiller J. J. Differential chemistry (structure), mechanism of action, and pharmacology of GLP-1 receptor agonists and DPP-4 inhibitors. J. American Pharm. Associ. (2009);49:S16–S29. doi: 10.1331/JAPhA.2009.09078. [DOI] [PubMed] [Google Scholar]
- 18.Yeom J. A., Kim S. E., Park H. S., Ham D. S., Sun C. L, Kim J. W., Cho J. H., Yoon K. H. Both sitagliptin analogue & pioglitazone preserve the β-cell proportion in the islets with different mechanism in non-obese and obese diabetic mice. BMB Rep. (2011);44(11):713–718. doi: 10.5483/BMBRep.2011.44.11.713. [DOI] [PubMed] [Google Scholar]
- 19.Chung H. S., Oh J. Y., Yoo S. B., Lee S. M., Cho H. S. The N-terminal alanine-extended GLP-1/IgG-Fc fusion protein confers resistance to DPP-IV and reduces serum glucose level in db/db mice. Regul. Pept. (2011);170:1–3. doi: 10.1016/j.regpep.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Son Y. K., Hong D. H., Kim D. J., Firth A. L., Park W. S. Direct effect of protein kinase C inhibitors on cardiovascular in ion channels. BMB Rep. (2011);44:559–565. doi: 10.5483/BMBRep.2011.44.9.559. [DOI] [PubMed] [Google Scholar]


