Abstract
The progression of androgenetic alopecia is closely related to androgen-inducible transforming growth factor (TGF)-β1 secretion by hair follicle dermal papilla cells (DPCs) in bald scalp. Physiological levels of androgen exposure were reported to increase reactive oxygen species (ROS) generation. In this study, rat vibrissae dermal papilla cells (DP-6) transfected with androgen receptor showed increased ROS production following androgen treatment. We confirmed that TGF-β1 secretion is increased by androgen treatment in DP-6, whereas androgeninducible TGF-β1 was significantly suppressed by the ROSscavenger, N-acetyl cysteine. Therefore, we suggest that induction of TGF-β1 by androgen is mediated by ROS in hair follicle DPCs. [BMB Reports 2013; 46(9): 460-464]
Keywords: Androgenetic alopecia, Androgen receptor, Dermal papilla, Reactive oxygen species, Transforming growth factors
INTRODUCTION
Androgenetic alopecia (AGA) is characterized by androgendependent progressive thinning and balding of scalp hair that follows a defined pattern in genetically predisposed subjects (1,2). Moreover, androgen affects hair growth (3), and the balding scalps exhibit higher levels of 5-alpha reductase and elevated androgen receptor (AR) expression in dermal papilla cells (DPCs) of hair follicles compared to normal hair-bearing scalps (4-6). In human hair follicles, it is generally accepted that androgen acts via the dermal papilla. Androgen regulates factors derived from DPCs, which are believed to influence the growth of hair follicle components (7-10). The role of androgens in hair growth regulation was previously reported in an in vitro co-culture system of human DPCs from AGA patients and keratinocytes (KC) (11). The study revealed that androgen-inducible transforming growth factor (TGF)-β1 secretion from balding DPCs inhibits epithelial cell growth.
Reactive oxygen species (ROS) are known to be profoundly increased in aged cells. In addition, high ROS levels, induced by oxidative stress, promote damage of cellular DNA, proteins, and lipids. Such damage can lead to cell-cycle arrest, cellular senescence, and even cellular death (12). Interestingly, the ROS hydrogen peroxide has been reported to induce TGF-β in epithelial cells (13). In addition, it was revealed that physiological levels of androgen exposure resulted in increased levels of ROS generation in androgen-sensitive human prostate cancer cells (14).
We postulated that androgen may play a significant role in regulating the ROS balance in DPCs, which, in turn, would influence the secretion of TGF-β1 and inhibit the proliferation of hair matrix epithelial cells. The aim of this study was to clarify the role of ROS in androgen-inducible TGF-β1 expression in DPCs. We investigated the effects of androgen on ROS production and on TGF-β1 secretion in DPCs, and then examined whether an antioxidant treatment could reverse the effects of ROS on androgen-induced TGF-β1 secretion by these cells.
RESULTS
Establishment of AR over-expressing rat DPCs (DP6-AR cells)
We transfected DP6 cells with rat AR cloning retroviral vector (p-Babe-rat AR-HA-puro). Western blot analysis revealed that AR was expressed on the DP6-AR cells, but not the control cells transfected with the puro vector only (data not shown).
Androgen markedly increased ROS generation by the DP6-AR cells
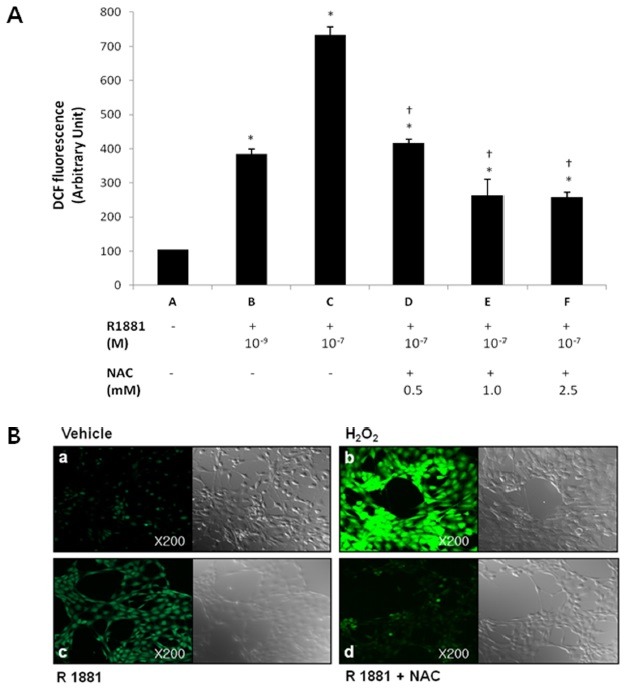
DP6-AR cells were incubated with 10-9 and 10-7 M R1881, and ROS generation was quantitatively measured by flow cytometry. ROS was generated by R1881 (Fig. 1A). Moreover, exposure to 10-9-10-7 M of dihydrotestosterone (DHT) also increased ROS generation (data not shown). The results indicate that the DP6-AR cells generated ROS in response to androgen. We also demonstrated that the androgen-induced ROS generation was suppressed by the ROS scavenger NAC (Fig. 1A). Laser scanning confocal microscopy revealed that exposure to 10-7 M R1881 produced a significant increase in ROS in DP6-AR cells; the effect was similar to that of H2O2 and was inhibited by NAC (Fig. 1B).
Fig. 1. Intracellular ROS production by DP6-AR cells stimulated with R1881, but then reduced following pre-treatment with NAC. (A) The amounts of ROS induced by R1881 were determined by FACS in DP6-AR cells (lane B, C). Pre-treatment with NAC at doses ranging from 0.5 mM to 2.5 mM for 2 h modulated ROS expression in response to 10-7 M R1881 in cultured DP6-AR cells (lane D, E, F). All results are the mean values of experiments performed in triplicate. Error bars indicate standard errors. ANOVA was performed at first and independent t-test was done for post hoc. *P < 0.05 versus control cells transfected with puro (mock) vector only (lane A). †P < 0.05 versus treatment with 10-7 M R1881 (lane C). ROS, reactive oxygen species; NAC, N-acetyl cysteine, a ROS scavenger; R1881, methyltrienolone, a synthetic androgen. (B) (a) Vehicle treatment. (negative control) (b) Treatment with 100 μM H2O2 (positive control) (c) Exposure of DP6-AR cells to 10-7 M R1881. (d) R1881 exposure after pre-treatment with NAC for 2 h.
Androgen-inducible ROS augmented TGF-b1 secretion from DPCs
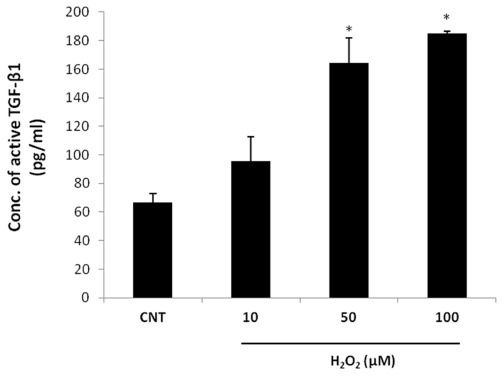
ROS has been reported to induce TGF-β in epithelial cells (13) and TGF-β1 is known to inhibit the proliferation of hair epithelial cells (11). To investigate the effects of oxidative stress, DP6-AR cells were treated with 10, 50, or 100 μM H2O2. The H2O2-treated DP6-AR cells showed 2-3-fold dose- dependent increases in the secretion of active TGF-β1 compared to the untreated cells (Fig. 2).
Fig. 2. Analysis of active TGF-β1 levels by ELISA after treatment with H2O2. The DP6-AR (5.0 × 105 cells/dish) cells were treated with H2O2 at 10, 50, or 100 μM for 24 h and the conditioned media were harvested. Concentrations of the active form of TGF-β1 in the conditioned media were measured using a specific ELISA. All results are the mean values of experiments performed in triplicate. Error bars indicate standard errors. ANOVA was performed at first and independent t-test was done for post hoc. *P < 0.05 versus control. CNT, control; TGF-β1, transforming growth factor-β1.
Androgen-induced TGF-β1 secretion was reversed by a ROS scavenger
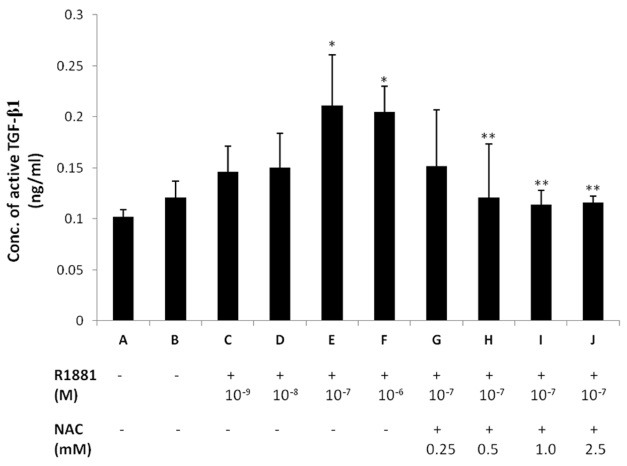
Next, we measured the levels of active TGF-β1 in conditioned media after treating the cells with various concentrations of R1881 for 48 h. We found that the levels of active TGF-β1 dose-dependently increased at R1881 concentrations of 10-9-10-7 M (Fig. 3). To determine whether ROS scavengers have suppressive effects on androgen-induced TGF-β1 regulation, we analyzed the effects of NAC on androgen-induced TGF-β1 secretion. Pre-treatment of DP6-AR cells with NAC at 0.25-2.5 mM for 2 h reduced the active TGF-β1 secretion when R1881 was added at 10-7 M compared to the control that was treated with only 10-7 M R1881 (Fig. 3). This result demonstrates that the ROS scavenger reversed the androgen-induced TGF-β1 secretion by the DPCs. These observations indicate that ROS is involved in the increased secretion of TGF-β1 from DPCs induced by androgen.
Fig. 3. Androgen-inducible ROS augmented the TGF-β1 secretion by dermal papilla cells. Increased active TGF-β1 secretions were observed after treatment with various concentrations of R1881 for 48 h (lane C, D, E, F). Comparatively, when the cells were initially pre-treated with NAC (0.25-2.5 mM) for 2 h, the active TGF-β1 secretions were reduced (lane G, H, I, J). Lane A represents the control cells transfected with the mock vector only. All results are the mean values of experiments performed in triplicate. Error bars indicate the standard errors. ANOVA was performed at first and independent t-test was done for post hoc. *P < 0.05 versus untreated DP6-AR cells (lane B). **P < 0.05 versus the control which was treated with only R1881 (lane E).
NAC suppressed the androgen-induced TGF-β1 secretion by the DPCs
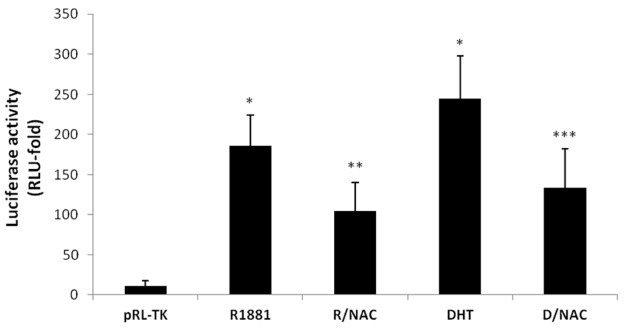
We performed promoter assays using the luciferase reporter gene to investigate the suppression of TGF-β1 by a ROS scavenger at the transcriptional level. DP6-AR cells co-cultured in the presence of 10-7 M R1881 or 10-9 M DHT stimulated the TGF-β1 promoter. Comparatively, luciferase reporter assays demonstrated that TGF-β1 promoter signaling was transcriptionally suppressed after the addition of 2.5 mM NAC in the presence of R1881 or DHT (Fig. 4). This observation suggests that the secretion of androgen-induced TGF-β1 in the DP6-AR cells is suppressed at the transcriptional level by pre-treatment with NAC. These results clearly demonstrate that antioxidants are able to block androgen-inducible TGF-β1 secretion from DPCs.
Fig. 4. Luciferase assays analyzing the TGF-β1 promoter levels after treatment with androgen and NAC. The DP6-AR cells were transiently transfected with the pGL3-TGF-β1-Luc reporter plasmid and the pRL-TK vector. First lane, pRL-TK vector only as a negative control; 2nd lane, 10-7 M R1881; 3rd lane, 10-7 M R1881 + 2.5 mM NAC; 4th lane, 1 nM DHT; 5th lane, 1 nM DHT + 2.5 mM NAC. At 48 h after treatment, the cells were harvested for the luciferase assays. The luciferase activities were measured using a luminometer. All results are the mean values of experiments performed in triplicate. Error bars indicate the standard errors. When the first, the second, and the third lane were compared, ANOVA was performed at first and independent t-test was done for post hoc. In comparison with the 2nd and the 3rd lane, or with the 4th and the 5th lane, independent t-test was done. *P < 0.05 versus a negative control. **P < 0.05 versus the control that was treated with only R1881. ***P < 0.05 versus the control that was treated with only DHT.
DISCUSSION
Balding scalp DPCs secret inhibitory factors that affect hair growth (15). In particular, the inhibitory role of androgens in keratinocyte growth was confirmed by Inui et al. (11). In addition, it has been reported that androgen-inducible TGF-β1 from balding DPCs is an inhibitory paracrine mediator in AGA (11,16). Thus, it has been well established that the progression of AGA is associated with androgen and TGF-β1 levels.
Studies by Ripple et al. (14) showed that androgens are capable of enhancing oxidative stress in androgen responsive LNCaP prostate carcinoma cells. Thus, it appears that androgens may play a significant role in regulating the cellular redox state, particularly with respect to ROS homeostasis in epithelial cells. In addition, ROS has been reported to induce TGF-β in epithelial cells (13), and therefore, androgens are involved in ROS generation, and ROS is related to TGF-β secretion. Moreover, these factors are involved in AGA. Consequently, we examined whether androgens are capable of increasing ROS production in dermal papilla cell lines, and if so, whether such mediation plays a role in AGA development.
During sub-cultivation of DPCs, sensitivity to androgens may be low because of the reduced expression level of AR (11). AR has been detected in the DPCs of human skin (5), and the DPCs of bald frontal scalps express higher levels of AR than those of non-balding occipital scalps (4). Therefore, to confirm our hypothesis, we used rat DPCs that over-express AR.
The ELISA and promoter assay showed that ROS induced TGF-β1 secretion in the DPCs, and that NAC inhibits androgen-induced TGF-β1 secretion at the transcriptional level (Fig. 2 and 4). Ascorbic acid, which is a well-known antioxidant, has been shown to stimulate DP cell growth and promote hair shaft elongation in vitro and in vivo in animal experiments (17). Moreover, green tea epigallocatechin-3-gallate, which is known to have potent antioxidant effects, was reported to promote hair growth (18). These findings, in conjunction with the present study illustrate the beneficial effects of antioxidants on hair growth, and offer a mechanistic basis for these effects.
In conclusion, the present study demonstrates that androgeninduced TGF-β1 regulation is mediated by ROS, and can be prevented by antioxidants in hair follicle DPCs. Moreover, our findings suggest the potential use of antioxidant therapy for the treatment of androgenetic alopecia.
MATERIALS AND METHODS
ROS generation assay and confocal microscopy
Intracellular H2O2 and hydroxyl radical levels were determined by measuring 2',7'-dichlorofluorescein diacetate (DCFDA; Molecular Probes, Eugene, OR) fluorescence. Non-fluorescent DCFDA is hydrolyzed to yield highly fluorescent dichlorofluorescein (DCF) in the presence of intracellular H2O2 and related peroxides.
After treatment with 10-9 and 10-7 M of the synthetic androgen, methyltrienolone (R1881; Sigma, St. Louis, MO), for 24 h, 1.0 × 106 DP6-AR cells were harvested, washed twice with PBS, resuspended in serum-free medium, and incubated with 10 μM DCF-DA in Hanks balanced salt solution (HBSS) at 37℃ for 30 min. They were then washed with ice-cold 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)/saline and placed on ice. Cellular fluorescence was measured by flow cytometry using a FACS Calibur flow cytometer (BD Biosciences, Mississanga, ON, Canada). As a positive control, 1.0 × 106 DP6-AR cells were treated with 100 μM H2O2 and then processed.
The cells were imaged using a Zeiss LSM 510 META confocal microscope (200×; Carl Zeiss MicroImaging, Inc, Thornwood, NY). The green fluorescence of DCF was excited at 488 nm using an argon laser and detected at 527 nm. Laser attenuation, pinhole diameter, and photomultiplier sensitivity and offset were maintained for all experiments. For imaging, glass coverslips were mounted in a recording chamber filled with HBSS.
TGF-β1 determination by ELISA
The DP6-AR (5.0 × 105 cells/dish) were treated with 10, 50, or 100 μM H2O2 for 24 h, and the conditioned media were harvested. After pre-incubation with N-acetyl cysteine (NAC) for 2 h, 1.0 × 105 DP6-AR cells/6-well plates were treated with R1881 (10-9, 10-8, 10-7, and 10-6 M) for 48 h, and the conditioned media were harvested. The concentration of TGF-β1 in the conditioned media was measured using ELISA, according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN), as previously described (19).
Others
Chemicals and reagents, as well as the protocols for the plasmid construction, cell culture, transfection, western blot, reporter gene assays, and statistical analyses are described in the supplementary materials and methods.
Acknowledgments
We thank Wendy Filsell (Unilever Research, Colworth House, Sharnbrook, Bedford MK44 1LQ, U.K.) for the kind gift of the rat vibrissa dermal papilla cell line (DPLTtsa 6) and Dr. Chawnshang Chang at the University of Rochester (USA) for his kind gift of the rat AR gene plasmid (pSG5-AR). This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (Grant No. A103017) and partially by a research agreement with AmorePacific Corporation, Republic of Korea.
References
- 1.Haber R. Pathogenesis and medical therapy of male and female pattern hair loss; in Hair transplantation, Haber, R. and Stough, D. (eds.) Elselvier Saunders; Philadelphia, USA: (2010). pp. 1–7. [Google Scholar]
- 2.Trueb R. M. Molecular mechanisms of androgenetic alopecia. Exp. Gerontol. (2002);37:981–990. doi: 10.1016/S0531-5565(02)00093-1. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman K. D. Androgen metabolism as it affects hair growth in androgenetic alopecia. Dermatol. Clin. (1996);14:697–711. doi: 10.1016/S0733-8635(05)70396-X. [DOI] [PubMed] [Google Scholar]
- 4.Hibberts N. A., Howell A. E., Randall V. A. Balding hair follicle dermal papilla cells contain higher levels of androgen receptors than those from non-balding scalp. J. Endocrinol. (1998);156:59–65. doi: 10.1677/joe.0.1560059. [DOI] [PubMed] [Google Scholar]
- 5.Randall V. A., Thornton M. J., Messenger A. G. Cultured dermal papilla cells from androgen-dependent human hair follicles (e.g. beard) contain more androgen receptors than those from non-balding areas of scalp. J. Endocrinol. (1992);133:141–147. doi: 10.1677/joe.0.1330141. [DOI] [PubMed] [Google Scholar]
- 6.Sawaya M. E., Price V. H. Different levels of 5alpha-reductase type I and II, aromatase, and androgen receptor in hair follicles of women and men with androgenetic alopecia. J. Invest. Dermatol. (1997);109:296–300. doi: 10.1111/1523-1747.ep12335779. [DOI] [PubMed] [Google Scholar]
- 7.Hamada K., Thornton M. J., Laing I., Messenger A. G., Randall V. A. The metabolism of testosterone by dermal papilla cells cultured from human pubic and axillary hair follicles concurs with hair growth in 5 alpha-reductase deficiency. J. Invest. Dermatol. (1996);106:1017–1022. doi: 10.1111/1523-1747.ep12338582. [DOI] [PubMed] [Google Scholar]
- 8.Obana N., Chang C. S., Uno H. Inhibition of hair growth by testosterone in the presence of dermal papilla cells from the frontal bald scalp of the postpubertal stumptailed macaque. Endocrinology. (1997);138:356–361. doi: 10.1210/endo.138.1.4890. [DOI] [PubMed] [Google Scholar]
- 9.Pan H. J., Uno H., Inui S., Fulmer N. O., Chang C. S. Roles of testosterone in the growth of keratinocytes through bald frontal dermal papilla cells. Endocrine. (1999);11:321–327. doi: 10.1385/ENDO:11:3:321. [DOI] [PubMed] [Google Scholar]
- 10.Won C. H., Kwon O. S., Kang Y. J., Yoo H. G., Lee D. H., Chung J. H., Kim K. H., Park W. S., Park N. H., Cho K., Kwon S. O., Choi J. S., Eun H. C. Comparative secretome analysis of human follicular dermal papilla cells and fibroblasts using shotgun proteomics. BMB Rep. (2012);45:253–258. doi: 10.5483/BMBRep.2012.45.4.253. [DOI] [PubMed] [Google Scholar]
- 11.Inui S., Fukuzato Y., Nakajima T., Yoshikawa K., Itami S. Androgen-inducible TGF-beta1 from balding dermal papilla cells inhibits epithelial cell growth: a clue to understand paradoxical effects of androgen on human hair growth. FASEB J. (2002);16:1967–1969. doi: 10.1096/fj.02-0043fje. [DOI] [PubMed] [Google Scholar]
- 12.Hogg N. Free radicals in disease. Semin. Reprod. Endocrinol. (1998);16:241–248. doi: 10.1055/s-2007-1016284. [DOI] [PubMed] [Google Scholar]
- 13.Park S. K., Kim J., Seomun Y., Choi J., Kim D. H., Han I. O., Lee E. H., Chung S. K., Joo C. K. Hydrogen peroxide is a novel inducer of connective tissue growth factor. Biochem. Biophys. Res. Commun. (2001);284:966–971. doi: 10.1006/bbrc.2001.5058. [DOI] [PubMed] [Google Scholar]
- 14.Ripple M. O., Henry W. F., Rago R. P., Wilding G. Prooxidant-antioxidant shift induced by androgen treatment of human prostate carcinoma cells. J. Natl. Cancer Inst. (1997);89:40–48. doi: 10.1093/jnci/89.1.40. [DOI] [PubMed] [Google Scholar]
- 15.Hamada K., Randall V. A. Inhibitory autocrine factors produced by the mesenchyme-derived hair follicle dermal papilla may be a key to male pattern baldness. Br. J. Dermatol. (2006);154:609–618. doi: 10.1111/j.1365-2133.2006.07144.x. [DOI] [PubMed] [Google Scholar]
- 16.Foitzik K., Lindner G., Mueller-Roever S., Maurer M., Botchkareva N., Botchkarev V., Handjiski B., Metz M., Hibino T., Soma T., Dotto G. P., Paus R. Control of murine hair follicle regression (catagen) by TGF-beta1 in vivo. FASEB J. (2000);14:752–760. doi: 10.1096/fasebj.14.5.752. [DOI] [PubMed] [Google Scholar]
- 17.Sung Y. K., Hwang S. Y., Cha S. Y., Kim S. R., Park S. Y., Kim M. K., Kim J. C. The hair growth promoting effect of ascorbic acid 2-phosphate, a long-acting Vitamin C derivative. J. Dermatol. Sci. (2006);41:150–152. doi: 10.1016/j.jdermsci.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Kwon O. S., Han J. H., Yoo H. G., Chung J. H., Cho K. H., Eun H. C., Kim K. H. Human hair growth enhancement in vitro by green tea epigallocatechin-3-gallate (EGCG). Phytomedicine. (2007);14:551–555. doi: 10.1016/j.phymed.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Yoo H. G., Kim J. S., Lee S. R., Pyo H. K., Moon H. I., Lee J. H., Kwon O. S., Chung J. H., Kim K. H., Eun H. C., Cho K. H. Perifollicular fibrosis: pathogenetic role in androgenetic alopecia. Biol. Pharm. Bull. (2006);29:1246–1250. doi: 10.1248/bpb.29.1246. [DOI] [PubMed] [Google Scholar]


